Aging is a complex multifactorial process, meaning that multiple pathways need to be targeted to effectively prevent or slow aging [1]. A number of molecular pathways are well known for influencing aging, but only a few have been successfully targeted with individual drugs, and these drugs do not individually target all aging pathways. However, combinations of these drugs might have the potential of effectively broadening the scope of aging targets. There are a number of drug combinations that could be combined based on different but overlapping pharmacological activities. Since the number one criterion for selecting drugs should be based on known anti-aging effects, for example, in preclinical mouse studies, the number of drugs available to consider is markedly reduced. Three drugs with well-validated anti-aging effects in laboratory animals, rapamycin [2,3], acarbose [4], and SS31 [5,6], are well suited to therapeutic multiplexing as a way to enhance healthy aging and stop the development of lesions associated with aging and physiological dysfunction based on interactive cellular mechanisms of each drug.
The inter-drug relationship of these three drugs can easily be perceived by explicitly defining the mechanism of action of each drug and how it overlaps and extends the mechanism of action of each of the other drugs in the complex. A plausible explanation then of how they could act as a multiplex in targeting molecular pathways in aged mice is as follows:
Acarbose given orally blocks intestinal alpha glucosidase so that carbohydrates are not broken down and absorbed. This results in lower blood glucose levels and prevents postprandial insulin spikes. The lower blood glucose and decreased need for insulin activate adenosine monophosphate-activated protein kinase, which tends to block mtorc1, the drug target for rapamycin. The lowered blood glucose provides less available substrate for mitochondrial metabolism thereby sensitizing mitochondria to increased electron transport chain (ETC) efficiency induced by SS31-bound cardiolipin. The decreased need for insulin helps alleviate insulin resistance induced by rapamycin-suppressed mtorc2.
Rapamycin blocks mtorc1 resulting in suppression of protein synthesis, suppression of mitochondrial metabolism, and activation of autophagy. The suppressed mitochondrial metabolic activity enhances the binding of SS31 peptide to cardiolipin thereby increasing ETC efficiency for vital ATP production, and decreases the generation of mitochondrial reactive oxygen species thought to play a role in progressive aging and age-related diseases. The suppression of protein synthesis helps conserve valuable cellular resources. Activation of autophagy helps eliminate dysfunctional and senescent cells thereby preventing additional cellular burden.
SS31 peptide binds to the phospholipase cardiolipin exclusively at the inner mitochondrial membrane [7]. This binding increases the hydrophobic interaction between cardiolipin and cytochrome c, thereby enhancing the function of cytochrome c as an electron carrier from complex III to complex IV on the ETC. This also results in a decrease in the peroxidase activities of cytochrome c. Thus, cardiolipin serves as a base for SS31 to optimize oxidative phosphorylation. Activation of autophagy helps eliminate dysfunctional and senescent cells thereby preventing additional cellular burden. The decreased substrate conditions triggered by acarbose and the downregulation of mitochondrial proteasome by rapamycin provide a highly sensitive environment in the mitochondria for the efficiency-enhancing effects of SS31 peptide.
The concept of drug multiplexing to slow aging looks good on paper, but drug combinations have yet to be tested in any meaningful way. Historically, testing single drugs in mouse lifespan studies has provided useful information, but it is costly and time consuming. More importantly, lifespan studies are difficult to recapitulate in humans, making translation of the preclinical information challenging. And especially relevant is the fact that lifespan studies in mice are not well-suited to testing drug combinations that could more effectively target multiple factors involved in aging. Thus, new paradigms for testing therapeutics aimed at slowing aging are needed. The Geropathology Grading Platform (GGP) was developed by the Geropathology Research Network to provide a grading system for lesions associated with aging [8]. One of the advantages of the GGP as a drug testing paradigm is that middle-age mice can be treated for as little as two months and see differences in lesion scores. For example, the platform was used to compare lesion grades in 26-month-old C57BL/6 mice treated with rapamycin for two months, starting at 24 months. Rapamycin-treated mice had significantly lower lesion scores compared to placebo treated mice [9].
The GGP measures biological aging aligned with mouse lifespan studies and physiological findings, and helps provide predictive power for antiaging effects that drug combinations might have in clinical trials. Thus, the GGP would be a critical tool in preclinical studies to determine if drug combinations slow aging (Figure 1). However, just like measuring lifespan in mice, the GGP is not something directly used in people because autopsies are not commonly done in clinical trials. Therefore, aging biomarkers are needed to determine if fundamental mechanisms of aging are being targeted. Surrogate aging biomarkers offer the potential to predict a subject’s outcome such as improved function, extended survival, or arrest of age-related disease. Preliminary observations suggest that proteins in the serum or peripheral blood cells correlate with the GGP [10]. The focus is on blood and serum, because these are readily available from patients and can be serially sampled with minimal risk. The translational impact emphasizes the importance of molecular markers as clinical end points suggesting the feasibility of identifying serum peptides and other molecular end points that reflect biological rather than chronological age.
Figure 1.
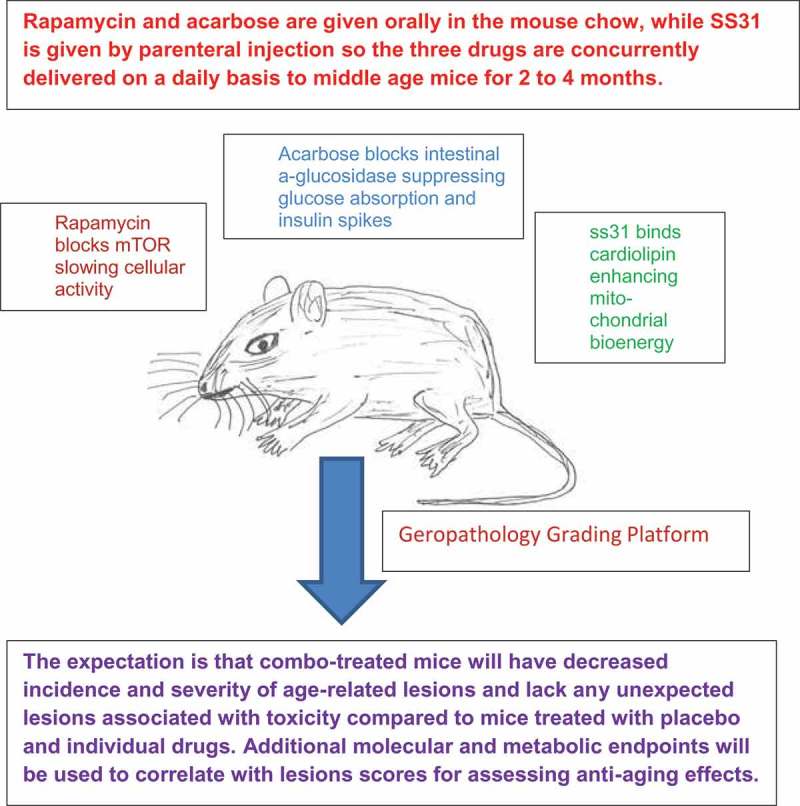
Slowing aging might best be achieved by drug combinations that target multiple interactive molecular pathways. However, very little testing of drug combinations has been done because of a lack of preclinical screening paradigms. This figure demonstrates how the interaction of a prototype drug combination, rapamycin, acarbose, and SS31, can be tested for anti-aging effects in a preclinical setting using the aged mouse and a geropathology grading system.
While the future for expanded use of drug combinations in treating various diseases and conditions, including aging, is highly promising the path toward eventual regulatory approval can be challenging and should be considered in any preclinical studies undertaken. The potential beneficial functional synergy gained from the logical and judicious use of rational drug combinations, such as rapamycin, acarbose and SS31, is obviously complicated by the fact that different drugs with different metabolic, pharmacokinetic, and toxicity profiles are being superimposed on top of one another. This may be of no consequence, but on the other hand could result in combinatorial enhancement of negative outcomes due to such things as imposition of conflicting metabolic pathways, altered absorption profiles, or additive toxicities. To address these concerns, the Food and Drug Administration provides a guidance document ‘Co-development of two or more new investigational drugs for use in combination’ [11]. This document provides an understanding of outcomes that investigators should be aware of during the preclinical development process. Focusing not just on the benefits of combination products but also the potential liabilities early on can speed the development process. It is thus reassuring that the GGP provides an informative system that enhances the combination drug development process by empowering concurrent assessment of both positive and negative effects.
In summary, the concept of drug multiplexing as a powerful platform to slow aging is promising but has not yet entered the mainstream of aging research. The combination of rapamycin, acarbose, and ss31 peptide, three drugs with individually documented anti-aging effects, is a logical approach designed to complement mechanisms of action of their molecular targets and robustly enhance a delay of aging and age-related disease not seen with mono-therapeutic approaches. Support for the preclinical investigation of this drug combination as well as other drug combinations is urgently needed to determine dosages, frequency of administration, and criteria for when to start administering the drugs, i.e. focus on treatment at older ages, or prevention at younger ages. These parameters are essential in correlating translational molecular end points from mouse to humans for drug testing in clinical trials.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Ladiges W. The quality control theory of aging. Pathobiol Aging Age Relat Dis. 2014;4 PubMed PMID: 24891937; PubMed Central PMCID: PMC4033319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012. August;11(4):675–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Flynn JM, O’Leary MN, Zambataro CA, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013. October;12(5):851–862. Epub 2013 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014. April;13(2):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Dai DF, Chen T, Szeto H, et al. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011. June 28;58(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014. April;171(8):2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Szeto HH, Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther. 2014. December;96(6):672–683. Epub 2014 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Ladiges W, Ikeno Y, Niedernhofer L, et al. The geropathology research network: an interdisciplinary approach for integrating pathology into research on aging. J Gerontol A Biol Sci Med Sci. 2016. April 4;71:431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Ladiges W, Snyder JM, Wilkinson E, et al. A new preclinical paradigm for testing anti-aging therapeutics. J Gerontol A Biol Sci Med Sci. 2017. June 1;72(6):760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Niedernhofer LJ, Kirkland JL, Ladiges W. Molecular pathology endpoints useful for aging studies. Ageing Res Rev. 2017;35:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research Codevelopment of Two or More New Investigational Drugs for Use in Combination; 2013. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM236669.pdf [Google Scholar]


