Abstract
Ex vivo‐expanded stem cells have long been a cornerstone of biotherapeutics and have attracted increasing attention for treating intractable diseases and improving tissue regeneration. However, using exogenous cellular materials to develop restorative treatments for large numbers of patients has become a major concern for both economic and safety reasons. Advances in cell biological research over the past two decades have expanded the potential for using endogenous stem cells during wound healing processes, and in particular, recent insight into stem cell movement and homing has prompted regenerative research and therapy based on recruiting endogenous cells. Inspired by the natural healing process, artificial administration of specific chemokines as signals systemically or at the injury site, typically using biomaterials as vehicles, is a state‐of‐the‐art strategy that potentiates stem cell homing and recreates an anti‐inflammatory and immunomodulatory microenvironment to enhance in situ tissue regeneration. However, pharmacologically coaxing endogenous stem cells to act as therapeutics in the field of biomedicine remains in the early stages; its efficacy is limited by the lack of innovative methodologies for chemokine presentation and release. This review describes how to direct the homing of endogenous stem cells via the administration of specific signals, with a particular emphasis on targeted signalling molecules that regulate this homing process, to enhance in situ tissue regeneration. We also provide an outlook on and critical considerations for future investigations to enhance stem cell recruitment and harness the reparative potential of these recruited cells as a clinically relevant cell therapy.
Keywords: stem cell homing, chemokines, controlled release, in situ tissue engineering, cell modification
Introduction
Our ageing population is facing an increasing burden of many age‐related degenerative and ischaemic diseases that currently are primarily treated with drugs designed to mitigate symptoms or with resection and reconstructive surgery in certain clinical scenarios 1, 2. Notably, the transplantation of tissues of either autogenic or allogenic origin has provided cures for various types of tissue deficiency, dysfunction and damage 3. Meanwhile, organ allotransplantation is routine and successful in clinical practice; this technique has saved the lives of numerous patients suffering from organ failure and improved the quality of life of many more (e.g. see 4, 5, 6, 7, 8, 9). Unfortunately, due to immunologic barriers and limited donor availability, such therapeutic strategies are applicable to only a small range of clinical scenarios or a small fraction of patients 10. Together with the ever‐increasing demand for organ transplants, the gap between supply and need continues to widen 11. For many conditions, even if tissue/organ allotransplantation is technically feasible and performed during the correct stage, morbidity and mortality due to treatment‐associated complications (e.g. graft‐versus‐host disease) remain unacceptably high, and the overall success rates are frustratingly low 12, 13. Therefore, innovative applications of novel cell therapies and tissue engineering to regenerate damaged tissue structures and to restore lost tissue function represent major frontiers for modern biomedicine, although the most satisfactory and successful stem cell therapy strategy remains to be explored and optimized 1, 14, 15, 16.
During the last decade, accumulating knowledge regarding endogenous mechanisms for the self‐repair of injured tissue has paved the way for the design of in situ regenerative approaches to achieve complete tissue repair 17, 18, 19. Although living tissues can possess inherent mechanisms that instruct stem cells to home to damaged areas to promote self‐repair, such staggering endogenous processes unfortunately cannot provide a universal regenerative solution 20. One key to potentiating and accelerating the body's own repair capacity is the proficient homing of endogenous stem cells into injury sites via the prolonged and controlled delivery of signalling molecules during the initial stage of wound healing 19, 21, 22. In this context, chemokines powerfully influence cell mobilization and homing, and artificially amplifying the doses or concentrations of particular chemokines at the site of damage represents an efficient approach to actively increasing the homing of host stem cells, thus augmenting in situ tissue regeneration 17, 19, 23, 24. The stem or progenitor cells in the local niche neighbouring the tissue defect are normally too few in quantity to strongly affect the intrinsic repair processes; therefore, in most cases of in situ tissue regeneration, it is advisable to actively mobilize mesenchymal stem cells (MSCs) from a central cell niche, such as the bone marrow (BMMSCs), into the peripheral blood system and to target these cells for therapeutic strategies by replenishing the local cell niche and/or for direct participation in regeneration 17, 18, 19, 21, 22, 23, 24, 25.
Similar to strategies applied to improve the homing and engraftment of exogenously transplanted cellular materials in recipient tissues (e.g. see 26, 27, 28), increasing the ability of the damaged site to recruit host cells and the extent to which the damaged site allows the recruited cells to exert their function are also critically important for ensuring the outcome of any endogenous regenerative procedures 29. Both goals can commonly be achieved by the implantation of a well‐devised material platform 18. The biological evidence underlying in vivo cell movement and its related mechanisms of action in self‐repair have been reviewed elsewhere; the readers are pointed to several previously published reviews for more information 18, 19, 21, 22, 23, 24, 25. In this context, protein delivery plays a critical role in the presentation and release of signalling molecules that target cell mobilization, homing and engraftment, together leading to tissue regeneration 30, 31. In this review, we briefly outline the identified and suggested signalling molecules that can affect the efficacy of cell migration, with a particular emphasis on how they are administered to direct stem cell homing and enhance the in situ regeneration process. We also critically evaluate their roles in biomaterials‐based stem cell homing and accommodation.
Steering endogenous cell populations for therapeutics
Given the roles of pluripotent and tissue‐restricted stem cells in maintaining and replenishing tissues, the potential activation of these cell populations for the development of novel therapies has fuelled a veritable explosion of studies in the emerging arena of biological therapeutics and regenerative medicine 16, 32, 33. The basic strategy of stem cell‐based regeneration is based on a combination of autologous or allogeneic stem cells with a matrix template incorporating suitable growth factors, thus yielding cell/tissue constructs that can be utilized for reparative procedures in patients 1 (Fig. 1A). However, in addition to the expensive and time‐consuming in vitro cell expansion procedures, several other technical hurdles must be addressed before the clinical utility of such stem cell therapies for combating human diseases can be realized 15, 34. As an alternative to cell transplantation, tissue regeneration can also be achieved using a cell‐free approach that obviates the need for delivering stem cells from an exogenous source, thereby qualifying this technique for broader applications (Fig. 1B) (e.g. see 35, 36, 37, 38). Increasing evidence indicates that the use of bioactive molecules and material matrices may harness the therapeutic potential of endogenous stem cells and, hence, unlock the innate power of the body to promote in situ tissue regeneration 17, 18, 19, 39, 40. To therapeutically target‐specific niche features (e.g. cell‐matrix and cell‐cell contacts), several cell‐free tissue‐engineering approaches have been developed based on biomaterials and the local or systemic administration of biologics or small molecules that may reverse the impaired regenerative microenvironment due to disease and/or induce homing of circulating stem cells for regeneration 40, 41. In these cases, endogenous cells residing in the central niches (e.g. the bone marrow) are activated in the circulation by extracellular signals and reach the injury site with the aid of blood flow. These stem cells can be arrested within the local vasculature in response to injury and transmigrate across the endothelium to replenish the local niche and/or participate in regeneration (Fig. 2A). In contrast, quiescent stem cells within local niches neighbouring the injury site can also be recruited to exert their reparative effects for the regeneration of new tissue. In this context, cells can reach the site of injury independently of blood flow (Fig. 2B) 19. During any regenerative event, the two types of directed cell movement and recruitment coexist, and this process has recently been nonmechanistically defined as stem cell homing 42. Notably, the molecules that signal for cell migration can be spontaneously released in response to injury; however, in many cases such as ageing and degenerative disease, sufficient homing and healing responses are unlikely to be achieved without artificial administration of one or several key homing factors 17, 23, 24. In the next section, we will review a number of selected cell mobilization and homing factors, as well as the mechanisms for their presentation and release for enhancing in situ tissue regeneration.
Figure 1.
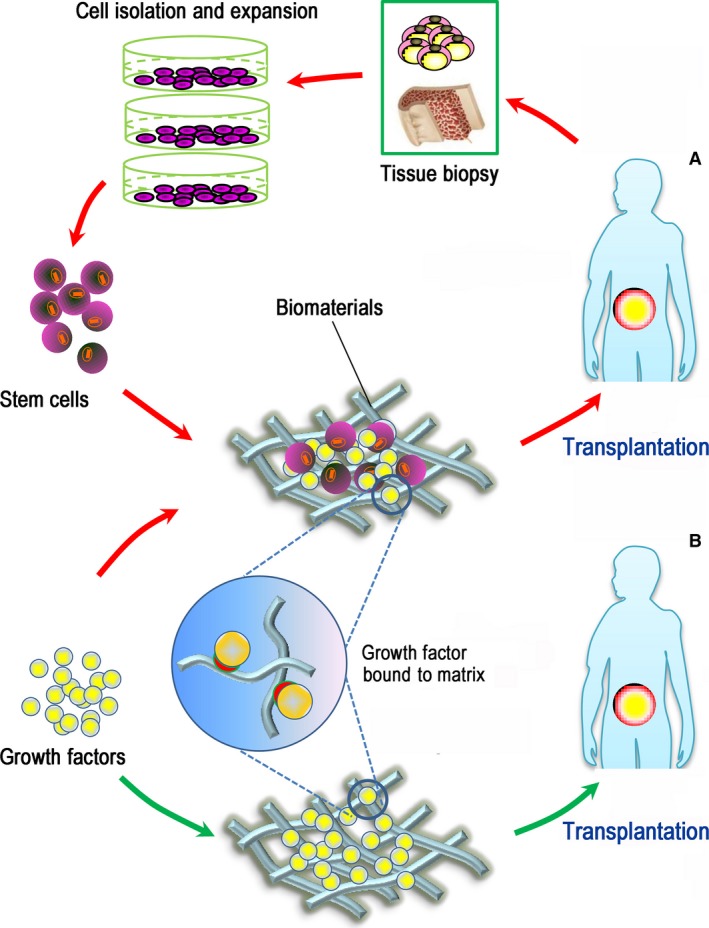
Schematic representation of cell‐based and cell‐free regenerative approaches. (A) A cell‐based approach (red arrows) involves harvesting stem cells from the tissue biopsy and expanding them in vitro; those cells, alone or in combination with biomaterials and selected signalling biomolecules, are then transplanted into the patient to regenerate damaged/diseased tissue. (B) In contrast, a cell‐free approach (green arrows) is used to harness endogenous stem cells for therapeutic regeneration using biomaterials bound with growth factors and thus does not require ex vivo cell manipulation and in vivo cell transplantation.
Figure 2.
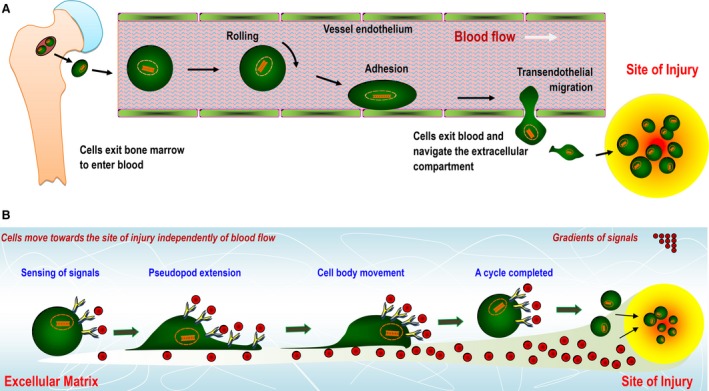
Schematic representation of stem cell movement and homing in the body in response to gradients of guidance cues (e.g. growth factors and/or chemokines) that are administered artificially or released by the tissue in response to injury or inflammation. (A) Cells are mobilized from the bone marrow and enter the blood. With the aid of blood flow, they traffic towards a distant target site and finally exit the microvascular systems via a multistep adhesion cascade. (B) Cells navigate extravascularly across the extracellular compartment to reach an injured site and participate in tissue regeneration.
Chemoattractants as potent cell mobilization and homing factors
Upon inflammation or insult, MSCs respond to signals emitted by the tissue and are recruited to the inflamed or injured sites requiring repair. Some of these cues have been identified; they include but are not limited to substance P, stromal‐derived factor (SDF)‐1α, stem cell factor (SCF), granulocyte colony‐stimulating factor (G‐CSF) and monocyte chemotactic proteins (MCPs) 43. Although stem cells may be guided to promote repair based on their inherent mechanisms in response to injury, these endogenous healing processes are typically insufficient to achieve complete regeneration. Mounting evidence indicates that chemoattractants such as substance P, SDF‐1α, SCF, G‐CSF and MCP‐3 are potent stem cell‐activating factors that may be used to prolong and potentiate endogenous stem cell homing and recruitment. In particular, a growing body of preclinical investigations indicates that increasing the concentrations of some of these specific chemokines systemically and/or at sites of tissue damage, such as via vehicle‐aided localized delivery or biomaterial presentation, is a state‐of‐the‐art strategy to amplify wound healing and tissue regeneration 22. With respect to in situ tissue regeneration, the therapeutic outcome of substance P, SDF‐1α, SCF, G‐CSF and MCP‐3 will be selectively discussed in this manuscript.
Substance P
When sensory nerves are injured, peripheral sensory neurons release substance P, which is an 11‐amino‐acid peptide and a nociceptive factor that functions as a neuromodulator and neurotransmitter and plays a role in reparative neovascularization via local and systemic actions 44. According to a study by Amadesi and colleagues 45, substance P‐based nociceptive signalling plays an important role in the recruitment of blood‐borne stem cells. They identified a novel regulatory mechanism triggered by limb ischaemia and involving the activation of substance P release from peripheral tissues into the circulation and the modulation (reduction) of substance P content in bone marrow 45. Substance P mediates its effects by preferentially binding and activating the tachykinin receptor NK1. Therefore, the creation of a substance P gradient between the circulation and the bone marrow facilitates the egress of neurokinin 1 (NK1)‐expressing populations from their niche into the peripheral blood; however, such reparative responses after ischaemia can be jeopardized by disruption of NK1 on bone marrow cells 45, 46. Hong et al. 46 showed strong evidence that substance P is a systemically acting messenger of injury that acts as a cell‐stimulating agent early in the tissue healing and repair process to induce the activation of stromal‐like CD29(+) cells from the bone marrow into the circulation in a corneal alkali burn model and to engage in tissue repair. Interestingly, a combination of the systemic injection of substance P and the local delivery of SDF‐1α from a biomatrix synergistically increased the trafficking and penetration of endogenous stem cells into the scaffold 47. In addition to systemic delivery, substance P has been widely applied in biomaterials to accelerate the endogenous recruitment of native cells for regeneration in various types of tissues 48. For in situ vascular regeneration, the controlled administration of substance P from the electrospun membranes of a vascular graft generated by mixing substance P‐bound poly (l‐lactide‐co‐ε‐caprolactone) (PLCL) and linear PLCL in appropriate proportions enhanced the recruitment of human BMMSCs, leading to the regeneration of abundant blood vessels in the explants 49. Similarly, in a rat knee model, self‐assembled peptide matrices coupled with substance P inhibited the progression of osteoarthritis by recruiting host MSCs to the site of insult 50. The rapid release of substance P along with the slow delivery of bone morphogenetic protein (BMP)‐2 was achieved using a heparin‐conjugated fibrin gel that enabled prompt cell recruitment during the first stage and long‐term in situ cell differentiation during the second stage; both features are key to ensuring effective bone regeneration 51. In a mouse model, substance P alone induced the recruitment of MSCs to the site of the ischaemic hindlimb; combined local and systemic substance P delivery resulted in a synergistic outcome, with greater cell recruitment compared with a single treatment, and effective regeneration was achieved without the injection of exogenous cells 52. Recently, substance P has been utilized in more targeted biomaterial designs. For example, a small‐diameter PLCL vascular graft with covalent binding of substance P and heparin was devised as a cell‐free strategy for in situ vascular regeneration; substance P was bound to recruit endogenous reparative cells, while heparin was conjugated to suppress thrombogenic responses by inducing microphages (Mφs) to polarize into the M2 phenotype 53. Aside from increasing cell migration and enhancing the egression of host MSCs into the peripheral blood, substance P increased cell proliferation and facilitated the large‐scale cultivation of cells, thereby shortening the in vitro propagation while sustaining the active state of MSCs 54. Furthermore, substance P exhibits the potential to rescue the weakened immunosuppressive functions of MSCs arising from long‐term culture. In this context, substance P may boost the ability of MSCs to produce TGF‐β1, thus eliminating alterations in the innate therapeutic potential of MSCs prior to their application in therapy 55. These findings suggest that substance P can play critical roles in the future in vitro expansion and manufacture of cellular materials.
SDF‐1α
Under in vivo physiological conditions, quiescent haematopoietic stem cells (HSCs) or haematopoietic progenitor cells are maintained in bone marrow stroma through chemokine signalling between the chemotactic factor SDF‐1α, also termed chemokine (C‐X‐C motif) ligand 12 (CXCL12), and the G protein‐coupled receptor C‐X‐C chemokine receptor 4 (CXCR4) 56. In addition to confining HSCs in their proper niche, the unique SDF‐1/CXCR4 signalling has been investigated frequently due to its pivotal role in the modulation of HSC homing and the subsequent engraftment following HSC transplantation. It is now clear that the interplay between SDF‐1 and CXCR4 is an essential factor that promotes the engraftment and survival of outside infused HSCs; such pleiotropic effects render this unique signalling initiator applicable not only for the restoration of haematopoiesis but also for the design of innovative therapies to achieve regeneration of damaged tissues 57. In fact, SDF‐1/CXCR4 signalling also contributes to the homing responses of many other mesenchymal cell populations. Because a number of progenitor/stem cells migrate towards the SDF‐1 gradient, SDF‐1 has been used as a representative chemotactic factor, alone or in combination, to induce the recruitment and homing of endogenous stem cells to sites of injury within the body (reviewed in 40). In a rat model of stroke, SDF‐1α expression was increased in the ischaemic region following systemic administration of polymeric micelles incorporating SDF‐1α, thereby leading to an increase in endothelial progenitor cell (EPC) homing 58. SDF‐1 is most often administered using biomaterials as local systems rather than using systemic approaches. In this context, the incorporation of exogenous SDF‐1 into a chitosan/poly(γ‐glutamic acid) (γ‐PGA) complex, for example, can generate a high concentration gradient that drives efficient stem cell migration into the biomaterial 59. Analogously, using a knitted silk‐collagen sponge as the SDF‐1 carrier when targeting tendon regeneration can improve local endogenous SDF‐1 expression at the target site can lead to increased recruitment of fibroblast‐like cells and tendon ECM production 60. Lim et al. 61 engineered a multifunctional biomaterial composed of injectable hydrogels and SDF‐1α‐loaded nanoparticles for injection into cavitary brain lesions. This device offered both chemotactic cues and structural support for recruiting endogenous neural progenitor cells and enhancing neural tissue repair/regeneration 61. For the sustained and long‐term release of SDF‐1α, we designed a platform featuring thermo‐responsive drug release properties due to poly(N‐isopropylacrylamide) (PNIPAAm) gates grafted on its outer pore surfaces that provided a swollen‐shrunken property in response to temperature changes 62 (Fig. 3). Recently, an SDF‐1α‐loaded silk fibroin scaffold was designed that could mediate dental pulp stem cell migration and improve de novo pulp regeneration in pulpectomized mature teeth in a canine model 63. Likewise, SDF‐1 effectively promoted the regeneration of cartilage defects when delivered via a radially oriented collagen scaffold 64. When SDF‐1α was engineered with a collagen‐binding domain, intramyocardial injection of the resultant recombinant chemokine led to improved cardiac function after myocardial infarction in rats because this fusion protein and its controlled release mobilized and recruited sufficient endogenous reparative cells to the ischaemic heart 65.
Figure 3.
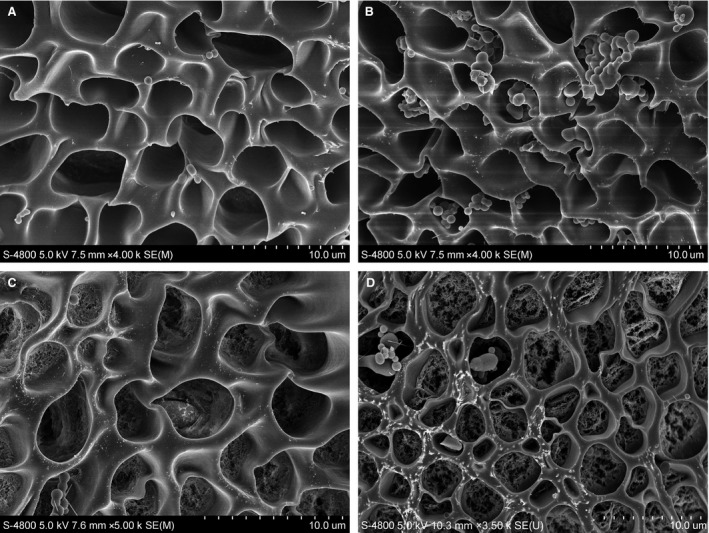
A platform featuring temperature‐controlled drug release properties due to thermo‐responsive gates grafted on their outer shell (representative SEM images of similar material devices with a tailored framework in terms of porosity and pore size as described in 62). (A) The macroporous pore structures of the platform; (B) growth factor‐loaded microparticles incorporated into the pore structures of the material; (C) surface engineering of pores with PNIPAAm gates; (D) opening of the engineered gates at temperatures above their lower critical solution temperature for drug release when PNIPAAm chains are in the shrunken state.
For in situ regeneration of a specific target, SDF‐1 is commonly combined with other therapeutic agents, and cooperative effects continue to be identified; some examples include the use of SDF‐1 in combination with TGF‐β1 and BMP‐2 for site‐targeted cell trafficking and tissue‐specific differentiation 66, with BMP‐7 for tooth and periodontal regeneration 67, with simvastatin for bone regeneration 68, with an angiogenic peptide (Ac‐SDKP) for chronic myocardial infarction regeneration 69 and with a Mφ recruitment agent for enhanced wound closure in a mouse skin wound defect 70. With the aid of biomaterials and drug delivery vehicles, multiple molecules can be embedded and released in a sequential and controlled manner; hence, a synergistic effect is expected. For example, by functionalizing the scaffold with SDF‐1 via physical adsorption, an initial quick release of the adsorbed homing agent can lead to a rapid homing of stem cells to the implanted sites during the first few days. To modulate cell differentiation and promote bone formation, slow and long‐term release of BMP‐2 from the scaffold is required. To achieve this goal, BMP‐2 can initially be loaded into particle delivery systems (e.g. microspheres), and these particles are subsequently introduced into the inner structure of the scaffold; such a design ensures the release of drug for as long as several weeks. In vivo data have shown promise that the sequential and controlled release of these two factors from well‐designed biomaterials may be able to regenerate calvarial critical size defects in rats without cell delivery 71. Although all studies have consistently suggested that SDF‐1 can effectively attract MSCs, in the in vivo milieu, this chemokine tends to be present in an inactive state due to protease cleavage, normally by CD26/dipeptidylpeptidase‐4 (DPP‐4) and matrix metalloproteinase‐2 (MMP‐2). To ensure the targeting of more reparative cells to damaged tissue, Kanki and colleagues created an engineered SDF‐1 in an MMP‐2/DPP‐4‐resistant form, termed SSDF‐1(S4V), and they found that this version of SDF‐1 could not be inactivated and was highly stable. In contrast to SDF‐1, which may be cleaved by DPP‐4, direct injection of protease‐resistant SSDF‐1(S4V) into an injured rat heart recruited more reparative cells to the damaged heart, leading to a dramatic improvement in ventricular function and angiogenesis 72. A similar study by Huber and colleagues suggested that as a DPP‐4 inhibitor, parathyroid hormone (PTH) can be used to prevent DPP‐4‐induced SDF‐1 inactivation and thereby promote SDF‐1‐driven cell mobilization and homing 73. With further tuning of the release profile of SDF‐1 from biomaterial devices and their therapeutic bioactivity, this chemotactic factor may have a clinically relevant impact on tissue repair and regeneration.
G‐CSF
G‐CSF, also termed granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), was initially developed for the treatment of neutropenia after cytotoxic therapy and is approved for use in patients to prevent infection‐associated complications (e.g. during antineoplastic therapy) 74. However, increasing evidence has suggested that G‐CSF possesses an autocrine protective signalling mechanism for neuroprotection in response to neural injury via inhibiting apoptosis and inflammation. Moreover, G‐CSF may participate in neural tissue repair through stimulating neurogenesis, indicating an important non‐hematopoietic function of this biofactor 75. In fact, G‐CSF is another stem cell mobilization‐accelerating factor that stimulates the activation and egress of HSCs and BMMSCs from their niche into the bloodstream 76. After myocardial infarction, G‐CSF treatment increased the number of resident cardiac cells but reduced the capacity of BMMSCs to migrate into ischaemic tissue 77, 78. To optimize the homing capacity of BMMSCs associated with improved survival and cardiac function, G‐CSF must be combined with other agents 79. In strategies focused on in situ tissue regeneration, beneficial outcome was achieved via either local injection of G‐CSF or the incorporation of this factor into the implanted biomaterial. Local administration of G‐CSF for 14 days induced circulating EPC mobilization and recruitment to the implanted small‐diameter heparinized decellularized vascular graft, thus facilitating the generation of endothelium in the graft and the inhibition of neointimal hyperplasia 80. Similarly, single intramuscular administration of a G‐CSF‐encapsulated PEG diacrylate‐poly(ethylene imine) hydrogel scaffold extended mononuclear cell mobilization and enhanced EPC mobilization into the blood 81. In combination with G‐CSF, plerixafor (AMD3100), a CXCR4 antagonist, was evaluated in murine and human systems; the synergistic effect of AMD3100 and G‐CSF on cell mobilization led to an enhanced number of mobilized cells 82 and to significant stimulation of angiogenesis for the treatment of acute hindlimb ischaemia 83. Nonetheless, after the onset of myocardial infarction, single‐dose AMD3100 administration also increased circulating counts of EPCs and augmented their recruitment to the neovasculature, thereby improving cardiac neovascularization and functional recovery 84. Recently, the release of G‐CSF or other inflammatory cytokines from polymer matrices was shown to mediate the accumulation of dendritic cells into the material, indicating that the immune response to biomaterials can be modulated via controlled G‐CSF delivery 85, 86.
SCF
SCF is a membrane‐bound soluble growth factor that is expressed on HSCs in either the primitive or the mature state. Like the c‐kit receptor CCL7 (i.e. chemokine (C‐C motif) ligand 7), this growth factor mediates signalling functions, including the proliferative response, cell survival and chemotactic activities. SCF synergizes with other therapeutic agents, such as G‐CSF, to augment cell mobilization and homing in vivo 87. SCF therapy alone can enhance CD34(+) cell yield, and when SCF therapy is combined with filgrastim, it correlates with manageable levels of toxicity for peripheral blood progenitor cell mobilization 88. Most likely via an autocrine pathway, the interplay between this factor and the tyrosine kinase receptor c‐kit plays pivotal roles in preventing vascular smooth muscle cell apoptosis, facilitating homing by circulating SCF(+) cells and developing the neointima with bone marrow‐derived c‐kit(+) progenitors 89, 90, 91. Ischaemia/reperfusion can stimulate cardiac stem cell (CSC) homing to the injured myocardium, and the accumulation of CSCs correlates with increased SCF expression 92. In this context, hyperhomocysteinemia decreases SCF expression via decreasing the activities of NF‐κB, ERK1/2 and p38, further inhibiting CSC recruitment into peri‐infarcted heart tissue 93. Recent work has increasingly revealed the capability of SCF to induce cell migration, angiogenesis and tissue remodelling, paving the way for its use as a potent homing agent in the regeneration of a wide variety of mesenchymal tissues (e.g. dental pulp) 94, 95. In combination with G‐CSF, SCF mobilized a sufficient number of BMMSCs in rat models of acute tubular necrosis and caused cell homing to the site of damage, thereby combating apoptosis and facilitating the regeneration of renal tubular epithelium following insult 79. Moreover, the precise recapitulation of native niche components, such as the covalent immobilization of SCF and SDF‐1α, has helped control the adhesion and spreading of HSCs towards their successful in vitro expansion 96, 97. Indeed, the modulation of SCF/c‐kit signalling controls MSC stemness and differentiation properties 98. Covalently immobilized SCF is a critical component that provides the appropriate sequence of environmental signals to selectively affect the growth of HSCs within a gelatin hydrogel in the laboratory, which is important for cell production in the treatment of blood diseases 99.
MCP‐3
MCP‐3 belongs to the MCP subgroup of the CC chemokine family 100. By binding to different receptors, MCP‐3 activates and promotes the chemotaxis of many types of immune cells, including all types of leucocytes, dendritic cells and natural killer cells 101. MCP‐3 may induce myocardial MSC homing; MCP‐3 overexpression in freshly infarcted myocardium can recruit MSCs and improve remodelling of the cardiac collagen matrix independently of cardiac myocyte regeneration 102. Further evidence suggests that MCP‐3 may be useful for improving cardiac repair by stimulating the migration of circulating angiogenic cells and angiogenesis 103. Recent evidence suggests that the local release of MCP‐1 from instructive, bioresorbable synthetic grafts can mediate the homing of circulating cells and thereby the regeneration of small‐diameter blood vessels in rats 104.
Regulation of stem cell migration for regeneration
The above discussion includes only a few examples of the currently investigated chemokines that demonstrate promise for inducing host stem cell recruitment. Although each signalling molecule may act on multiple cell types, stromal‐like cells in the bone marrow are more likely to be activated by substance P 45, 46, while SDF‐1α contributes largely to the homing and engraftment of HSCs in their central niche 56, 57. In contrast, G‐CSF may stimulate the release of both HSCs and BMMSCs from their niches into the bloodstream 76, 77, 78, whereas MCP‐3 induces the homing of myocardial MSCs 89, 90, 91. Specifically, SCF plays a crucial role in facilitating the homing of circulating SCF(+) cells 102, 103. Many other growth factors or biological agents, such as BMPs, TGF‐βs, insulin, fibroblast growth factors and hepatocyte growth factor, can also stimulate MSC recruitment, alone or in various combinations 66, 67, 68, 105, 106. In recent years, two reviews have specifically discussed the recruitment and homing outcome of various types of chemoattractants, including chemokines and growth factors 22, 43. In addition to those previously described chemoattractants with decisive roles in tissue‐specific reparative processes, distinct homoeostatic chemokines have also been implicated in vasculogenesis and tissue development, where they serve as guiding cues that orchestrate directional stem and progenitor cell activation and migration 107. Human MSCs migrate upon activation by CXCL8 (interleukin‐8, IL‐8) but not CCL2 (MCP‐1). Based on a 96‐well chemotaxis assay, CXCR4 and CXCR1/2 ligands (SDF‐1 and IL‐8), but not the CC chemokine receptor 2 (CCR2) ligand CCL2, have dose‐dependent chemotactic effects on human MSC recruitment 108. Using an in vitro model of peripheral tissue (human pancreatic islets), Sordi and colleagues showed that the released factors present in islet supernatants can stimulate chemotaxis of BMMSCs; this stimulation was further demonstrated to be largely regulated by chemokine (C‐X3‐C motif) ligand 1 (CX3CL1) and CXCL12 (SDF‐1α) 109. In cell biological assays, a distinct set of biofunctional ligands (CCL2/4/5/20, CXCL8/12 and CX3CL1) attracted BMMSCs; however, only CXCL12 (SDF‐1α) could induce cytoskeletal F‐actin polymerization 110. In addition to CCR4/7/10 and CXCR5, CXCR4 mRNA is also expressed in primary isolates of CD34(‐) progenitors and immortalized MSC lines; this SDF‐1 receptor was not detected on the surfaces of those cells 111. Apart from the SDF‐1 receptor CXCR4, MSCs isolated from bone marrow also express tyrosine kinase receptors, such as receptors for platelet‐derived growth factor and insulin‐like growth factor (IGF), as well as the RANTES and Mφ‐derived chemokine receptors CCR2/3/4 112. Based on microarray analysis, molecules such as CXCL1‐3/6/8, post‐transcriptional gene silencing 2 (PTGS2), phosphodiesterase 4B (PDE4B) and transglutaminase 2 (TGM2) are directly involved in cell migration. Other factors, such as phospholipase D1 (PLD1) and IGF‐binding protein 1 (IGFBP1), may participate in membrane and cytoskeletal reorganization. Additionally, PLD1 contributes to cell polarity, while CXCL1‐3/8 and PDE4B contribute to chemotaxis and the recruitment of cells from the bone marrow 113. Furthermore, chemotaxis assays have revealed that the chemokines CXCL11 and CXCL10 significantly attract human MSCs, while CCL16, CCL18 and CCL27 demonstrated no chemotactic effects on MSCs 114. Recently, CCL20/25 and CXCL9/16 were found to significantly enhance the transendothelial migration of MSCs across aortic endothelial cells in rats, and the transmigrated MSCs exhibited a downregulation of receptors such as CCR6, CCR9, CXCR3 and CXCR6 115.
Collectively, human BMMSCs show detectable chemotaxis towards a wide variety of chemokines, including CCL2/3/5/7/17/19–22/CCL25/CCL28, CXCL8–13/16 and CX3CL1 [43, 109, 110, 111, 112, 113, 114, 116]. Specifically, the chemokine CXCL12 (SDF‐1α) is constitutively detected in the bone marrow and probably represents the most prominent cell homing factor, attracting a wide range of stem or progenitor cells. Although the action of CXCL12 (SDF‐1α) via CXCR4 in the bone marrow milieu is key for retaining HSCs in a quiescent state and maintaining a number of distinct cell populations, the molecular events involved in cellular chemotaxis and migration remain largely unknown 117. Currently, 30 differentially expressed genes (6 repressed and 24 induced) have been detected by microarray analysis, and 11 of these differentially expressed genes are involved in the molecular pathways of cytokine‐cytokine receptor interactions and cellular movement 118. Notably, CXCL12 (SDF‐1α) also signals through the receptor CXCR7; however, no evidence supports the involvement of CXCR7 in signalling pathways that mediate cell migration 119.
Regulation of the selective mobilization of subsets of progenitors from the bone marrow, such as endothelial and stromal progenitors, depends on the cytokine microenvironment, which modulates cell retention and proliferation. By disrupting the CXCR4/SDF‐1α axis, for example, G‐CSF stimulates HPC release into the bloodstream 76. Following pre‐treatment of mice with vascular endothelial growth factor (VEGF), the CXCR4/SDF‐1α retention axis was not disrupted, and HPCs were not mobilized, but the entry of these cells into the cell cycle was stimulated via VEGF receptor 1 120. In contrast, enhanced EPC mobilization via VEGF receptor 2 occurs in response to CXCR4 antagonism following VEGF pre‐treatment. Furthermore, in VEGF‐pre‐treated mice, administration of a CXCR4 antagonist produced detectable stromal progenitor cell mobilization, whereas G‐CSF administration did not 120, 121. The regulatory mechanisms underlying cell activation and recruitment from bone marrow can be further exploited to develop efficacious therapeutic paradigms that harness discrete populations of stem/progenitor cells for tissue regeneration.
In the last decade, much of our current knowledge on stem cell homing and accommodation has been limited to how soluble biofactors, such as chemokines and cytokines, influence resident cells. Strategies for the administration of chemoattractants and chemical signals systemically and/or at the desired destination are critically important for enhancing the endogenous regeneration process because the presentation and release of signalling factors make the site of injury and/or inflammation more attractive to stem cells and hence can dictate stem cell homing 40, 122, 123 (Fig. 4). Guan and colleagues developed a peptidomimetic ligand named LLP2A that demonstrates high specificity and affinity for activated α4β1 integrin 124. Integrins are critical for cell movement and play essential roles in directing HSCs to bone 125. Therefore, the injection of the ligand LLP2A may drive MSCs to the surface of bone. When this ligand was further conjugated to alendronate to engineer the hybrid compound LLP2A‐Ale and tested in animals, the rate of bone formation in both xenotransplantation studies and immunocompetent mice was significantly increased via a single intravenous administration of LLP2A‐Ale 124. Because alendronate is a bisphosphonate with high affinity for bone, it functions as the bone‐tracking constituent that can recruit LLP2A to bone 126, 127. Indeed, the promotion of cell recruitment and regeneration can also be achieved via the co‐delivery of silicon ions and growth factors, which may exert a synergistic effect on endogenous stem/progenitor cells 128.
Figure 4.

Schematic representation of strategies employed for the systemic administration of chemoattractants and chemical signals to mobilize and recruit stem cells from the circulatory system and/or the localized presentation and release of homing factors at the site of injury. Localized administrated signals can coax stem cell migration from neighbouring healthy tissue based on signalling gradients that are commonly established by the implantation of a well‐designed biomaterial platform. Endogenous cells recruited from either the circulatory system or a local cell niche can participate in tissue regeneration at the injured site.
Recently, extraordinary accomplishments have been achieved in the development of biomaterial vehicles that are capable of incorporating and releasing selected chemokines for therapeutic tissue regeneration based on cell recruitment and homing 17, 22. However, scientific and technological challenges in material design and drug delivery must be overcome to obtain an accurate mimic of the natural wound healing cascade. Although a wide range of developed drug delivery systems may also be used for the presentation and release of homing factors (e.g. see Fig. 5) 62, 129, 130, new insights into the effects of the doses of chemokines and their concentration gradients on cell mobilization and trafficking will enable the precise development of future endogenous regenerative therapies 131. Moreover, the complexity of the in vivo milieu underscores the importance of considering not only the correlation among cargo release kinetics, optimal time‐points and cell recruitment efficacy but also other environmental factors such as ECM stiffness, architecture and composition, which can jointly impact stem cell fates. Indeed, new findings continue to generate concern regarding how biomaterial cues may control cell geometry and how a diverse array of biophysical factors may be transmitted into the cell 132. Thus, regulating cell activity requires the reestablishment of an in vivo environment with a suitable hierarchical structure and topography at the nanoscale level and with proper mechanical properties 133.
Figure 5.
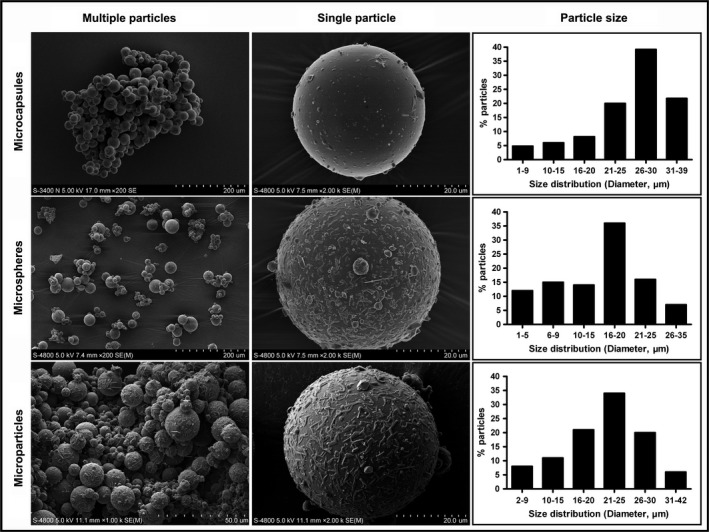
Microparticulate delivery systems for the controlled presentation and release of various bioactive factors for scaffold development and/or regenerative therapy applications (representative SEM images of similar microparticulates fabricated in our laboratory with tailored particle sizes as described in 19, 62, 129, 130).
Increasing the surface sensitivity of stem cells to homing factors
As an alternative to delivering chemical signals, ions or other chemokines through a biomaterial platform, augmented cell homing can be achieved by making the surfaces of the targeted cells more responsive to specific homing inducers, typically via chemical modification and/or genetic engineering 134, 135, 136. The development of tools for enhancing cell retention is feasible for increasing targeting efficiencies and has become the research focus of many current endeavours in cell‐based therapy 137, 138. Although cell modification cannot be incorporated into in situ tissue‐engineering strategies without transplanting ex vivo‐expanded stem cells, this strategy offers an important tool for studying or regulating in vivo cell homing processes and would be useful for the future design of endogenous regenerative approaches 139. On the other hand, it is clear that homing of the patient's own cells may be restricted by ageing, inflammation or disease. In the ageing population, for example, the number of resident reparative cells is intrinsically insufficient for mobilization, and those cells may intrinsically have poor migration and regenerative potentials for tissue regeneration. In such cases, the delivery of outside expanded cells would be necessary to achieve a therapeutically regenerative solution 74. Interestingly, there is evidence that cell transplantation can act as an initiator to trigger endogenous regenerative process, typically via the secretion of growth factors or various other cytokines that enhance migration of endogenous stem cells to the lesion site and improve their function for integration, angiogenesis and neovascularization 140, 141, 142. In this respect, cell transplantation would not be excluded from the field of in situ tissue regeneration.
Systemic infusion is a typical and convenient strategy that maximizes practical aspects of repeated doses and minimizes the invasiveness of cellular therapy; however, this delivery mode cannot guarantee engraftment efficiency 137, 138. For clinical applications of adoptive cellular therapy to be successful, the ability to deliver a sufficient number of viable cells to a predetermined anatomical compartment with a high efficiency of cell engraftment or the ability to drive the recruitment of blood‐borne cells to sites where they are needed is critical and presents a substantial challenge 143. In local implantation models, the expression of specific homing factors has also shown promise for enhancing the homing of culture‐expanded osteogenic cells into bone fracture sites 144. In some cases, cells are genetically modified via the introduction of specific gene sequences prior to transplantation; once these cells live in an in vivo milieu, they may express factors that induce recruitment, subsequently promoting the homing of endogenous stem cells 40. For example, the use of an adenovirus to overexpress SDF‐1 in stem cells increased the effectiveness of native cell trafficking to the bone defect and hence led to enhanced bone regeneration at the site of fracture by promoting osteogenic differentiation and bone production 145. Analogously, SDF‐1 has a stimulatory effect on stem cell recruitment to the bone defect when using adipose tissue grafts that were adenovirally activated to express SDF‐1α and/or BMP‐2 146. Further, gene‐activated scaffolds delivering both VEGF and BMP‐2 produced much greater vascularization and bone regeneration than did either a single delivery or a blank control system 147. Although cells overexpressing other factors have been used as therapeutics in a number of similar studies, insufficient evidence supports enhanced tissue regeneration as an outcome of increased recruitment of host cells in response to their transplantation 69, 148, 149. Indeed, genetically engineered cells that exhibit prolonged expression of therapeutic agents themselves may have an enhanced regenerative potential at the site of action 150.
Although modification of cellular binding sites may be a viable approach to facilitating cell homing to a tissue of interest, current strategies, such as gene transfection, are practically complex and have potential safety concerns 137. In addition, these approaches do not offer a universal solution; that is, they are either not adaptable to different type of cells or unable to accommodate a vast array of ligand molecules. Immense effort is currently focused on making genetic modification simple and safe. Using lipid vesicles, Sarkar and colleagues reported a simple method to transiently modify cell surfaces, resulting in the ability to efficiently immobilize molecular ligands on cell surfaces for targeting to the inflamed site following systemic administration 134. Likewise, preconditioning stem cells with Ro‐31‐8425, an identified hit from a small‐molecule screen, increases firm cell adhesion to an ICAM‐1‐coated substrate in vitro. Further experiments demonstrated that such chemical modification enabled the systemic infusion and targeted recruitment of MSCs to an inflamed region in vivo in a CD11a‐dependent (and other ICAM‐1‐binding domain‐dependent) manner 151. Both studies imply that surface modifications of MSCs may help the cells find their way to a tissue of interest or to the bone marrow and potentially yield high‐efficiency targeting of other cell types to specific tissue areas via the bloodstream 135.
For stem cells to successfully home to sites of injury, a sequence of coordinated and regulatory interplays between a cell and its microenvironment provides signs that the signal is reaching the cell along its journey. Chemokines are the most important factors that regulate cell migration in vitro and in vivo, and MSCs move in response to a CXCL12 (SDF‐1α) gradient 22, 117. However, the in vitro chemotactic response of human MSCs to thymus‐expressed chemokine (TECK), or CCL25, is more than 10‐fold greater than that to CXCL12 (SDF‐1α), indicating that TECK is a more potent in vitro chemoattractant for MSCs 113. MSCs without preconditioning show very low CXCR4 surface expression; hence, the in vitro SDF‐1‐directed migration potential for human MSCs is quite low 111. However, the chemokine receptor expression and chemotaxis of MSCs can be increased by shear stress or by hypoxic/proinflammatory preconditioning 112. Hypoxic preconditioning of BMMSCs before transplantation increases the expression of CXCR4/7; facilitates in vitro cell adhesion, migration and survival; and ameliorates the cells’ capacity to survive and engraft at the site of interest 152. Further results indicate that hypoxia inducible factor (HIF)‐1α plays very important roles in hypoxia‐induced cell activation and movement, most likely acting via its downstream genes SDF‐1α and VEGF 153. Additionally, short‐term stimulation of BMMSCs with combinations of various cytokines demonstrated up‐regulated cell surface and intracellular CXCR4 expression, leading to improved cell trafficking potential in vitro and in vivo 154. Although numerous investigations have revealed that either a hypoxic or an inflammatory stimulus may increase the capacity for cell migration, dual stimuli combined with hypoxia and inflammation do not necessarily lead to a synergistic effect 155, 156. Further strategies to increase and control cell migration in vivo and to control this process may bring us closer to reaching the original goals of stem cell therapy.
Challenges and opportunities
The rising incidence of tissue insult caused by trauma, infection and/or destructive disease has led to an increasing demand for new, safe, effective and practical therapies for clinical use; this situation is exacerbated by an increasingly ageing population. In this context, the last two decades have witnessed remarkable laboratory‐based and preclinical success in the development of tissue‐engineering therapies 157, 158, 159. Unfortunately, very few of these novel therapeutics are being used in clinical applications 160. For clinical translation and population‐wide applications, constructs engineered in the laboratory based on stem cells in combination with signalling factors and/or material scaffolds will probably be deemed unrealistic because of the time, cost and indeed numerous regulatory issues 122, 161. In situ tissue regenerative procedures offer great potential for broader clinical application and, more importantly, would only rely on off‐the‐shelf scaffolds without the need for ex vivo‐manipulated stem cells, thus reducing the time and cost in comparison with the classical tissue‐engineering approach 24, 159. With the aid of signalling molecules, such biomaterial devices should mimic the native nanoenvironment of regenerating tissues as closely as possible and thereby unlock the innate regenerative capacities of the body for therapeutic regeneration applications 159, 161, 162.
Functionally, issues remaining to be addressed include the chemotaxis of reparative stem cells following material transplantation, the amplification of these cells (via proliferation) as a transient pool, and the local functions of these cells within the materials, particularly considering secretion, concerted differentiation and remodelling 22. Increasing the migratory capacity of reparative cells, the sensitivity of the target sites and hence the efficiency of stem cell homing is important for accelerating tissue repair and regeneration. Unfortunately, we still do not know which cell type is most likely to be recruited to a site of injury or how different cell types collaborate during cell homing and regeneration. Therefore, further investigation into the mechanisms underlying both stem cell homing and cell‐mediated regeneration may not only facilitate the development of effective yet simple endogenous regenerative therapies but also eventually expedite progress in many related fields, such as biomaterials science and in situ tissue engineering 74. Intensive effort has been and continues to be directed towards this field, but success is not a simple proposition. In terms of chemokine presentation, we need to control the interaction between the incorporated protein and the material in order to slow their diffusive release, considering the protein itself, the vehicle for delivery and the microenvironment targeted for therapy 162, 163. With respect to cell movement, numerous static and dynamic (transendothelial) in vitro migration models have been used to study cell chemotaxis; however, a discrepancy between primary and ex vivo‐expanded MSCs in terms of mobilization or homing has been identified based on the observation that the cell phenotype undergoes dramatic changes during the in vitro expansion phase 164. Currently, no innovative culture system can better characterize different cell types in their native and in vitro environments, and we still lack a reliable approach for in vivo monitoring of cell trafficking to an injury site. Based on in vivo microscopy, labelled cells can now be tracked in an animal after transplantation. Intravital microscopy can visualize labelled cells in a given tissue or organ after surgical dissection, thus revealing the in vivo chemokine‐directed interactions between MSCs and the endothelium and the subsequent cell migration within the ECM 22, 165. However, we still cannot identify native MSCs and track their chemotaxis in situ, and the actual roles of endogenously recruited cell populations in tissue regeneration remain unclear 166. Advancements in in vitro and in vivo model investigations will be rewarded with increased potential across the arena of stem cell biology and therapy 137, 167.
As the implantation of outside devices into the in vivo milieu often produces inflammatory states, insights into the impacts of a variety of environmental stimuli generated by implants on Mφ phenotype and function may offer new information for future materials design 168, 169, 170, 171. Because the cellular response to chemokines is affected by the pathophysiological state and the status of the tissue insult, another exciting and emerging area related to the endogenous regeneration paradigm is immuno‐engineering, in which material devices can be utilized to modulate immune responses (i.e. the balance between immunosuppressive and immunostimulatory responses), to therapeutically stimulate receptor complexes and cells, or as vaccines for the delivery of multiple immunomodulatory molecules 172. Directed stem cell homing thus provides a new avenue for research into regeneration, and the combination of immuno‐engineering and in situ tissue engineering will continue to provide new and more effective and treatments to address the problems currently encountered in the clinic 161, 169.
Conclusions
The local use of signalling molecules at the area of injury to drive stem cell homing in vivo is a new therapeutic strategy that holds great potential for achieving functional tissue regeneration without the need for the delivery of ex vivo‐expanded cells. To exploit homing mechanisms therapeutically, we must identify the complex interactions and pathways underlying the cascades of cellular events involved in wound healing and regeneration. Additionally, suitable animal models for tracking and evaluating endogenous reparative cells in vivo must be established to reveal the chemokine signalling and activity underlying directed cell recruitment. For this strategy to be successful, further investigations are required to ensure that the recruited cells propagate and divide into appropriate cell types for regeneration. In conclusion, the regulation of stem cell homing as a new paradigm for regeneration is still in its infancy. Further optimization of current material platforms that deliver potent signals for stem cell mobilization and homing and a better understanding of the priming or upstream signals involved in functional in situ tissue regeneration are necessary before an effective therapeutic outcome can be translated into the clinic for patient health care.
Authors’ contributions
All authors participated in the drafting, writing and revision of the manuscript and gave final approval for publication of the work.
Ethics approval and consent to participate
Not applicable.
Conflict of interests
The authors confirm that there are no conflict of interests.
Acknowledgements
None.
Funding source: This work was supported in part by the Changjiang Scholars Program of Ministry of Education of the People’s Republic of China (2016) and the National Natural Science Foundation of China (Nos. 81530050 and 81471791).
References
- 1. Griffith LG, Naughton G. Tissue engineering–current challenges and expanding opportunities. Science. 2002; 295: 1009–14. [DOI] [PubMed] [Google Scholar]
- 2. Morrissey MB, Herr K, Levine C. Public health imperative of the 21st century: innovations in palliative care systems, services, and supports to improve health and well‐being of older Americans. Gerontologist. 2015; 55: 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016; 53: 86–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeannet M, Pinn VW, Flax MH, et al Humoral antibodies in renal allotransplantation in man. N Engl J Med. 1970; 282: 111–7. [DOI] [PubMed] [Google Scholar]
- 5. Starzl TE, Fung J, Tzakis A, et al Baboon‐to‐human liver transplantation. Lancet. 1993; 341: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones JW, Gruber SA, Barker JH, et al Successful hand transplantation. One‐year follow‐up. Louisville hand transplant team. N Engl J Med. 2000; 343: 468–73. [DOI] [PubMed] [Google Scholar]
- 7. John R, Rajasinghe H, Chen JM, et al Impact of current management practices on early and late death in more than 500 consecutive cardiac transplant recipients. Ann Surg. 2000; 232: 302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo S, Han Y, Zhang X, et al Human facial allotransplantation: a 2‐year follow‐up study. Lancet. 2008; 372: 631–8. [DOI] [PubMed] [Google Scholar]
- 9. Siemionow M, Papay F, Alam D, et al Near‐total human face transplantation for a severely disfigured patient in the USA. Lancet. 2009; 374: 203–9. [DOI] [PubMed] [Google Scholar]
- 10. Daar AS. The future of replacement and restorative therapies: from organ transplantation to regenerative medicine. Transplant Proc. 2013; 45: 3450–2. [DOI] [PubMed] [Google Scholar]
- 11. Wilkinson D, Savulescu J. Should we allow organ donation euthanasia? Alternatives for maximizing the number and quality of organs for transplantation. Bioethics. 2012; 26: 32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma A, Armstrong AE, Posner MP, et al Graft‐versus‐host disease after solid organ transplantation: a single center experience and review of literature. Ann Transplant. 2012; 17: 133–9. [DOI] [PubMed] [Google Scholar]
- 13. Pai S‐Y, Logan BR, Griffith LM, et al Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014; 371: 434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007; 213: 341–7. [DOI] [PubMed] [Google Scholar]
- 15. Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012; 10: 740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012; 10: 362–9. [DOI] [PubMed] [Google Scholar]
- 17. Wu R‐X, Yin Y, He X‐T, et al Engineering a cell home for stem cell homing and accommodation. Advanced Biosystems. 2017; 1: 1700004 https://doi.org/10.1002/adbi.201700004. [DOI] [PubMed] [Google Scholar]
- 18. Yin Y, Li X, He XT, et al Leveraging stem cell homing for therapeutic regeneration. J Dent Res. 2017; 96: 601–9. https://doi.org/10.1177/0022034517706070. [DOI] [PubMed] [Google Scholar]
- 19. Chen FM, Wu LA, Zhang M, et al Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011; 32: 3189–209. [DOI] [PubMed] [Google Scholar]
- 20. Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009; 8: 457–70. [DOI] [PubMed] [Google Scholar]
- 21. Miller FD, Kaplan DR. Mobilizing endogenous stem cells for repair and regeneration: are we there yet? Cell Stem Cell. 2012; 10: 650–2. [DOI] [PubMed] [Google Scholar]
- 22. Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine‐guided stem cell recruitment. Trends Biotechnol. 2014; 32: 483–92. [DOI] [PubMed] [Google Scholar]
- 23. Chen FM, An Y, Zhang R, et al New insights into and novel applications of release technology for periodontal reconstructive therapies. J Control Release. 2011; 149: 92–110. [DOI] [PubMed] [Google Scholar]
- 24. Ko IK, Lee SJ, Atala A, et al In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013; 45: e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011; 32: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007; 21: 3917–27. [DOI] [PubMed] [Google Scholar]
- 27. Meleshko A, Prakharenia I, Kletski S, et al Chimerism of allogeneic mesenchymal cells in bone marrow, liver, and spleen after mesenchymal stem cells infusion. Pediatr Transplant. 2013; 17: E189–94. [DOI] [PubMed] [Google Scholar]
- 28. Bentzinger CF, von Maltzahn J, Dumont NA, et al Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. J Cell Biol. 2014; 205: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ezquer FE, Ezquer ME, Vicencio JM, et al Two complementary strategies to improve cell engraftment in mesenchymal stem cell‐based therapy: increasing transplanted cell resistance and increasing tissue receptivity. Cell Adh Migr. 2017; 11: 110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santo VE, Gomes ME, Mano JF, et al Controlled release strategies for bone, cartilage, and osteochondral engineering‐Part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev. 2013; 19: 308–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santo VE, Gomes ME, Mano JF, et al Controlled release strategies for bone, cartilage, and osteochondral engineering‐Part II: challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng Part B Rev. 2013; 19: 327–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bajada S, Mazakova I, Richardson JB, et al Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008; 2: 169–83. [DOI] [PubMed] [Google Scholar]
- 33. Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012; 481: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez RA, Choi S‐J, Han C‐M, et al Biomaterials control of pluripotent stem cell fate for regenerative therapy. Prog Mater Sci. 2016; 82: 234–93. [Google Scholar]
- 35. Lee CH, Cook JL, Mendelson A, et al Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010; 376: 440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang F, Leong W, Su K, et al A Transduced living hyaline cartilage graft releasing transgenic stromal cell‐derived factor‐1 inducing endogenous stem cell homing in vivo . Tissue Eng Part A. 2013; 19: 1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee CH, Rodeo SA, Fortier LA, et al Protein‐releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med. 2014; 6: 266ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee CH, Lee FY, Tarafder S, et al Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 2015; 125: 2690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adam C. Endogenous musculoskeletal tissue engineering‐a focused perspective. Cell Tissue Res. 2012; 347: 489–99. [DOI] [PubMed] [Google Scholar]
- 40. Herrmann M, Verrier S, Alini M. Strategies to stimulate mobilization and homing of endogenous stem and progenitor cells for bone tissue repair. Front Bioeng Biotechnol. 2015; 3: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014; 32: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009; 4: 206–16. [DOI] [PubMed] [Google Scholar]
- 43. Vanden Berg‐Foels WS. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Eng Part B Rev. 2014; 20: 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hong HS, Kim DY, Yoon KJ, et al A new paradigm for stem cell therapy: substance‐P as a stem cell‐stimulating agent. Arch Pharm Res. 2011; 34: 2003–6. [DOI] [PubMed] [Google Scholar]
- 45. Amadesi S, Reni C, Katare R, et al Role for substance p‐based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation. 2012; 125: 1774–86, s1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hong HS, Lee J, Lee E, et al A new role of substance P as an injury‐inducible messenger for mobilization of CD29(+) stromal‐like cells. Nat Med. 2009; 15: 425–35. [DOI] [PubMed] [Google Scholar]
- 47. Ko IK, Ju YM, Chen T, et al Combined systemic and local delivery of stem cell inducing/recruiting factors for in situ tissue regeneration. FASEB J. 2012; 26: 158–68. [DOI] [PubMed] [Google Scholar]
- 48. Kim SH, Hur W, Kim JE, et al Self‐assembling peptide nanofibers coupled with neuropeptide substance P for bone tissue engineering. Tissue Eng Part A. 2015; 21: 1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shafiq M, Jung Y, Kim SH. In situ vascular regeneration using substance P‐immobilised poly(L‐lactide‐co‐epsilon‐caprolactone) scaffolds: stem cell recruitment, angiogenesis, and tissue regeneration. Eur Cell Mater. 2015; 30: 282–302. [DOI] [PubMed] [Google Scholar]
- 50. Kim SJ, Kim JE, Kim SH, et al Therapeutic effects of neuropeptide substance P coupled with self‐assembled peptide nanofibers on the progression of osteoarthritis in a rat model. Biomaterials. 2016; 74: 119–30. [DOI] [PubMed] [Google Scholar]
- 51. Noh SS, Bhang SH, La WG, et al A dual delivery of substance P and bone morphogenetic protein‐2 for mesenchymal stem cell recruitment and bone regeneration. Tissue Eng Part A. 2015; 21: 1275–87. [DOI] [PubMed] [Google Scholar]
- 52. Kim JE, Jung KM, Kim SH, et al Combined treatment with systemic and local delivery of substance P coupled with self‐assembled peptides for a hind limb ischemia model. Tissue Eng Part A. 2016; 22: 545–55. [DOI] [PubMed] [Google Scholar]
- 53. Shafiq M, Jung Y, Kim SH. Covalent immobilization of stem cell inducing/recruiting factor and heparin on cell‐free small‐diameter vascular graft for accelerated in situ tissue regeneration. J Biomed Mater Res A. 2016; 104: 1352–71. [DOI] [PubMed] [Google Scholar]
- 54. Dubon MJ, Park K‐S. Substance P enhances the proliferation and migration potential of murine bone marrow‐derived mesenchymal stem cell‐like cell lines. Exp Ther Med. 2015; 9: 1185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jin Y, Hong HS, Son Y. Substance P enhances mesenchymal stem cells‐mediated immune modulation. Cytokine. 2015; 71: 145–53. [DOI] [PubMed] [Google Scholar]
- 56. Sugiyama T, Kohara H, Noda M, et al Maintenance of the hematopoietic stem cell pool by CXCL12‐CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006; 25: 977–88. [DOI] [PubMed] [Google Scholar]
- 57. Sharma M, Afrin F, Satija N, et al Stromal‐derived factor‐1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev. 2011; 20: 933–46. [DOI] [PubMed] [Google Scholar]
- 58. Kim DH, Seo YK, Thambi T, et al Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor‐1α using a dual ionic pH‐sensitive copolymer. Biomaterials. 2015; 61: 115–25. [DOI] [PubMed] [Google Scholar]
- 59. Goncalves RM, Antunes JC, Barbosa MA. Mesenchymal stem cell recruitment by stromal derived factor‐1‐delivery systems based on chitosan/poly(gamma‐glutamic acid) polyelectrolyte complexes. Eur Cell Mater. 2012; 23: 249–60. [DOI] [PubMed] [Google Scholar]
- 60. Shen W, Chen X, Chen J, et al The effect of incorporation of exogenous stromal cell‐derived factor‐1 alpha within a knitted silk‐collagen sponge scaffold on tendon regeneration. Biomaterials. 2010; 31: 7239–49. [DOI] [PubMed] [Google Scholar]
- 61. Lim TC, Rokkappanavar S, Toh WS, et al Chemotactic recruitment of adult neural progenitor cells into multifunctional hydrogels providing sustained SDF‐1α release and compatible structural support. FASEB J. 2013; 27: 1023–33. [DOI] [PubMed] [Google Scholar]
- 62. Chen FM, Lu H, Wu LA, et al Surface‐engineering of glycidyl methacrylated dextran/gelatin microcapsules with thermo‐responsive poly(N‐isopropylacrylamide) gates for controlled delivery of stromal cell‐derived factor‐1alpha. Biomaterials. 2013; 34: 6515–27. [DOI] [PubMed] [Google Scholar]
- 63. Yang JW, Zhang YF, Wan CY, et al Autophagy in SDF‐1α‐mediated‐mediated DPSC migration and pulp regeneration. Biomaterials. 2015; 44: 11–23. [DOI] [PubMed] [Google Scholar]
- 64. Chen P, Tao J, Zhu S, et al Radially oriented collagen scaffold with SDF‐1 promotes osteochondral repair by facilitating cell homing. Biomaterials. 2015; 39: 114–23. [DOI] [PubMed] [Google Scholar]
- 65. Sun J, Zhao Y, Li Q, et al Controlled release of collagen‐binding SDF‐1α improves cardiac function after myocardial infarction by recruiting endogenous stem cells. Sci Rep. 2016; 6: 26683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chim H, Miller E, Gliniak C, et al Stromal‐cell‐derived factor (SDF) 1‐alpha in combination with BMP‐2 and TGF‐beta1 induces site‐directed cell homing and osteogenic and chondrogenic differentiation for tissue engineering without the requirement for cell seeding. Cell Tissue Res. 2012; 350: 89–94. [DOI] [PubMed] [Google Scholar]
- 67. Kim K, Lee CH, Kim BK, et al Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010; 89: 842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu YS, Ou ME, Liu H, et al The effect of simvastatin on chemotactic capability of SDF‐1alpha and the promotion of bone regeneration. Biomaterials. 2014; 35: 4489–98. [DOI] [PubMed] [Google Scholar]
- 69. Song M, Jang H, Lee J, et al Regeneration of chronic myocardial infarction by injectable hydrogels containing stem cell homing factor SDF‐1 and angiogenic peptide Ac‐SDKP. Biomaterials. 2014; 35: 2436–45. [DOI] [PubMed] [Google Scholar]
- 70. Kim YH, Tabata Y. Recruitment of mesenchymal stem cells and macrophages by dual release of stromal cell‐derived factor‐1 and a macrophage recruitment agent enhances wound closure. J Biomed Mater Res A. 2016; 104: 942–56. [DOI] [PubMed] [Google Scholar]
- 71. Shen X, Zhang Y, Gu Y, et al Sequential and sustained release of SDF‐1 and BMP‐2 from silk fibroin‐nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials. 2016; 106: 205–16. [DOI] [PubMed] [Google Scholar]
- 72. Kanki S, Segers VF, Wu W, et al Stromal cell‐derived factor‐1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011; 4: 509–18. [DOI] [PubMed] [Google Scholar]
- 73. Huber BC, Brunner S, Segeth A, et al Parathyroid hormone is a DPP‐IV inhibitor and increases SDF‐1‐driven homing of CXCR4(+) stem cells into the ischaemic heart. Cardiovasc Res. 2011; 90: 529–37. [DOI] [PubMed] [Google Scholar]
- 74. Ishikawa K, Tanaka H, Matsuoka T, et al Recombinant human granulocyte colony‐stimulating factor attenuates inflammatory responses in septic patients with neutropenia. J Trauma. 1998; 44: 1047–54. [DOI] [PubMed] [Google Scholar]
- 75. Solaroglu I, Jadhav V, Zhang JH. Neuroprotective effect of granulocyte‐colony stimulating factor. Front Biosci. 2007; 12: 712–24. [DOI] [PubMed] [Google Scholar]
- 76. Petit I, Szyper‐Kravitz M, Nagler A, et al G‐CSF induces stem cell mobilization by decreasing bone marrow SDF‐1 and up‐regulating CXCR4. Nat Immunol. 2002; 3: 687–94. [DOI] [PubMed] [Google Scholar]
- 77. Brunner S, Huber BC, Fischer R, et al G‐CSF treatment after myocardial infarction: impact on bone marrow‐derived vs cardiac progenitor cells. Exp Hematol. 2008; 36: 695–702. [DOI] [PubMed] [Google Scholar]
- 78. Huber BC, Beetz NL, Laskowski A, et al Attenuation of cardiac hypertrophy by G‐CSF is associated with enhanced migration of bone marrow‐derived cells. J Cell Mol Med. 2015; 19: 1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bi L, Wang G, Yang D, et al Effects of autologous bone marrow‐derived stem cell mobilization on acute tubular necrosis and cell apoptosis in rats. Exp Ther Med. 2015; 10: 851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhou M, Liu Z, Li K, et al Beneficial effects of granulocyte‐colony stimulating factor on small‐diameter heparin immobilized decellularized vascular graft. J Biomed Mater Res A. 2010; 95: 600–10. [DOI] [PubMed] [Google Scholar]
- 81. Liang Y, Jensen TW, Roy EJ, et al Tuning the non‐equilibrium state of a drug‐encapsulated poly(ethylene glycol) hydrogel for stem and progenitor cell mobilization. Biomaterials. 2011; 32: 2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Broxmeyer HE, Orschell CM, Clapp DW, et al Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005; 201: 1307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Capoccia BJ, Shepherd RM, Link DC. G‐CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006; 108: 2438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jujo K, Hamada H, Iwakura A, et al CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010; 107: 11008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ali OA, Tayalia P, Shvartsman D, et al Inflammatory cytokines presented from polymer matrices differentially generate and activate DCs in situ . Adv Funct Mater. 2013; 23: 4621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Verbeke CS, Mooney DJ. Injectable, pore‐forming hydrogels for in vivo enrichment of immature dendritic cells. Adv Healthc Mater. 2015; 4: 2677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McNiece IK, Briddell RA. Stem cell factor. J Leukoc Biol. 1995; 58: 14–22. [DOI] [PubMed] [Google Scholar]
- 88. Glaspy JA, Shpall EJ, LeMaistre CF, et al Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood. 1997; 90: 2939–51. [PubMed] [Google Scholar]
- 89. Hollenbeck ST, Sakakibara K, Faries PL, et al Stem cell factor and c‐kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004; 120: 288–94. [DOI] [PubMed] [Google Scholar]
- 90. Wang CH, Anderson N, Li SH, et al Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ Res. 2006; 99: 617–25. [DOI] [PubMed] [Google Scholar]
- 91. Wang CH, Verma S, Hsieh IC, et al Stem cell factor attenuates vascular smooth muscle apoptosis and increases intimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2007; 27: 540–7. [DOI] [PubMed] [Google Scholar]
- 92. Guo J, Jie W, Kuang D, et al Ischaemia/reperfusion induced cardiac stem cell homing to the injured myocardium by stimulating stem cell factor expression via NF‐kappaB pathway. Int J Exp Pathol. 2009; 90: 355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wan J, Deng Y, Guo J, et al Hyperhomocysteinemia inhibited cardiac stem cell homing into the peri‐infarcted area post myocardial infarction in rats. Exp Mol Pathol. 2011; 91: 411–8. [DOI] [PubMed] [Google Scholar]
- 94. Pan S, Dangaria S, Gopinathan G, et al SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling‐potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev. 2013; 9: 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ruangsawasdi N, Zehnder M, Patcas R, et al Effects of stem cell factor on cell homing during functional pulp regeneration in human immature teeth. Tissue Eng Part A. 2017; 23: 115–23. [DOI] [PubMed] [Google Scholar]
- 96. Cuchiara ML, Horter KL, Banda OA, et al Covalent immobilization of stem cell factor and stromal derived factor 1alpha for in vitro culture of hematopoietic progenitor cells. Acta Biomater. 2013; 9: 9258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cuchiara ML, Coskun S, Banda OA, et al Bioactive poly(ethylene glycol) hydrogels to recapitulate the HSC niche and facilitate HSC expansion in culture. Biotechnol Bioeng. 2016; 113: 870–81. [DOI] [PubMed] [Google Scholar]
- 98. Suphanantachat S, Iwata T, Ishihara J, et al A role for c‐Kit in the maintenance of undifferentiated human mesenchymal stromal cells. Biomaterials. 2014; 35: 3618–26. [DOI] [PubMed] [Google Scholar]
- 99. Mahadik BP, Pedron Haba S, Skertich LJ, et al The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials. 2015; 67: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Menten P, Wuyts A, Van Damme J. Monocyte chemotactic protein‐3. Eur Cytokine Netw. 2001; 12: 554–60. [PubMed] [Google Scholar]
- 101. Proost P, Wuyts A, Van Damme J. Human monocyte chemotactic proteins‐2 and‐3: structural and functional comparison with MCP‐1. J Leukoc Biol. 1996; 59: 67–74. [DOI] [PubMed] [Google Scholar]
- 102. Schenk S, Mal N, Finan A, et al Monocyte chemotactic protein‐3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007; 25: 245–51. [DOI] [PubMed] [Google Scholar]
- 103. Bousquenaud M, Schwartz C, Leonard F, et al Monocyte chemotactic protein 3 is a homing factor for circulating angiogenic cells. Cardiovasc Res. 2012; 94: 519–25. [DOI] [PubMed] [Google Scholar]
- 104. Talacua H, Smits AI, Muylaert DE, et al In situ tissue engineering of functional small‐diameter blood vessels by host circulating cells only. Tissue Eng Part A. 2015; 21: 2583–94. [DOI] [PubMed] [Google Scholar]
- 105. Filova E, Rampichova M, Litvinec A, et al A cell‐free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int J Pharm. 2013; 447: 139–49. [DOI] [PubMed] [Google Scholar]
- 106. van de Kamp J, Paefgen V, Woltje M, et al Mesenchymal stem cells can be recruited to wounded tissue via hepatocyte growth factor‐loaded biomaterials. J Tissue Eng Regen Med. 2016; Epub ahead of print. https://doi.org/10.1002/term.2201. [DOI] [PubMed] [Google Scholar]
- 107. Anders HJ, Romagnani P, Mantovani A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol Med. 2014; 20: 154–65. [DOI] [PubMed] [Google Scholar]
- 108. Ringe J, Strassburg S, Neumann K, et al Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007; 101: 135–46. [DOI] [PubMed] [Google Scholar]
- 109. Sordi V, Malosio ML, Marchesi F, et al Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005; 106: 419–27. [DOI] [PubMed] [Google Scholar]
- 110. Honczarenko M, Le Y, Swierkowski M, et al Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006; 24: 1030–41. [DOI] [PubMed] [Google Scholar]
- 111. Von Luttichau I, Notohamiprodjo M, Wechselberger A, et al Human adult CD34− progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005; 14: 329–36. [DOI] [PubMed] [Google Scholar]
- 112. Ponte AL, Marais E, Gallay N, et al The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007; 25: 1737–45. [DOI] [PubMed] [Google Scholar]
- 113. Binger T, Stich S, Andreas K, et al Migration potential and gene expression profile of human mesenchymal stem cells induced by CCL25. Exp Cell Res. 2009; 315: 1468–79. [DOI] [PubMed] [Google Scholar]
- 114. Kalwitz G, Andreas K, Endres M, et al Chemokine profile of human serum from whole blood: migratory effects of CXCL‐10 and CXCL‐11 on human mesenchymal stem cells. Connect Tissue Res. 2010; 51: 113–22. [DOI] [PubMed] [Google Scholar]
- 115. Chamberlain G, Smith H, Rainger GE, et al Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shear. PLoS ONE. 2011; 6: e25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sasaki M, Abe R, Fujita Y, et al Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008; 180: 2581–7. [DOI] [PubMed] [Google Scholar]
- 117. Wynn RF, Hart CA, Corradi‐Perini C, et al A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004; 104: 2643–5. [DOI] [PubMed] [Google Scholar]
- 118. Stich S, Haag M, Haupl T, et al Gene expression profiling of human mesenchymal stem cells chemotactically induced with CXCL12. Cell Tissue Res. 2009; 336: 225–36. [DOI] [PubMed] [Google Scholar]
- 119. Rankin SM. Chemokines and adult bone marrow stem cells. Immunol Lett. 2012; 145: 47–54. [DOI] [PubMed] [Google Scholar]
- 120. Pitchford SC, Furze RC, Jones CP, et al Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009; 4: 62–72. [DOI] [PubMed] [Google Scholar]
- 121. Bruns AF, Bao L, Walker JH, et al VEGF‐A‐stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co‐ordinated trafficking and proteolysis. Biochem Soc Trans. 2009; 37: 1193–7. [DOI] [PubMed] [Google Scholar]
- 122. Mao JJ, Stosich MS, Moioli EK, et al Facial reconstruction by biosurgery: cell transplantation versus cell homing. Tissue Eng Part B Rev. 2010; 16: 257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mendelson A, Ahn JM, Paluch K, et al Engineered nasal cartilage by cell homing: a model for augmentative and reconstructive rhinoplasty. Plast Reconstr Surg. 2014; 133: 1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Guan M, Yao W, Liu R, et al Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012; 18: 456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zanjani ED, Flake AW, Almeida‐Porada G, et al Homing of human cells in the fetal sheep model: modulation by antibodies activating or inhibiting very late activation antigen‐4‐dependent function. Blood. 1999; 94: 2515–22. [PubMed] [Google Scholar]
- 126. Yao W, Guan M, Jia J, et al Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells. 2013; 31: 2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yao W, Lay YE, Kot A, et al Improved mobilization of exogenous mesenchymal stem cells to bone for fracture healing and sex difference. Stem Cells. 2016; 34: 2587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dashnyam K, Jin GZ, Kim JH, et al Promoting angiogenesis with mesoporous microcarriers through a synergistic action of delivered silicon ion and VEGF. Biomaterials. 2017; 116: 145–57. [DOI] [PubMed] [Google Scholar]
- 129. Chen FM, Wu ZF, Sun HH, et al Release of bioactive BMP from dextran‐derived microspheres: a novel delivery concept. Int J Pharm. 2006; 307: 23–32. [DOI] [PubMed] [Google Scholar]
- 130. Chen FM, Zhao YM, Wu H, et al Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin‐like growth factor‐I from dextran‐co‐gelatin microspheres. J Control Release. 2006; 114: 209–22. [DOI] [PubMed] [Google Scholar]
- 131. Hocking AM. The role of chemokines in mesenchymal stem cell homing to wounds. Adv Wound Care (New Rochelle). 2015; 4: 623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Biondi M, Ungaro F, Quaglia F, et al Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008; 60: 229–42. [DOI] [PubMed] [Google Scholar]
- 133. Guilak F, Cohen DM, Estes BT, et al Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009; 5: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sarkar D, Vemula PK, Zhao W, et al Engineered mesenchymal stem cells with self‐assembled vesicles for systemic cell targeting. Biomaterials. 2010; 31: 5266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sarkar D, Spencer JA, Phillips JA, et al Engineered cell homing. Blood. 2011; 118: e184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Liang X, Huang X, Zhou Y, et al Mechanical stretching promotes skin tissue regeneration via enhancing mesenchymal stem cell homing and transdifferentiation. Stem Cells Transl Med. 2016; 5: 960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ansboro S, Greiser U, Barry F, et al Strategies for improved targeting of therapeutic cells: implications for tissue repair. Eur Cell Mater. 2012; 23: 310–8. [DOI] [PubMed] [Google Scholar]
- 138. Ratajczak MZ, Suszynska M. Emerging strategies to enhance homing and engraftment of hematopoietic stem cells. Stem Cell Rev. 2016; 12: 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005; 106: 1901–10. [DOI] [PubMed] [Google Scholar]
- 140. Weil BR, Canty JM Jr. Stem cell stimulation of endogenous myocyte regeneration. Clin Sci (Lond). 2013; 125: 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Dong F, Caplan AI. Cell transplantation as an initiator of endogenous stem cell‐based tissue repair. Curr Opin Organ Transplant. 2012; 17: 670–4. [DOI] [PubMed] [Google Scholar]
- 142. Horie N, Hiu T, Nagata I. Stem cell transplantation enhances endogenous brain repair after experimental stroke. Neurol Med Chir (Tokyo). 2015; 55: 107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sackstein R. Glycoengineering of HCELL, the human bone marrow homing receptor: sweetly programming cell migration. Ann Biomed Eng. 2012; 40: 766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shinohara K, Greenfield S, Pan H, et al Stromal cell‐derived factor‐1 and monocyte chemotactic protein‐3 improve recruitment of osteogenic cells into sites of musculoskeletal repair. J Orthop Res. 2011; 29: 1064–9. [DOI] [PubMed] [Google Scholar]
- 145. Ho CY, Sanghani A, Hua J, et al Mesenchymal stem cells with increased stromal cell‐derived factor 1 expression enhanced fracture healing. Tissue Eng Part A. 2015; 21: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zwingenberger S, Yao Z, Jacobi A, et al Enhancement of BMP‐2 induced bone regeneration by SDF‐1alpha mediated stem cell recruitment. Tissue Eng Part A. 2014; 20: 810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Curtin CM, Tierney EG, McSorley K, et al Combinatorial gene therapy accelerates bone regeneration: non‐viral dual delivery of VEGF and BMP2 in a collagen‐nanohydroxyapatite scaffold. Adv Healthc Mater. 2015; 4: 223–7. [DOI] [PubMed] [Google Scholar]
- 148. Zhang Y, Cheng N, Miron R, et al Delivery of PDGF‐B and BMP‐7 by mesoporous bioglass/silk fibrin scaffolds for the repair of osteoporotic defects. Biomaterials. 2012; 33: 6698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Koc A, Finkenzeller G, Elcin AE, et al Evaluation of adenoviral vascular endothelial growth factor‐activated chitosan/hydroxyapatite scaffold for engineering vascularized bone tissue using human osteoblasts: in vitro and in vivo studies. J Biomater Appl. 2014; 29: 748–60. [DOI] [PubMed] [Google Scholar]
- 150. Lin CY, Chang YH, Sung LY, et al Long‐term tracking of segmental bone healing mediated by genetically engineered adipose‐derived stem cells: focuses on bone remodeling and potential side effects. Tissue Eng Part A. 2014; 20: 1392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Levy O, Mortensen LJ, Boquet G, et al A small‐molecule screen for enhanced homing of systemically infused cells. Cell Rep. 2015; 10: 1261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu H, Xue W, Ge G, et al Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF‐1alpha in MSCs. Biochem Biophys Res Commun. 2010; 401: 509–15. [DOI] [PubMed] [Google Scholar]
- 153. Liu L, Yu Q, Lin J, et al Hypoxia‐inducible factor‐1alpha is essential for hypoxia‐induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev. 2011; 20: 1961–71. [DOI] [PubMed] [Google Scholar]
- 154. Shi M, Li J, Liao L, et al Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007; 92: 897–904. [DOI] [PubMed] [Google Scholar]
- 155. Yu Y, Bi CS, Wu RX, et al Effects of short‐term inflammatory and/or hypoxic pretreatments on periodontal ligament stem cells: in vitro and in vivo studies. Cell Tissue Res. 2016; 366: 311–28. [DOI] [PubMed] [Google Scholar]
- 156. Yu Y, Wu RX, Gao LN, et al Stromal cell‐derived factor‐1‐directed bone marrow mesenchymal stem cell migration in response to inflammatory and/or hypoxic stimuli. Cell Adh Migr. 2016; 10: 342–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Anitua E, Sanchez M, Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev. 2010; 62: 741–52. [DOI] [PubMed] [Google Scholar]
- 158. Zhang Z, Gupte MJ, Ma PX. Biomaterials and stem cells for tissue engineering. Expert Opin Biol Ther. 2013; 13: 527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rambhia KJ, Ma PX. Controlled drug release for tissue engineering. J Control Release. 2015; 219: 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chen FM, Zhao YM, Jin Y, et al Prospects for translational regenerative medicine. Biotechnol Adv. 2012; 30: 658–72. [DOI] [PubMed] [Google Scholar]
- 161. Sengupta D, Waldman SD, Li S. From in vitro to in situ tissue engineering. Ann Biomed Eng. 2014; 42: 1537–45. [DOI] [PubMed] [Google Scholar]
- 162. Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009; 21: 3269–85. [DOI] [PubMed] [Google Scholar]
- 163. Pakulska MM, Miersch S, Shoichet MS. Designer protein delivery: from natural to engineered affinity‐controlled release systems. Science. 2016; 351: aac4750. [DOI] [PubMed] [Google Scholar]
- 164. Bara JJ, Richards RG, Alini M, et al Concise review: bone marrow‐derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014; 32: 1713–23. [DOI] [PubMed] [Google Scholar]
- 165. Ruster B, Gottig S, Ludwig RJ, et al Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006; 108: 3938–44. [DOI] [PubMed] [Google Scholar]
- 166. Zhao D, Lei L, Wang S, et al Understanding cell homing‐based tissue regeneration from the perspective of materials. J Mater Chem B. 2015; 3: 7319–33. [DOI] [PubMed] [Google Scholar]
- 167. Sridharan R, Karp JM, Zhao W. Bioengineering tools to elucidate and control the fate of transplanted stem cells. Biochem Soc Trans. 2014; 42: 679–87. [DOI] [PubMed] [Google Scholar]
- 168. McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015; 72: 1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yu Y, Wu R‐X, Yin Y, et al Directing immunomodulation using biomaterials for endogenous regeneration. J Mater Chem B. 2016; 4: 569–84. [DOI] [PubMed] [Google Scholar]
- 170. Pei Y, Yeo Y. Drug delivery to macrophages: challenges and opportunities. J Control Release. 2016; 240: 202–11. [DOI] [PubMed] [Google Scholar]
- 171. Alvarez MM, Liu JC, Trujillo‐de Santiago G, et al Delivery strategies to control inflammatory response: modulating M1‐M2 polarization in tissue engineering applications. J Control Release. 2016; 240: 349–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Purwada A, Roy K, Singh A. Engineering vaccines and niches for immune modulation. Acta Biomater. 2014; 10: 1728–40. [DOI] [PubMed] [Google Scholar]


