Abstract
Objectives
We aimed to investigate the prevalence of dyslipidemia in patients with osteoarthritis (OA) and whether OA and dyslipidemia are associated.
Methods
We performed a systematic literature review and a meta-analysis, including cross-sectional, cohort and case–control studies, to assess the number of patients with OA and/or dyslipidemia. We calculated the mean (±SD) prevalence of dyslipidemia in patients with and without OA and the risk of dyslipidemia (OR, 95% CI) among patients with OA.
Results
From 605 articles screened, 48 were included in the analysis (describing 29 cross-sectional, 10 cohort and 9 case–control studies). The mean prevalence of dyslipidemia was 30.2%±0.6% among 14 843 patients with OA and 8.0%±0.1% among 196 168 without OA. The risk of dyslipidemia was greater with than without OA overall (OR 1.98,95% CI 1.43 to 2.75, p<0.0001) and with knee OA (OR 2.27, 1.33 to 3.89, p=0.003) and hand OA (OR 2.12, 1.46 to 3.07), p<0.0001).
Conclusion
The risk of dyslipidemia was twofold greater with than without OA, so lipid disturbances could be a risk factor for OA. Such a result supports the individualisation of the metabolic syndrome-associated OA phenotype.
Keywords: Osteoarthritis, Dyslipidemia, Cholesterol, Metabolic Syndrome, Meta-analysis
Key messages.
What is already known about this subject?
Metabolic disturbances such as obesity or diabetes mellitus are associated with osteoarthritis (OA), but data about the link between OA and lipid disturbances remain conflicting.
What does this study add?
This is the first systematic review and meta-analysis demonstrating an association between OA and dyslipidemia. This result reinforces the concept of the metabolic syndrome-associated OA phenotype.
How might this impact on clinical practice?
This study emphasises the need to screen and manage cardiovascular comorbidities, especially lipid disturbances in patients with OA in clinical daily practice.
Introduction
Osteoarthritis (OA) is the most common joint disease and a major cause of pain and disability. It is currently considered a disease with multiple distinguishable phenotypes: post-traumatic, ageing-related, genetic and metabolic syndrome (MetS)-associated OA.1 Metabolic OA, the most commonly studied phenotype, is defined by the association between OA and MetS, associating obesity, hyperglycaemia with insulin resistance, dyslipidemia and hypertension.2 Metabolic OA mainly affects middle-aged people (45–65 years) and leads to knee, hand and generalised OA. The association between OA and MetS has been reported in several epidemiological studies.3 4 The pathophysiological link between both diseases could be chronic low-grade systemic inflammation occurring in both conditions.5
The association of OA with each MetS component has been investigated.6 Obesity and overweight are independently linked to hand OA, with a twofold increased risk.7 This association suggests the release of inflammatory mediators by adipose tissue adipokines. We recently reported an association between OA and diabetes mellitus, with a 1.46-fold increased risk of OA with diabetes mellitus and a 1.41-fold increased risk of diabetes mellitus with OA.8 The link between both pathologies could be explained by the action of pro-inflammatory cytokines and oxidative stress occurring in both diseases.9–12
The link between OA and the other components of MetS remains debated. Experimental studies have suggested that lipid disturbances could be involved in OA pathophysiology,13 but epidemiological studies revealed heterogeneous results.
With a systematic literature review and meta-analysis, we aimed to investigate the prevalence of dyslipidemia in patients with OA and assess whether OA and dyslipidemia are associated.
Methods
The systematic review was registered on PROSPERO (CRD: 42016037290).
Literature search
We performed a systematic search of articles in MEDLINE via PubMed, EMBASE and the Cochrane library. The keywords used for the PubMed search were (((‘Dyslipidemias’[Mesh] OR ‘Hypertriglyceridemia’[Mesh]) OR ‘Hypercholesterolemia’[Mesh]) OR ‘HDL’[All Fields] OR ‘LDL’[All Fields] OR ‘Triglycerides’[All Fields] OR ‘Hyperlipidemias’[Mesh]) OR ‘Cholesterol’[Mesh] OR ‘Metabolic Syndrome X’[Mesh] AND ‘Osteoarthritis’[Mesh] AND (‘humans’[MeSH Terms] AND (English[lang] OR French[lang])). No time limit was set for publication date, and articles published up to 1 January 2016 were searched. We also searched the abstracts from international meetings of the American College of Rheumatology (ACR), European League Against Rheumatism, Société Française de Rhumatologie, European Society of Cardiology, Endocrine Society’s Annual Meeting and European Congress of Endocrinology.
Study selection
We selected articles published in English or French that described observational studies of adults (>18 years of age) with cohort, case–control and cross-sectional designs. Studies were included if they specified the number of patients with OA and dyslipidemia and/or the prevalence or incidence of OA in patients with dyslipidemia and/or dyslipidemia in patients with OA, and/or the mean values of parameters of dyslipidemia in patients with and without OA and/or the existence or not of an association between OA and dyslipidemia. We excluded non-observational studies (therapeutic trials, reviews, letters and case reports). Articles that did not mention the number of patients with OA or dyslipidemia and those that did not evaluate the link between the two diseases were excluded. The selection of articles was based on titles and abstracts, then full texts.
Data synthesis
We extracted the following data: publication data (title of the article, first author, journal and publication date), study design (type of study, year(s) of inclusion, study quality score), population (total number of patients included, mean age and sex of patients), methodology of articles (the definition used for OA and dyslipidemia, OA location) and data needed for statistical analysis (number of patients with OA and/or dyslipidemic patients; mean total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglyceride (TG) levels (mg/dL or mmol/L); and number of patients receiving statins, number with MetS and number with obesity or mean body mass index (BMI) in kg/cm2). The quality of the study was estimated by using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) scale, the score expressed in percentage of positive answers in relation to the number of items selected.14
Statistical analysis
First, we performed a descriptive analysis of the prevalence of dyslipidemia in patients with and without OA and used the number of patients with dyslipidemia and total number with and without OA. To estimate this prevalence from cohort longitudinal prospective studies, we used baseline data. Prevalence was expressed as mean±SD Second, we calculated the mean TC, LDL, HDL and TG levels in patients with and without OA. Third, for studies examining an association between OA and dyslipidemia, we calculated the risk of dyslipidemia with OA by estimating the overall OR with 95% CIs. The data were extracted from studies examining the number of dyslipidemic patients with and without OA. We used Revman V.5.3 for the meta-analysis with a fixed-effects model. Heterogeneity was assessed by the I² index; with I²>50% (high heterogeneity), we used a random-effects model, and with I2 <50% (low heterogeneity), we used a fixed-effects model. With strong heterogeneity, we used a randomised-effects analysis. To investigate potential publication bias, we have performed the funnel plot. The association was considered positive with OR >1, and the result was considered statistically significant with p≤0.05. We performed sensitivity and subgroup analyses.
Results
Characteristics of studies included
The selection of articles is reported in the flow chart (figure 1). We identified 605 publications; 48 articles (including 13 abstracts) from 43 studies were included (2 articles from the SEKOIA study, 4 from the FRAMINGHAM study and 2 from the National Health and Nutrition Examination Survey III). One abstract15 was obtained from the EMBASE database and not from screening congress abstracts. The 48 articles described 29 cross-sectional, 10 cohort and 9 case–control studies. Among them, 29 articles involved the OA population and 19 the general population (table 1). We did not find any studies based on a cohort of patients with dyslipidemia, which explains why the prevalence or relative risk of OA in patients with dyslipidemia was not calculated. Table 2 shows the definitions of OA and dyslipidemia in selected studies.
Figure 1.
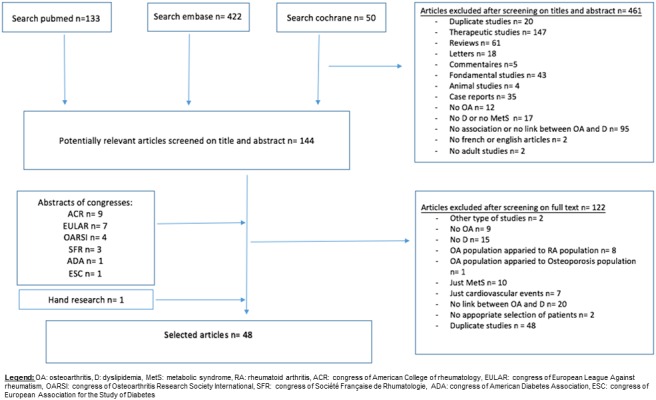
Flow chart of articles in the study
Table 1.
Description of the 48 articles studies selected for analysis
| Osteoarthritis population | General population | |||
| Type of study | Author | Year | Author | Year |
| Cross-selectional | Stürmer et al25 | 1998 | Davis et al26 | 1988 |
| Racaza et al65 | 2012 | Han et al27 | 2013 | |
| Erb et al66 | 2004 | Dahaghin et al41 | 2007 | |
| Eymard et al28 | 2015 | Haugen et al42 | 2015 | |
| Shea et al29 | 2015 | Inoue et al30 | 2011 | |
| Salamon et al50 | 2015 | Cemeroglu et al22 | 2014 | |
| Abourazzak et al20 | 2015 | Meek et al51 | 2014 | |
| Juge et al17* | 2015 | Al-Arfaj31 | 2003 | |
| Rollefstad et al23* | 2014 | Suri et al48 | 2010 | |
| Saunders et al53* | 2013 | Puenpatom et al4 | 2009 | |
| Nuñez et al32* | 2012 | Hart et al19 | 1995 | |
| Shukurova et al67* | 2014 | Maddah et al24 | 2015 | |
| Salaru et al33* | 2013 | Engström et al34 | 2009 | |
| Kemta Lekpa et al35* | 2014 | Yoshimura et al3 | 2012 | |
| Niu et al36* | 2015 | Nielen et al54 | 2012 | |
| Haugen et al43* | 2013 | Marshall et al44 | 2015 | |
| Courties et al45* | 2014 | Hussain et al37 | 2014 | |
| Cohort | Gandhi et al49 | 2014 | Sowers et al21 | 2009 |
| Laires et al38* | 2015 | Massengale et al46 | 2012 | |
| Thelier–Deloison et al15* | 2012 | |||
| Case–control | Soran et al16 | 2008 | ||
| Cheras et al18 | 1997 | |||
| Mishra et al39 | 2012 | |||
| Oliviero et al52 | 2012 | |||
| Addimanda et al47 | 2012 | |||
| Philbin et al55 | 1996 | |||
| Irshad et al56 | 2014 | |||
| Zayed et al40 | 2013 | |||
| Cheng et al57* | 2013 | |||
*Data from a congress.
Table 2.
Characteristics of the 48 included articles: definitions of osteoarthritis (OA) and dyslipidemia, outcomes and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) study quality
| Author | OA definition | Dyslipidemia definition | Outcome | STROBE study quality (%) |
| Stürmer et al25 | Arthroplasty or KL≥2 | TC≥240 mg/dL and/or statin therapy | MV in OA+ Association of OA and dyslipidemia |
53 |
| Racaza et al65 | ACR or Cq and Rx | – | NPD in OA+ | 42 |
| Erb et al66 | Cq and Rx | – | MV in OA+ | 50 |
| Eymard et al28 | ACR Cq and Rx KL scale |
History of dyslipidemia | NPD in OA+ | 82 |
| Shea et al29 | Cq and Rx | – | NPS in OA+ MV in OA+ |
78 |
| Salamon et al50 | ACR | – | NPD in OA+ MV in OA+ |
72 |
| Abourazzak et al20 | KL≥2 | HDL<50 mg/dL TG≥150 mg/dL |
NPD in OA+ | 66 |
| Juge et al17* | Rx | – | NPD in OA+ | NA |
| Rollefstad et al23* | History of OA | – | MV in OA+ and OA– Association of OA and dyslipidemia |
NA |
| Saunders et al53* | KL scale | TC>4 mmol/L | NPD in OA+ Association of MV and KL scale |
NA |
| Nuñez et al32* | – | Hypercholesterolemia (ND) | NPD in OA+ | NA |
| Shukurova et al67* | - | Hypercholesterolemia (ND) | NPD in OA+ | NA |
| Salaru et al33* | ACR | – | NPD in OA+ | NA |
| Kemta Lekpa et al35* | ACR | – | NPD in OA+ | NA |
| Niu et al36* | Arthroplasty or KL≥2 | HDL<40 mg/dL in M;<50 mg/dL in W TG>150 mg/dL |
Association of OA and dyslipidemia | NA |
| Haugen et al43* | KL≥2 | Low HDL and HTG (ND) | NPD in OA+ Association of OA and dyslipidemia |
NA |
| Courties et al45* | KL≥2 | – | NPD in OA+ | NA |
| Gandhi et al49 | Cq and Rx | HDL<35 mg/dL in M,<40 mg/dL in W; TG≥150 mg/dL | NPD in OA+ | 52 |
| Laires et al38* | – | – | NPD in OA+ | NA |
| Thelier–Deloison et al15* | History of OA | – | NPD in OA+ and OA– Association of OA and dyslipidemia |
NA |
| Soran et al16 | Cq and Rx | – | MV in OA+ and OA– Association of OA and dyslipidemia |
65 |
| Cheras et al18 | Cq and Rx | – | MV in OA+ and OA– Association of OA and dyslipidemia |
75 |
| Mishra et al39 | KL scale ACR |
– | MV in OA+ and OA– Association of OA and dyslipidemia |
58 |
| Oliviero et al52 | ACR | – | MV in OA+ and OA– Association OA and dyslipidemia |
67 |
| Addimanda et al47 | Cq KL scale |
LDL≥130 mg/dL and/or CT≥240 mg/dL and/or statin therapy | NPD in OA+ and OA– Association of OA and dyslipidemia |
75 |
| Philbin et al55 | Questionnaire Radiological Danielson scale |
LDL≥160 mg/dL and/or HDL≤35 mg/dL | NPD in OA+ and OA– NPS in OA+ and OA– Association of OA and dyslipidemia MV in OA+ and OA– |
73 |
| Irshad et al56 | KL scale | TC≥200 mg/dL and/or TG≥150 mg/dL | NPD in OA+ and OA– Association of OA and dyslipidemia MV in OA+ and OA– |
47 |
| Zayed et al40 | ACR | – | MV in OA+ and OA– Association of OA and dyslipidemia |
56 |
| Cheng et al57* | – | – | Association of OA and dyslipidemia | NA |
| Davis et al26 | Rx | – | MV in OA+ and OA– Association of OA and dyslipidemia |
67 |
| Han et al27 | History of OA by physician | HDL<40 mg/dL in M,<50 mg/dL in W; TG≥150 mg/dL | MV in OA+ and OA– Association of OA and dyslipidemia |
84 |
| Dahaghin et al41 | KL≥2, ACR, Cq | – | MV in OA+ and OA– Association of OA and dyslipidemia |
69 |
| Haugen et al42 | KL≥2 | – | NPS in OA+ MV in OA+ |
84 |
| Inoue et al30 | KL≥2 | HDL<40 mg/dL in M,<50 mg/dL in W; TG≥150 mg/dL | NPD in OA+ and OA– Association of OA and dyslipidemia MV in OA+ and OA– |
69 |
| Cemeroglu et al22 | ≥3 articulations with KL≥2 | TC>200 mg/dL LDL>100 mg/dL HDL<40 mg/dL TG>150 mg/dL | NPD in OA+ and OA– MV in OA+ and OA– NPS in OA+ and OA– Association of OA and dyslipidemia |
59 |
| Meek et al51 | Codes | – | MV in OA+ NPS in OA+ |
78 |
| Al-Arfaj31 | KL≥2 | TC≥220 mg/dL | NPD in OA+ and OA– Association of OA and dyslipidemia |
50 |
| Suri et al48 | Pathria and Weishaupt scale | TC≥240 mg/dL | NPD in OA+ and OA– Association of OA and dyslipidemia |
72 |
| Puenpatom et al4 | Codes Rx History of OA by physician |
Codes or HDL<40 mg/dL in M,<50 mg/dL in W; or TG≥150 mg/dL | NPD in OA+ and OA– Association of OA and dyslipidemia |
69 |
| Hart et al19 | KL≥2 | – | Association of OA and dyslipidemia | 78 |
| Maddah et al24 | KL≥2 | TC≥5 mmol/L and TG≥2 mmol/L and HDL≤1 mmol/L in M, ≤1.1 mmol/L in W | NPD in OA+ and OA– Association of OA and dyslipidemia MV in OA+ and OA– |
72 |
| Engström et al34 | Codes: arthroplasty for hip or knee OA | HDL<1.03 mmol/L in M,<1.29 mmol/L in W; TG≥1.7 mmol/L or statin therapy | NPD in OA+ and OA– Association of OA and dyslipidemia |
79 |
| Yoshimura et al3 | KL≥2 | HDL≤40 mg/dL | MV in OA+ and OA– Association of OA and dyslipidemia |
91 |
| Nielen et al54 | Codes | Codes: hypercholesterolemia | NPD in OA+ and OA– NPS in OA+ and OA– |
81 |
| Marshall et al44 | KL scale | Codes | NPD in OA+ NPS in OA+ |
74 |
| Hussain et al37 | Joint replacement | HDL<1.03 mmol/L in M,<1.29 mmol/L in W; HTG≥1.7 mmol/L | NPD in OA+ and OA– Association of OA and dyslipidemia |
85 |
| Sowers et al21 | KL≥2 | HDL≤45 mg/dL or LDL>160 mg/dL or TG>200 mg/dL | MV in OA+ and OA– Association of OA and dyslipidemia |
70 |
| Massengale et al46 | – | TC≥240 mg/dL | NPD in OA+ and OA– | 78 |
ACR, American College of Rheumatology; Cq, clinical; HDL, high-density lipoprotein; HTG, hypertriglyceridemia; KL, Kellgren and Lawrence; LDL, low-density lipoprotein; M, men; MV, mean values of lipid profile; NA, if the data were issued only from congress; ND, not defined; NPD, number of patients with dyslipidemia; NPS, number of patients with statin therapy; OA+, patients with osteoarthritis; OA–, patients without osteoarthritis; Rx, radiography; TC, total cholesterol; TG, triglycerides; W, women.
*Data from a congress.
The median STROBE quality score was 69.1% (range 42%–91%). Nine articles had a STROBE quality score <60% (table 3).
Table 3.
Characteristics of the population on the 48 included articles: number, age, gender, overweight proportion
| Author | Sample size (N = number of total patients; n= number of patients with OA) | Mean age (years) in OA+ and OA– patients | Gender in OA+ and OA– patients (% of F) | Overweight proportion (%) or BMI (kg/m2) in OA+ and OA– |
| Stürmer et al25 | n=809 | – | OA+: F: 62.3% | – |
| Racaza et al65 | n=859 | OA+: 62.9 | OA+: F: 74.5% | – |
| Erb et al66 | N=250 n=64 |
OA+: 57.3±10.1 | OA+: F: 62.5% | OA+: 30.9±7.6 kg/m2 |
| Eymard et al28 | n=559 | OA+: 62.8 | OA+: F: 70.1% | |
| Shea et al29 | n=791 | OA+: 74.25±4.5 | OA+: F: 62.3% | OA+: 27.28 kg/m2 |
| Salamon et al50 | N=927 n=344 |
– | OA+: F: 83.4% | OA+: 29.5 kg/m2 |
| Abourazzak et al20 | n=130 | OA+: 56.7±8.1 | OA+: F: 100% | OA+: 32.54±2.9 kg/m2 |
| Juge et al17 * | n=147 | OA+ : 75.8±10 | OA+: F: 68.7% | OA+: 27.2 kg/m2 |
| Rollefstad et al23 * | N=626 n=469 |
OA+: 64.1±8.6 OA–: 63.3±9.3 |
OA+: F: 73.1% OA–: F: 58% |
– |
| Saunders et al53 * | n=57 | – | – | – |
| Nuñez et al32 * | n=260 | OA+: 69.8±8 | OA+: F: 79.2% | – |
| Shukurova et al67 * | n=1243 | OA+: 56.1±7.9 | – | OA+: 61.6% of OP |
| Salaru et al33 * | n=61 | OA+: 64.9±2.7 | OA+: F: 77% | OA+: 60.6% of OP |
| Kemta Lekpa et al35 * | n=148 | OA+: 57±10.6 | OA+: F: 75% | OA+: 53% of OP 30.8±5.6 kg/m2 |
| Niu et al36 * | n=1091 | OA+: 62 | OA+: F: 55.5% | – |
| Haugen et al43 * | n=748 | OA+: 58.1 | – | OA+65.7% of OP |
| Courties et al45 * | n=869 | OA+: 54±7 | OA+: F : 72% | – |
| Gandhi et al49 | n=1502 | OA+: 55.3±15.5 | OA+: F: 48.8% | OA+: 27.3 kg/m2 |
| Laires et al38 * | n=197 | OA+: 67±8.6 | OA+: F: 79.2% | – |
| Thelier–Deloison et al15 * | n=112 n=26 |
– | – | OA+: 100% of OP |
| Soran et al16 | N=66 n=36 |
OA+: 40.9±2.5 OA–: 39±4.7 |
OA+: F: 72.2% OA–: F : 66.7% |
OA+: 29.9±3.3 kg/m2
OA–: 27.6±3.8 kg/m2 |
| Cheras et al18 | N=96 n=44 |
OA+: 69±9 OA–: 68±7 |
OA+: F 40.9% OA–: F 40.4% |
OA+: 25.8 kg/m2
OA–: 24.8 kg/m2 |
| Mishra et al39 | N=100 n=28 |
OA+: 49.1±1.4 OA–: 49.6±1.3 |
OA+: M: F: 71.4% OA–: M: F: 69.4% |
OA+: 23.4±0.6 kg/m2
OA–: 22.9±0.6 kg/m2 |
| Oliviero et al52 | N=77 n=16 |
OA+: 54.7±11.5 OA–: – |
OA+: F: 68.7% OA–: – |
– |
| Addimanda et al47 | N=753 n=446 |
OA+: 68±8 OA–: 63.9±9 |
OA+: F: 92.8% OA– : F : 97.4% |
OA+: 25.1±3.8 kg/m2
OA–: 24.9±3.9 kg/m2 |
| Philbin et al55 | N=69 n=46 |
OA+: 65.8±9.3 OA–: 67.9±6.7 |
OA+: F: 56.5% OA–: F: 65.2% |
OA+: 31.2±5.9 kg/m2
OA–: 24.6±3.2 kg/m2 |
| Irshad et al56 | N=100 n=50 |
– | – | – |
| Zayed et al40 | N=80 n=40 |
OA+: 43.5±3.7 OA–: 44.4±3.9 |
OA+: F: 87.5% OA–: F: 87.5% |
OA+: 37.3±5.9 kg/m2
OA–: 23.5±1.3 kg/m2 |
| Cheng et al57 * | N=56 607 n=23 530 |
– | – | – |
| Davis et al26 | N=3885 n=301 |
– | – | – |
| Han et al27 | N=10 839 n=270 |
OA+: 64.5±10.1 OA–: 53.2±11 |
OA+: F: 84.8% OA–: F: 50% |
– |
| Dahaghin et al41 | n=3585 | – | – | OA+26.3±3.5 kg/m2 |
| Haugen et al42 | N=1348 n=726 |
– | – | – |
| Inoue et al30 | N=795 n=251 |
OA+: 66.3 OA–: 55.5 |
OA+: F: 79.3% OA–: F : 54.7% |
OA+: 23.8 kg/m2
OA–: 22.8 kg/m2 |
| Cemeroglu et al22 | N=61 n=39 |
– | OA+: F: 100% OA–: F: 100% |
– |
| Meek et al51 | N=858 n=206 |
OA+: 59.2±11 | OA+: F: 79.1% | – |
| Al-Arfaj31 | N=246 n=122 |
– | – | – |
| Suri et al48 | N=441 n=310 |
OA+: 57.8±10.6 OA–: 46.7±9.7 |
OA+: F: 49% OA–: F: 39% |
– |
| Puenpatom et al4 | N=7714 n=975 |
OA+: 69.6 OA–: 41.3 |
OA+: F: 61.3% OA–: F: 51.3% |
OA+: 66.9% of OP OA–: 34.8% of OP |
| Hart et al19 | N=979 n=118 |
– | OA+: F: 100% | |
| Maddah et al24 | N=625 n=244 |
OA+: 61.2 OA–: 48.0 |
OA+: F: 89.8% OA–: F: 73.8% |
|
| Engström et al34 | N=5194 n=209 |
OA+: 59.9 OA–: 57.6 |
OA+: F: 66.5% OA–: F: 58.4% |
OA+: 27.9 kg/m2
OA–: 25.37 kg/m2 |
| Yoshimura et al3 | N=1690 n=71 |
OA+: 67.3±8.2 OA–: 58.2±11.8 |
OA+: F: 74.6% OA–: F: 58.6% |
OA+: 23.6±2.9 kg/m2
OA–: 22.4±3.2 kg/m2 |
| Nielen et al54 | N=175 956 n=4040 | OA+: 69.8 OA–: 51 |
OA+: F: 68.7% OA–: F: 50.4% |
– |
| Marshall et al44 | N=1076 n=341 |
OA+: 69.0 | OA+: F: 80.4% | – |
| Hussain et al37 | N=20 430 n=1222 |
OA+: 68.3±7.7 OA–: 64.8±8.6 |
OA+ : F: 66.2 OA–: F: 59.5% |
OA+: 76.8% of OP, 28.6±5.0 kg/m2
OA–: 62.6% of OP, 26.8±4.5 kg/m2 |
| Sowers et al21 | N=664 n=53 |
OA+: 50±5 OA–: 47±8 |
OA+: F: 100% OA–: F: 100% |
OA+: 35.6±11.1 kg/m2
OA–: 27.3±8.4 kg/m2 |
| Massengale et al46 | N=2477 n=466 |
– | OA+: F: 58.2% OA–: F: 46.6% |
– |
*Data from a congress. BMI, body mass index; F, female; M, male; OA, osteoarthritis.
In total, 30 articles assessed the association of OA and dyslipidemia, 30 assessed the prevalence of dyslipidemia among patients with OA and 22 assessed mean lipid level values among patients with OA (table 3).
Patient characteristics
This study involved 306 044 patients. The mean age range was 39.0±4.716 to 77.5±9.0 years.17 The mean proportion of females was 53.2% (range 40.6%18 to 100%19–22). The localisation was the knee in 23 articles,3 15 16 19–21 24–40 hand in 9,15 22 41–47 generalised OA in 3,25 31 47 hip in 3,25 34 37 spine in 248 49 and shoulder in 1.17 MetS was reported in nine articles,4 20 24 28 30 36 40 43 50 the prevalence of MetS ranged from 5%24 to 97.5%.40 The prevalence of obesity ranged from 7.8%51 to 100%15 40 and BMI from 22.3±2.730 to 37.3±5.9 g/cm2.40 Seven articles described the use of statin treatment (table 3).
Prevalence of dyslipidemia among patients with and without OA (table 4)
Table 4.
Main results of prevalence of dyslipidemia and mean lipid-level values in patients with osteoarthritis (OA) and non-OA patients
| Prevalence of dyslipidemia | Mean CT level (mg/dL) | Mean high-density lipoprotein level (mg/dL) | Mean low-density lipoprotein level (mg/dL) | Mean triglyceride level (mg/dL) | |
| OA+ population | 30.2%±0.7% n=14 823 n=28 |
245±25.1 n=6037 n=14 |
54.4±8.9 n=5856 n=18 |
126.5±20.7 n=656 n=9 |
137.3±80.3 n=2406 n=15 |
| OA– population | 8.0%±0.1% n=196 168 n=13 |
233.1±17.5 n=3763 n=3 |
53.1±7.5 n=412 n=7 |
136.9±15.9 n=451 n=2 |
131±27.3 n=3460 n=6 |
The mean prevalence of dyslipidemia was 30.2%±0.6% among 14 843 patients with OA and 8.0%±0.1% among 1 96 168 without OA. The mean prevalence with knee OA was 27.6%±1.4%,15 20 24 25 28 30–35 37 38 hand OA 37.6%±1.6%,22 43–47 generalised OA 30.5%±3.9%,25 31 47 hip OA 20%±2.1%25 34 37 and symptomatic OA was 21%.28 44
Mean lipid-level values with and without OA (table 4)
The mean lipid-level values for patients with and without OA were for TC, 245±25.1 and 233.1±17.5 mg/dL; LDL, 126.5±20.7 and 136.9±15.9 mg/dL; HDL, 54.4±8.9 and 53.1±7.5 mg/dL; and TG, 137.3±80.3 and 131±27.3 mg/dL.
Association between dyslipidemia and OA
Overall, 30 articles indicated the presence or the absence of an association between OA and dyslipidemia; 21 (70%) showed a positive association between OA and dyslipidemia3 4 15 18 19 21 23 24 25 30 31 39 40 47 48 52–57; 12/18 articles (67%) with STROBE score >60% found a positive association.3 4 18 19 21 24 30 47 48 52 54 55 In addition, 4/7 articles19 25 31 47 that reported an OR adjusted on age and BMI found a positive association. Among the three with negative association findings after adjustment, two had a STROBE score >60%.34 37
Overall risk of dyslipidemia with OA: meta-analysis
Among 204 148 patients from 13 articles,4 15 22 24 30 31 34 37 47 48 54–56 the overall OR was 1.98 (95% CI 1.43 to 2.75, p<0.0001; I2=94%), evaluated by a random-effects model (figure 2).
Figure 2.
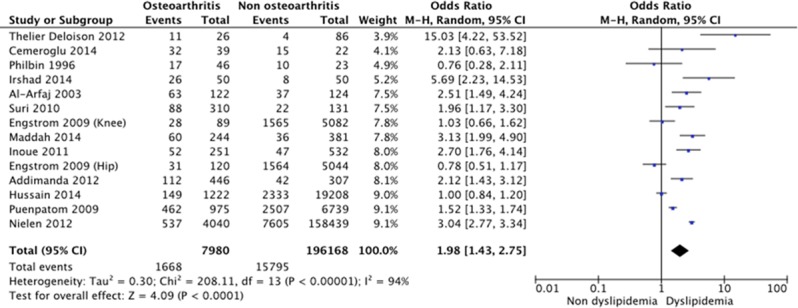
Forest plot for dyslipidemia among patients with and without osteoarthritis (OA).
Risk of dyslipidemia with OA: sensitivity analyses
To strengthen our results, we performed four sensitivity analyses. First, we removed the studies that did not use ACR criteria or Kellgren-Lawrence grading for OA diagnosis: among 2568 patients from the six remaining articles,22 24 30 31 47 56 the risk of dyslipidemia was increased with than without OA (OR 2.64, 95% CI 2.14 to 3.26, p<0.00001, I2=0%). Second, we excluded studies with a STROBE score <60%: among 203 629 patients from the nine remaining articles,4 24 30 34 37 47 48 54 55 the risk of dyslipidemia remained increased with than without OA (OR 1.63, 1.13 to 2.36, p=0.009, I2=95%). Third, we excluded studies that specified the use of statin treatment because the definition of dyslipidemia in these studies was based on only lipid values and did not account for statin treatment. Among 41 539 patients from the 10 remaining articles,4 15 24 30 31 34 37 47 48 56 the risk of dyslipidemia remained increased with than without OA (overall OR 1.93, 1.42 to 2.61, p<0.0001, I2=87%). Fourth, we pooled the results of the articles that reported an age-adjusted and BMI-adjusted OR. Among 31 764 patients, from the four articles,31 34 37 47 there was no association between dyslipidemia and OA (OR 1.31, 95% CI 0.88 to 1.95, p<0.0001, I2=83%).
Risk of dyslipidemia with OA: subgroup analyses
We performed a subgroup analysis by OA localisation. The increased risk of dyslipidemia with OA persisted with knee OA (among 26 805 patients, OR 2.27, 1.33 to 3.89, p=0003, I2=88%)15 24 30 31 34 37 and hand OA (among 814 patients, OR 2.12, 1.46 to 3.07, p<0.0001, I2=0%)22 47 but not hip OA (among 24 934 patients, OR 0.86, 0.69 to 1.08, p=0.18, I2=0%).34 37
Discussion
We investigated the potential association between OA and dyslipidemia with a systematic review and meta-analysis and found a 30% prevalence of dyslipidemia with OA, which seems much higher than in the non-OA population (8.0%). Furthermore, the meta-analysis revealed an increased risk of dyslipidemia, by 1.98, with than without OA and was observed with knee as well as hand OA.
The mean prevalence of dyslipidemia in hand OA was 37.6%±1.6%, much higher than the mean prevalence of 30.2%±0.6% with OA overall. Moreover, the risk of dyslipidemia was increased twofold with hand OA (OR 2.12, 95% CI 1.46 to 3.07). These results again confirm the systemic metabolic component of hand OA, as recently reported in the NEO study.58 The pathophysiological link between hand OA and MetS might be explained by the action of the adipose-tissue source of proinflammatory cytokines and the action of visceral fat.58
Hip OA, defined by joint replacement, was not associated with dyslipidemia possibly because of a selection bias of patients: cardiovascular comorbidities often associated with dyslipidemia might have restricted the indication for surgery due to the perioperative period. Furthermore, mechanical stress is more involved than metabolic stress in this joint.
For knee OA, the mean prevalence of dyslipidemia was 27.6%±1.4% and the association between knee OA and dyslipidemia was confirmed with increased risk of dyslipidemia (OR 2.27, 95% CI 1.33 to 3.89). The association between knee OA and MetS is sometimes conflicting. Han et al,27 Inoue et al,30 and Hussain et al37 did not find any positive association possibly because of different OA definitions. A recent study showed that the most important risk factor of knee OA was mechanical stress (before and after adjustment for metabolic factors), which limits the identification of a systemic metabolic component in knee OA.
Our meta-analysis has some limitations. The heterogeneity between studies was high, probably because of differences in OA localisations, definition of OA and dyslipidemia, statin therapy could not have been taken into account, and types and quality of studies. Dyslipidemia referred to lipid abnormalities such as hypercholesterolemia, low HDL level, high LDL level or hypertriglyceridemia. Because of the different definitions of dyslipidemia, we chose to define dyslipidemia first by high LDL level, then low HDL level, then hypercholesterolemia and hypertriglyceridemia. To counteract this heterogeneity, we performed sensitivity analyses to check whether the association between OA and dyslipidemia persisted after removing studies with poor methodology and found that the association persisted in all sensitivity analyses. Moreover, the heterogeneity of the studies was assessed by the I² index and we adapted the method to its value. The results of the meta-analysis are not modified by removing the most heterogeneous studies (data not shown). We were not able to integrate confounding factors such as age, BMI, HTA, smoking and physical activity in the overall statistical analysis. Obesity is a major risk factor of development and progression of OA. Obesity increases the risk of OA of the weightbearing joints due to excessive mechanical stress but is also associated with dyslipidemia in MetS.59 We identified seven articles accounting for confounding factors of dyslipidemia and OA: four showed a positive association after adjustment on age and BMI. However, when we meta-analysed the seven articles that reported an age-adjusted and BMI-adjusted OR, there was no association between dyslipidemia and OA, but raw data before adjustment on age and BMI are used. Finally, the impact of statin treatment could not be assessed because of the lack of data concerning its prescription. In fact, we have no details about statin use in dyslipidemic and non-dyslipidemic patients. However, Riddle et al did not find beneficial effect of statins on the structural progress at patients monitored for a knee osteoarthritis.60
In this funnel plot, the distribution of common values is not heterogeneous. Likewise, we can consider that there is no major publication bias in our meta-analysis.
We demonstrated an association between dyslipidemia and OA, but the pathophysiological explanation for the causal relationship has not been clearly defined. Experimental studies suggest the existence of lipid metabolism dysfunction in OA. Mice with altered HDL metabolism showed knee OA despite abnormal weight gain.61 Gierman et al showed that dietary cholesterol intake increased spontaneous cartilage damage in mice.62 High LDL levels promote synovial inflammation and ectopic bone formation in mouse OA models.63 Oxidised-LDL (oxLDL) could be involved in the development and progression of OA by stimulating synovial cells (macrophages, synovial fibroblasts and endothelial cells) and chondrocytes. A treatment strategy that lowers the level of oxLDL could be interesting.64
In conclusion, this is the first systematic review and meta-analysis demonstrating an association between OA and dyslipidemia, which illustrates the role of metabolic disturbances beyond glucose metabolism in OA pathophysiology. Such a study emphasises the need to screen and manage cardiovascular comorbidities in patients with OA in clinical practice.
rmdopen-2017-000442supp001.docx (234.9KB, docx)
Acknowledgments
Laura Smales (BioMed Editing, Toronto, Canada)
Footnotes
Twitter: @Larhumato
Contributors: PB, KL, FB and JS were involved in conception and design. PB and KL were involved in acquisition of data and statistical analysis. PB, KL, CM, FB and JS were involved in analyses and interpretation of data, drafting of the manuscript, revision of the manuscript and final approval, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. 10.1016/S0140-6736(11)60243-2 [DOI] [PubMed] [Google Scholar]
- 2.Mertens I, Van Gaal LF. New International Diabetes Federation (IDF) and National Cholesterol Education Program adult treatment panel III (NCEP-ATPIII) criteria and the involvement of hemostasis and fibrinolysis in the metabolic syndrome. J Thromb Haemost 2006;4:1164–6. 10.1111/j.1538-7836.2006.01919.x [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura N, Muraki S, Oka H, et al. . Accumulation of metabolic risk factors such as overweight, hypertension, Dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217–26. 10.1016/j.joca.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 4.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med 2009;121:9–20. 10.3810/pgm.2009.11.2073 [DOI] [PubMed] [Google Scholar]
- 5.Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine 2013;80:568–73. 10.1016/j.jbspin.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Zhuo Q, Yang W, Chen J, et al. . Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 2012;8:729–37. 10.1038/nrrheum.2012.135 [DOI] [PubMed] [Google Scholar]
- 7.Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. . Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010;69:761–5. 10.1136/ard.2008.106930 [DOI] [PubMed] [Google Scholar]
- 8.Louati K, Vidal C, Berenbaum F, et al. . Association between Diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 2015;1:e000077 10.1136/rmdopen-2015-000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Postgrad Med J 2012;88:240–2. 10.1136/pgmj.2010.146399rep [DOI] [PubMed] [Google Scholar]
- 10.Verzijl N, Bank RA, TeKoppele JM, et al. . AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol 2003;15:616–22. 10.1097/00002281-200309000-00016 [DOI] [PubMed] [Google Scholar]
- 11.Laiguillon MC, Courties A, Houard X, et al. . Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthritis Cartilage 2015;23:1513–22. 10.1016/j.joca.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 12.Hamada D, Maynard R, Schott E, et al. . Suppressive effects of insulin on tumor necrosis Factor-Dependent early osteoarthritic changes associated with obesity and type 2 Diabetes Mellitus. Arthritis Rheumatol 2016;68:1392–402. 10.1002/art.39561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwers H, von Hegedus J, Toes R, et al. . Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best Pract Res Clin Rheumatol 2015;29:741–55. 10.1016/j.berh.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. . The strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 15.Thelier-Deloison N, Chevalier X, Oppert J-M, et al. . Prevalence of clinical digital osteoarthritis (Heberden and bouchard nodes) in a selected population of patients with severe obesity: a prospective study. Osteoarthritis Cartilage 2012;20:S174–S296. 10.1016/j.joca.2012.02.264 [DOI] [Google Scholar]
- 16.Soran N, Altindag O, Cakir H, et al. . Assessment of paraoxonase activities in patients with knee osteoarthritis. Redox Rep 2008;13:194–8. 10.1179/135100008X308911 [DOI] [PubMed] [Google Scholar]
- 17.Juge P, et al. . Are metabolic factors associated with shoulder osteoartritis? A multicentric study [abstract 331]. ACR Congr 2015. [Google Scholar]
- 18.Cheras PA, Whitaker AN, Blackwell EA, et al. . Hypercoagulability and hypofibrinolysis in primary osteoarthritis. Clin Orthop Relat Res 1997:57–67. [PubMed] [Google Scholar]
- 19.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol 1995;22:1118–23. [PubMed] [Google Scholar]
- 20.Abourazzak F, Talbi S, Lazrak F, et al. . Does metabolic syndrome or its individual components affect pain and function in knee osteoarthritis women? Curr Rheumatol Rev 2015:8–14. 10.2174/1573397111666150522093337 [DOI] [PubMed] [Google Scholar]
- 21.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, et al. . Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum 2009;61:1328–36. 10.1002/art.24739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cemeroglu O, Aydın HI, Yasar ZS, et al. . Hand and heart, hand in hand: is radiological hand osteoarthritis associated with atherosclerosis? Int J Rheum Dis 2014;17:299–303. 10.1111/1756-185X.12251 [DOI] [PubMed] [Google Scholar]
- 23.Rollefstad S, et al. . Patients with Osteoarthritis DO NOT have increased risk of Cardiovascular Disease in Ullensaker Community in Norway [abstract 1274]. ACR Congr 2014. [Google Scholar]
- 24.Maddah S, Mahdizadeh J. Association of metabolic syndrome and its Components with Knee Osteoarthritis. Acta Med Iran 2015;53:743–8. [PubMed] [Google Scholar]
- 25.Stürmer T, Sun Y, Sauerland S, et al. . Serum cholesterol and osteoarthritis. the baseline examination of the Ulm Osteoarthritis Study. J Rheumatol 1998;25:1827–32. [PubMed] [Google Scholar]
- 26.Davis MA, Ettinger WH, Neuhaus JM. The role of metabolic factors and blood pressure in the association of obesity with osteoarthritis of the knee. J Rheumatol 1988;15:1827–32. [PubMed] [Google Scholar]
- 27.Han CD, Yang IH, Lee WS, et al. . Correlation between metabolic syndrome and knee osteoarthritis: data from the korean National Health and Nutrition Examination survey (KNHANES). BMC Public Health 2013;13:603 10.1186/1471-2458-13-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eymard F, Parsons C, Edwards MH, et al. . Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage 2015;23:851–9. 10.1016/j.joca.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 29.Shea MK, Kritchevsky SB, Hsu FC, et al. . The association between vitamin K status and knee osteoarthritis features in older adults: the Health, Aging and Body Composition Study. Osteoarthritis Cartilage 2015;23:370–8. 10.1016/j.joca.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue R, Ishibashi Y, Tsuda E, et al. . Medical problems and risk factors of metabolic syndrome among radiographic knee osteoarthritis patients in the japanese general population. J Orthop Sci 2011;16:704–9. 10.1007/s00776-011-0157-9 [DOI] [PubMed] [Google Scholar]
- 31.Al-Arfaj AS. Radiographic osteoarthritis and serum cholesterol. Saudi Med J 2003;24:745–7. [PubMed] [Google Scholar]
- 32.Nuñez M, et al. . Locus of control of pain in patients with knee osteoarthritis [abstract]. Ann Rheum Dis 2012;71:633.22387730 [Google Scholar]
- 33.Salaru V, et al. . The relationship between co-morbidity and quality of life in patients with knee osteoarthritis [abstract 246]. Eur J Intern Med 2013. [Google Scholar]
- 34.Engström G, Gerhardsson de Verdier M, Rollof J, et al. . C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage 2009;17:168–73. 10.1016/j.joca.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 35.Kemta Lekpa F, et al. . La gonarthrose est-elle associée au syndrome métabolique ? etude transversale dans une population sub-saharienne au Cameroun [abstract Di.153]. SFR Congr 2014. [Google Scholar]
- 36.Niu J, et al. . The metabolic syndrome, its elements and knee osteoarthritis : the Framingham osteoarthritis (OA) Study [abstract 949]. ACR Congr 2015. [Google Scholar]
- 37.Monira Hussain S, Wang Y, Cicuttini FM, et al. . Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin Arthritis Rheum 2014;43:429–36. 10.1016/j.semarthrit.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 38.Laires PA, Laíns J, Miranda LC, et al. . Factors associated with inadequate pain Relief in patients with primary knee osteoarthritis in Portugal: an analysis from the survey of Osteoarthritis Real World Therapies (Sort). Value Health 2015;18:A652–A766. 10.1016/j.jval.2015.09.2349 [DOI] [Google Scholar]
- 39.Mishra R, Singh A, Chandra V, et al. . A comparative analysis of serological parameters and oxidative stress in osteoarthritis and rheumatoid arthritis. Rheumatol Int 2012;32:2377–82. 10.1007/s00296-011-1964-1 [DOI] [PubMed] [Google Scholar]
- 40.Zayed HS, Younis G, Bader R, et al. . Prevalence of preclinical renal dysfunction in obese egyptian patients with primary knee osteoarthritis, preliminary data. The Egyptian Rheumatologist 2013;35:239–44. 10.1016/j.ejr.2013.06.002 [DOI] [Google Scholar]
- 41.Dahaghin S, Bierma-Zeinstra SM, Koes BW, et al. . Do metabolic factors add to the effect of overweight on hand osteoarthritis? the Rotterdam Study. Ann Rheum Dis 2007;66:916–20. 10.1136/ard.2005.045724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haugen IK, Ramachandran VS, Misra D, et al. . Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann Rheum Dis 2015;74:74–81. 10.1136/annrheumdis-2013-203789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haugen IK, et al. . The association between metabolic syndrome and hand osteoarthritis - Data from the Framingham Study [abstract 243]. ACR Congr 2013. [Google Scholar]
- 44.Marshall M, Nicholls E, Kwok WY, et al. . Erosive osteoarthritis: a more severe form of radiographic hand osteoarthritis rather than a distinct entity? Ann Rheum Dis 2015;74:136–41. 10.1136/annrheumdis-2013-203948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courties A, et al. . La cardiopathie ischémique : une comorbidité associée à l’arthrose digitale. Résultats ancillaires de l’essai SEKOIA [abstract O.37]. SFR Congr 2014. [Google Scholar]
- 46.Massengale M, Reichmann WM, Losina E, et al. . The relationship between hand osteoarthritis and serum leptin concentration in participants of the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2012;14:R132 10.1186/ar3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Addimanda O, Mancarella L, Dolzani P, et al. . Clinical associations in patients with hand osteoarthritis. Scand J Rheumatol 2012;41:310–3. 10.3109/03009742.2012.656699 [DOI] [PubMed] [Google Scholar]
- 48.Suri P, Katz JN, Rainville J, et al. . Vascular disease is associated with facet joint osteoarthritis. Osteoarthritis Cartilage 2010;18:1127–32. 10.1016/j.joca.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhi R, Woo KM, Zywiel MG, et al. . Metabolic syndrome increases the prevalence of spine osteoarthritis. Orthop Surg 2014;6:23–7. 10.1111/os.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Šalamon L, Morović-Vergles J, Marasović-Krstulović D, et al. . Differences in the prevalence and characteristics of metabolic syndrome in rheumatoid arthritis and osteoarthritis: a multicentric study. Rheumatol Int 2015;35:2047–57. 10.1007/s00296-015-3307-0 [DOI] [PubMed] [Google Scholar]
- 51.Meek IL, Vonkeman HE, van de Laar MA. Hyperuricaemia: a marker of increased cardiovascular risk in rheumatic patients: analysis of the ACT-CVD cohort. BMC Musculoskelet Disord 2014;15:174 10.1186/1471-2474-15-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliviero F, Lo Nigro A, Bernardi D, et al. . A comparative study of serum and synovial fluid lipoprotein levels in patients with various arthritides. Clin Chim Acta 2012;413:303–7. 10.1016/j.cca.2011.10.019 [DOI] [PubMed] [Google Scholar]
- 53.Saunders FR, Yoshida K, Barr RJ, et al. . Biomarkers of osteoarthritis progression. Osteoarthritis Cartilage 2013;21:S78–S312. 10.1016/j.joca.2013.02.171 [DOI] [Google Scholar]
- 54.Nielen MM, van Sijl AM, Peters MJ, et al. . Cardiovascular disease prevalence in patients with inflammatory arthritis, diabetes mellitus and osteoarthritis: a cross-sectional study in primary care. BMC Musculoskelet Disord 2012;13:150 10.1186/1471-2474-13-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philbin EF, Ries MD, Groff GD, et al. . Osteoarthritis as a determinant of an adverse coronary heart disease risk profile. J Cardiovasc Risk 1996;3:529–33. 10.1097/00043798-199612000-00008 [DOI] [PubMed] [Google Scholar]
- 56.Irshad K, Afzal MN. Comparison of serum lipid levels among patients suffering from osteoarthritis in Pakistan. Rawal Med J 2014;39:6–9. [Google Scholar]
- 57.Cheng K-H, Chu C-S, Lee K-T, et al. . Osteoarthritis is an independent risk factor for Major adverse cardiovascular events-nationwide case-control studies. Eur Heart J 2013;34:P5173 10.1093/eurheartj/eht310.P5173 [DOI] [Google Scholar]
- 58.Visser AW, de Mutsert R, le Cessie S, et al. . The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis 2015;74:1842–7. 10.1136/annrheumdis-2013-205012 [DOI] [PubMed] [Google Scholar]
- 59.Courties A, Sellam J. Obésité Et arthrose : données physiopatologiques. Rev Rhum Monogr 2016;83:18–24. [Google Scholar]
- 60.Riddle DL, Moxley G, Dumenci L. Associations between statin use and changes in pain, function and structural progression: a longitudinal study of persons with knee osteoarthritis. Ann Rheum Dis 2013;72:196–203. 10.1136/annrheumdis-2012-202159 [DOI] [PubMed] [Google Scholar]
- 61.Triantaphyllidou IE, Kalyvioti E, Karavia E, et al. . Perturbations in the HDL metabolic pathway predispose to the development of osteoarthritis in mice following long-term exposure to western-type diet. Osteoarthritis Cartilage 2013;21:322–30. 10.1016/j.joca.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 62.Gierman LM, Kühnast S, Koudijs A, et al. . Osteoarthritis development is induced by increased dietary cholesterol and can be inhibited by atorvastatin in APOE*3leiden.CETP mice--a translational model for atherosclerosis. Ann Rheum Dis 2014;73:921–7. 10.1136/annrheumdis-2013-203248 [DOI] [PubMed] [Google Scholar]
- 63.de Munter W, van den Bosch MH, Slöetjes AW, et al. . High LDL levels lead to increased synovial inflammation and accelerated ectopic bone formation during experimental osteoarthritis. Osteoarthritis Cartilage 2016;24:844–55. 10.1016/j.joca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 64.de Munter W, van der Kraan PM, van den Berg WB, et al. . High systemic levels of low-density lipoprotein cholesterol: fuel to the flames in inflammatory osteoarthritis? Rheumatology 2016;55:16–24. 10.1093/rheumatology/kev270 [DOI] [PubMed] [Google Scholar]
- 65.Racaza GZ, Salido EO, Penserga EG. Clinical profile of Filipino patients with osteoarthritis seen at two arthritis clinics. Int J Rheum Dis 2012;15:399–406. 10.1111/j.1756-185X.2012.01758.x [DOI] [PubMed] [Google Scholar]
- 66.Erb N, Pace AV, Douglas KM, et al. . Risk assessment for coronary heart disease in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol 2004;33:293–9. 10.1080/03009740410006899 [DOI] [PubMed] [Google Scholar]
- 67.Shukurova S. Osteoarthritis place in rheumatic diseases hospitalization structure [abstract P-260]. MCR Congr 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2017-000442supp001.docx (234.9KB, docx)


