Abstract
Pain associated to mechanical and chemical irritation of the eye surface is mediated by trigeminal ganglia mechano- and polymodal nociceptor neurons while cold thermoreceptors detect wetness and reflexly maintain basal tear production and blinking rate. These neurons project into two regions of the trigeminal brain stem nuclear complex: ViVc, activated by changes in the moisture of the ocular surface and VcC1, mediating sensory-discriminative aspects of ocular pain and reflex blinking. ViVc ocular neurons project to brain regions that control lacrimation and spontaneous blinking and to the sensory thalamus. Secretion of the main lacrimal gland is regulated dominantly by autonomic parasympathetic nerves, reflexly activated by eye surface sensory nerves. These also evoke goblet cell secretion through unidentified efferent fibers. Neural pathways involved in the regulation of Meibonian gland secretion or mucins release have not been identified.
In dry eye disease, reduced tear secretion leads to inflammation and peripheral nerve damage. Inflammation causes sensitization of polymodal and mechano-nociceptor nerve endings and an abnormal increase in cold thermoreceptor activity, altogether evoking dryness sensations and pain. Long-term inflammation and nerve injury alter gene expression of ion channels and receptors at terminals and cell bodies of trigeminal ganglion and brainstem neurons, changing their excitability, connectivity and impulse firing. Perpetuation of molecular, structural and functional disturbances in ocular sensory pathways ultimately leads to dysestesias and neuropathic pain referred to the eye surface. Pain can be assessed with a variety of questionaires while the status of corneal nerves is evaluated with esthesiometry and with in vivo confocal microscopy.
Keywords: Pain, Neuropathic pain, Dry eye disease, Ocular surface dryness, Peripheral sensory nerves, TRP channels, Cold receptors, Polymodal nociceptors, Mechano-nociceptors, Trigeminal brainstem nuclear complex, Central nervous system, Sensation, Corneal esthesiometry, In vivo corneal confocal microscopy
1. Introduction
The objective of the TFOS DEWS II Pain and Sensation Subcommittee was to highlight the neurobiological mechanisms that underpin discomfort accompanying dry eye disease (DED). In 2013, the TFOS International Workshop on Contact Lens Discomfort produced a report of its Subcommittee on Neurobiology [1] that included a detailed description of the morphological and immunocytochemical characteristics of ocular surface sensory innervation in experimental animals and in humans. It also covered the basic molecular, cellular and integrative mechanisms underlying the detection and processing of environmental and endogenous stimuli acting on the eye at various levels of the brain and spinal cord, which lead ultimately to conscious sensory experiences and behavioral and autonomic adaptive responses. This report has been updated here with new data on the genetic and molecular signature of corneal sensory neurons and their peripheral nerve branches, and recent information on the changes that take place in ocular surface sensory pathways as a result of the corneal and conjunctival disturbances during DED, including the cross talk between immune and neural elements. New experimental and clinical data on the psychophysical characteristics and possible neural mechanisms underlying conscious pain and discomfort sensations in DED are reported, discussing their similarities and differences with the various types of pain experienced in other human pathologies. Finally, the current report describes the methods available for the experimental and clinical exploration in humans of the neurobiological parameters involved in DED symptoms.
The use of the term ‘pain’ in eye care has been traditionally limited to a small number of pathological conditions, because conscious sensations originating at the ocular surface do not generally have a diagnostic interest. In fact, most of the prevalent sight-threatening eye diseases, like open angle glaucoma, cataract or retinal pathologies, occur and progress without pain [2]. Moderately unpleasant sensations accompany many common ocular surface diseases (allergic conjunctivitis, DED), but they have been described clinically, in most cases, with terms such as ‘dryness,’ ‘discomfort,’ and ‘itch,’ without making a direct, explicit association with pain sensations. Until a few decades ago, the term pain as a symptom of eye pathology was generally reserved for the sensations accompanying predominantly traumatic or infectious keratitis, iridocyclitis, angle closure glaucoma, and other entities [2]. This evolution of our understanding of ocular pain has parallels to pain in general, which was initially described by Celsus, a Roman scholar, as one of the signs (dolor) of inflammation, and has evolved ever since [3].
Reports of pain after photorefractive surgery procedures and the ‘discomfort’ experienced by contact lens wearers finally directed the attention of eye care practitioners and researchers toward the origin and mechanisms of the unpleasant, and sometimes overtly painful, sensations arising from the ocular surface. This interest extended to ‘dryness’ sensations experienced by patients suffering DED and by patients experiencing severe ocular surface symptoms, but with minimal or no clinical signs on slit-lamp examination. The underlying neurobiological mechanisms producing these sensations appear to be consistent with those mediating ocular pain in other eye pathologies, and unpleasant dry eye sensations should be considered and studied as a specific form of eye pain occurring in this particular disease.
The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” [4]. Various types of pain have been distinguished based on etiology, duration or clinical features.
Nociceptive pain is the pain that arises from actual or threatened damage to tissues and is due to the activation of nociceptors. Nociceptors are sensory receptors of the peripheral somatosensory nervous system that are capable of transducing and encoding potentially tissue-damaging noxious mechanical, thermal and chemical stimuli, thereby signaling the location, size, intensity and duration of tissue injury.
Neuropathic pain is defined by the IASP as pain caused by a lesion or disease of the somatosensory nervous system, in contrast with nociceptive pain produced by the normal function of nociceptors. Neuropathic pain is a clinical description, which requires a demonstrable lesion or a disease of the somatosensory nervous system that satisfies established neurological diagnostic criteria [4], and is also commonly referred to as pathological pain or pain without biological value. Neuropathic pain has been categorized etiologically (eg degenerative, traumatic, infectious, metabolic, and toxic) and anatomically (into peripheral vs central) because this class of pain is generated by a functional disturbance that may occur at different levels of the neuroaxis. The nature of the injury to peripheral sensory nerves (peripheral neuropathic pain) determines the development of ectopic activity and abnormal excitability of peripheral nerve terminals and nociceptive neurons of sensory ganglia. Altered gene expression triggered by peripheral axotomy, cell body damage and/or by local inflammation is a common feature of peripheral neuropathic pain [5]. The abnormal activity caused by peripheral neuronal injury may produce anatomical and functional changes in the distribution and efficacy of synaptic connections arriving from the periphery to the central nervous system, altering the excitability of second-order and higher-order projection neurons of the pain pathways. Excitability of central pain pathways is further enhanced by local activation of microglia and impairment of the inhibitory descending modulation arriving from higher CNS areas [6]. Central neuropathic pain also may be generated by malfunction of central somatosensory nervous system structures due to lesion or disease, for example trauma, stroke or genetic abnormality.
Acute nociceptive pain results from high intensity stimulation of nociceptors and usually persists as long as stimulus is applied. Inflammation develops in parallel to tissue injury or infection, reflecting the activation of the immune system. Inflammatory mediators modify the normal responsiveness of nociceptors (‘sensitization’) by decreasing their threshold for activation and/or increasing suprathreshold responses, often causing spontaneous discharges. Inflammatory pain has an adaptive role, offering a longer term protection during the healing period. When pain persists past the normal time of healing, it becomes chronic or persistent pain [7]. The definition of chronic pain is not precise and may last less than one month, or more frequently, over six months. Among its salient characteristics are the absence of apparent biological value and the frequent tendency to become severe and intractable [4,7].
Itch is a complex unpleasant sensation, often present in the eye with many elements in common with pain, although its sensory quality and associated urge to scratch make it a distinct perceptual entity. Experimental evidence in the skin indicates that itch is evoked by activation of specific receptors on peripheral sensory fibers of sensory neurons (pruriceptive neurons) in the trigeminal and dorsal root ganglia (DRG) [8]. These sensory neurons are functionally distinct from nociceptors are often subdivided into chemically-sensitive and mechanically-sensitive itch neurons with characteristic cell and molecular signatures and separate functional sensory pathways. Peripheral itch-mediating pathways are segregated from those signaling acute pain, although the pathways interact at various levels of the CNS to finally evoke conscious sensations of either itch or pain [9]. In the skin, pruritus accompanies a large variety of chronic cutaneous and systemic diseases like xerosis (dry skin) [10], while ocular itch is often a pathognomonic sign of allergic conjunctivitis [11]. Finally, it is important to note that the above mechanisms do not necessarily occur exclusively, but may be present concurrently, and thus can be challenging to discern for clinicians.
2. Neurobiological features in normal/non dry eye disease
2.1. Peripheral afferent pathways for transduction of physical and chemical stimuli at the ocular surface
The eye and periocular tissues are potential sources of pain and itch resulting from pathological processes affecting directly or indirectly trigeminal sensory nerves. In DED, umpleasant dryness sensation, the most precocious and main symptom of the disease [12], implies activation of sensory nerves subserving nociception at the ocular surface and the subsequent sensory processing of this information.
The ocular surface and contiguous areas of the upper and lower eyelids are supplied by sensory fibers of the trigeminal nerve. Of these, the cornea is the most richly innervated of all ocular structures and the most densely innervated surface epithelium in the human body, while the conjunctiva and eyelid margins receive comparatively a less dense innervation.
2.1.1. Trigeminal ganglion
2.1.1.1. Nerve projections to the eye
Sensory neurons that supply the ocular surface have their cell bodies in the ophthalmic and maxillary regions of the trigeminal ganglion (TG) [13–16]. Estimates in mice, rats, cats and monkeys indicate that between 50 and 450 TG neurons supply the cornea and these constitute about 2% of all TG neurons [13,17–19]. Sensory nerves to the cornea and the anterior bulbar conjunctiva travel to the eye initially via the nasociliary branch of the ophthalmic nerve, and then via the long ciliary nerves and the communicating branch to the ciliary ganglion. The ciliary ganglion gives rise to the short ciliary nerves, which contain both sensory and autonomic fibers. The long and short ciliary nerves pierce the sclera at the back of the eye and run forward to the anterior segment in the suprachoroidal space [20,21]. The ciliary nerves divide to form multiple branches that arrive at the corneal limbus at equidistant intervals around its circumference where they form the circumferentially arranged pericorneal plexus [22]. The sensory and autonomic nerve fibers supplying the cornea and limbal conjunctiva exit anteriorly from this plexus. The supratrochlear, supraorbital, infratrochlear, and lacrimal branches of the ophthalmic nerve as well as the infraorbital nerve branch of the maxillary nerve supply the innervation of the remaining bulbar conjunctiva, entire palpebral conjunctiva, and the skin covering the eyelid margins (Fig. 1) [20,23–25].
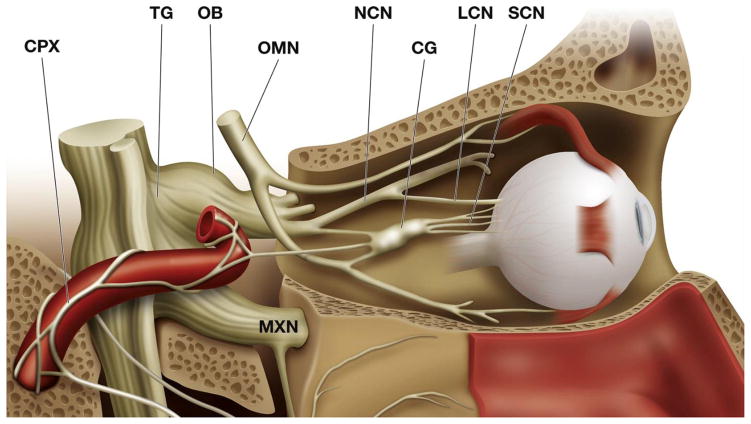
Fig. 1.
Medial view of the orbit, showing the sensory and autonomic innervation of the eye. The lacrimal gland has been removed for clarity. The ophthalmic branch (OB) of the trigeminal ganglion (TG) gives the nasociliary nerve (NCN) that sends long (LCN) and short (SCN) ciliary nerves to the eye ball. Frontal (FN) and lacrimal (LN) nerves appear as cut in this picture. Sympathetic fibers from the superior cervical ganglion, travelling in the carotid plexus (CPX) and parasympathetic branches of the ciliary (CG) and the pterigopalatine ganglion (PPG) join the short the ciliary nerves. OMN: Oculomotor nerve. MXN: Maxillary nerve. Modified from Netter, F, Atlas of Human Anatomy, 2nd Edition. Icon Learning Systems, 1997.
2.1.1.2. Molecular, genetic and electrophysiological diversity of TG ocular neurons
Few studies have investigated the membrane properties and ion channel currents of corneal TG neurons using intracellular, whole cell or calcium imaging recordings. These investigations have been performed in dissociated TG neurons [26–28] and in neurons of intact TGs isolated in vitro [29,30] or recorded in vivo in anesthetized mice [31]. In summary, the electrophysiological studies have demonstrated that corneal sensory neurons are either thinly myelinated (Aδ-type) or unmyelinated (C-type) and have heterogeneous passive and active membrane properties [2].
The heterogeneity of TG neurons innervating the ocular surface is also reflected in the variable expression of cellular markers for molecules with a relevant role in sensory transduction and signaling such as transient receptor potential (TRP) ion channels or neuropeptides. The unique transcriptional programs of the different classes of primary sensory neurons lead to differential expression of specific ion channels underlying stimulus transduction and encoding properties. These gene expression patterns also determine the central distribution at higher-order neurons of nerve terminals belonging to each sensory neuron lineage. In recent years, great progress has been made in combining transcriptome-based neuron typing performed with single-cell RNA-sequencing, with the sensory phenotyping of DRG and TG neurons according to size, stimulus modality, and expression of neuropeptides, ion channels or protein receptors [32,33]. This allows sen sory neuron types to be classified into a larger number of subtypes with distinct transcriptional profiles, molecular markers and functional properties, some of which are associated with pathological signs [34]. Such approaches have not yet been applied to TG neurons innervating the ocular surface but would be expected to greatly expand the limited knowledge of the relationship between ocular sensory neurons and their contribution to sensations and autonomic responses in DED.
2.1.2. Sensory innervation of the cornea
2.1.2.1. Architecture of axons and terminals in the cornea
In animals, approximately 20–30% of the axons supplying the cornea are thinly myelinated (Aδ) and the remainder are unmyelinated [35,36]. However, myelinated fibers lose their myelin sheath within about a millimeter of entering the corneal stroma [22,37]. Soon after entering the cornea, the nerve bundles branch and anastomose with neighboring bundles to form the stromal plexus concentrated in the anterior one-third of the stroma [38]. The most superficial layer of the stromal nerve plexus, located immediately below the Bowman’s layer, is known as the subepithelial plexus [38].
The majority of axons entering the corneal stroma penetrate Bowman’s layer from the subepithelial plexus and terminate as unencapsulated nerve endings in the corneal epthelium [37]. However, a small population of axons terminates in the stroma [38], while others form close anatomical relationships with stromal keratocytes and macrophages [37,39]. The major innervation of the corneal epithelium originates from 200 to 500 fine stromal nerve bundles that penetrate Bowman’s layer for the most part in the peripheral and intermediate cornea [37,38]. In addition at its periphery, the epithelium receives inputs from nerves that enter directly from the pericorneal plexus [38]. On entering the epithelium, each stromal nerve bends at an acute angle and branches into multiple nerve fascicles that form the subbasal nerve fibers at the interface between Bowman’s layer and the basal epithelial cells. The composite nerve structure formed by the subbasal nerve fibers arising from each stromal nerve is termed an epithelial leash [40,41]. Individual subbasal nerve fibers run parallel to one another and to the ocular surface, for up to 6–8 mm [38]. Human sub-basal nerve fibers contain as many as 40 axons [42] that lose their Schwann cell envelope when they enter the epithelium [37].
Adjacent epithelial leashes anastomose with one another extensively forming a dense, mesh-like subbasal nerve plexus. This plexus constitutes the densest layer of the human corneal innervation and is readily visualized by in vivo confocal microscopy and quantified in terms of nerve density [43,44]. This approach has allowed the effects of conditions such as DED, diabetes, keratoconus, herpetic infections, as well as normal aging on corneal innervation to be assessed (see Section 6.3.2). When viewed in its entirety, the subbasal nerve fibers in the human subbasal nerve plexus form a whorl-like pattern or “vortex” [38,43,45], whose center is located approximately 2–3 mm inferonasal to the corneal apex. Similar whorl-like patterns of subbasal nerve fibers are present in other, but not all, mammalian corneas, including mice and rats [46–48].
Each subbasal nerve fiber gives rise to numerous intra-epithelial terminals that are distributed throughout all layers of the corneal epithelium [38,40,48]. Some axons end with small boutons within the basal epithelium. Those that terminate more superficially in the epithelium arise perpendicularly from the subbasal nerves and project up to within a few micrometers of the surface of the epithelium, where their terminations can be described on the basis of their branching pattern as simple, ramifying or complex (Fig. 2) [48]. Simple terminals do not branch after leaving the subbasal nerves and end with a single, bulbar swelling in or just below the squamous cell layer of the epithelium. Ramifying terminals branch within or just below the squamous cell layer of the epithelium into a number (usually 3–4) of horizontal fibers that run parallel to the surface for up to 100 μm. Each branch ends in a single bulbar swelling similar to those of simple terminals. The axons forming complex terminals start to branch within the wing cell layer of the cornea and form a cluster of highly branched fibers that have endings in both the wing and squamous cell layers. Each of the many branches in complex terminals has multiple bulbar endings that are often larger than those associated with the simple and ramifying terminals.
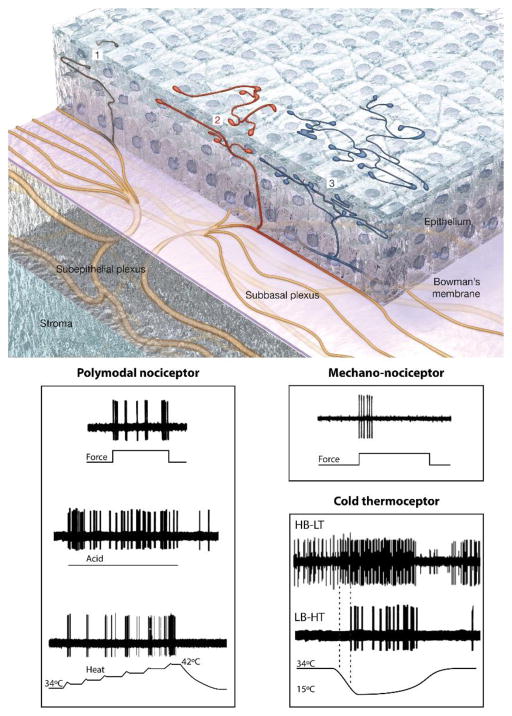
Fig. 2.
Reconstruction of superficial nerve terminals in the mouse corneal epithelium showing examples of simple (1,black), ramifying (2, red) and complex (3, blue) nerve terminals and impulse activity recorded from the different functional types of corneal nerve terminals in response to their specific stimuli (Modified from Ivanusic et al., [48] Gallar et al. [61] and Belmonte et al. [59,60]).
Epithelial nerve terminal arborizations are not static structures and undergo continuous structural remodeling [49]. This normally occurs in the healthy cornea due to the continuous shedding of corneal epithelium cells, a process that results in full renewal in a matter of days. When stromal and/or epithelial nerve branches are damaged by trauma or disease there is also regrowth of axons to repopulate the denervated tissues [50]. Corneal surgery (cataract, photorefractive correction, glaucoma) is unavoidably accompanied by nerve damage followed by a variable degree of regeneration, depending on the location and extent of the injury [51]. Nerve damage often accompanies metabolic diseases like diabetes and viral, parasitic or bacterial infections [52–54].
2.1.2.2. Molecular and functional characteristics of corneal sensory innervation
Electrophysiological recordings of single sensory nerve fibers innervating the cornea have revealed the existence of different functional types of ocular sensory neurons. These can be broadly classified as polymodal nociceptor neurons, cold thermoreceptor neurons and selective mechano-nociceptor neurons [55–57]. There is now significant evidence, at least in animals, supporting the notion that these three broad classes can be identified on the basis of their molecular phenotype and morphology [36,48,58]. Molecular heterogeneity has been described both at the level of the nerve terminals in the corneal epithelium and also in the soma of corneal sensory neurons in the TG.
2.1.2.2.1. Polymodal nociceptors
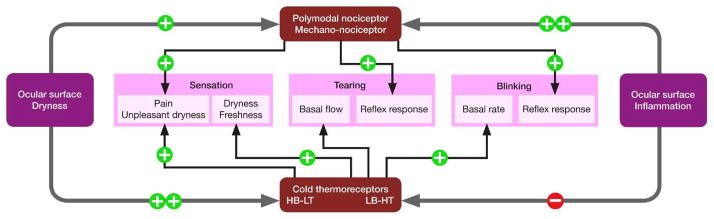
The majority of the sensory nerve fibers innervating the cornea are polymodal nociceptors. They are activated over a wide range of stimulus intensities that include near-noxious or noxious mechanical energy, heat, and chemical irritants. They are also sensitive to endogenous chemical mediators released by damaged corneal tissue and by resident and migrating inflammatory cells, or leaking from limbal blood vessels [59–62]. Polymodal nociceptors have a mechanical threshold slightly lower than pure mechano-nociceptors (described below) and produce a sustained discharge in response to maintained mechanical indentation of the cornea [55]. When stimulated with heat, they begin to fire at temperatures higher than 37 °C [61]. Acidic solutions of pH 5.0–6.5 or gas jets containing CO2 (which forms carbonic acid at the corneal surface) also activate corneal polymodal nociceptors [59–61,63,64], as do other chemical agents known to excite polymodal nociceptors in other tissues (eg prostaglandins, bradykinin, capsaicin) [60–62,65–67] (Fig. 2). Corneal polymodal nociceptors can be sensitized by repeated noxious heat stimuli and by inflammatory mediators [59–61], and this has been demonstrated to occur in animals with allergic keratoconjunctivitis [68]. In psychophysical studies, activation of corneal polymodal receptors with an acidic stimulus (0–80% CO2 in air) evokes stinging and burning pain sensations [64,66].
The TRP cation channel subfamily V member 1 (TRPV1) is important for sensory transduction in polymodal nociceptors and has been used extensively as a molecular marker for this cell class. It is activated by capsaicin, low pH (pH 6), noxious heat (>42 °C) [69–71] and hyperosmlarity [72]. TRPV1 knockout mice display altered responses to these stimuli and reduced thermal hypersensitivity in the context of inflammation [69]. Capsaicin activates polymodal nociceptors in the cornea [60,65,73] and upon application to the human eye produces pain [74,75]. TRPV1 activation by heat, protons and inflammatory mediators enhances excitability of polymodal nociceptors [76].
In rat and guinea pig TG, substantial proportions (25–45%) of corneal afferent neurons express TRPV1 [58,77,78]. TRPV1 expressing corneal afferent neurons do not express Piezo2 (a putative marker for low-threshold mechanoreceptors [36], see below) and only ~6% co-express TRP cation channel subfamily M member 8 (TRPM8) channels (a putative marker for cold thermoreceptor neurons, see below) [58]. It is possible that the population of neurons that co-express TRPV1 and TRPM8 represent a subpopulation of cold thermoreceptor neurons that display a paradoxical response to noxious heating [79]. TRPV1 is also expressed in intra-epithelial nerve terminal endings in the corneal epithelium [58,77,78]. It should be noted that TRPV1 expression is not restricted to neural elements in the eye, but is also found in supporting cells throughout the different layers of the cornea [80]. It is not known if activation of TRPV1 in support cells contributes to corneal nerve activation.
Other transducer channels appear also to contribute to chemical sensitivity of corneal polymodal nociceptors. In a fraction of them, sensitivity to acidic stimulation remains after complete blockade of capsaicin-induced activation of TRPV1 channels with capsazepine [65,68]. This may be explained by the expression of acid-sensing ion channels (ASICs) as responses of corneal polymodal nociceptors to pH 6.6 solutions are abolished by ASIC blockers [27]. TRP cation channel subfamily A member 1 (TRPA1) is an extremely broadly tuned chemo-nocisensor channel [81,82]. TRPA1 is expressed in the TG and ocular instillation of a selective TRPA1 agonist produces neuronal activation of trigeminal brainstem neurons that is enhanced in an animal model for DED [83]. The contribution of TRPA1 activation to the enhanced excitability of polymodal nociceptors produced by inflammatory mediators seems to be more modest than for TRPV1 [68].
There is evidence that multiple subpopulations of corneal poly-modal nociceptors exist with different molecular phenotypes, nerve terminal morphologies and epithelial distribution within the cornea. In guinea pig corneal epithelium, TRPV1 expressing nerve terminals can be divided into 3 populations [58]. One population displays terminals with ramifying morphology in the squamous cell layer. These terminals do not contain calcitonin gene-related peptide (CGRP), but they do express the glial cell line-derived neurotrophic factor family receptor alpha3 (GFRα3). The other two populations end with simple endings, one in the wing cell layers and the other in the subbasal plexus. Those nerves terminating in wing cell layers express both CGRP and GFRα3, whereas those terminating in the subbasal plexus express CGRP, but not GFRα3. Importantly, the molecular phenotype of these neurons is maintained both at the level of the nerve terminals in the epithelium and at their somas in the TG [58]. Whether this molecular heterogeneity in corneal polymodal nociceptors is reflected in differences in function has yet to be determined. The neuropeptides contained in some polymodal receptors (substance P and CGRP) contribute to the inflammatory response (‘neurogenic inflammation’) [84] and promote corneal epithelial maintenance and physiological renewal by activating cellular pathways that stimulate epithelial cell proliferation, migration, adhesion and differentiation [85–88]. Thus, peptidergic polymodal nociceptors are likely to have an important role in maintaining corneal integrity. There is increasing evidence that GFRα3 signaling is involved in sensitization of peripheral sensory neurons and that the TRPV1 ion channel is involved in this process [89–92].
2.1.2.2.2. Cold thermoreceptors
The cold thermoreceptors represent 10–15% of the total population of corneal sensory neurons. At stable temperatures close to that of the ocular surface (34–35 °C), most corneal cold thermoreceptors fibres continuously generate action potentials and their activity is increased and decreased by moderate cooling and heating, respectively. [60,61,93–95], thus ressembling the canonical cold thermoreceptors reported in other body tissues. This population of cold thermoreceptors has been named high background, low threshold (HB-LT) corneal cold thermoreceptors [96]. HB-LT cold thermoreceptors change their activity at different static temperatures and are much more strongly modulated by dynamic changes in temperature [28,61]. With both cooling and heating, the magnitude of the change in action potential generation in HB-LT cold thermoreceptors is strongly dependent on the rate of change of temperature [94,95]. HB-LT cold thermoreceptors detect and encode very precisely the intensity of a temperature variation by their impulse frequency, responding to tem perature drops of 0.5 °C or less [61,96–98], explaining the perception of cooling produced by 1–2 °C reductions in temperature at the corneal surface [99]. (Fig. 2).
In addition to sensing changes in temperature, the HB-LT corneal cold thermoreceptors detect mild to moderate changes in osmolarity [61,67,97,100,101]. At constant temperature, increases in osmolarity accelerate action potential generation in these receptors. In mouse cornea, there was a significant increase in activity of cold thermoreceptors when osmolarity was raised from 310 mOsm/L (control) to values greater than 340 mOsm/L [67]. This modulation of nerve activity is observed when the solutions are made hyperosmotic by the addition of NaCl [67,97,102,103] or by the addition of mannitol or sucrose [97,101,103,104]. Thus it is the change in osmolarity rather than the change in ionic composition of the solution that modulates nerve activity, although an additional direct surface charge effect produced by a high sodium ion concentration cannot be excluded. The activity of HB-LT cold thermoreceptors is also inhibited by hypo-osmotic solutions [101]. This finding suggests that under basal conditions tear film osmolarity provides a stimulus to the cold thermoreceptors that contributes to maintaining their ongoing nerve activity [67,105].
TRPM8 is a cation channel that is activated by cooling and menthol and is important for cold sensation [98,106,107], including cold pain [108]. A recent report demonstrates that TRPM8 channels are also activated by increases in osmolarity from about 200 mOsmol/l [101]. Indeed the sensitivity of cells heterologously expressing TRPM8 to changes in osmolarity is very similar to that of corneal cold thermoreceptors in mouse cornea [101]. In TRPM8 knockout mice, nerve endings with high levels of ongoing nerve activity characteristic of HB-LT cold thermoreceptors were not detected in the cornea [98]. Furthermore, in wild-type mice, blockade of TRPM8 with BCTC (N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropy-razine-1(2H)-carbox-amide – a TRPM8 antagonist) markedly reduced or silenced the ongoing activity of HB-LT cold thermoreceptors and inhibited their response to cooling [98]. Together these findings indicate that TRPM8 channels transduce both cold and osmotic stimuli in corneal HB-LT cold thermoreceptors and generate a background depolarizing current that drives their ongoing nerve activity. This sub-population of cold thermoreceptors further exhibits a prominent expression of hyperpolarization-activated cyclic-nucleotide-modulated (HCN) channels, which modulates the depolarizing current thereby contributing to tune the regular spiking exhibited by HB-LT cold thermoreceptors [109].
Whilst most (around 70%) of corneal cold thermoreceptors belong to the HB-LT type, there is a subpopulation (30%) of cold sensitive corneal nerve fibers with very low ongoing activity at basal temperature, higher thermal threshold (requiring temperature drops >4 °C for activation) and weaker response to cooling [104,110] named low background, high threshold (LB-HT) cold thermoreceptors [110] (Fig. 2).
In the TG, approximately 10–20% of corneal afferent neurons express TRPM8 [36,48,58]. Isolated trigeminal neurons with the phenotype of cold thermoreceptors exhibit a wide range of thermal and menthol thresholds [111]. The differences in threshold of cold thermoreceptor neurons and peripheral terminals have been attributed to variable expression of TRPM8 and of cold-sensitive slowly inactivating K+ channels whose activity opposes cold-induced depolarization [111,112].
Morphologically, TRPM8 expressing nerve terminals in the corneal epithelium are almost exclusively of the complex type and have axon endings in both the wing and squamous cell layers of the epithelium [48]. A similar complex morphology has been demon strated for nerve terminals expressing green fluorescent protein in the corneal epithelium of TRPM8 reporter mice [98], reinforcing the idea that endings with this morphology are associated with cold thermoreceptor neurons.
2.1.2.2.3. Mechano-nociceptors
In electrophysiological studies, about 20–30% of the peripheral axons innervating the cornea are selective mechano-nociceptors that respond only to mechanical forces at an order of magnitude close to that required to damage corneal epithelial cells [60,62]. The receptors of this class have conduction velocities in the Aδ-fiber range and fire one or a few nerve impulses in response to brief or sustained indentations of the corneal surface, and often when the stimulus is removed [60,62]. Thus, the mechano-nociceptors are phasic sensory receptors that signal the presence of the stimulus and, to a very limited degree, its intensity and duration (Fig. 2). The threshold force required to activate corneal mechano-nociceptors is relatively low (about 0.6 mN) in comparison with the force that activates mechano-nociceptor fibers in the skin, but is higher than the threshold force required for activation of corneal polymodal nociceptors [55]. The corneal mechano-nociceptors are probably responsible for the immediate, sharp sensation of pain produced by touching or scratching of the corneal surface. Acute sensitization of corneal mechano-nociceptors when repeatedly stimulated is not obvious, although there is experimental evidence of a transient reduction of their mechanical threshold during allergic kerato-conjunctivitis [68].
Recently, expression of a newly identified mechanically sensitive ion channel, Piezo2, has been demonstrated in some DRG and TG sensory neurons [36,113–115]. To date, Piezo2 expression has not been demonstrated in intra-epithelial nerve terminals in the cornea. However, Piezo2 expression assessed with in situ hybridization is present in approximately 30% of corneal sensory neurons in the TG [36,58]. Piezo2-expressing corneal sensory neurons do not express TRPV1, CGRP and/or TRPM8 and are thus unlikely to be corneal polymodal nociceptors or cold thermoreceptor neurons [36,58]. They are medium to large size neurons and express NF200 (a marker for myelinated neurons), which is consistent with their classification as being thinly myelinated Aδ fiber neurons. Thus Piezo2 appears to be a marker for the corneal mechano-nociceptor neurons.
Approximately 80% of corneal afferent neurons in the TG express TRPV1, TRPM8 or Piezo2 as seen by immuno-labelling and/or in situ hybridization [58]. There is functional evidence that TRA1 channels are also expressed by some TRPV1 expressing corneal polymodal nociceptor nerve fibers [110,116], but immunocytochemical identification in TG corneal neurons or nerve terminals is still lacking. The identity of the remaining 20% of corneal afferent neurons remains to be determined. It is possible that some members of the three functional subpopulations of corneal afferent neurons do not express their respective molecular marker under normal conditions, but switch on de novo expression in pathological conditions (such as inflammation). This would be consistent with the classification of so-called ‘silent nociceptors’, which under normal conditions are not activated by any stimuli at all, but after inflammation, become sensitive to one or more stimulus types [117]. Alternatively, it is possible that unlabeled neurons represent another as yet undefined subpopulation of corneal afferent neurons, or even polymodal nociceptors that do not express TR-PV1.
2.1.2.3. Correspondence between functional properties of somata and terminals
The correspondence between the different functional characteristics of corneal nerve terminals and the electrophysiological prop erties of their cell bodies in the TG are still incompletely understood. A majority of Aδ-fiber neurons and virtually all C-fiber neurons supplying the cornea have long action potentials with an inflexion (hump) on their falling phase [29,30] and display partial or complete resistance to the voltage-gated Na+ (NaV) channel blocker tetrodotoxin (TTX), reflecting the expression of TTX-resistant NaV channels typical of corneal nociceptor neurons [118]. Additionally, neurons with these properties are depolarized by capsaicin, low pH, heat and mechanical stimuli, thus likely correspond to the polymodal nociceptor type (see Section 2.1.2.2.1). Another, distinct group of neurons exhibit a fast action potential without a hump, that is blocked by TTX, and respond phasically to mechanical stimulation, thus are likely mechano-nociceptors (see Section 2.1.2.2.3). Finally, a smaller number of neurons show a relatively fast action potential with either no or a very small shoulder on the falling phase, high input resistance and a tonic response to depolarizing pulses. They are depolarized by cold and menthol and are presumably cold thermoreceptor neurons [29,31,112] (see Section 2.1.2.2.2).
2.1.3. Sensory innervation of the conjunctiva and eyelid margin
2.1.3.1. Morphology of sensory axons and terminals of the conjunctiva and eyelid margin
Much less is known about the sensory innervation of the conjunctiva and eyelid margin than of the cornea. Conjunctival sensory neurons have small diameter myelinated and unmyelinated axons with peripheral endings that are mostly unencapsulated (free endings) and often contain CGRP and/or substance P (markers of peptidergic sensory neurons) [23,119–125]. In the conjunctiva, the peptidergic free nerve endings are located mostly around blood vessels in the stroma, but can also be found to a lesser degree in the epithelium or around the acini of meibomian glands and lymph follicles [15,121–123,125]. The presence of non-peptidergic sensory neurons innervating the conjunctiva has not yet been described. There is a single report of complex corpuscular endings associated with myelinated axons in the conjunctiva [126]. They are mostly located around the limbal conjunctiva in humans and appear morphologically similar to Krause endings.
In the eyelid margin, the morphology of sensory nerve terminals is more diverse and includes abundant Meissner corpuscles, Merkel disc endings and dermal and intra-epithelial free nerve endings [120]. In addition, there are complex lanceolate, circular Ruffini, Merkel and free nerve endings around the eyelashes [120]. Many of these types of endings are specialized for the detection of very low intensity mechanical stimuli, thus explaining why mechanical threshold values in the lid border are similar or lower than in the cornea [127]. The molecular phenotype of sensory neurons that innervate the eyelid margin has not been examined.
2.1.3.2. Functional characteristics of the sensory innervation of the conjunctiva
Only one study has directly investigated the response characteristics of sensory nerves supplying the bulbar and palpebral conjunctiva [128]. In recordings from axons in the nasociliary nerves supplying the guinea pig conjunctiva, 53% were only activated by mechanical stimulation (mechano-sensory units), 41% percent were activated by mechanical stimuli, heating and application of irritant chemicals (polymodal units), and 5% were activated selectively by cooling (cold-sensitive units). So the response characteristics of conjunctival sensory nerves appear to be similar to those of the cornea. Indeed, in cats, the receptive fields of some corneal Aδ-fiber mechano-sensory and polymodal receptors extend into the episcleral tissue that includes the limbus and surrounding bulbar conjunctiva [60]. In the episcleral tissue of cats, there are also cold thermoreceptors [61]. Consistent with these findings, psychophysical studies in humans demonstrate that the conjunctiva is sensitive to mechanical, acidic and cooling stimuli [99,129,130].
Comparisons of corneal and conjunctival sensitivity to mechanical or acidic stimuli demonstrate that the conjunctiva is less sensitive to both stimuli [129,130]. As yet we are not aware of any reported differences between the response characteristics of sensory neurons supplying the bulbar and palpebral conjunctiva. In the human conjunctiva, application of low and moderate mechanical stimuli generated minimal irritation, but their intensity could be detected [99]. This finding is consistent with the conjunctiva containing low threshold mechanoreceptors that generate innocuous sensations. Stronger mechanical stimuli produced irritation and pain, but their intensity was less than for the same stimuli applied to the cornea [99]. Activation of conjunctival polymodal receptors with acidic stimulation evoked only irritation and pain [99]. In conjunctiva, unlike cornea, cooling stimuli produced purely cold sensations [99].
No electrophysiological studies have reported the characteristics of sensory neurons supplying the eyelid margins.
2.1.4. Contribution of ocular sensory neurons to sensing eye wetness
The eye surface of terrestrial animals, including humans, is exposed to continuous oscillations in environmental humidity and temperature that may reach extreme values [131]. Under comfortable ambient conditions these changes remain unnoticed, yet they can still cause subtle changes to the tear film and the outermost corneal and conjunctival epithelium cells [132], and are potential stimuli for ocular surface nerve endings.
2.1.4.1. Cold thermoreceptors
Considerable evidence suggests an involvement of the two classes of cold thermoreceptors (HB-LT and LB-HT), both in regulating tear formation to maintain the adequate moistness of the ocular surface and in generating the sense of irritation produced acutely by excessive drying of the ocular surface [133]. The ability of cornea HB-LT cold thermoreceptors to detect relatively small changes in both temperature and osmolarity makes it likely that their primary role is to detect changes in the tear film produced by evaporation - the primary contributor to tear film thinning [134]. In humans, during a blink, the temperature at the surface of the cornea rises by 0.5–1.0 °C in less than a second and then the temperature declines at ~0.05 °C/s between blinks due primarily to evaporative cooling of the tears [135–138]. In guinea pig cornea, the activity of HB-LT cold thermoreceptors is reduced by about 50% when the temperature of the cornea is increased by 1 °C at a rate of ~0.2 °C/s [95]. During a blink, the rise in temperature at the surface of the cornea is much faster and it can be predicted that this would strongly inhibit the ongoing activity of cold thermoreceptors. During cycles of heating and cooling, the activity of cold thermoreceptors increases very rapidly when cooling starts [95]. In addition, the increase in tear film osmolarity produced by evaporation will increase the activity of cold thermoreceptors [67]. Therefore it can be predicted that the activity of HB-LT cold thermoreceptors will be strongly modulated by cyclical changes in the tear film produced by blinking.
In accord with their role in sensing changes in tear film, corneal HT-LT cold thermoreceptors are strongly activated by a drying stimulus that would both cool the ocular surface and increase tear film osmolarity [79,97,104,133], consistent with the hypothesis that cold thermoreceptors contribute to the reflex control of blinking and basal tear production [139]. In support of this hypothesis, both blinking and the basal level of tear production are reduced in TRPM8 knockout mice compared to wild type animals [98,101], while low concentrations of the TRPM8 agonist menthol increases tear production in wild type mice, but have no effect in TRPM8 knockout mice [140]. Menthol also stimulates tear production in guinea pigs [28]. Importantly, in these animal studies, the concentrations of menthol that stimulated tear production did not induce nocifensive behaviors (eye swiping and lid closure) that are associated with noxious chemical stimuli known to activate polymodal receptors. In humans studied at elevated ambient temperatures (~43 °C), a stimulus known to inhibit the activity of cold thermoreceptors in animals, basal tear formation is reduced [98]. Together these findings support a role for corneal HB-LT cold thermoreceptors in providing information about the dryness level of the eye surface and in the reflex regulation of basal blinking and tear production. The possibility that dysfunction of corneal cold thermoreception contributes to the etiology of DED has been suggested [28,98,139], but remains to be demonstrated.
Selective activation of cold thermoreceptors also evokes conscious sensations in humans. In psychophysical experiments, cooling the corneal surface by 1–2 °C elicits a sense of cooling, whereas cooling the corneal surface by 4–5 °C elicits a sense of irritation [64,99]. Similarly, humans exposed to menthol vapor that increased its concentration in tears to ~5 μM reported a sense of cooling, while those exposed to a higher level of menthol vapor that increased the concentration to ~40 μM reported irritation [28]. When tested in guinea pigs, concentrations of menthol up to 200 μM increased the activity of cold thermoreceptors, but did not activate polymodal receptors [28]. The finding that weak stimulation of cold thermoreceptors causes a sense of cooling while a stronger stimulation elicits a sense of irritation can potentially be explained by the recruitment of cold thermoreceptors with higher thermal thresholds (LB-HT cold thermoreceptors; see Section 2.1.2.4). This evidence suggests that the expected strong activation of cold thermoreceptors during excessive drying of the eye surface elicits the sense of irritation [139].
2.1.4.2. Polymodal and mechano-nociceptors
In intact eyes of anesthetized animals [60,61], spontaneous nerve impulse activity is absent or of very low frequency in conjunctival and corneal mechano-nociceptors and polymodal neurons, indicating that these cell types do not signal under resting conditions. Corneal polymodal receptors are insensitive to innocuous cooling, although they are activated by hyperosmotic solutions [61,67]. However, in comparison with cold thermoreceptors, the sensitivity of polymodal neurons to increases in osmolarity is much lower, with an activation threshold around 600 mOsm/L [67]. Therefore, they are unlikely to be activated by this stimulus under normal conditions. Mechanical stress of the cornea surface produced by tear film breakup and dryness could potentially be detected by both the polymodal receptors and mechano-nociceptors [141]. Also the marked increases in tear film osmolarity that are suggested to occur with tear film breakup [142] would be sufficient to activate polymodal receptors and thereby contribute to the ocular discomfort that is experienced with an acute, excessive drying of the ocular surface.
2.2. Interactions between primary sensory neurons and the immune system
The nervous and immune systems have been traditionally considered independent entities serving separate functions and with limited cross-talk. In general, the inflammatory response following tissue injury involves activation of mast cells and resident sentinel immune cells such as dendritic cells (DC) in the cornea and microglia in the CNS, as well as infiltration of the injury site by circulating immune cells (neutrophils, lymphocytes and macrophages). Together these immune cells neutralize infective pathogens and contribute to tissue repair. In response to tissue injury, the peripheral sensory neurons transduce and transmit information about tissue damage to evoke sensations and reflex responses. In addition, in the periphery, some sensory neurons release neuropeptides that interact with immune cells and other tissue elements to contribute to the induction and spread of inflammation (neurogenic inflammation) [57]. By contrast, recent evidence indicates significant and complex interactions between the nervous and immune systems that extend both to peripheral and central nervous systems [143,144]. Neuro-immune crosstalk likely plays a significant role in ocular homeostasis following tissue damage and infection (see Section 5).
As a tissue in continuous contact with the outside world, the cornea has evolved as an immune privileged site in order to maintain transparency and preserve vision [145]. For that purpose, the local immune system is actively controlled through complex regulatory mechanisms to prevent inflammation, which is essential to protect tissues from infectious agents, but can result in corneal scarring and vision loss. Corneal immune privilege is based on several pillars, including lack of blood and lymphatic vessels, paucity of resident antigen presenting cells (APCs), low to minimal expression levels of major histocompatibility complexes (MHC) [146] and expression of neuropeptides and immunomodulatory factors. Recent research has demonstrated that sensory innervation contributes to maintaining the cornea’s immune privilege by suppressing adaptive immune responses, preventing blood- and lymph-angiogenesis, and the expression of pro-inflammatory cytokines, thereby maintaining immune tolerance [147]. In the cornea, a close physical co-location of resident bone marrow (BM)-derived cells with corneal nerves is well established [39,144,147–151]. Resident corneal macrophages have been shown to be in close contact with up to 10 separate axons in the subbasal plexus in the central cornea, and enwrap nerve bundles in the limbus and stroma [150].
Peptidergic polymodal nociceptor terminals of the cornea contain the sensory neuropeptides substance P and CGRP [19] that play a critical role in the induction of neurogenic inflammation following tissue injury [56]. This occurs because the peripheral axon terminals of the peptidergic nociceptors are highly branched and action potentials arising in one nerve terminal branch propagate both centrally to the brainstem and antidromically into all the other nerve terminal branches evoking the release of CGRP and substance P (the so called ‘axon reflex’). These neuropeptides act locally on pericorneal blood vessels and resident and infiltrating immune cells, extending and amplifying the inflammatory reaction induced by tissue injury [152]. Both CGRP and substance P also contribute to the maintenance of corneal immune privilege; CGRP has immunosuppressive effects while substance P acts as a potent pro-inflammatory neuropeptide [153,154]. Mature and immature DCs express CGRP type 1 receptors [155], with signaling through these receptors decreasing HLA-DR (Human Leukocyte Antigen - antigen D Related) and co-stimulatory marker molecule expression. Thus, CGRP release inhibits antigen presentation [156] and decreases T cell proliferation in response to antigens [155]. Furthermore, CGRP has been shown to attract DCs towards peripheral nerves, where higher concentrations result in arrest of their movement [157]. Conversely, increased levels of substance P disable regulatory T cells, which normally suppress the activity of effector immune cells involved in immune responses [158]. These observations suggest a role for sensory neuropeptides in the maintenance of corneal immunoprivilege under normal circumstances. However, the roles of CGRP and substance P in the neuro-immune cross-talk at the ocular surface in DED are yet to be elucidated. Other neuropeptides (cholecystokinin, gastrin) have been also detected in the cornea and TG neurons [159]. However, their functional role remains enigmatic.
Fractalkine (FKN, also known as chemokine (C-X3-C motif) lig-and 1 (CX3CL1) is a structurally unique chemotactic cytokine of the CX3C class and is produced by primary sensory neurons [160]. Unlike other non-selective cytokines, it binds only to its receptor CX3CR1, which is expressed by sensory ganglion satellite glial cells and leukocytes [161], and is a potent chemoattractant for immune cells [162,163]. There is extensive evidence to support a role for FKN/CX3CR1 signaling in the maintenance of homeostasis and that its disruption results in the induction of inflammation [164]. In the cornea, a role for FKN signaling in the recruitment of putative macrophages and MHC class II expressing DCs to the corneal epithelium has been demonstrated [165]. In addition, the dissociation of macrophages from nerves in the corneal stroma after injury has been shown to be in part CX3CR1 dependent [39]. Interestingly, while soluble FKN is not present in tears of healthy patients, it is up-regulated in patients with DED [166].
Finally, the crosstalk between neurons and immune cells contributes to the maintenance of peripheral nerve integrity and influences regeneration and degeneration processes. In humans, the increased numbers of resident and infiltrating immune cells in the cornea during infectious keratitis are highly correlated with reduced numbers of subbasal nerves in the central cornea, suggesting a causal relationship [148]. In contrast, activation of a T cell-dependent inflammatory cascade, involving IL-17, neutrophils, platelets and vascular endothelial growth factor (VEGF)-A enhances corneal nerve regeneration [167]. Also, the cytokine erythropoietin (EPO) acting on the innate repair receptor (IRR) activates anti-inflammatory and tissue repair pathways, favoring healing and tissue repair, and regeneration of injured peripheral sensory nerves [168].
Nerve growth factor (NGF) is essential for the development and maintenance of peripheral sensory neurons. Several cell types present in the cornea including epithelium, endothelium, keratocytes, and nerves express NGF and/or the NGF receptors TrkA and p75NTR. Likewise, NGF is also expressed by some of the various subsets of BM-derived cells reported in the cornea [151,169,170]. Another group of molecular regulators that potentially mediate crosstalk between the immune and nervous systems in the cornea are the “immune semaphorins” (i.e., Sema3A, 4A, 4D, 6D, and 7A). Unlike many conventional semaphorins that act as repulsive axon guidance factors, “immune semaphorins” regulate immune cell contacts and promote axon outgrowth [171,172]. For example, Sema7A acts as a neurotrophic factor in the cornea that can also influence inflammatory processes, while Sema3A is a negative regulator of innervation that counterbalances the positive neuromodulatory function of VEGF [173]. A more complete identification and functional characterization of the molecules that mediate neuro-immune crosstalk is likely to aid in defining the cellular processes that regulate the innervation of the ocular surface in normal tissue and loss of innervation in chronic inflammation.
Experimental evidence suggests that satellite glial cells in the TG release a variety of molecules that modulate TG neuron excitability under normal circumstances and increase excitability when they are persistently activated by noxious stimuli [174]. However, the role that satellite glial cells play in modifying the excitability of TG neurons that supply the ocular surface has not yet been investigated. At spinal levels, persistent activity of DRG neurons activates microglia in the dorsal horn that, in turn, modifies spinal neurons that integrate noxious sensory information [5,175]. Activated microglia release pro-inflammatory cytokines and other factors that amplify synaptic transmission in the dorsal horn and thereby induce central sensitization. The potential role of microglia activation in DED associated discomfort and pain is discussed in Section 5.2.
In a healthy eye, bidirectional communication between nerves and the immune system forms a negative feedback loop that keeps both systems in check. Inflammation may disrupt neuro-immune communication in DED, resulting in altered sensory nerve activity and the unpleasant sensations associated with this condition (see Section 5.1.1).
2.3. Central pathways and sensory processing
2.3.1. Trigeminal brain stem nuclear complex
TG neurons supplying the ocular surface and surrounding periocular tissues send branches centrally to terminate at multiple rostrocaudal levels of the trigeminal brain stem nuclear complex (TBNC) [176–180]. The TBNC is composed of the principal trigeminal nucleus (Vp) in the pons and the spinal trigeminal nucleus in the medulla that is further subdivided into subnucleus oralis (Vo), interpolaris (Vi) and caudalis (Vc), based on anatomical and functional properties. Second-order neurons that respond to ocular surface stimuli are found at multiple levels of the TBNC, an organization that is unique to the trigeminal system and has no spinal equivalent [181]. The majority of ocular surface-responsive TG neurons terminate at two spatially discrete regions of the lower TBNC: the transition region between caudal Vi and Vc (ViVc transition) and at the Vc/upper cervical cord junction (VcC1 region); however, a smaller number of afferent fibers terminate in Vp and Vo [179,180]. TG neurons that supply the eyelids [16,182], lacrimal gland [183,184] and meibomian glands [177,185] display a similar terminal pattern in the TBNC. The significance of eye representation at multiple regions of the TBNC may reflect redundancy to preserve eye function or alternatively, may reflect cell groupings that serve different aspects of ocular function [186].
The role of CNS neurons in somatosensory function is predicted using neurophysiological methods and is based largely on: i) encoding properties to adequate stimuli, ii) effects of analgesic drugs and iii) efferent fiber projection targets. Based on these lines of inquiry, current evidence suggests that corneal neurons at the ViVc transition and VcC1 region (i.e., “ocular neurons”) serve different aspects of ocular function.
2.3.1.1. Ocular neurons at the ViVc transition
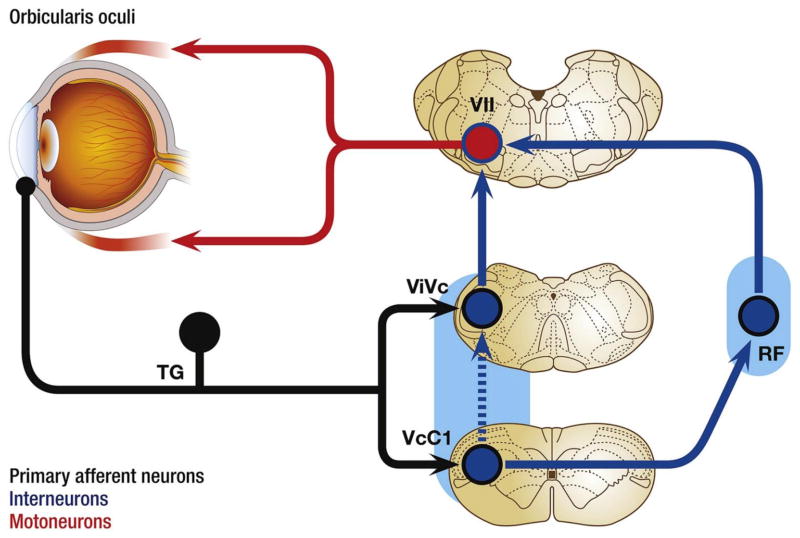
Ocular neurons recorded at the ViVc transition encode the intensity of mechanical, thermal and chemical stimulation of the ocular surface [100,187,188]. Ocular neurons at the ViVc transition neurons are excited by bright light [189] and are sensitive to changes in the moisture status of the ocular surface, a feature not seen by neurons in other TBNC regions [190]. The receptive field for most ViVc transition neurons includes the entire ocular surface. Many ViVc transition neurons also respond to innocuous and noxious stimulation of periorbital skin; however, nearly 50% of neurons at this region respond only to ocular surface stimulation [187,191]. Small diameter myelinated (Aδ fibers) and unmyelinated (C fibers) corneal nerve fibers terminate at the ViVc transition. Repetitive ocular surface stimulation often causes desensitization or fatigue of ViVc transition neurons, while systemic morphine administration enhances corneal-evoked responses in about 30% of recorded neurons, a feature that may contribute to opioid analgesia-induced ocular itch [192]. Ocular neurons at the ViVc transition project to brain regions that control lacrimation (superior salivatory nucleus) and eye blink (facial motor nucleus) as well as to the sensory thalamus [193,194]. Blockade of synaptic activity at the ViVc transition prevents reflex lacrimation due to bright light [195] and reduces eye blink behavior evoked by hypertonic saline [196]. Collectively, these properties suggest that ocular neurons at the ViVc transition play a significant role in maintaining ocular homeostasis and a lesser role in sensory-discriminative aspects of ocular pain.
2.3.1.2. Ocular neurons at the VcC1 region
Ocular neurons at the VcC1 region encode the intensity of mechanical, thermal and chemical stimulation of the ocular surface, and at similar thresholds as ViVc transition neurons [187,188]. VcC1 neurons often respond to multiple classes of chemical irritants [197,198]. However, there are significant differences in encoding properties for ocular neurons at these two regions. Unlike neurons at the ViVc transition, the receptive field for most VcC1 ocular neurons includes only a portion of the ocular surface and all neurons are activated by noxious stimulation of periorbital skin [187,188]. Many VcC1 neurons receive convergent input from the cornea and the dura, suggesting a role in headache [199,200]. Repetitive ocular surface stimulation sensitizes VcC1 neurons, while systemic morphine administration inhibits corneal-evoked responses of all VcC1 neurons in a dose-dependent manner [192]. Although VcC1 ocular neurons are also activated by bright light, synaptic blockade of this region does not alter light-evoked lacrimation [189]. The efferent projections of VcC1 ocular neurons include the facial motor nucleus, pontine parabrachial nucleus, sensory thalamus and hypothalamus [187,192,194,201]. (Fig. 3). Synaptic blockade of the VcC1 region causes a transient reduction in/of saline- or light-evoked eye blink [196]. Collectively, current data suggest that ocular neurons at the VcC1 region behave similar to nociceptive neurons found in dorsal horn of the spinal cord and likely are critical for sensory-discriminative aspects of ocular pain.
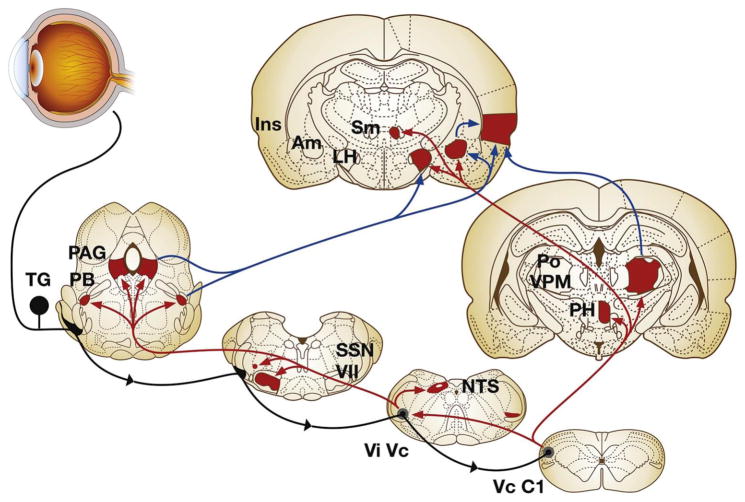
Fig. 3.
Major ascending brain pathways for trigeminal sensory fibers that supply the eye. The cell somata of sensory fibers are found within the trigeminal ganglion and project centrally to terminate in two spatially discrete regions of the trigeminal brainstem complex, the trigeminal subnucleus interpolaris/caudalis transition region (ViVc) and the caudalis/upper cervical cord junction (VcC1). Second-order ocular neurons in ViVc and VcC1 project to brain regions that mediate eyeblink (facial motor nucleus, VII), lacrimation (superior salivatory nucleus, SSN), and cardiovascular reflexes (nucleus tractus solitarius, NTS). Projections to higher centers such as the periaqueductal gray (PAG), PBA (PB), lateral hypothalamus (LH), posterior hypothalamus (PH), and amygdala (Am) contribute to the affective and modulatory aspects of ocular pain, while projections to posterior thalamus (posterior nuclear group, Po; ventral posteromedial nucleus, VPM) and insular cortex (Ins) mediate sensory-discriminative aspects. Note that a small group of ocular responsive neurons also are found in the contralateral ViVc; the source of input to this group is not well defined. Primary afferent fibers are drawn in black, second-order projections in red and third-order projections in blue. (Reproduced from Stapleton et al. [1]). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
There are scattered reports of neurons at Vp and Vo regions responding to periocular stimulation [202–204]; however, their contribution to ocular function is not known. Damage to the lateral pons and medulla, as occurs in some stroke patients, can result in paroxysmal or “salt and pepper” sensations in the eye consistent with a role for the Vp/Vo region in ocular sensation [205].
2.3.1.3. TBNC intersubnuclear communication
Rostral and caudal regions of the TBNC are connected through a dense longitudinal fiber system [206–209]. Evidence that intersubnuclear pathways serve a “feed forward” facilitatory function has been reported in models for acute dental pain [210], headache [211] and evoked eye blinks [212]; however, a role in DED has not been determined. Intersubnuclear pathways also may contribute to opioid-induced modulation of ocular pain since localized injections of opioid receptor agonists into the VcC1 region markedly alters corneal-evoked responses of ocular neurons at the ViVc transition [194].
2.3.2. Representation of the eye at supraspinal brain levels
The representation of the ocular surface and periocular skin at higher levels of the neuroaxis has not been well examined. Earlier mapping studies identified neurons with periorbital receptive fields in the most posterior and medial portions of the somatosensory thalamus [194,213], somatotopically appropriate for representation of the ophthalmic branch of the trigeminal nerve [214,215]. There are ocular neurons at the ViVc transition and VcC1 region with projections to the parabrachial area and the posterior thalamus, and not to the main sensory thalamic areas [187,192,216]. Since these areas have strong connections with the amygdala, insular cortex and other limbic brain areas, this suggests a role in affective and/or autonomic aspects of pain [217,218]. Interestingly, stimulation of insular cortex evokes tingling and pain sensations on the face and around the eye [219] whereas, in their original mapping study in 1937 using electrical stimulation of primary somatosensory cortex, Penfield and Boldrey [220] could not elicit ocular sensations. Neurons with a corneal or periorbital receptive field have been recorded in primary somatosensory cortex [221,222] and neuroimaging has identified an increase in signal in primary somatosensory cortex after painful bright light stimulation in humans [223]. These studies suggest that the eye is poorly represented at thalamic and cortical areas closely associated with sensory-discriminative aspects of pain, whereas strong connections to brain regions associated with affective and autonomic aspects of pain are found. A schematic representation of the ascending neural pathways associated to the eye at different levels of the neuroaxis is represented in Fig. 3 (see also Fig. 6).
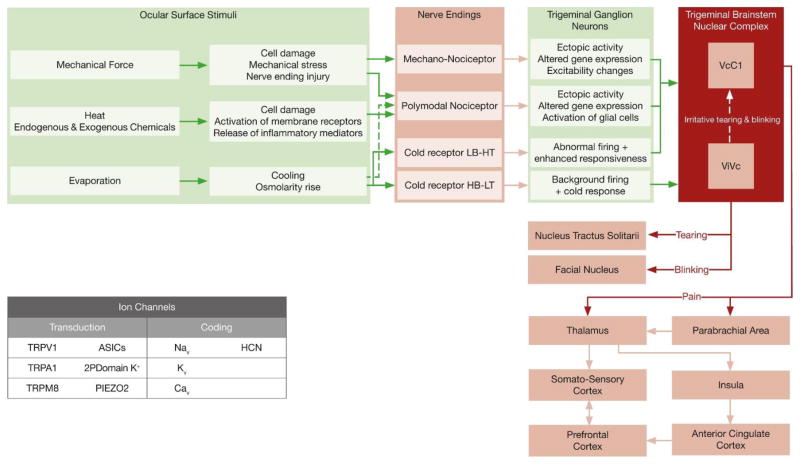
Fig. 6.
Peripheral and central neural mechanisms involved in the sensory and autonomic responses evoked by eye surface dryness. The main types of ion channels involved in the transduction and coding of mechanical, thermal and chemical stimuli are represented in the inset.
3. Neural regulation of tear production
The components of the tear film are produced by the main and accessory lacrimal glands, meibomian glands, goblet cells, stratified squamous epithelial cells and corneal epithelium. The neural regulation of secretion by each of these tissues is distinct.
The aqueous tear producing tissues receive a parasympathetic innervation that originates from the parasympathetic pterygopalatine or ciliary ganglia and a sympathetic innervation that originates from the superior cervical ganglia. In addition, most of these tissues receive a peptidergic sensory innervation from TG that potentially subserves an efferent function. In the sections below describing the innervation of specific glandular tissues, immunohistochemistry was used to define the parasympathetic, sympathetic and sensory nerve fibers. In most studies, immunoreactivity (IR) to the neuropeptide Vasoactive Intestinal Peptide (VIP) was used to identify parasympathetic nerve fibers, but these fibers were also localized by their IR for the enzyme acetyl-cholinesterase that inactivates released acetylcholine and the vesicular acetylcholine transporter. Sympathetic nerve fibers were identified by their IR for Neuropeptide Y (NPY), or for the enzymes Tyrosine Hydroxylase (TH) or Dopamine β-Hydroxylase (DBH) that are required for the synthesis of the sympathetic neurotransmitter norepinephrine. Peptidergic sensory nerve fibers were identified by their IR for the neuropeptides CGRP or Substance P.
3.1. Lacrimal gland
The lacrimal gland is the main producer of electrolytes, water, and protein in the tear film. The lacrimal gland synthesizes and secretes multiple proteins many of which are anti-bacterial [224–229]. These proteins are stored in secretory granules in the acinar cells until released by the appropriate stimuli. Only a small percentage of granules are released by exocytosis in response to a given stimulus, a mechanism of secretion known as merocrine [230]. Water and electrolytes are secreted by the coordinated activation of ion channels and pumps [231–233]. Electrolytes and water come from the blood supply and are transported across the basolateral membranes into the lacrimal gland cells and then across the apical membrane into the duct system. The ionic composition of the fluid produced by the acini is modified within the ducts [234]. The ducts also secrete proteins that are different from those produced by acinar cells [235].
Autonomic nerves regulate the secretory activity of the lacrimal gland. The activity of these efferent nerves is regulated by reflexes initiated by activation of sensory neurons supplying the ocular surface. As described in Section 2.1.4.1, recent evidence indicates that corneal cold thermoreceptors sense changes in the dryness of the ocular surface and elicit a reflex that contributes to the regulation of basal aqueous tear formation. The overflow tearing initiated by damaging or potentially damaging stimuli is elicited by activation of mechano- and polymodal nociceptor sensory nerves in the cornea and conjunctiva (Section 2.1.4.2).
While the lacrimal gland receives both a sympathetic and parasympathetic innervation, the latter is most extensive. VIP-IR nerve fibers (parasympathetic) are localized densely around the basal surfaces of the acini with rare appearance around ducts and blood vessels, and in the interstitial stroma and connective tissue [236–241]. Acetyl-cholinesterase- and vesicular acetylcholine transporter-IR nerve fibers are also localized around the ducts, acini and blood vessels, and in the interstitial stroma and interlobular tissue [239,242,243]. Thus the distribution of nerve fibers expressing these proteins is similar to those containing VIP [239].
Exogenous application of muscarinic receptor agonists to the lacrimal gland increases both protein and aqueous fluid (water and electrolyte) secretion from the lacrimal gland [244–248]. In addition, there is consistent evidence that lesioning the pre- or post-ganglionic parasympathetic nerves to the lacrimal gland cause a marked reduction in lacrimal gland secretion [249,250]. A similar reduction in lacrimal gland secretion is produced by in vivo administration of muscarinic antagonists [251,252], demonstrating the importance of neurally released acetylcholine in mediating the actions of the parasympathetic nerves. Evidence indicates that acetylcholine mediates its effects in the lacrimal gland by activating M3 muscarinic receptors [253,254]. IR and gene expression for M3-muscarinic receptors were demonstrated in lacrimal acinar, myoepithelial and duct cells [255,256]. While these findings clearly support a role of muscarinic receptors in stimulating lacrimal secretion, prolonged application of the muscarinic agonist carbachol to lacrimal gland acinar cells in vitro results in a marked reduction in protein secretion [257,258]. This reduction, which may play a role in dysfunction of the lacrimal gland, is not accompanied by changes in membrane expression of M3 muscarinic receptors and has been attributed to down regulation of post receptor-signaling mediators and effectors [259]. In humans, the VIP receptor 1 was identified on the basolateral membrane of the acinar cells, whereas the VIP receptor 2 was localized to the myoepithelial cells. Application of VIP to isolated rabbit acinar cells or to the isolated porcine lacrimal gland increases protein secretion [237,260–263]. When both muscarinic and adrenergic receptors were blocked, application of a VIP receptor antagonist produced a small reduction in protein secretion evoked by electrical stimulation of the nerves supplying the isolated porcine lacrimal gland [261].
In general, the sympathetic innervation is located predominately around the vasculature. Nerve fibers with IR for NPY (sympathetic) are found around the arteries and arterioles and in the interstitial stroma between lacrimal gland acini [239]. Similarly nerve fibers with IR to TH or DBH are seen around the blood vessels and in the interlobular connective tissue and in the stroma between acini [239,241–243]. One study demonstrated more TH-IR nerve fibers than NPY-IR nerve fibers [239], which may indicate that the gland is innervated by more than one sub-population of postganglionic sympathetic neuron as has been described in salivary glands [264].
Sympathetic nerves can affect lacrimal gland secretion in two different ways. First they can alter blood flow, with vasodilation increasing electrolyte and water secretion, and with vasoconstriction decreasing them [265]. Second, sympathetic neurotransmitters can directly induce protein, electrolyte and water secretion [243,261,266,267]. Exogenous application of α1- or β-adrenergic receptor agonists increases lacrimal gland protein secretion [268,269]. In rat lacrimal gland, the stimulatory action of α1-adrenergic receptor agonists is mediated via α1D-adrenergic receptors [268]. Mouse lacrimal gland tissue showed IR for α1- and β1-adrenergic receptors, with the acinar cells expressing both types of receptor. Within the acinar cells, IR for α1-adren-ergic receptors was present both at the cell surface and in the cytoplasm, whereas that for β1-adrenergic receptors was localized primarily to the cytoplasm [269]. In addition, IR for α1-adrenergic receptors is localized to the blood vessels and ducts in the mouse lacrimal gland [269]. The actions of exogenously applied NPY have only been investigated in porcine lacrimal gland where it also increases protein secretion [261]. Despite the findings that exogenous delivery of sympathomimetics stimulates protein secretion, the physiological role of the sympathetic innervation to the lacrimal gland has been questioned because its function was not altered in rabbits when innervation from the superior cervical ganglion was interrupted [270].
ATP, which is potentially released from both parasympathetic and sympathetic nerve terminals in lacrimal gland, can stimulate protein secretion via activation of P2X3- and P2X7-purinoceptors [271,272]. In addition, evidence suggests that there is a complex synergistic interaction between muscarinic and P2X7 receptors in stimulating protein secretion, and that activation of α1D-adrenergic receptors triggers the release of ATP from the acini [273,274].
The lacrimal gland is also innervated by CGRP-IR fibers (pepidergic, sensory) that are found predominantly around the lacrimal ducts, but are also associated with the arteries and arterioles in the lacrimal gland [236,239]. Substance P-IR nerve fibers have a similar distribution to the CGRP-IR nerve fibers, but the numbers of CGRP-IR fibers is higher [239]. Sensory dennervation of the rabbit lacrimal gland increases its protein secretion in response to exogenous application of the β-adrenergic receptor agonist isoproternenol or the muscarinic receptor agonist carbachol [270]. In addition, sensory dennervation results in a massive accumulation of vesicles within the lacrimal gland acini. Together these findings suggest that the sensory nerves play an efferent role in regulating lacrimal gland secretion.
3.2. Accessory lacrimal glands
Like the main lacrimal gland, the accessory lacrimal glands contribute electrolytes, water and protein to tears. Very little is known about the neural control of accessory lacrimal glands, but it appears to be similar to the main lacrimal gland [275]. Electron microscopy studies have demonstrated the presence of non-myelinated axons containing vesicles close to cells in the secretory epithelium and intralobular ducts of the glands [276]. VIP-IR nerve fibers (parasympathetic) have a similar distribution within the accessory lacrimal glands [240]. Electron microscopy revealed a few axons with small and large dense cored vesicles indicative of sympathetic axons, but those with small clear vesicles and large dense core vesicles indicative that parasympathetic axons were much more prevalent [276]. Human accessory lacrimal glands express muscarinic receptors (M1 and M3), VIP receptors (1 and 2) and adrenergic receptors (α1A, α2A, and β2) [275].
3.3. Meibomian gland
The meibomian gland produces meibum, which contains the major lipid components of tears. These lipids are synthesized by the meibocyte acinar cells and accumulate in these cells as they mature and migrate toward the center of the acinus. Secretion occurs when the cells rupture and release their contents into the lumen of the duct system (holocrine secretion). There appear to be no studies examining the role of nerves and their neurotransmitters in regulating the holocrine secretion of the meibomian gland. Studies have, however, noted the proximity of nerve fibers to the meibomian glands in a wide range of animal species including humans [125,185,240,277–283].
VIP-IR nerve fibers (parasympathetic) are present in the meibomian glands [240,277,280,284] and are mostly associated with the acini and central duct [123,183], but there is some debate surrounding their association with the meibomian gland vasculature [125,185,284,285]. Evidence exists to support the presence of VIP receptors 1 and 2 and muscarinic receptors M1, M2, M3, M4, and M5 within the meibomian gland acini, ducts, and basal epithelium [286–288]. In immortalized human meibomian gland cells, application of the muscarinic agonist carbachol or VIP increases cytoplasmic Ca2+ concentration and stimulates cell proliferation [288]. Application of VIP in combination with either 3-isobutyl-1-methylxanthine or forskolin also causes a significant elevation in intracellular cAMP content [288].
DBH- and NPY-IR (sympathetic) nerve fibers are predominately associated with the vasculature and are found more sparsely in the meibomian gland acini [123,125,183,185,277,289]. Immunolabeling has demonstrated that NPY receptor 1 is localized to the nuclear membrane of the acinar cells and to the cell membrane of the meibomian gland duct and acinar cells in mice [287].
CGRP-IR nerve fibers (peptidergic, sensory) were localized mostly to the meibomian gland vasculature, but are also associated with the ducts where their density is highest close to the orifice [121,123,125,183,185,277]. Substance P-IR nerve fibers have a very sparse presence in the meibomian glands, but they are also associated with the acini, ducts and vasculature [122,123,125,185,277,285]. The Substance P receptor (neurokinin 1 receptor) was also localized to the cell membrane of duct and acini cells in the mouse meibomian gland [287].
3.4. Conjunctival goblet cells
Conjunctival goblet cells secrete the gel forming mucin MUC5AC, electrolytes and water [290–292]. The secretory proteins including mucins are synthesized in the goblet cells and stored in secretory granules. Upon an appropriate stimulus, the secretory granules fuse with each other and with the apical membrane to simultaneously release most of the secretory granules in a given cell (apocrine secretion). Activation of sensory nerves supplying the rat cornea evokes goblet cell mucous secretion that is dependent on activation of nerves within the conjunctiva [293]. However the efferent nerve type(s) involved in mediating this reflex response remains to be established.
Not all goblet cell clusters appear to have nerve fibers near their basal membranes [124]. Where present, parasympathetic nerve fibers revealed by VIP-IR have been found at the epithelial-stroma junction and around the basolateral aspect of goblet cells in a variety of aminals [124,240,294]. IR for M1, M2 and M3 muscarinic receptors has been demonstrated in goblet cells from mice and humans, whereas rats only expressed M2 and M3 muscarinic receptors [294,295]. These receptors, as well as the VIP receptor 2, are located subjacent to the secretory granules within the goblet cells. Exogenous application of either VIP or the muscarinic receptor agonist carbachol stimulates goblet cell secretion, indicating the importance of the parasympathetic innervation [295,296].
DBH- and TH-IR (sympathetic) nerve fibers surround the goblet cells [124,294]. Immunolabelling studies indicate that goblet cells in humans express α1A- and β3-adrenergic receptors, whereas in mice and rats β1- and β2- adrenergic receptors were demonstrated [294]. Conclusions regarding β1- and β2-adrenergic receptor expression in human goblet cells cannot be made, because suitable antibodies are currently not available. In rats, CGRP-IR nerve fibers (peptidergic sensory) are present in the conjunctiva, but were not found close to the goblet cells [124].
As described for the lacrimal gland, ATP is another potential signaling molecule that can be released from both parasympathetic and sympathetic nerve terminals, and by other cell types. This purine elicits mucin secretion from goblet cells in rabbit and human conjunctiva via activation of P2Y2 purinoceptors [297].
3.5. Conjunctival stratified squamous cells
Conjunctival stratified squamous cells produce the membrane-spanning mucins MUC1, MUC4, and MUC16 [298–300]. These cells also secrete electrolytes and water into the tear film [290]. The mucins are synthesized within the squamous cells and are then inserted into the apical membrane where the extracellular domain forms the glycocalyx that reaches into the tear film. The extracellular domains of MUC1, MUC4 and MUC16 can be released into the tear film by ectodomain shedding [301]. MUC4 can also be routed via an intra-cellular pathway where it is secreted as a small soluble mucin into the tear film [302]. Several non-neural processes regulate the release of mucins from stratified squamous cells, but to date no regulatory role for nerves or neurotransmitters has been identified.
In mice, VIP-IR (parasympathetic) nerve fibers are located at the basal surface of the stratified conjunctival squamous epithelial cells, whereas TH-IR (sympathetic) nerve fibers are located at the base of the epithelium [294]. By contrast, in humans, VIP-IR nerve fibers are located at the base of the epithelium and TH-IR nerve fibers are located at the basal surface of the squamous epithelial cells [294]. IR for α1A- and β3-adrenergic receptors was detected in squamous epithelial cells of human conjunctiva, whereas in mice IR for α1A-, β1- and β2-adrenergic receptors were detected in these cells [294]. In humans, M1, M2 and M3 muscarinic receptor subtypes were detected in epithelial cells, with a high expression of M2 and M3 receptors in the basal epithelial cell layer of the conjunctiva [294]. In mice and rats, M1 and M2 muscarinic receptors were detected in squamous epithelial cells [294,295].
As indicated above, both goblet cells and stratified conjunctival squamous epithelial cells secrete electrolyte and water into the aqueous tear layer. To date this secretion has only been studied in whole tissue segments of conjunctiva that contain both goblet cells and stratified squamous epithelial cells. However, as there are many more stratified squamous cells than goblet cells, it is likely that these studies have primarily measured electrolyte and water secretion from the squamous cells [290,303]. While there is no direct evidence that nerves regulate conjunctival epithelial fluid secretion, exogenous application of the α- and β-adrenergic receptor agonist epinephrine stimulates the electrolyte secretory activity of the isolated rabbit conjunctiva [303,304]. This effect of epinephrine was attributed to stimulation of β2-adrenergic receptors and the elevation of intracellular cAMP (cyclic adenosine monophosphate) levels [304]. As it has a large surface area compared to the cornea, the conjunctiva can supply the pre-corneal, tear film.
3.6. Corneal epithelium
The corneal epithelium also secretes mucins, electrolytes and water into the tear film, but its contribution is limited. The autonomic nerve fibers innervating the corneal epithelium are predominantly sympathetic, but there is also a minor contribution of parasympathetic nerve fibers [305]. As indicated in Section 2.1.2, a significant proportion of polymodal nociceptor sensory nerve fibers of the cornea are peptidergic and release neuropeptides when activated by noxious stimuli [306]. Their influence on corneal epithelium tear fluid secretion is unknown.
As with the conjunctiva, epinephrine stimulates the electrolyte secretory activity of the corneal epithelium [307]. Furthermore, surgical removal of the superior cervical ganglion reduces corneal epithelial Cl− transport and increases sensitivity to epinephrine [307]. These findings clearly indicate a functional role for sympathetic nerves in regulation of corneal electrolyte secretion. As with the conjunctiva, the stimulatory effect of epinephrine on corneal electrolyte secretion is attributed to activation of β-adrenergic receptors and the elevation of intracellular cAMP levels [307].
4. Neural regulation of eye blinks
The neural pathways involved in reflex blinking are better known than for spontaneous blinks. The orbicularis oculi (OO) muscle acts to close the upper eyelid during the blink. Ocular surface activation of the OO arises in its sensory nerves, which project to the motor neurons of facial nerve (Cranial nerve VII) through trigeminal sensory fibers [193,308]. There are at least two major circuits involved in the downward phase of blinking that are associated with a stimulus evoking reflex blinks. The short-latency (R1) response originates in the caudalis subdivision of the TBNC and is pre-programmed to produce increasing OO motoneuron activity with increasing stimulus amplitude that is relatively insensitive to sensory feedback. The long-latency circuit (R2) is slower and is sensitive to sensory feedback, including sensory neurons from the ocular surface. Unlike the R1 response, the R2 response is not pre-programmed, but rather can modify the blink to fit the stimulus [309,310].
The reflex blink pathway has three basic components in its simplest form: (1) primary corneal afferents, (2) second order TBNC neurons, and (3) OO motoneurons (Fig. 4). Anatomical studies in rats show that the Vi/Vc transition region and the VcC1 region project to the facial motor nucleus [182,194] and that damage to the caudal trigeminal brainstem complex severely impairs the generation of cornea-evoked reflex blinks in humans [311]. The discharge pattern of Vi/Vc neurons appears to initiate blinking, sets blink amplitude and peak velocity of corneal reflex blinks, while neurons at the VcC1 region modify the activity of ViVc transition neurons and eye blinks [212,312]. (see Section 2.3.1).
Fig. 4.
The corneal eye blink reflex is initiated by the free nerve endings in the cornea and involves the trigeminal nerve and ganglion (TG), the brainstem nuclei (VcC1: caudalis/upper cervical cord junction and ViVc: interpolaris/caudalis transition region), interneurons in the reticular formation (RF), motor neurons in the facial nucleus (VII) and nerve, and the orbicularis oculi. As the afferent information is distributed bilaterally to facial motor neurons by the reticular formation interneurons, the eye blink response is consensual, that is, both eye lids will close to stimulation of the cornea of either eye.
The main downward force of the blink is generated by the sphincter-like OO, but there is an additional passive downward force generated by the spring-like qualities of the stretched tendons associated with the levator palpebrae (LP) muscle. Thus, the downward phase of the blink is very rapid and occurs immediately following a burst of OO motoneuron activity, during which LP motoneurons temporarily cease firing. For example, downward lid saccades may reach a velocity of 294° per sec, while the down phase of the blink can easily surpass that speed at 840° per sec. All of the other extraorbital muscles transiently co-contract with the blink, with the exception of the superior oblique, so that the eye is drawn transiently upward during the blink [313].
The main upward force of the blink comes from the LP muscle, with some input from Müller’s muscle. Motoneurons of the facial nerve innervate the OO and show little or no tonic activity. Instead, these motoneurons are characterized by high frequency bursts of activity with the down phase of the blink. In contrast, LP motoneurons like most of the other extraocular muscles in the orbit are innervated by the oculomotor nerve (Cranial Nerve III) and exhibit tonic activity, which increases with upward gaze. Thus, the open eye condition is maintained by tonic activity of the LP, balanced by passive downward forces provided by the LP aponeurosis, the LP tendon and palpebral ligaments [314]. The up phase of the blink is slower than the downphase and is due to a burst exceeding the tonic activity of the LP. Müller’s muscle has a slight additive effect on the up phase, opening the eye wider with sympathetic nerve activation in, for example, a state of fear or surprise. When sympathetic innervation is affected, as in Horner’s syndrome, lack of input from the Müller’s muscle results in ptosis of the eyelid [313].
The ocular surface input for spontaneous blinking is less well understood than for réflex blinking. Many studies have shown increased spontaneous blinking when the ocular surface is stimulated [315–317]. Recently, Wu et al. showed a linear increase in blink rate with increasing air flows applied on the ocular surface [318]. This stimulus enhances cold thermoreceptor activity, thus favoring the interpretation that spontaneous blinking is maintained, at least in part, by the continuous nerve impulse firing of corneal cold thermoreceptors (see section 2.1.4.1) [139]. Indeed, genetic silencing of cold thermoreceptors in mice or ocular instillation of a local anesthetic in humans, reduces blink rate [101,315,319]. Hence, these studies suggest that the afferent input from ocular surface sensory nerves, in particular cold thermoreceptors, contribute to maintain the spontaneous blink rate through modulation of the spontaneous blink generator. The association of dry eye condition with increased blinking is presumably due to an enhanced activity of ocular surface sensory nerve terminals evoked by the irritation provided by an unstable tear film [28,315,320–322]. Wearing contact lenses, which may stimulate the ocular surface, is also associated with an increased blink rate [323,324].
Central dopamine levels are also known to affect the spontaneous blink rate. For example, patients with schizophrenia exhibit a higher blink rate, while those with Parkinson’s Disease show a lower blink rate, most likely due to differences in dopamine levels in those conditions [325–327]. Cognitive state can also vary the blink rate [328–330]. Reading, working on computers, or other visual tasks requiring concentration are known to decrease blink frequency [316,321,324,331,332]. Thus, it is clear that a number of factors acting at various levels of the neuroaxis combine to affect the spontaneous blink rate.
Recently, Kaminer et al. [333] hypothesized that a spontaneous blink generator that acts to set the spontaneous blink rate was located in the spinal trigeminal complex. They found that both rats and humans exhibited a periodicity in the blink rate over time, with both species displaying a similar temporal organization in spontaneous blink pattern. They were able to show modulation of the blink rate in DED and by altering dopamine levels in the brain in a rat model. These data suggest an essential role for the spinal trigeminal complex, already known to play a part in reflex blinking, in determining the spontaneous blink pattern. Thus, spontaneous blink patterns are likely to be modified by multiple neural sources including corneal afferents, the secretion of dopamine by basal ganglia and cortical input that can be altered by mood or task.
Blinks also can occur in clusters or flurries [321,334], where relatively innocuous stimuli can evoke multiple blinks, termed blink oscillations, instead of a single reflex blink [335]. Blink oscillations are also common in Parkinson’s Disease [336], a condition characterized by loss of dopamine neurons in basal ganglia. In aging, the blink oscillations may occur due to a normal, age-related loss of dopamine neurons [335,337]. Blink ocillations also increase in blepharospasm that may be driven, in part, by dry eye symptoms and ocular surface irritation that enhance the activation of Aδ- and C-fiber sensory nerve inputs [337]. Peshori et al. hypothesized that the purpose of these blink oscillations is to improve the ocular surface tear film to compensate for increasing eye dryness with age [335].
5. Neurobiological changes in dry eye disease
It is well established that the sensory nerves of the ocular surface display structural and functional changes in DED and underlie the development of adverse symptoms that range in intensity from mild discomfort and dryness to burning pain. In chronic DED, it is likely that multiple levels of the neuroaxis are involved resulting in aggravation of adverse symptoms as well as altered tissue trophism, regulation of tear production, blinking and reflex vascular responses.
5.1. Effects of eye surface dryness on peripheral sensory neurons
Reduced tear secretion leaves the corneal epithelium exposed to adverse environmental conditions. The osmolarity of the tear film increases while excessive evaporation causes rapid cooling of the ocular surface. Both of these events cause stress to the ocular mucosal epithelium, leading ultimately to local inflammation and a variable level of peripheral nerve damage (see TFOS DEWS II Pathophysiology report). In other tissues, it has been shown that local inflammation and nerve injury evoke profound short- and long-term genetic and molecular changes that modify the electrophysiological characteristics of the peripheral terminals, parent axons and cell bodies of the primary sensory neurons [338]. In the longer term, these changes lead to abnormal peripheral and central nerve terminal sprouting, aberrant impulse activity and alterations in the central synaptic transmission, resulting in dysregulated transmission and processing of pain signals leading to chronic pain [338]. While the mechanisms that lead to the abnormal sensations of discomfort and pain referred to the eye with chronic tear deficiency have not been precisely identified, it is likely that they involve similar mechanisms to those demonstrated for other chronic pain conditions.
5.1.1. Local inflammation
Inflammation plays a key role in the pathogenesis and chronicity of DED [339] and represents a major driving force in sensitization, damage and regeneration of the peripheral sensory neurons [338,340,341]. Persistent stress from desiccation stimulates the local release of a variety of chemical mediators from epithelial cells, keratocytes and resident or infiltrating immune cells (such as APCs, neutrophils, monocytes, mast cells and platelets). In DED, the lacrimal gland can also become inflamed and is another potential source of chemical mediators that contribute to the inflammatory environment at the ocular surface [342,343]. The chemical mediators released include eicosanoids [prostaglandins (PGs), leukotrienes (LTs), thromboxanes (TXs)], bradykinin (BK), 5-hydroxytryptamine (5-HT), histamine (HIS), purines [adenosine, adenosine triphosphate (ATP)], hydrogen ions (H+), nitric oxide (NO), platelet-activating factor (PAF), neurotrophins (eg. NGF, GDNF), endocannabinoids, proteases as well as pro-inflammatory cytokines such as interleukins (IL-2, IL-6, IL-8, IL-10, IL-17), macrophage inflammatory protein-1α, tumor necrosis factor (TNF)-α and granulocyte-macrophage colony stimulating factor (GM-CSF) [56,57,338,344–351]. Each of these chemical mediators potentially contributes to increasing activation of sensory nerve terminals, either by reducing their threshold for activation by sensory stimuli (sensitization) and/or by directly inducing or increasing their ongoing nerve activity.
The events triggered by proinflammatory agents are diverse and complex [347,352]. Activation of G protein coupled receptors (GPCR) located in the membrane of nociceptive terminals by the inflammatory agents is one of the general mechanisms for modulation of nociceptor’s excitability employed by numerous proinflamamtory substances. Activated GPCRs couple to specific G proteins that mediate the recruitment of one or multiple signaling pathways (protein kinase C, phospholipase C (PLC), cAMP–protein-kinase-A, release of calcium from intracellular stores) [353,354]. Other chemical mediators, like growth factors act on tyrosine kinase receptors, which then activate a variety of intracellular signaling pathways (PLC, phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK)) [354]. The kinases activated by these signaling pathways in turn phosphorylate existing protein targets in the nociceptors. These include ion channels that contribute critically to the activation of sensory nerve endings by stimuli, such as transduction channels that convert the sensory stimulus into a membrane depolarization (receptor potential) and voltage-gated, ligand-gated and background (or leak) ion channels that regulate the resting membrane potential and action potential firing properties. In general, phosphorylation increases the function of protein targets such as TRPV1 [69] and leak (TREK) K+ channels [355] as well as NaV channels [356] by reducing their threshold for activation and/or by affecting their activation kinetics.
Excitation and sensitization are the main consequences of the interaction of proinflammatory mediators with their target membrane receptor protein in nociceptors. However, there is also a group of bioactive lipids mediators produced at sites of injury and inflammation that antagonize the effects of pain promoting (proalgesic) agents and modulate pain initiation. They include endogenous cannabinoids, a family of arachidonic acid derivatives of which anandamide and 2-arachidonoyl-sn-glycerol are best known [357]. These endogenous analgesic agents are produced during injury by hydrolysis of phospho-lipid precursors in cell membranes and they activate CB1 and CB2 cannabinoid receptors, a class of Gi/o-coupled GPCR. These in turn inhibit both TRPV1 sensitization and the activation of voltage-gated Ca2+ channels and ASICs [357], thus counteracting the inflammatory process to restore homeostasis in damaged tissues.
Acute sensitization of nociceptor terminals is the short-term consequence of the activation by proinflammatory agents of intracellular signaling cascades leading to augmented activity of ion channels mediating transduction and action potential firing. However, altered gene expression is another major cellular process that induces persistent nociceptor sensitization. It is initiated by the electrical and molecular signals sent from the periphery to the cell body as a result of sustained nociceptor stimulation [358]. As a result, there ensues an upregulation of proteins (receptors and signaling molecules, ion channels) that are transported back to nerve terminals, wherein they mediate the increased responsiveness to peripheral stimuli. Altered gene expression may include either induction of novel genes and/or upregulated expression of constitutively expressed genes. Typically, in nociceptors this includes increased expression of TRPV1 channels and various NaV channels [358]. Neurotrophins (eg NGF, GDNF) that are generated by tissue injury/inflammation are key retrograde messengers that induce changes in gene expression [89,359,360]. Recently, changes in expression of transcription regulating micro RNAs have been demonstrated in DRG and TG in response to peripheral inflammation, and it has been speculated that these contribute to modulating the expression of pro-and/or anti-nociceptive molecules in acute and chronic pain states [361,362].
Knowledge of the cellular and molecular inflammatory mechanisms activated by ocular dryness and the effects on corneo-conjunctival peripheral nociceptor terminals is incomplete compared with other tissues like the skin. In experimental animals, acute appli cation of prostaglandin E2, bradykinin, or of a mixture of proinflammatory agents that contains both these substances together with 5-hy-droxytryptamine, histamine and substance P (‘inflammatory soup’), to the cornea produces enhanced spontaneous and stimulus-evoked nerve impulse activity in polymodal nociceptors [59,363]. Likewise, corneal inflammation induced experimentally by an ocular allergic challenge [68] or by exposure of the eye surface to UV radiation [116], elicits spontaneous firing and a reduction in mechanical threshold of corneal mechano-nociceptor fibers, while spontaneous firing and sensitivity to acidic stimulation is enhanced in polymodal nociceptors. This sensitization of polymodal receptors appears to involve TRPV1 channels as it was reversed by blockade of these ion channels [68]. Hence, the available experimental evidence suggests that in DED the interaction of mechano-nociceptors and polymodal nociceptors with pro-inflammatory substances released during dryness-evoked inflammation is likely to induce both an increase in ongoing nerve activity and an enhanced sensitivity to sensory stimuli [139]. In contrast, the activity of corneal cold thermoreceptors is reduced by exposure to ‘inflammatory soup’, an effect mediated via activation of Gq-coupled GPCR and a direct inhibitory action of the G-protein subunit Gαq on TRPM8 channels [364].
5.1.2. Nerve injury
The high density and the superficial location of the sensory nerve terminals between the epithelial cells at the surface of the cornea, makes them particularly vulnerable to injury by adverse environmental conditions (such as air pollution, low humidity), trauma (cataract and refractive surgery) and disease (pterygium, conjunctivochalasis, keratoconus) [365–367]. Also, in animals in which aqueous tear secretion or composition is disturbed experimentally, the density and architecture of subbasal nerve leashes and epithelial nerve terminals are greatly altered [28,103]. Likewise, in humans, confocal microscopy of corneal nerves in patients with aqueous tear deficiency of variable ethiology reveals changes in number, tortuosity, and branching pattern of subbasal plexus nerve fibers [368–372]. It is reasonable to assume that when the tear film covering the eye surface is thinned, the mechanical stress generated by blinking movements on superficial epithelium cells becomes abnormally high, injuring terminal nerve branches. This initiates a cycle of degeneration and regeneration leading to the altered architecture of corneal and conjunctival nerve fibers.
It is well established that damage to the peripheral nerve endings of nociceptive sensory neurons elicits profound changes in their spontaneous and stimulus-evoked firing pattern [341]. As with inflammation, these changes in nerve activity are the consequence of alterations in the activation threshold and kinetics of the transduction ion channels and voltage-gated ion channels in the axonal membrane, and a change in the expression and trafficking of these proteins. For example, altered expression of various transduction channels (such as TRPV1, TRPA1, and TRPM8) has been demonstrated in experimental models of nerve injury-induced pain [373]. Also, the density and distribution of NaV (eg NaV1.3, NaV1.6, and NaV1.9 channels) and voltage-gated calcium (CaV3) channels that are involved in the generation and propagation of action potentials can be markedly altered by nerve injury [374–376]. Moreover, the expression of voltage-activated K+ channels (KCNQ, Kv3, Kv7), hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and leak (TREK) K+ channels that are involved in setting the resting membrane potential and in regulating neuronal excitability can change markedly [6,376]. Together, these disturbances lead to increased responsiveness to natural stimulation and to the initiation of spontaneous action potentials at sites in the sensory neuron (in peripheral axon or soma) that do not normally generate impulses (ectopic activity). Altered ion channel expression in response to nerve injury also extends to the centrally projecting presynaptic terminals of the sensory neurons in the dorsal horn of the spinal cord. In this location increased expression of voltage-activated Ca2+ channels, in particularly the CaV3 channels that are responsible for the low-voltage-activated ‘T’-type current, has been shown to contribute to the generation of hyperalgesia [376].
It is worth noting that nerve damage also occurs in response to continued exposure to inflammatory agents and, conversely, acute nerve injury triggers an immediate local inflammatory reaction with macrophage infiltration in the surroundings of the injured and entrapped axons [341]. Therefore, the genetic, molecular and functional consequences of inflammation and injury overlap to produce increased activity of peripheral sensory nerve fibers and the generation of unpleasant and painful sensations. Typically, resolution of inflammation and repair of damaged axons would result in the reversal of effects in early stages of DED. However, sensory hypersensitivity can persist for prolonged periods of time, even after the original cause of the changes (eg infection, surgery, toxicity) has resolved and can worsen if damage to the nervous elements persists through chronic inflammation [5]. Although inflammation and changes to the innervation of the cornea indicative of nerve damage have been demonstrated in DED, the exact contribution of these changes to the generation of abnormal sensations referred to the ocular surface remains to be resolved.
5.1.3. Dryness-induced disturbances in the activity of peripheral ocular sensory fibers
The effects of a chronic deficiency in tear production on the behavior of the different classes of corneal sensory receptors have been studied in animals following lacrimal gland removal [28,100].
5.1.3.1. Cold thermoreceptors
In both rats and guinea pigs, prolonged reduction in tear production sensitizes HB-LT cold thermoreceptors, with both a shift in the cooling threshold to warmer values and an increased peak frequency of nerve activity evoked during cooling ramps [28,100]. In addition, in rats there is an increased likelihood that the cold thermoreceptors will respond to noxious heating than in sham-operated rats [377]. In guinea pigs, there is a gradual increase in the level of ongoing nerve activity of cold thermoreceptors and the sensitization of cold thermoreceptors induced by tear deficiency is associated with morphological changes to the corneal innervation, suggestive of nerve damage [28]. The hyperexcitability of cold thermoreceptors could partially explain the characteristic unpleasant dryness sensations reported by patients with DED (see Section 2.1.4.2).
Electrophysiological investigation of corneal cold-sensitive neurons isolated from TGs of guinea pigs with deficient tears demonstrated no changes in the cold-induced inward current [28]. This finding suggests that sensitization is not attributable to increased expression of TRPM8 channels. Instead, there are increases in NaV channel currents and decreases in K+ channel currents in corneal cold thermoreceptive neurons; changes that would increase neuron excitability by reducing the voltage threshold for action potential initiation. The underlying mechanism(s) that produce these changes are unknown.
5.1.3.2. Polymodal and mechano-nociceptors
Mechanical sensitivity of the cornea assessed behaviorally is increased by chronic tear deficiency in rats [378], but whether this change involves sensitization of polymodal receptors and/or mechano-nociceptors to mechanical stimuli is unknown. The effects of chronic tear deficiency on the activity of corneal polymodal receptors and mechano-nociceptors have been investigated in guinea pigs [28] and rats [377]. In guinea pigs, there was a transient increase in the spontaneous activity of both polymodal receptors and mechano-nociceptors that returned to normal 4 weeks following lacrimal gland removal. However, the mechanical threshold of both the polymodal receptors and mechanonociceptors was not changed by lacrimal gland removal. In contrast, the response of the polymodal receptors to acidic stimulation was increased, consistent with a moderate degree of sensitization [28]. The possibility that tear film deficiency sensitizes corneal polymodal receptors to hyperosmotic stimuli has not been explored. However, acute topical application of a combination of inflammatory mediators to the cornea increased the ongoing activity of polymodal receptors, but did not increase their sensitivity to hyperosmotic stimuli [67]. Electrophysiological investigation of retrogradely labelled capsaicin-sensitive corneal neurons (presumed polymodal receptors) isolated from TG of guinea pigs with deficient tears, revealed increases in NaV channel currents and decreases in some K+ channel currents [28]. These changes would increase the excitability of the polymodal receptors and explain their moderate degree of sensitization.
Fig. 5 summarizes the interactive influences of dryness and inflammation on the activity of ocular surface sensory receptors on sensation, blinking and tearing.
Fig. 5.
Schematic diagram summarizing how ocular inflammation of various etiologies or ocular surface dryness in DED, provoke variable increases (+) or decreases (−) of nerve impulse activity in polymodal- and mechano-nociceptors and in cold thermoreceptors of the high background, low threshold (HB-LT) and low background, high threshold (LB-HT) types. Together these changes evoke conscious sensations of different quality, as well as changes in tear flow and in spontaneous and reflex blinking.
5.2. Central mechanisms of sensitization
The abnormal impulse activity generated by peripheral inflammation and/or nerve injury represents by itself an altered sensory message transmitted to higher relay stations in the CNS. It also has additional consequences on the excitability, synaptic efficacy and connection pattern of the presynaptic nerve terminals of sensory neurons in the dorsal horn and brainstem, modifying transmission of signals to second-order neurons.
Chronic pain and central sensitization often begin following an initial insult to peripheral nerves and once established, are maintained by even low levels of peripheral nerve activity [5,379]. The concept of an initial insult that sensitizes pain pathways in the brain often is referred to as “hyperalgesic priming” and is well established for other pain models [380–382], but is poorly defined in animal models for DED. Although a wide variety of peripheral factors associated with ocular surface disease are capable of activating corneal sensory nerves [133,139,383], it is not known which factors or combinations of factors must be present and for how long, to induce central sensitization and chronic ocular pain in DED.
Central sensitization of spinal cord neurons is maintained by persistent activity in peripheral nerves together with changes in their activation and modulation by local circuit neurons and microglia, and altered descending controls from higher brain centers [5,175,379]. Nerve injury or inflammation enhances the release of neurotransmitters, such as substance P and glutamate, from primary afferent fibers that bind to neurokinin and N-methyl-D-aspartate (NMDA) glutamate receptors on second-order neurons in the spinal dorsal horn, resulting in an increase neuronal excitability [338]. Sensitized spinal dorsal horn neurons project to higher brain centers and are necessary to engage thalamocortical and limbic pathways that underlie the discriminative and affective aspects of pain [384]. Considerable evidence suggests that second-order ocular neurons at the ViVc transition and VcC1 region behave, in many respects, as spinal dorsal horn neurons and are critical for the development of persistent ocular pain. In animal models for anterior uveitis [198] or photokeratitis [385], in which ocular inflammation is prominent, neurons at the VcC1 region, but not at the ViVc transition, develop hypersensitivity to ocular surface stimuli, while ocular neurons at both regions display increased convergent input from periocular skin, consistent with allodynia reported by some DED patients. Enlarged cutaneous receptive field areas of dorsal horn neurons after nerve injury or inflammation are thought to be due strictly to central brain mechanisms and to reflect spatial summation, a key component of central sensitization [386]. In a rat model for tear deficiency, ocular neurons at both the ViVc transition and VcC1 region display enhanced responsiveness to activation of ocular surface sensory neurons with hypertonic saline and enlarged convergent cutaneous receptive field areas [322]. The cellular and molecular mechanisms for hyperalgesia of ocular neurons at the ViVc transition and VcC1 region in models for DED are not known. Hypersensitivity of CNS neurons is thought to derive from a combination of increased excitatory synaptic drive and/or loss of inhibitory controls [5,338]. Central administration of a selective NMDA receptor antagonist reduces acute corneal-evoked activation of ocular neurons in both regions [387], while blockade of substance P receptors preferentially reduces activation of ocular neurons at the VcC1 region [388]. Local microinjection of the GABAA receptor agonist, muscimol, greatly reduces corneal-evoked neural activity at the ViVc transition and VcC1 region [208]. Even brief stimulation of sensory nerves at C fiber strength is sufficient to induce prolonged activation of microglia in spinal dorsal horn [389], however, similar studies have not assessed the role of microglia in animal models for ocular pain. Collectively, these studies suggest that second-order neurons at the ViVc transition and VcC1 region contribute to ocular-related hyperalgesia in addition to mediating more specialized aspects of ocular function such as lacrimation and eye blink. In future studies, it will be critical to determine how persistent tear reduction influences excitatory and inhibitory synaptic mechanisms of ocular neurons in the TBNC.
Central sensitization can develop in the setting of ongoing afferent nociceptive traffic that occurs with peripheral sensitization [390,391]. Anatomically, corneal nociceptors have their cell bodies in the TG and synapse in two main areas of the TBNC, the ViVc transition and the spinomedullary junction or the Vc/C1-2 region [100,392,393]. Dry-responsive neurons have been identified in the ViVc transition, and a subgroup of these neurons receive additional converging input from corneal afferents sensitive to other stimuli such as acidity, heat and noxious chemicals [100,190]. Cornea-responsive neurons at the Vi/Vc transition and at the VcC1 region receive both innocuous and noxious sensory information and display increased responsiveness in an animal model for tear deficient DED [322]. This suggests that central sensitization occurs at multiple levels of the TBNC in DED.
On a molecular level, NMDA receptor activation may partially underlie the phenotypic changes seen in neurons during central sensitization. For example, NMDA receptor activation can lead to the progressive increase in the firing of second order neurons of the TBNC, even with sub-threshold noxious stimuli, clinically manifesting as hyperalgesia and allodynia [394]. In vitro, co-culture experiments have identified NMDA receptors as important in the communication between corneal epithelial cells and TG sensory neurons [395]. In a rat model for ocular nociception, NMDA receptors located on peripheral neurons or on postsynaptic neurons in the TBNC play a key role in transmission of nociceptive signals from the primary afferent neurons to central pain pathways [387].
Interactions between glial cells and neurons likely have an important role in the pathophysiology of chronic pain [396,397]. Preclinical studies have found that activated microglia and astrocytes mediate the generation and maintenance of several pain states [398] in a fashion modulated by specific genetic polymorphisms and circulating pro-inflammatory cytokines [399]. Glial activation in the brain as a consequence of stress (eg traumatic brain injury or systemic inflammatory responses) can induce the expression of pro-inflammatory cytokines that directly amplify spinal cord synaptic transmission and induce central sensitization to pain via signal amplification [397]. Peripheral and systemic inflammatory responses can also lead to microglial activation and depression via monoaminergic, glutamatergic and neurotrophic mechanisms [400].
5.3. Descending mechanisms
The activity in ascending excitatory nociceptive pathways is modulated by descending control pathways from higher brain centers that may exert facilitatory or inhibitory effects on spinal and trigeminal sensory input [384,401]. However, the role of descending control systems in DED is not known. Normally, interneurons within the central pain pathway release neurotransmitters including gamma amino butyric acid (GABA) and glycine, which are involved in the inhibition of nociceptive signaling [402]. However, after a noxious insult, the ensuing inflammatory cascade in the spinal cord may reduce the GABA-mediated inhibitory influence on the ascending pathway or even make the GABA inputs excitatory [5]. In a rat model for ocular nociception, application of muscimol, a GABA receptor agonist, inhibited corneal input to both ViVc transition and VcC1 neurons [208]. A loss of the inhibitory GABA-mediated chloride current may allow for an upregulation of ascending pain pathway signals and thus a chronic neuropathic pain state [403,404].
Quantitative sensory testing can assess abnormalities in the ascending and descending pain pathways. Chronic pain patients (not involving the eye) often display greater temporal summation following repetitive presentations of a noxious stimulus (“wind up”) [405,406] and reduced descending controls, or conditioned pain modulation, as compared to normal subjects [407].
5.4. Contribution of peripheral and central mechanisms to DED discomfort and pain
Peripheral and central neural mechanisms participate in the development of adverse symptoms of discomfort, dryness or burning pain in DED patients and their relief is the main reason for them to seek medical attention [404,408,409]. However, efforts to manage symptoms in chronic moderate to severe DED by ocular treatments alone have been inadequate [410,411]. Peripherally mediated DED pain or discomfort symptoms are presumed to originate by noxious stimulation of sensory neurons supplying the ocular surface (see Section 2.1). In DED, an inadequate or unstable tear film is the likely cause of tear hyperosmolarity and local surface drying leading to damage of the ocular surface tissues, including nerve terminals. Indeed, tear breakup is associated with increased sensation [141,412] and repeated episodes of tear breakup have been shown to lead to DED-like symptoms of ocular irritation [413]. Noxious stimulation arising from an inadequate tear film may also lead to inflammation which results in sensitization of the sensory terminals at the ocular surface (see Section 5.1), rendering previously non-noxious or low power stimuli able to evoke sensation [414]. If the underlying cause is not addressed and ameliorated, the increased activity of peripheral sensory neurons may lead to central sensitization. Although in most cases ocular surface pain has a proximate physical cause, it may be reported in the absence of tissue damage or any likely pathophysiological cause, but still should be accepted as pain [415]. Indeed, the weak correlation between signs and symptoms in DED [416–418] is consistent with the notion that sensitization of eye sensory pathways is triggered by events that may occur well before the patient enters the clinic. When peripheral nerve injury/inflammation due to disturbances of ocular surface homeostasis generates functional and anatomical alterations at higher levels of eye pain pathways, central pain largely independent of the original cause may develop and persist without an obvious relationship with the peripheral nociceptive input. Instillation of a topical anesthetic at the surface of the eye has been suggested as a simple and immediate way to differentiate pain arising from activation of peripheral sensory nerve terminals from that arising at a more proximal site in the sensory neuron or in the CNS [419].
Fig. 6 summarizes the peripheral and central neural mechanisms involved in the generation of perceptual, autonomic and motor responses in DED.
Clinical identification of the neurological mechanisms underlying pain is important to define therapies. This is required to distinguish nociceptive from neuropathic pain, whose definition has been restricted to lesion or disease affecting the somatosensory system [420]. Precise identification of the neural mechanism underlying discomfort or pain reported by patients with a diagnosis of DED is often difficult with the exploration tools available today to evaluate the neurobiology of the ocular surface and the functional state of central neural pathways involved in eye pain (see TFOS DEWS II Diagnostic Methodology report).
6. Evaluation of ocular surface neurobiology
6.1. Patient-reported characteristics (surveys, questionnaires)
Numerous questionnaires have been developed for DED (see TFOS DEWS II Diagnostic Methodology and Epidemiology reports). Most of these DED questionnaires were developed from a clinical perspective, aiming to understand the symptoms associated with the condition and develop diagnostic tools based on symptoms. Using this approach, the discomfort category of DED symptoms has been characterized as ocular dryness, irritation, soreness, grittiness, scratchiness or achiness, but may also contain questions about burning and stinging. Many DED questionnaires also include questions on foreign body sensation (feeling like ‘something is in the eye’) and light sensitivity [421–424]. Interestingly, dryness is not always queried in DED questionnaires [421]. It is also important to note that the words chosen as symptoms in these questionnaires depend heavily on language, which may take seemingly different meanings when translated. For example, “tired eyes” has been reported as the most common symptom of DED in Japan [425], but is included in only a few questionnaires in English [422,426].
Ocular pain and discomfort have also been measured by other questionnaires that were not specifically developed for DED. The National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) queries pain and discomfort around the eyes and vision-related quality of life, which is appropriate for the dry eye condition due to its effects on vision [427,428]. In one study, the ocular pain subscale of the NEI VFQ-25 was shown to be substantially lower (worse) for patients with DED than for eight other ophthalmic conditions [428]. The Eye Sensation Scale was developed to measure ocular pain, specifically to assess pain relief following corneal transplantation surgery [429]. Recently, a new questionnaire, the Ocular Pain Assessment Survey (OPAS), was validated to assess ocular pain in a variety of ocular conditions, from corneal ulcers to DED [430]. In addition to the eye, the OPAS queries the location of pain elsewhere in the body, supporting recent findings that DED symptoms closely align with non-ocular pain [409,431]. A grading system based on clinical judgement, to define the level of certainty that the pain in an individual is neuropathic in nature was proposed in 2008 by a Special Interest group of IASP [432] and revised recently [420]. This system distinguishes Possible, Probable and Definite Neuropathic Pain grades. An extension of these criteria to DED or other pain conditions in the eye has not been yet made, but may serve as a tool to distinguish definite ocular neuropathic pain from the discomfort and pain experienced in many eye pathologies.
6.2. Psychophysical characteristics
While corneal and conjunctival sensory function has been evaluated using electrophysiological recording in animals, this experimental approach cannot be used in humans. Consequently, the evaluation of subjective responses to controlled stimulation of the ocular surface has been applied to capture human ocular sensory information. The use of mechanical, chemical or thermal stimuli has been enabled by various aesthesiometer designs, including the Cochet Bonnet aesthesiometer and the gas jet aesthesiometer [433–436].
Depending on the experimental or psychophysical paradigm and instrument used, different qualities of the sensitivity or sensation response can be determined. Measurement of detection thresholds is the most common method, allowing ease of comparison between conditions and analysis of change. Thresholds to mechanical, chemical and thermal stimuli have been measured using a variety of psychophysical techniques, including method of limits [437], method of constant stimuli [129] and staircasing techniques [438]. As with other sensory systems, which may show adaptation or sensitization, different psychophysical methods result in different threshold values. Discrepancies in threshold may also result from physiological variations in sensitivity, variations due to disease as well as method related variations including stimulus duration, distance of probe from the ocular surface and characteristics of the gas stimulus.
A number of investigators have also utilized subjective grading of suprathreshold stimuli in order to determine the relationship between the magnitude of the stimulus presented and its perceived intensity [63,64,317,435,439], and some have made observations of the quality and attributes of the evoked sensations [63,64,435,439–441]. Other suprathreshold approaches have included grading of just noticeable differences in sensation [442], threshold differences in sensation [443], matching detection thresholds to the discomfort experienced and grading the intensity or assigning a descriptor to suprathreshold stimuli. Sensations evoked can be evaluated quantitatively and qualitatively and compared with the receptor properties established in animal models [63], providing a clearer understanding of the processes involved in human ocular surface sensitivity.
Psychophysical studies have demonstrated that the eyelid margins are sensitive to mechanical stimuli [444–446], but responses to other types of stimuli have not been assessed. Careful assessment of the tactile sensitivity of the eyelid margins indicates that the occlusional surface has lower sensitivity than the marginal angle where the eyelid margin contacts the surface of the eye [445]. Studies also indicate that the tactile sensitivity of lower eyelid margin is higher than that of upper eyelid margin [445,446]. Interestingly, in healthy subjects the tactile sensitivity of the lower eyelid margin was positively correlated with tear osmolarity [446], however, neither eyelid sensitivity nor tear osmolarity correlated with symptoms of DED.
6.2.1. Ocular surface sensitivity and DED
Table 1 summarizes studies that have evaluated ocular surface sensitivity in various populations of DED subjects, and the associations between sensitivity and symptoms reported. While subject numbers are frequently low in individual studies, corneal sensitivity to a pure mechanical stimulus, such as the Cochet Bonnet instrument, is consistently reduced in DED [372,447–453]. Of the different subclasses of DED patients, those presumed as demonstrating aqueous deficient DED consistently present with reduced corneal sensitivity using the Cochet Bonnet instrument, and this has been attributed to the greater corneal epithelial disruption assessed by corneal staining [454]. In Sjögren syndrome, the decrement in corneal sensitivity to mechanical stimuli was similarly associated with the degree of corneal staining [455]. In a cohort with DED defined by clinical signs and symptoms, the degree of reduction in corneal mechanical sensitivity was associated with severity of clinical signs, including tear film signs [453].
Table 1.
Corneal sensitivity and symptoms report in DED.
| Author(s)/Year | Subjects | Sensitivity (with ↑symptoms) | Aesthesiometer | Symptoms questionnaire |
|---|---|---|---|---|
| Xu et al. (1996) [447] | SS (n = 15) + DED (n = 44) | ↓ | COBO | not stated |
| Versura et al. (2007) [448] | SS (n = 66) + DED (n = 59) | ↓ | COBO | OSDI |
| Barboza et al. (2008) [449] | SS (n = 17) | ↓ | COBO | OSDI |
| Toker and Asfuroglu (2010) [450] | SS (n = 23) + DED (n = 14) | ↓cornea + conjunctiva | COBO | OSDI |
| Bourcier et al. (2005) [466] | SS (n = 14) + DED (n = 30) | ↓* | BGE | burning, itching, stinging |
| Benitez-del-Castillo et al. (2007) [460] | SS (n = 11) + DED (n = 10) | ↓* | BGE | not stated |
| De Paiva and Pflugfelder (2004) [457] | DED (n = 20) | ↑ | modified BGE | 11 items |
| Situ et al. (2008) [456] | DED (n = 43) | ↓* cornea + conjunctiva | modified BGE (20 °C) | OSDI, SeSOD |
| Tuisku et al. (2008) [371] | SS (n = 20) | ↑* | modified BGE | OSDI |
| Labbe et al. (2012) [452] | DED (n = 12) | ↓ | COBO | not measured |
| Kim et al. (2012) [451] | RA DE (n = 106) | ↓ | COBO | OSDI |
| Labbe et al. (2013) [372] | DED (n = 43) | ↓ | COBO | OSDI |
| Nepp and Wirth (2015) [453] | DED (n = 46) | ↓ | COBO | not measured |
| Rahman et al. (2015) [454] | MGD (n = 11) SS (n = 3) DED (n = 7) CC (n = 12) |
↓ (DED only) | COBO and Jet aesthesiometer (28 °C) | OSDI, VAS |
| Spierer et al. (2016) [458] | DED (n = 129) | ↑ | modified BGE (23–26 °C) | DEQ5, OSDI |
| Kaido et al. (2016) [462] | DED (n = 21) | No change in touch sensitivity ↑ pain sensitivity ↑ blink sensitivity |
COBO | 12 item questionnaire |
Key: SS, Sjögren’s Syndrome; DED, dry eye disease; DGE, Belmonte’s gas aesthesiometer; COBO, Cochet-Bonnet aesthesiometer; MGD, meibomian gland dysfunction; OSDI, ocular surface disease index; CC, conjunctivochalasis.
Some studies, particularly those using a gas jet aesthesiometer with mechanical stimuli delivered either at eye temperature or at room temperature have shown an increased [371,456–458] or decreased [454,459,460] corneal sensitivity in DED. This apparent dichotomy could be related to the type of stimulus, where a gas jet aesthesiometer delivers a more complex stimulus than the pure mechanical stimulus of the Cochet Bonnet aesthesiometer; gas jet and contact aesthesiometers vary in stimulus composition and mode of stimulation and are therefore likely to assess different aspects of the neural response [461]. Aside from a mechanical stimulus, the gas jet stimulus may induce a cooling/evaporative/tear thinning effect that may stimulate cold thermoreceptors in addition to mechano-nociceptors and polymodal nociceptors, even with stimuli delivered at eye temperature. Given the likely relevance of corneal cold themoreceptors in DED, it is conceivable that augmented activity of these fibres in DED is associated with an increased corneal sensitivity to cooling stimuli.
An additional cause of the differences in corneal sensitivity among DED patients is that sensitivity may vary between DED subtypes or with disease severity. Recent studies have shown that patients exhibiting high DED symptom severity scores and neuropathic pain symptom inventory scores have lower mechanical thresholds and pain thresholds measured with a gas esthesiometer [458]. Also, in patients showing DED symptoms, touch sensitivity measured with the Cochet-Bonnet esthesiometer was not significantly altered, but the mechanical threshold required to evoke blinking and to report pain was lower than in asymptomatic subjects [462]. These observations suggest that in DED the reduction of corneal sensitivity, caused by damage to the sensory nerve endings, may be accompanied by central sensitization due to abnormal ongoing activity in injured corneal nerve fibers, trigeminal neurons and higher order neurons of the central ocular pain pathways leading to neuropathic pain symptoms. This mechanism may also underpin the report of eye discomfort symptoms in the presence of reduced corneal sensitivity to external stimuli. The relationship between corneal sensitivity in DED and disease severity is often confounded by a lack of knowledge of the time of disease onset [463]. For example, persistent ocular surface damage may ultimately cause central nervous system sensitization and the involvement of neuropathic mechanisms.
Corneal sensitivity has been discussed as a potential biomarker in DED [464]. One study has demonstrated an improvement in corneal sensitivity following cyclosporine therapy [450]. There are however concerns for the repeatability of corneal sensitivity measurements in DED over a three month period without intervention [453], although in normal subjects, good repeatability has been demonstrated [465].
6.2.2. Neuropathic pain
Neuropathic pain (neuralgia) is pain caused by damage or disease affecting the somatosensory nervous system and is often chronic in nature [467]. This pain can be associated with any part of the body including the eyes, but as it is not caused by the pathophysiology of DED (see TFOS DEWS II Pathophysiology report), it should not be diagnosed as DED (see TFOS DEWS II Diagnostic Methodology report). As commented above (Section 5.1.2) neuro-sensory dysfunction is a recognized feature of DED [404,468], but this aspect of the disease is not routinely evaluated or considered in clinical practice. DED-related neuro-sensory dysfunction may account for the lack of association between signs and symptoms and those DED patients who remain symptomatic despite adherence to therapy.
6.3. Objective metrics
6.3.1. Blink parameters
Blinking is commonly quantified by measuring the blink rate [315,320,321,323,329,331,469] or its reciprocal value, the inter-blink interval [318,324,333]. Neurologically, the blink rate is theoretically set by an endogenous spontaneous blink generator located in the spinal trigeminal complex that is modulated by afferent input from the cornea, dopamine levels in the brain and cognitive state [333]. (see Section 4).
It is well established that stimulation of the ocular surface leads to an increased blink rate [315,318], whereas surface anesthesia leads to a decreased blink rate [319]. This observation has been used to explain the increased blink rate in DED, presumably caused by ocular surface irritation due to surface dryness or an unstable tear film [196,333]. Experimental evidence in animals has shown that chronic reduction of basal tearing produced by surgical removal of the main lacrimal gland increases background activity of corneal cold thermoreceptors [28]; conversely, basal blinking of TRPM8 null mice whose cold thermoreptor background activity is absent is very low [101]. These findings indicate that cold thermoreceptors contribute to the peripheral tonic drive maintaining basal blinking. Although corneal nerve terminals in DED patients are often reported to be less sensitive to external mechanical, thermal and chemical stimuli [455,459], this does not exclude the possibility that they display in parallel enhanced spontaneous firing (see Section 5.1.2). An increase in spontaneous activity may explain ongoing discomfort and an augmented basal blink rate in DED patients [28,196,333], while a low blink rate may be a causative factor in DED [470]. The Ocular Protection Index (OPI) is based on the idea that the blink rate can be too slow to compensate for more rapid tear breakup [471]. Thus, changes in the blink rate can be considered both a cause and an effect of DED.
Aside from blink rate, other blink parameters include the amplitude, duration and velocity of the upward and downward phases of the blink (see section 4). Regardless of whether the blinks are spon taneous, reflex or voluntary, all blinks show a similar pattern. The down phase is very rapid and the up phase is slower, with the maximum velocity of the down phase roughly double that of the up phase [314]. In a group of normal subjects, Evinger et al. [314] showed that the maximum velocity of the down and up phase show a linear relationship to blink amplitude, meaning that a fuller blink tends to be faster and vice versa. In addition, they found that the duration of most blinks changed little with blink amplitude.
However, some factors are known to alter blink duration. Wu et al. found that ocular surface stimulation by air tended to increase down phase blink duration and some subjects demonstrated cluster blinking [318]. This supports the hypothesis that ocular surface irritation can prolong blink duration and enhance blink excitability, perhaps for protective purposes [312]. However, concentration on a task descreased blink duration, presumably to minimize interruption of vision by the lid. In addition, an increased variability in the relationship between maximum blink velocity and amplitude for DED subjects has been reported, suggesting that individual subjects may respond differently [318].
Partial or low amplitude blinking is common and some studies have found that blink amplitude and blink rate decreased with concentration on a visual task [324,332]. 29% of blinks were partial or lower than 100% amplitude, but most (71%) covered the pupil, suggesting that blinks are necessary to wet the cornea over the pupil to provide a smooth tear film surface for good vision [317].
Blinking can be affected by several factors other than ocular surface stimulation or spontaneous firing of ocular surface nerves. The effect of cognitive input on the blink rate is marked, especially during a visual task, when the blink rate can markedly slow [316,320,321,331,472]. Blink rate can be altered by task, lighting, time of day and time period of data collection. Given the large percentage of the population that works on computers, this is likely to be a major cause of the increasing incidence of ergonomic dry eye complaints. Engaging in conversation and daydreaming can also affect blink rate [328–330], so that any clinical measure of blink rate should include information about task and lighting because blink rate varies so widely with mood and task. Time of day should also be included as blink rate is known to vary diurnally, presumably due to changing dopamine levels over the day [333]. Another aspect that affects measurement of the blink rate is the length of the time period of data collection. Kaminer et al. showed that shorter periods of data collection generated a higher blink rate than did longer periods, attributed to the increased probability of smaller inter-blink intervals when short periods of eye blink data were data collected [333].
6.3.2. In vivo confocal microscopy of corneal nerves and immune cells
With increased focus on corneal neurobiology in DED, there is an urgent need for the development of new biomarkers in this area. However, objective assessment of ocular surface neurobiology has been challenging for clinicians, given that corneal nerves cannot be visualized in detail by slit-lamp examination and that accurate functional tests are not widely available. Corneal in vivo confocal microscopy (IVCM), allows high-resolution in vivo visualization of sub-basal corneal nerves and immune cells at a cellular level, providing an image resolution closer to the one obtained with histochemical methods. In particular, the Heidelberg Retina Tomograph with the Rostock Cornea Module (HRT/RCM, Heidelberg Engineering, Heidelberg, Germany), is a laser-scanning IVCM that uses a 670 nm diode laser [473], that allows real-time imaging of the cornea, generating a 400 × 400 μm images and a lateral resolution of 1μm/pixel. Recent studies demonstrated that there are no significant differences in the mean nerve and immune cell densities in the central cornea between representative standard IVCM images and wide-field composite images, confirming that standard images can be used in clinical studies to accurately assess cellular structures [474]. IVCM allows detection of changes in the subbasal nerve plexus in patients with corneal neuropathy and corneal neuralgia from DED or other ocular and systemic conditions, which can be monitored for disease severity and response to treatment [475,476].
6.3.2.1. Corneal nerves
There are several published qualitative and quantitative IVCM studies of the central corneal nerve plexus in patients with DED that attempt to elucidate alterations in corneal innervation and their clinical significance [477,478]. These studies have demonstrated rather conflicting results regarding nerve density. Most studies have reported a decrease in nerve density in both Sjögren and non-Sjögren DED patients [452,460,474,479], correlating to decrease in corneal sensitivity [372,447,452,460,480]. In contrast Hoşal et al. [481] and Tuominen et al. [368] observed no change in subbasal nerve density in DED patients compared with controls, while Zhang et al. reported increased corneal nerve density in patients with Sjögren’s syndrome (Table 2) [482]. The latter study corresponds to studies showing hypersensitivity of the cornea [371,483]. The discrepancies in findings related to changes in nerve density may be attributed to either different stages and severity of DED that induce different degeneration/regeneration patterns of nerves, levels of inflammation, or levels of corneal hyperalgesia and allodynia after repeated insults to corneal nerves. More consistency has been shown regarding other morphological nerve parameters, such as increased tortuosity, reflectivity and beading [368,369,460,480,482,484,485]. These changes are believed to arise from initial damage and subsequent regeneration of subbasal corneal nerves.
Table 2.
Nerve morphology changes described in DED and Sjögren syndrome and associations with sensitivity.
| Author(s)/Year | Subjects | Control | Nerve morphology parameters | Association with sensitivity | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Density/number | Tortuosity | Branching | Beading | Other | ||||
| Tuominen et al., 2003 [368] | SS (n = 10) | normal (n = 10) | – | altered | – | – | ↑sprouting | – |
| Benitez del Castillo et al., 2004, 2007 [369,460] | DED (n = 10) SS (n = 11) |
normal (n = 20) | ↓ | ↑ | no Δ | ↑ | no Δ thickness, reflectivity | Sensitivity associated with density (BGE) |
| Zhang et al., 2005 [484] | DED (n = 30) SS (n = 8) |
normal (n = 30) | no Δ (DED) (SS) | ↑ | ↑ | no Δ | no Δ thickness, | – |
| Hosal et al., 2005 [481] | DED (n = 6) SS (n = 10) | normal (n = 10) | no Δ | no Δ | – | – | no Δ thickness, reflectivity | – |
| Villani et al., 2007 [485] | SS (n = 35) | normal (n = 20) | ↓ | ↑ | – | – | – | Sensitivity associated with tortuosity (COBO) |
| ErDEDlyi et al., 2007 [370] | DED (n = 10) | normal (n = 10) | no Δ | – | no Δ | no Δ | – | – |
| Tuisku et al., 2008 [371] | SS (n = 20) | normal (n = 10) | no Δ | – | – | – | ↑ sprouting | No association (modified BGE) |
| Zhang et al., 2011 [482] | DED (n = 40) | normal (n = 20) | – | ↑ | – | – | Subbasal nerve rupture (moderate/severe DED) | – |
| Labbe et al., 2012 [452] | DED (n = 12) | normal (n = 10) | ↓ | no Δ | no Δ | no Δ | no Δ thickness, reflectivity | Sensitivity associated with density (COBO) |
Key: SS, Sjögren Syndrome; DED, dry eye disease; BGE Belmonte aesthesiometer; COBO, Cochet-Bonnet aesthesiometer.
Correlation of nerve alterations by IVCM to clinical signs and symptoms has been shown in several studies. Benitez et al. found that subbasal nerve density and corneal sensation correlated with Schirmer’s test results [460]. Further, Zhang et al. demonstrated that beading of corneal nerves was inversely related to corneal damage assessed with Rose Bengal staining [484]. Moreover, Labbe et al. revealed that both subbasal nerve density and corneal sensitivity were negatively correlated with the severity of DED [372]. Finally, a recent randomized clinical trial demonstrated that only patients with near-normal corneal nerve density showed improvements in both symptoms and signs after one-month therapy with artificial tears or the topical steroid loteprednol, while patients with low corneal nerve density did not demonstrate changes in signs or symptoms, providing one possible explanation for the variability in therapeutic response [474].
Corneal nerve injury due to inflammatory processes, followed by altered excitability in regenerated nerves [475,476,486], may result in the development of hyperalgesia or allodynia in patients with DED. These findings potentially explain the variability of their response to therapy and the different effects of DED on subbasal nerve density observed in various IVCM studies. In these patients, the formation of microneuromas, abrupt swellings of injured nerve endings formed during regeneration, is caused by sprouting from endbulbs [368,371,404].
A new and highly promising use for IVCM is the clinically challenging differentiation between DED-induced discomfort and light sensitivity from corneal neuralgia or photoallodynia in patients with corneal neuropathy, given their similar symptomatic presentation and potential clinical overlap [404,408,409,468]. Particularly in patients with ocular pain, with notoriously poor correlation between clinical signs and symptoms [417,487,488], IVCM may allow the diagnosis of corneal neuropathy with quantifiable changes in corneal subbasal nerve metrics [475,476,486]. IVCM in patients with corneal neuropathy demonstrates the presence of microneuromas, increased beading and reflectivity, as well as a more profound loss of subbasal nerves [475,476]. In recent studies, the treatment of patients with corneal neuropathy-induced photoallodynia or neuralgia with autologous serum tears demonstrated restoration of nerve topography through nerve regeneration, correlating with decreased symptoms of photoallodynia and pain scores [475,476]. While IVCM does not distinguish causality from secondary effects, additional IVCM studies in more homogenous populations would shed light on the pathophysiology of corneal neuropathic disease and DED. IVCM also shows promise in monitoring the corneal neurogenerative response to treatment. Methods to automate quantitative IVCM meausures would greatly enhance research methodology and interpretion of results.
6.3.2.2. Corneal immune cells
Recent studies show that inflammation plays a significant role in the development of DED [349]. One of the major participants of the immune system in DED are the APCs that induce T cell activation and thereby initiate an inflammatory cascade [489,490]. Among APCs, corneal DCs are involved in the development of DED [349,491,492]. To evaluate immune cell alterations in patients with DED, IVCM has recently been used to visualize DCs in the cornea. Results from these studies are consistent with immunohistochemical findings [493,494] showing that epithelial DCs are primarily located in close proximity to the subbasal nerve plexus [148].
Several IVCM studies have assessed the density and distribution of DCs and other immune cells in DED and demonstrated an increased density of DCs [371,477–480,495–500]. Lin et al. also showed that central and peripheral corneal DCs were significantly increased in both non-Sjögren and Sjögren syndrome DED, as compared to normal subjects [495]. Further, they showed putative activation of DCs as documented by the increased presence of dendrites on these cells. Similarly, increased density of purportedly mature DCs in DED patients with underlying systemic immune diseases has been reported [371,474]. Moreover, comparison between patients with presumed aqueous-deficient and evaporative DED showed that DC density is significantly higher in aqueous-deficient DED [474].
Alterations of epithelial DC density correlate with clinical signs and symptoms of DED [499,501]. Thus IVCM may serve as a useful supplementary assessment tool for clinical diagnosis of DED and for determining the need for anti-inflammatory therapy. Further, IVCM can be used serially to objectively assess the therapeutic success of an anti-inflammatory therapy [499,501]. However, additional studies are required to validate utility of IVCM imaging of DC in clinical practice, including the development of analytical tools to automate and standardize image analysis. Evaluation of DCs could also be used for treatment stratification and measurement of therapeutic efficacy when used with clinical tests (see TFOS DEWS II Management report).
6.3.3. Biomarkers in tears
Biomarkers in tears can potentially be used as an indicator of the status of ocular surface innervation, DED severity or as a measure of disease progression or response to treatment.
6.3.3.1. Nerve growth factor
NGF and its receptors are upregulated following damage to the ocular surface or its innervation [502,503] and the levels return to normal following wound healing [504]. Tear levels of NGF are elevated immediately post-laser-assisted in situ keratomileusis (LASIK) and remain increased until at least 6 months post-surgery [505]. Table 3 shows the levels of NGF in tears are also elevated in non-Sjögren DED [506] and in contact lens related DED [507].
Table 3.
Changes in tear concentration of neuromediators after refractive surgery and with DED compared with normal eyes.
| Tear neuropeptide levels | |||||
|---|---|---|---|---|---|
|
| |||||
| Nerve Growth Factor | Substance P | CGRP | Neuropeptide Y | Vasoactive intestinal peptide | |
| Refractive surgery | Increased with PRK > LASIK Mean NGF/total tear protein PRK 89.2.8 ± 10.2 pg/μg, LASIK 55.4 ± 11.7 pg/μg at 1 month [505] | Increased after LASIK 9.6 ± 2.6 ng/ml at 1 month [516] | Increased with PRK 377 ± 83.2 ng/min at day 7 [517] No change with LASIK 93.7 ± 59.6/ml [516] |
n/a | n/a |
| Non-Sjögren syndrome | Increased 186.5 ± 64.8 pg/ml [507,518] | No change [518] | Reduced 3.0 ± 1.7 ng/ml [518] | No change 4.6 ± 3.9 ng/ml [518] | No change [518] |
| Sjögren syndrome | No change 54.5 ± 61.8 pg/ml [518] | No change [518] | No change 6.0 ± 2.4 ng/ml [518] | Reduced 1.5 ± 0.3 ng/ml [518] | No change [518] |
| Ocular cicatrizing pemphigoid | Increased 120.8 ± 53.3 pg/ml [518] | Not reported [518] | Reduced 2.3 ± 1.2 ng/ml [518] | Reduced 1.5 ± 2.0 ng/ml [518] | No change [518] |
| Normal eyes | Mean 64.7 ± 48.0 pg/ml [518] Mean NGF/total tear protein 32.8 ± 6.2 pg/μg [505] |
Mean 2300 pg/ml [518] Range 306–332 7.5 ± 1.7 ng/ml [516] |
77.4 ± 75.7 ng/ml [516] 6.0 ± 2.2 ng/ml [518] 198 ± 36.6 ng/min [517] |
4.3 ± 1.9 ng/ml [518] | Not reported [518] |
CGRP, calcitonin gene-related peptide; PRK, photorefractive keratectomy; LASIK, laser-assisted in situ keratomileusis.
6.3.3.2. Substance P and calcitonin gene-related peptide
A substantial percentage of the sensory neurons supplying the ocular surface contain neuropeptides including substance P, CGRP and galanin [19,56,78,508]. These neuropeptides modulate epithelial and immune cell function in normal and damaged cornea [56], and play a role in local inflammation, wound healing and in the initiation and maintenance of pain (see Section 2.4) [509]. At the ocular surface specifically, CGRP induces epithelial cell differentiation and substance P stimulates epithelial cell proliferation [86].
One study has shown that tear levels of CGRP are reduced in DED patients and that tear CGRP levels are inversely associated with DED severity, corneal fluorescein staining and Schirmer test results (shown in Table 3) [506]. Exogenously delivered CGRP facilitates corneal epithelium repair in vivo in animals and in vitro in cell culture models [510,511], which may be consistent with reduced tear levels and increased ocular surface damage in DED. There are no reported studies in humans that have evaluated the effects of exogenous CRGP on nerve morphology, corneal sensitivity or DED.
In contrast, the role of substance P in human DED is equivocal. As can be seen in Table 3, there are a small number of studies available with varying methodology and generally small sample sizes. No studies have determined relationships between tear levels of substance P and disease severity. The level of substance P in tears appears to be unchanged in both Sjögren and non-Sjögren DED [506], but it is reduced in patients with corneal hyperaesthesia [512], compared to those with normal corneal sensitivity [506,513,514].
One study has investigated the levels of VIP and NPY in tears of DED patients and found no differences in VIP levels, but reduced levels of NPY compared to those of subjects with normal eyes [506]. NPY is released from sympathetic nerves and it is not known whether the changes in tear NPY levels reflect changes in the lacrimal gland (or other glandular tissues contributing the tears) or arise as a consequence of ocular surface damage. NPY inhibits T cell type I-driven inflammatory responses [515]. The lack of change in VIP with DED may reflect the very limited innervation of the cornea by VIP expressing parasympathetic nerves [511].
There are other potential tear markers of inflammation and/or ocular surface demage that have not yet been assessed in DED. Tear levels of TNF-α, transforming growth factor (TGF-β), VEGF and hepatocyte growth factor (HGF) are increased immediately after surface refractive surgery procedures and decline thereafter [519–521]. These bio-markers are all likely to be relevant in wound healing, and VEGF in particular may be relevant for corneal reinnervation as sequestration of this growth factor reduced recovery of subbasal fibers in a mouse model of corneal injury [522].
7. Future directions
Understanding the characteristics of adverse sensations associated with the ocular surface is important since symptom presentation is often the driving force behind treatment in DED [523]. Research to date has uncovered the complexity of peripheral and central neural mechanisms associated with ocular surface sensations and tissue homeostasis; however, a thorough understanding of this complexity in relation to DED is still not fully achieved. Application to the eye of the technological and conceptual advances to the understanding of pain made at the genetic, molecular, cellular and integrative level in other tissues pathologies could help to extend our knowledge of neural mechanisms underlying DED dysesthesias. Also development of adequate animal models aimed at reproducing this pathology and analyzing experimentally integrative neural mechanisms will help to refine the present knowledge on how unpleasant sensations originate in DED. New instruments and procedures designed to quantify eye sensations and pain need to be developed both in experimental animals and in humans, to correlate experimental and clinical data and to obtain reliable, objective information on the psychophysical parameters of normal and pathological sensations.
A second area of research important in furthering the understanding of pain and sensation at the ocular surface is the definition and characterization of pain referred to the eye with a neuropathic origin. The lack of correlation between signs and symptoms of DED has made research and clinical practice challenging for many [417]. Differentiating DED-evoked nociceptive pain from peripheral and central neuropathic ocular pain is important in successful treatment of patients and in defining research approaches. The investigation of these questions using basic research technology will help to better understand the molecular and cellular modifications taking place in the peripheral and central ocular pain pathways in DED and how they develop, progress and eventually perpetuate. From a clinical perspective, neuropathic pain from a wide range of etiologies has been studied in large samples of patients collected in multinational pain research networks, and classified according to their intrinsic pain-related sensory symptoms and signs which were associated with pathophysiological mechanisms [420,524]. To extend this approach to neu ropathic pain associated to ocular sensory pathways could help to extend to eye pain the advances in diagnosis and therapy made for neuropathic pain affecting other body territories.
Neuropathic pain should not be diagnosed as DED (See section 6.2.2), but management when it manifests as dry eye symptoms, needs further research. For patients who report pain in multiple body parts as well as the eye, management of or referral for neuropathic pain interventions should be evoked such as neuropathic pain therapy [408,419], a diet rich in anti-oxidants [525,526], systemic pharmacological agents traditionally used to manage pain (such as anticonvulsants, tricyclic antidepressants, opioids) [486,527,528,530] topical analgesia [531], neuromodulators (such as diclofenac, gabapentin and pregabalin) [528,532–540], GABAergics in late stage pain [529], and using stimulation therapies and psychological treatments (including exercise, acupuncture, “scrambler” or peripheral stimulation therapy, transcranial magnetic stimulation, transcranial direct current stimulation and cognitive behavior therapy) [541–543]. These have mostly not been investigated specifically for ocular neuropathic pain, but there is evidence of effectiveness in neuropathic pain and/or chronic pain syndromes, so further research is warranted.
8. Summary
The TFOS DEWS II Pain and Sensation Subcommittee report provides a perspective of DED focused on pain. Pain can be divided into nociceptive and neuropathic pain. Nociceptive pain occurs in response to actual or threatened damage to tissues. However, neuropathic pain occurs due to a lesion within the somatosensory nervous system and does not have biological value.
Pain associated with DED is transmitted via the peripheral axons of TG neurons innervating the cornea and conjunctiva. They form a subepithelial nerve plexus in the stroma whose ascending branches penetrate Bowman’s layer and ramify extensively to terminate within the surface epithelium layers. Functionally, corneal sensory neurons can be classified as polymodal nociceptors, selective mechano-nociceptors or cold thermoreceptor neurons. Polymodal nociceptors are normally silent and respond to chemical, mechanical and thermal stimuli. They become sensitized by the inflammatory mediators released by ocular surface injury. TRPV1 channels are important for sensory transduction and sensitization of polymodal nociceptors. Mechano-nociceptors are normally silent at rest and respond only to mechanical forces through mechanosensitive ion channels such as piezo2. Most cold thermoreceptors discharge continuously at the normal eye surface temperature with an increase or decreasing the firing frequency upon cooling or warming, respectively. TRPM8 is the main transduction channel for cooling or cold and is also sensitive to changes in osmolarity. Inter-blink tear evaporation causes discrete cooling of the ocular surface and tear osmolarity rises, thereby augmenting basal activity of cold thermoreceptors. This is consistent with the hypothesis that cold-sensitive fibers contribute to the reflex control of basal tear production and blinking (Figs. 5 and 6).
The TG neurons that supply the ocular surface project primarily into two spatially discrete regions within the TBNC: the transition region between caudal Vi and Vc (ViVc transition) and at the Vc/upper cervical cord junction (VcC1 region). Evidence suggests that the VcC1 region plays a dominant role in sensory-discriminative aspects of ocular pain. ViVc transition and VcC1 neurons are excited by bright light while whereas only ViVc transition neurons are activated by changes in the moisture status of the ocular surface. Ocular neurons at the ViVc transition are more likely to project to brain regions that control lacrimation (superior salivatory nucleus) and eye blink (facial motor nucleus), while cornea-responsive neurons in both re gions project to the sensory thalamus. Thus, it is suggested that ocular neurons at the ViVc transition play a significant role in maintaining ocular surface homeostasis, while neurons at VcC1 may be more concerned with the expression of adverse symptoms (Fig. 6).
Autonomic sympathetic and parasympathetic nerves, whose activity is regulated by reflex influences from sensory neurons supplying the ocular surface, regulate the secretory activity of the main lacrimal gland. Parasympathetic innervation of the main lacrimal gland is extensive, while little is known about the neural control of accessory lacrimal glands. While nerves are present around the meibomian glands, there are no studies examining the role of sensory or autonomic nerves and their neurotransmitters in regulating the holocrine secretion of the meibomian gland. Activation of sensory nerves supplying the rat cornea evokes goblet cell mucous secretion; however efferent nerve type(s) involved in this reflex response remain to be established. Several non-neural processes regulate the release of mucins from stratified squamous cells, but to date no regulatory role for nerves or neurotransmitters has been identified.
In addition to regulation of tear production, ocular surface nerves mediating sensations contribute to blinking behavior. It has been suggested that spontaneous blinking is maintained, at least in part by the continuous nerve impulse firing of eye surface cold thermoreceptors, an effect likely mediated by the connections of TG neurons with brainstem ViVc neurons which in turn project to the facial motoneurons (Cranial nerve VII). Nociceptive sensory input to neurons at the VcC1 region initiates reflex eye blinking and, through their projections to ViVc transition neurons, sets blink amplitude and peak velocity of corneal reflex blinks.
In DED, reduced tear secretion leaves the corneal epithelium exposed to adverse environmental conditions that may result in inflammation of the ocular surface and peripheral nerve damage. Inflammation may sensitize polymodal nociceptors and mechano-nociceptors, while depressing cold thermoreceptor activity. However, in experimental models of DED, sensitization of nociceptor fibers is minor, whereas a prominent and abnormal increase in cold thermoreceptor nerve activity occurs that parallels the morphological changes in corneal innervation. In trigeminal brainstem, ocular surface-responsive neurons at both ViVc and VcC1 regions display enhanced excitability.
Several questionnaires are available to assess pain and sensation associated with DED. These questionnaires vary widely in wording, symptom investigated, and scaling. In addition to questionnaires, aesthesiometry can be used to assess the functional status of the corneal nerves.
In vivo confocal microscopy allows for visualization of nerves and inflammatory cells in the corneal surface. DED is associated with morphological abnormalities in nerve terminals, such as increased tortuosity, reflectivity and beading, while changes in nerve density are not consistent. In addition, an increased density of inflammatory cells in DED has been reported. Tear components may also help to objectively assess DED. Nerve growth factor is increased in DED while CGRP is reduced. Substance P, neuropeptide Y and VIP appear to be unchanged.
Abbreviations
- list
5-HT, 5-hydroxytryptamine
- APC
antigen presenting cells
- ASIC channels
acid-sensing ion channels
- BCTC
(N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-carbox-amide
- BGE
Belmonte’s gas aesthesiometer
- BK
Bradkinin
- BM derived cells
Bone marrow derived cells
- CaV3 channels
The subfamily of voltage-gated calcium channels that generate T-type calcium currents
- CC
conjunctivochalasis
- CGRP
calcitonin gene-related peptide
- COBO
Cochet-Bonnet aesthesiometer
- CX3C
a class of cytokine that includes Fractalkine
- CX3CR1
a receptor for Fractalkine
- DBH
Dopamine β-Hydroxylase
- DC
Dendritic cell
- DED
Dry eye disease
- DRG
Dorsal root ganglion – dorsal root ganglia
- EPO
Erythropoietin
- FKN
Fractalkine (also known as chemokine (C-X3-C motif) ligand 1 (CX3CL1)
- GABA
gamma amino butyric acid
- GDNF
glial cell line-derived neurotrophic factor
- GFRα3
glial cell line-derived neurotrophic factor family receptor alpha3
- GPCR
G protein coupled receptors
- HB-LT cold thermoreceptors
high background, low threshold cold thermoreceptors
- HIS
histamine
- HLA-DR
Human Leukocyte Antigen - antigen D Related
- HPC
hepatocyte growth factor
- HRT/RCM
Heidelberg Retina Tomograph with the Rostock Cornea Module
- IASP
The International Association for the Study of Pain
- IR
immunoreactivity
- IRR
innate repair receptor
- IVCM
in vivo confocal microscopy
- LASIK
laser-assisted in situ keratomileusis
- LB-HT cold thermoreceptors
low background, high threshold cold thermoreceptors
- LP
levator palpebrae
- MAPK
mitogen-activated protein kinase
- MGD
meibomian gland dysfunction
- MHC
major histocompatibility complexes
- NaV channels
voltage-gated Na+ channel
- NEI VFQ-25
The National Eye Institute Visual Functioning Questionnaire
- NGF
Nerve growth factor – changes needs
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NPY
Neuropeptide Y
- OO
orbicularis oculi
- OPAS
Ocular Pain Assessment Survey
- OPI
Ocular Protection Index
- OSDI
ocular surface disease index
- PI3K
phosphoinositide 3-kinase
- PLC
phospholipase C
- PRK
photorefractive keratectomy
- R1 blinking response
Short latency blinking response
- R2 blinking response
Long latency blinking response
- SS
Sjögren’s Syndrome
- TBNC
trigeminal brain stem nuclear complex
- TG
trigeminal ganglion
- TGF-β
transforming growth factor-β
- TH
Tyrosine hydroxylase
- TNF-α
tumor necrosis factor-α granulocyte-macrophage colony stimulating factor
- TREK
A member of the two-pore-domain family of potassium channels
- TRP channels
transient receptor potential channels
- TRPA1 channels
transient receptor potential cation channel subfamily A member 1
- TRPM8 channels
transient receptor potential cation channel subfamily M member 8
- TRPV1 channels
transient receptor potential cation channel subfamily V member 1
- TTX
tetrodotoxin
- Vc
caudalis region of the spinal trigeminal nucleus
- VcC1 region
the junction between the caudalis region of the spinal trigeminal nucleus and the upper cervical spinal cord
- VEGF
vascular endothelial growth factor EPO – erythropoietin
- Vi
interpolaris region of the spinal trigeminal nucleus
- VIP
Vasoactive Intestinal Peptide
- ViVc transition
the transition region between caudal interpolaris region and the caudalis region of the spinal trigeminal nucleus
- Vo
subnucleus oralis region of the spinal trigeminal nucleus
- Vp
principal trigeminal nucleus
Footnotes
Disclosures
| Carlos Belmonte | Avizorex (P, C, I), Matrix Biology Institute, Cooper Vision (F) |
| Jason J. Nichols | Alcon, Allergan, Vistakon, (F); Alcon (R) |
| Stephanie M. Cox | Alcon, Allergan, BruderHealthcare, ElevenBiotherapeutics, Oculus, Kala, Shire, TearScience, Vistakon (F) |
| James A. Brock | None |
| Carolyn G. Begley | Coopervision, Santen Pharmaceutical (F); Johnson & Johnson Vision Care (C) |
| David A. Bereiter | None |
| Darlene A. Dartt | Allergan, Coopervision (C) |
| Anat Galor | Allergan, Shire (C), Veterans Affaies, NIH grants (F) |
| Pedram Hamrah | Coopervision (F), Eyegate Pharma, Jade Therapeutics, Novabay, Revision Optics, Santen, Valeant (C), Allergan, Bausch & Lomb, Dompe, GlaxoSmithKline, Shire (F, C). |
| Jason J. Ivanusic | None |
| Deborah Jacobs | BostonSight (E); TECLens (C) |
| Nancy A. McNamara | None |
| Mark I. Rosenblatt | Alcon, Roche, Santen (C), Laurel Therapeutics (F, C), Weill Cornell Medical College, University of Illinois at Chicago (P), Sarentis Therapeutics (F,I,C) |
| Fiona Stapleton | None |
| James S. Wolffsohn | Alcon, Aston EyeTech, Bausch & Lomb, BetterVision Ltd, CooperVision, Eaglet Eye, European Union, Eyebag, EMPharma, EyeDocs, Gelflex, Innovate UK, Johnson & Johnson Vision Care, Lenstec, Medmont, Rayner, Théa, Optimec, Visioncare Research (F); Aston EyeTech (I); British Contact Lens Association, University of Houston, Visioncare Research, CooperVision (C); Portable Aberrometer, Contrast Sensitivity Chart (P); Johnson & Johnson (R) |
References
- 1.Stapleton F, Marfurt C, Golebiowski B, Rosenblatt M, Bereiter D, Begley C, et al. TFOS international Workshop on contact lens discomfort, the TFOS international Workshop on contact lens discomfort: report of the subcommittee on neurobiology. Investig Ophthalmol Vis Sci. 2013;54(11):TFOS71–TFOS97. doi: 10.1167/iovs.13-13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmonte C, Tervo TT. Pain in and around the eye. In: McMahon SB, Koltzenburg M, Tracey I, Turks DC, editors. Wall and Melzack’s Textbook of Pain. 6. Philadelphia, PA, USA: Elsevier Saunders; 2013. [Google Scholar]
- 3.Wilson N. The semantics of pain in Greco-Roman antiquity. J Hist Neurosci. 2013;22(2):129–143. doi: 10.1080/0964704X.2012.684610. [DOI] [PubMed] [Google Scholar]
- 4.IASP. The International Association for the Study of Pain [Google Scholar]
- 5.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tulleuda Astrid, Cokic Barbara, Callejo Gerard, Saiani Barbara, Serra Jordi, Gasull Xavier. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain. 2011;7:1744–8069. doi: 10.1186/1744-8069-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonica JJ. The management of pain of cancer. J - Mich State Med Soc. 1953;52:284–290. [PubMed] [Google Scholar]
- 8.Hoon MA. Molecular dissection of itch. Curr Opin Neurobiol. 2015;34:61–66. doi: 10.1016/j.conb.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16(8):1016–1023. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valtcheva Manouela V, Samineni Vijay K, Golden Judith P, Gereau Robert W, Davidson Steve. Enhanced nonpeptidergic intraepidermal fiber density and an expanded subset of chloroquine-responsive trigeminal neurons in a mouse model of dry skin itch. J Pain. 2015;16(4):346–356. doi: 10.1016/j.jpain.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonardi Andrea, Castegnaro Angela, Valerio Alvise La Gloria, Lazzarini Daniela. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2015;15(5):482–488. doi: 10.1097/ACI.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 12.Nichols KK, Begley CG, Caffery B, Jones LA. Symptoms of ocular irritation in patients diagnosed with dry eye. Optom Vis Sci Of Publ Am Acad Optom. 1999;76(12):838–844. doi: 10.1097/00006324-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig Ophthalmol Vis Sci. 1989;30(3):461–472. [PubMed] [Google Scholar]
- 14.Bratton GR, Klemm WR, Hudson LC, Sherry CJ, Dziezyc J. Innervation of the feline eyelids. Neurol Res. 1992;14(5):369–374. doi: 10.1080/01616412.1992.11740087. [DOI] [PubMed] [Google Scholar]
- 15.van der Werf F, Baljet B, Prins M, Ruskell GL, Otto JA. Innervation of the palpebral conjunctiva and the superior tarsal muscle in the cynomolgous monkey: a retrograde fluorescent tracing study. J Anat. 1996;189(Pt 2):285–292. [PMC free article] [PubMed] [Google Scholar]
- 16.May PJ, Porter JD. The distribution of primary afferent terminals from the eyelids of macaque monkeys. Exp Brain Res. 1998;123(4):368–381. doi: 10.1007/s002210050582. [DOI] [PubMed] [Google Scholar]
- 17.Morgan CW, Nadelhaft I, de Groat WC. Anatomical localization of corneal afferent cells in the trigeminal ganglion. Neurosurgery. 1978 May-Jun;2(3):252–258. doi: 10.1227/00006123-197805000-00012. [DOI] [PubMed] [Google Scholar]
- 18.LaVail JH, Johnson WE, Spencer LC. Immunohistochemical identification of trigeminal ganglion neurons that innervate the mouse cornea: relevance to intercellular spread of herpes simplex virus. J Comp Neurol. 1993;327(1):133–140. doi: 10.1002/cne.903270111. [DOI] [PubMed] [Google Scholar]
- 19.De Felipe C, Gonzalez GG, Gallar J, Belmonte C. Quantification and immunocytochemical characteristics of trigeminal ganglion neurons projecting to the cornea: effect of corneal wounding. Eur J Pain. 1999;3:31–39. doi: 10.1053/eujp.1998.0100. [DOI] [PubMed] [Google Scholar]
- 20.Bron AJ, Tripathi R, Tripathi B. Wolff’s Anatomy of the Eye and Orbit. 8. Chapman & Hall Medical; London: 1997. [Google Scholar]
- 21.May CA. Description and function of the ciliary nerves-some historical remarks on choroidal innervation. Exp eye Res. 1997;65(1):1–5. doi: 10.1006/exer.1997.0312. [DOI] [PubMed] [Google Scholar]
- 22.Zander E, Weddell G. Observations on the innervation of the cornea. J Anat. 1951;85:68–99. [PMC free article] [PubMed] [Google Scholar]
- 23.ten Tusscher MPM, Klooster J, van der Want JJL, Lamers WPMA, Vrensen GFJM. The allocation of nerve fibres to the anterior eye segment and peripheral ganglia of rats. I. The sensory innervation. Brain Res. 1989;494(1):95–104. doi: 10.1016/0006-8993(89)90147-9. [DOI] [PubMed] [Google Scholar]
- 24.Oduntan O, Ruskell G. The source of sensory fibres of the inferior conjunctiva of monkeys. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und Exp Ophthalmol. 1992;230(3):258–263. doi: 10.1007/BF00176301. [DOI] [PubMed] [Google Scholar]
- 25.Oduntan AO. Cellular inflammatory response induced by sensory denervation of the conjunctiva in monkeys. J Anat. 2005;206(3):287–294. doi: 10.1111/j.1469-7580.2005.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veiga Moreira TH, Gover TD, Weinreich D. Electrophysiological properties and chemosensitivity of acutely dissociated trigeminal somata innervating the cornea. Neuroscience. 2007;148(3):766–774. doi: 10.1016/j.neuroscience.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callejo G, Castellanos A, Castany M, Gual A, Luna C, Acosta MC, et al. Acid-sensing ion channels detect moderate acidifications to induce ocular pain. Pain. 2015;156(3):483–495. doi: 10.1097/01.j.pain.0000460335.49525.17. [DOI] [PubMed] [Google Scholar]
- 28.Kovács I, Luna C, Quirce S, Mizerska K, Callejo G, Riestra A, et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain. 2016;157(2):399–417. doi: 10.1097/j.pain.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López de Armentia M, Cabanes C, Belmonte C. Electrophysiological properties of identified trigeminal ganglion neurons innervating the cornea of the mouse. Neuroscience. 2000;101(4):1109–1115. doi: 10.1016/s0306-4522(00)00440-1. [DOI] [PubMed] [Google Scholar]
- 30.Cabanes C, López de Armentia M, Viana F, Belmonte C. Postnatal changes in membrane properties of mice trigeminal ganglion neurons. J Neurophysiol. 2002;87(5):2398–2407. doi: 10.1152/jn.2002.87.5.2398. [DOI] [PubMed] [Google Scholar]
- 31.Boada MD. Relationship between electrophysiological signature and defined sensory modality of trigeminal ganglion neurons in vivo. J Neurophysiol. 2013;109(3):749–757. doi: 10.1152/jn.00693.2012. [DOI] [PubMed] [Google Scholar]
- 32.Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012;35(6):373–381. doi: 10.1016/j.tins.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 2016;26(1):83–102. doi: 10.1038/cr.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 35.De Felipe C, Belmonte C. c-Jun expression after axotomy of corneal trigeminal ganglion neurons is dependent on the site of injury. Eur J Neurosci. 1999;11(3):899–906. doi: 10.1046/j.1460-9568.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 36.Bron R, Wood RJ, Brock JA, Ivanusic JJ. Piezo2 expression in corneal afferent neurons. J Comp Neurol. 2014;522(13):2967–2979. doi: 10.1002/cne.23560. [DOI] [PubMed] [Google Scholar]
- 37.Müller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Investig Ophthalmol Vis Sci. 1996;37(4):476–488. [PubMed] [Google Scholar]
- 38.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp eye Res. 2010;90(4):478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Seyed-Razavi Y, Chinnery HR, McMenamin PG. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Investig Ophthalmol Vis Sci. 2014;55(3):1313–1320. doi: 10.1167/iovs.13-12995. [DOI] [PubMed] [Google Scholar]
- 40.Rózsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14(2):105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 41.Schimmelpfennig B. Nerve structures in human central corneal epithelium. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und Exp Ophthalmol. 1982;218(1):14–20. doi: 10.1007/BF02134093. [DOI] [PubMed] [Google Scholar]
- 42.Ueda S, del Cerro M, LoCascio JA, Aquavella JV. Peptidergic and cate-cholaminergic fibers in the human corneal epithelium. An immunohistochemical and electron microscopic study. Acta Ophthalmol Suppl. 1989;192:80–90. doi: 10.1111/j.1755-3768.1989.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 43.Patel DV, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Investig Ophthalmol Vis Sci. 2005;46(12):4485–4488. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 44.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010 Sep-Nov;25(5–6):171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp eye Res. 2010;91(4):513–523. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu CQ, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Investig Ophthalmol Vis Sci. 2007;48(4):1535–1542. doi: 10.1167/iovs.06-1192. [DOI] [PubMed] [Google Scholar]
- 47.Dvorscak L, Marfurt CF. Age-related changes in rat corneal epithelial nerve density. Investig Ophthalmol Vis Sci. 2008;49(3):910–916. doi: 10.1167/iovs.07-1324. [DOI] [PubMed] [Google Scholar]
- 48.Ivanusic JJ, Wood RJ, Brock JA. Sensory and sympathetic innervation of the mouse and Guinea pig corneal epithelium. J Comp Neurol. 2013;521(4):877–893. doi: 10.1002/cne.23207. [DOI] [PubMed] [Google Scholar]
- 49.Harris LW, Purves D. Rapid remodeling of sensory endings in the corneas of living mice. J Neurosci Of J Soc Neurosci. 1989;9(6):2210–2214. doi: 10.1523/JNEUROSCI.09-06-02210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rózsa AJ, Guss RB, Beuerman RW. Neural remodeling following experimental surgery of the rabbit cornea. Investig Ophthalmol Vis Sci. 1983;24(8):1033–1051. [PubMed] [Google Scholar]
- 51.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014 May-Jun;59(3):263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Cillà S, Ranno S, Carini E, Fogagnolo P, Ceresara G, Orzalesi N, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Investig Ophthalmol Vis Sci. 2009;50(11):5155–5158. doi: 10.1167/iovs.09-3384. [DOI] [PubMed] [Google Scholar]
- 53.Kurbanyan K, Hoesl LM, Schrems WA, Hamrah P. Corneal nerve alterations in acute Acanthamoeba and fungal keratitis: an in vivo confocal microscopy study. Eye Lond Engl. 2012;26(1):126–132. doi: 10.1038/eye.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamrah P, Cruzat A, Dastjerdi MH, Prüss H, Zheng L, Shahatit BM, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120(1):40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belmonte C, Garcia-Hirschfeled J, Gallar J. Neurobiology of ocular pain. Prog Retin Eye Res. 1996;16:117–156. [Google Scholar]
- 56.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp eye Res. 2004;78(3):513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 57.Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocular Surf. 2004;2(4):248–253. doi: 10.1016/s1542-0124(12)70112-x. [DOI] [PubMed] [Google Scholar]
- 58.Alamri A, Bron R, Brock JA, Ivanusic JJ. Transient receptor potential cation channel subfamily V member 1 expressing corneal sensory neurons can be subdivided into at least three subpopulations. Front Neuroanat. 2015;9:71. doi: 10.3389/fnana.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat’s cornea. J Physiol. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat’s cornea. J Physiol. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacIver MB, Tanelian DL. Structural and functional specialization of A delta and C fiber free nerve endings innervating rabbit corneal epithelium. J Neurosci Of J Soc Neurosci. 1993;13(10):4511–4524. doi: 10.1523/JNEUROSCI.13-10-04511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X, Gallar J, Pozo MA, Baeza M, Belmonte C. CO2 stimulation of the cornea: a comparison between human sensation and nerve activity in poly-modal nociceptive afferents of the cat. Eur J Neurosci. 1995;7(6):1154–1163. doi: 10.1111/j.1460-9568.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 64.Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001;534(Pt. 2):511–525. doi: 10.1111/j.1469-7793.2001.t01-1-00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, Belmonte C, Rang HP. Capsaicin and carbon dioxide act by distinct mechanisms on sensory nerve terminals in the cat cornea. Pain. 1997;70(1):23–29. doi: 10.1016/s0304-3959(96)03256-3. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Gallar J, Belmonte C. Reduction by antiinflammatory drugs of the response of corneal sensory nerve fibers to chemical irritation. Investig Ophthalmol Vis Sci. 1997;38(10):1944–1953. [PubMed] [Google Scholar]
- 67.Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain. 2014;155(8):1481–1491. doi: 10.1016/j.pain.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 68.Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J. Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain. 2013;154(11):2353–2362. doi: 10.1016/j.pain.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 69.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 70.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 71.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 72.Straub RH. TRPV1, TRPA1, and TRPM8 channels in inflammation, energy redirection, and water retention: role in chronic inflammatory diseases with an evolutionary perspective. J Mol Med Berlin Ger. 2014;92(9):925–937. doi: 10.1007/s00109-014-1175-9. [DOI] [PubMed] [Google Scholar]
- 73.Gover TD, Kao JP, Weinreich D. Calcium signaling in single peripheral sensory nerve terminals. J Neurosci Of J Soc Neurosci. 2003;23(12):4793–4797. doi: 10.1523/JNEUROSCI.23-12-04793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupuy B, Thompson H, Beuerman RW. Capsiacin: a psycophysical tool to stduy corneal sensitivity. Investig Ophthalmol Vis Sci. 1988;29(Supp):454. [Google Scholar]
- 75.Zollman TM, Bragg RM, Harrison DA. Clinical effects of oleoresin capsicum (pepper spray) on the human cornea and conjunctiva. Ophthalmology. 2000;107(12):2186–2189. doi: 10.1016/s0161-6420(00)00463-2. [DOI] [PubMed] [Google Scholar]
- 76.Immke DC, Gavva NR. The TRPV1 receptor and nociception. Semin Cell & Dev Biol. 2006;17(5):582–591. doi: 10.1016/j.semcdb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11(3):946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 78.Murata Y, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006;1085(1):87–94. doi: 10.1016/j.brainres.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 79.Hirata H, Fried N, Oshinsky ML. Quantitative characterization reveals three types of dry-sensitive corneal afferents: pattern of discharge, receptive field, and thermal and chemical sensitivity. J Neurophysiol. 2012;108(9):2481–2493. doi: 10.1152/jn.00523.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mergler S, Valtink M, Takayoshi S, Okada Y, Miyajima M, Saika S, et al. Temperature-sensitive transient receptor potential channels in corneal tissue layers and cells. Ophthalmic Res. 2014;52(3):151–159. doi: 10.1159/000365334. [DOI] [PubMed] [Google Scholar]
- 81.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 82.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: a gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katagiri A, Thompson R, Rahman M, Okamoto K, Bereiter DA. Evidence for TRPA1 involvement in central neural mechanisms in a rat model of dry eye. Neuroscience. 2015;290:204–213. doi: 10.1016/j.neuroscience.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mantelli F, Micera A, Sacchetti M, Bonini S. Neurogenic inflammation of the ocular surface. Curr Opin allergy Clin Immunol. 2010;10(5):498–504. doi: 10.1097/ACI.0b013e32833e16cc. [DOI] [PubMed] [Google Scholar]
- 85.Reid TW, Murphy CJ, Iwahashi CK, Foster BA, Mannis MJ. Stimulation of epithelial cell growth by the neuropeptide substance P. J Cell Biochem. 1993;52(4):476–485. doi: 10.1002/jcb.240520411. [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp eye Res. 1994;59(5):597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- 87.Tran MT, Ritchie MH, Lausch RN, Oakes JE. Calcitonin gene-related peptide induces IL-8 synthesis in human corneal epithelial cells. J Immunol Baltim Md 1950. 2000;164(8):4307–4312. doi: 10.4049/jimmunol.164.8.4307. [DOI] [PubMed] [Google Scholar]
- 88.Tran MT, Lausch RN, Oakes JE. Substance P differentially stimulates IL-8 synthesis in human corneal epithelial cells. Investig Ophthalmol Vis Sci. 2000;41(12):3871–3877. [PubMed] [Google Scholar]
- 89.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci Of J Soc Neurosci. 2006;26(33):8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, et al. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci Of J Soc Neurosci. 2006;26(33):8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malin SA, Davis BM. Postnatal roles of glial cell line-derived neurotrophic factor family members in nociceptors plasticity. Sheng li xue bao. Acta Physiol Sin. 2008;60:571–578. [PMC free article] [PubMed] [Google Scholar]
- 92.Elitt CM, Malin SA, Koerber HR, Davis BM, Albers KM. Overexpression of artemin in the tongue increases expression of TRPV1 and TRPA1 in trigeminal afferents and causes oral sensitivity to capsaicin and mustard oil. Brain Res. 2008;1230:80–90. doi: 10.1016/j.brainres.2008.06.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanelian DL, Beuerman RW. Responses of rabbit corneal nociceptors to mechanical and thermal stimulation. Exp Neurol. 1984;84(1):165–178. doi: 10.1016/0014-4886(84)90013-x. [DOI] [PubMed] [Google Scholar]
- 94.Carr RW, Pianova S, Fernandez J, Fallon JB, Belmonte C, Brock JA. Effects of heating and cooling on nerve terminal impulses recorded from cold-sensitive receptors in the Guinea-pig cornea. J general Physiol. 2003;121(5):427–439. doi: 10.1085/jgp.200308814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brock J, Acosta MC, Al Abed A, Pianova S, Belmonte C. Barium ions inhibit the dynamic response of Guinea-pig corneal cold receptors to heating but not to cooling. J Physiol. 2006;575(Pt 2):573–581. doi: 10.1113/jphysiol.2006.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.González-González O, Bech F, Gallar J, Merayo-Lloves J, Belmonte C. Functional properties of sensory nerve terminals of the mouse cornea. Investig Ophthalmol Vis Sci. 2017;58(1):404–415. doi: 10.1167/iovs.16-20033. [DOI] [PubMed] [Google Scholar]
- 97.Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Investig Ophthalmol Vis Sci. 2010;51(8):3969–3976. doi: 10.1167/iovs.09-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16(12):1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 99.Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Investig Ophthalmol Vis Sci. 2001;42(9):2063–2067. [PubMed] [Google Scholar]
- 100.Kurose M, Meng ID. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. J Neurophysiol. 2013;109(10):2517–2522. doi: 10.1152/jn.00889.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quallo T, Vastani N, Horridge E, Gentry C, Parra A, Moss S, et al. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat Commun. 2015;6:7150. doi: 10.1038/ncomms8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirata H, Rosenblatt MI. Hyperosmolar tears enhance cooling sensitivity of the corneal nerves in rats: possible neural basis for cold-induced dry eye pain. Investig Ophthalmol Vis Sci. 2014;55(9):5821–5833. doi: 10.1167/iovs.14-14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hirata H, Mizerska K, Marfurt CF, Rosenblatt MI. Hyperosmolar tears induce functional and structural alterations of corneal nerves: electrophysiological and anatomical evidence toward neurotoxicity. Investig Ophthalmol Vis Sci. 2015;56:8125–8140. doi: 10.1167/iovs.15-18383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirata H, Oshinsky ML. Ocular dryness excites two classes of corneal afferent neurons implicated in basal tearing in rats: involvement of transient receptor potential channels. J Neurophysiol. 2012;107(4):1199–1209. doi: 10.1152/jn.00657.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olivares E, Salgado S, Maidana JP, Herrera G, Campos M, Madrid R, et al. TRPM8-Dependent dynamic response in a mathematical model of cold thermoreceptor. PloS One. 2015;10:e0139314. doi: 10.1371/journal.pone.0139314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 107.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 108.Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, et al. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci Off J Soc Neurosci. 2013;33(7):2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orio P, Parra A, Madrid R, González O, Belmonte C, Viana F. Role of Ih in the firing pattern of mammalian cold thermoreceptor endings. J Neurophysiol. 2012;108(11):3009–3023. doi: 10.1152/jn.01033.2011. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez-Gonzalez O, Bech F, Gallar J, Merayo-Lloves J, Belmonte C. Functional properties of sensory nerve terminals of the mouse cornea. Investig Ophthalmol Vis Sci. 2017;58(1):404–415. doi: 10.1167/iovs.16-20033. [DOI] [PubMed] [Google Scholar]
- 111.Madrid R, de la Peña E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci Off J Soc Neurosci. 2009;29(10):3120–3131. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viana F, de la Peña E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5(3):254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- 113.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Sci (New York, NY) 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483(7388):209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J. Corneal sensory nerve activity in an experimental model of UV keratitis. Investig Ophthalmol Vis Sci. 2014;55(6):3403–3412. doi: 10.1167/iovs.13-13774. [DOI] [PubMed] [Google Scholar]
- 117.Michaelis M, Häbler HJ, Jäenig W. Silent afferents: a separate class of primary afferents? Clin Exp Pharmacol Physiol. 1996;23(2):99–105. doi: 10.1111/j.1440-1681.1996.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 118.Brock James A, McLachlan Elspeth M, Belmonte Carlos. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in Guinea-pig cornea. J Physiol. 1998;512(1):211–217. doi: 10.1111/j.1469-7793.1998.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oppenheimer DR, Palmer E, Weddell G. Nerve endings in the conjunctiva. J Anat. 1958;92:321–352. [PMC free article] [PubMed] [Google Scholar]
- 120.Munger BL, Halata Z. The sensorineural apparatus of the human eyelid. Am J anatomy. 1984;170(2):181–204. doi: 10.1002/aja.1001700205. [DOI] [PubMed] [Google Scholar]
- 121.Luhtala J, Palkama A, Uusitalo H. Calcitonin gene-related peptide immunoreactive nerve fibers in the rat conjunctiva. Investig Ophthalmol Vis Sci. 1991;32(3):640–645. [PubMed] [Google Scholar]
- 122.Luhtala J, Uusitalo H. The distribution and origin of substance P immunoreactive nerve fibres in the rat conjunctiva. Exp eye Res. 1991;53(5):641–646. doi: 10.1016/0014-4835(91)90224-3. [DOI] [PubMed] [Google Scholar]
- 123.Elsås T, Edvinsson L, Sundler F, Uddman R. Neuronal pathways to the rat conjunctiva revealed by retrograde tracing and immunocytochemistry. Exp eye Res. 1994;58(1):117–126. doi: 10.1006/exer.1994.1201. [DOI] [PubMed] [Google Scholar]
- 124.Dartt DA, McCarthy DM, Mercer HJ, Kessler TL, Chung EH, Zieske JD. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr eye Res. 1995;14(11):993–1000. doi: 10.3109/02713689508998520. [DOI] [PubMed] [Google Scholar]
- 125.Chung CW, Tigges M, Stone RA. Peptidergic innervation of the primate meibomian gland. Investig Ophthalmol Vis Sci. 1996;37(1):238–245. [PubMed] [Google Scholar]
- 126.Lawrenson JG, Ruskell GL. The structure of corpuscular nerve endings in the limbal conjunctiva of the human eye. J Anat. 1991;177:75–84. [PMC free article] [PubMed] [Google Scholar]
- 127.Lowther GE, Hill RM. Sensitivity threshold of the lower lid margin in the course of adaptation to contact lenses. Am J Optom Arch Am Acad Optom. 1968;45(9):587–594. doi: 10.1097/00006324-196809000-00003. [DOI] [PubMed] [Google Scholar]
- 128.Aracil A, Gallar J, Belmonte C. Functional properties of the conjunctival innervation. Opthalmic Res. 2001;33(Suppl 1):101. [Google Scholar]
- 129.Stapleton F, Tan ME, Papas EB, Ehrmann K, Golebiowski B, Vega J, et al. Corneal and conjunctival sensitivity to air stimuli. Br J Ophthalmol. 2004;88(12):1547–1551. doi: 10.1136/bjo.2004.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Acosta MC, Alfaro ML, Borrás F, Belmonte C, Gallar J. Influence of age, gender and iris color on mechanical and chemical sensitivity of the cornea and conjunctiva. Exp eye Res. 2006;83(4):932–938. doi: 10.1016/j.exer.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 131.Belmonte C, ed la Pena E. Thermosensation. In: Galizia GCG, Lledo PM, editors. Neurosciences. Springer Verlag; Berlin, Germany: 2013. pp. 303–319. [Google Scholar]
- 132.Xiao B, Wang Y, Reinach PS, Ren Y, Li J, Hua S, et al. Dynamic ocular surface and lacrimal gland changes induced in experimental murine dry eye. PloS One. 2015;10:e0115333. doi: 10.1371/journal.pone.0115333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Investig Ophthalmol Vis Sci. 2011;52(6):3888–3892. doi: 10.1167/iovs.09-5119. [DOI] [PubMed] [Google Scholar]
- 134.Kimball SH, King-Smith PE, Nichols JJ. Evidence for the major contribution of evaporation to tear film thinning between blinks. Investig Ophthalmol Vis Sci. 2010;51(12):6294–6297. doi: 10.1167/iovs.09-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Purslow C, Wolffsohn J. The relation between physical properties of the anterior eye and ocular surface temperature. Optom Vis Sci Off Publ Am Acad Optom. 2007;84(3):197–201. doi: 10.1097/OPX.0b013e3180339f6e. [DOI] [PubMed] [Google Scholar]
- 136.Li W, Graham AD, Selvin S, Lin MC. Ocular surface cooling corresponds to tear film thinning and breakup. Optom and Vis Sci Off Publ Am Acad of Optom. 2015;92(9):e248–e256. doi: 10.1097/OPX.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 137.Pattmöller J, Wang J, Zemova E, Seitz B, Eppig T, Langenbucher A, et al. Correlation of corneal thickness, endothelial cell density and anterior chamber depth with ocular surface temperature in normal subjects. Z fur Med Phys. 2015;25(3):243–250. doi: 10.1016/j.zemedi.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 138.Versura P, Giannaccare G, Fresina M, Campos EC. Subjective discomfort symptoms are related to low corneal temperature in patients with evaporative dry eye. Cornea. 2015;34(9):1079–1085. doi: 10.1097/ICO.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 139.Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What causes eye pain? Curr Ophthalmol Rep. 2015;3(2):111–121. doi: 10.1007/s40135-015-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robbins A, Kurose M, Winterson BJ, Meng ID. Menthol activation of corneal cool cells induces TRPM8-mediated lacrimation but not nociceptive responses in rodents. Investig Ophthalmol Vis Sci. 2012;53(11):7034–7042. doi: 10.1167/iovs.12-10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Begley C, Simpson T, Liu H, Salvo E, Wu Z, Bradley A, et al. Quantitative analysis of tear film fluorescence and discomfort during tear film instability and thinning. Investig Ophthalmol Vis Sci. 2013;54(4):2645–2653. doi: 10.1167/iovs.12-11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, et al. A link between tear instability and hyperosmolarity in dry eye. Investig Ophthalmol Vis Sci. 2009;50(8):3671–3679. doi: 10.1167/iovs.08-2689. [DOI] [PubMed] [Google Scholar]
- 143.Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, Riol-Blanco L, Barreiro O, von Andrian UH. The regulation of immunological processes by pe ripheral neurons in homeostasis and disease. Trends Immunol. 2015;36(10):578–604. doi: 10.1016/j.it.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu K, Harris DL, Yamaguchi T, von Andrian UH, Hamrah P. A dual role for corneal dendritic cells in herpes simplex keratitis: local suppression of corneal damage and promotion of systemic viral dissemination. PloS One. 2015;10:e0137123. doi: 10.1371/journal.pone.0137123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 146.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74(2):172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 147.Yamaguchi T, Harris DL, Higa K, Shimazaki J, von Andrian U, Hamrah P. Neurogenic immune homeostasis: peripheral innervation maintains avascularity and immune privilege of the cornea. Investig Ophthalmol Vis Sci. 2015;56:4034. [Google Scholar]
- 148.Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Investig Ophthalmol Vis Sci. 2011;52(8):5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, et al. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Investig Ophthalmol Vis Sci. 2014;55(6):3603–3615. doi: 10.1167/iovs.14-14307. [DOI] [PubMed] [Google Scholar]
- 150.Blanco T, Saban DR. The cornea has “the nerve” to encourage immune rejection. Am J Transplant. 2015;15(6):1453–1454. doi: 10.1111/ajt.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jamali A, Lopez MJ, Sendra V, Harris DL, Hamrah P. Plasmacytoid dendritic cells demonstrate vital neuro-protective properties in the cornea and induce corneal nerve regeneration. Investig Ophthalmol Vis Sci. 2015;56:4355. [Google Scholar]
- 152.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Micera A, Lambiase A, Bonini S. The role of neuromediators in ocular allergy. Curr Opin allergy Clin Immunol. 2008;8(5):466–471. doi: 10.1097/ACI.0b013e32830e6b17. [DOI] [PubMed] [Google Scholar]
- 154.Launay PS, Reboussin E, Liang H, Kessal K, Godefroy D, Rostene W, et al. Ocular inflammation induces trigeminal pain, peripheral and central neuroinflammatory mechanisms. Neurobiol Dis. 2016;88:16–28. doi: 10.1016/j.nbd.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 155.Carucci JA, Ignatius R, Wei Y, Cypess AM, Schaer DA, Pope M, et al. Calcitonin gene-related peptide decreases expression of HLA-DR and CD86 by human dendritic cells and dampens dendritic cell-driven T cell-proliferative responses via the type I calcitonin gene-related peptide receptor. J Immunol. 2000;164(7):3494–3499. doi: 10.4049/jimmunol.164.7.3494. [DOI] [PubMed] [Google Scholar]
- 156.Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363(6425):159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 157.Dunzendorfer Stefan, Kaser Arthur, Meierhofer Christian, Tilg Herbert, Wiedermann Christian J. Cutting edge: peripheral neuropeptides attract immature and arrest mature blood-derived dendritic cells. J Immunol. 2001;166(4):2167–2172. doi: 10.4049/jimmunol.166.4.2167. [DOI] [PubMed] [Google Scholar]
- 158.Paunicka KJ, Mellon J, Robertson D, Petroll M, Brown JR, Niederkorn JY. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15(6):1490–1501. doi: 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gonzalez-Coto AF, Alonso-Ron C, Alcalde I, Gallar J, Meana Á, Merayo-Lloves J, et al. Expression of cholecystokinin, gastrin, and their receptors in the mouse cornea. Investig Ophthalmol Vis Sci. 2014;55(3):1965–1975. doi: 10.1167/iovs.13-12068. [DOI] [PubMed] [Google Scholar]
- 160.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20(5):1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 161.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mizoue LS, Bazan JF, Johnson EC, Handel TM. Solution structure and dynamics of the CX3C chemokine domain of fractalkine and its interaction with an N-terminal fragment of CX3CR1. Biochemistry. 1999;38(5):1402–1414. doi: 10.1021/bi9820614. [DOI] [PubMed] [Google Scholar]
- 163.Owlasiuk P, Zajkowska JM, Pietruczuk M, Pancewicz SA, Hermanowska-Szpakowicz T. Fractalkine–structure, functions and biological activity. Polski Merkuriusz Lekarski organ Pol Tow Lek. 2009;26:253–257. [PubMed] [Google Scholar]
- 164.Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci. 2014;8:121. doi: 10.3389/fncel.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, Mc-Menamin PG. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Investig Ophthalmol Vis Sci. 2007;48(4):1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carreño E, Enríquez-de-Salamanca A, Tesón M, García-Vázquez C, Stern ME, Whitcup SM, et al. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol. 2010;88(7):e250–e258. doi: 10.1111/j.1755-3768.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- 167.Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol. 2011;178(3):1106–1116. doi: 10.1016/j.ajpath.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dahan A, Brines M, Niesters M, Cerami A, van Velzen M. Targeting the innate repair receptor to treat neuropathy. PAIN Reports. 2016;1:e566. doi: 10.1097/PR9.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hamrah P, Dana R. Antigen-presenting cells in the eye and ocular surface. In: Dart DA, editor. Encyclopedia of the Eye. Academic Press; Oxford: 2010. pp. 120–127. [Google Scholar]
- 170.Sarkar J, Chaudhary S, Jassim SH, Ozturk O, Chamon W, Ganesh B, et al. CD11b+GR1+ myeloid cells secrete NGF and promote trigeminal ganglion neurite growth: implications for corneal nerve regeneration. Investig Ophthalmol Vis Sci. 2013;54(9):5920–5936. doi: 10.1167/iovs.13-12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Okuno T, Nakatsuji Y, Kumanogoh A. The role of immune semaphorins in multiple sclerosis. FEBS Lett. 2011;585(23):3829–3835. doi: 10.1016/j.febslet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 172.Takamatsu H, Kumanogoh A. Diverse roles for semaphorinplexin signaling in the immune system. Trends Immunol. 2012;33(3):127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 173.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Stern-berg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282(36):26294–26305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- 174.Goto T, Oh SB, Takeda M, Shinoda M, Sato T, Gunjikake KK, et al. Recent advances in basic research on the trigeminal ganglion. J Physiol Sci. 2016;66(5):381–386. doi: 10.1007/s12576-016-0448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the tranganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;203(4):785–798. doi: 10.1002/cne.902030414. [DOI] [PubMed] [Google Scholar]
- 177.Panneton WM, Burton H. Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;199(3):327–344. doi: 10.1002/cne.901990303. [DOI] [PubMed] [Google Scholar]
- 178.Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol. 1987;261(3):450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- 179.Marfurt CF, Echtenkamp SF. Central projections and trigeminal ganglion location of corneal afferent neurons in the monkey, Macaca fascicularis. J Comp Neurol. 1988;272(3):370–382. doi: 10.1002/cne.902720307. [DOI] [PubMed] [Google Scholar]
- 180.Panneton WM, Hsu H, Gan Q. Distinct central representations for sensory fibers innervating either the conjunctiva or cornea of the rat. Exp eye Res. 2010;90(3):388–396. doi: 10.1016/j.exer.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bereiter DA, Hargreaves KM, Hu JW. Trigeminal mechanisms of nociception: peripheral and brainstem organization. In: Basbaum A, Bushnell MC, editors. Science of Pain. Elsevier; New York: 2009. pp. 435–460. [Google Scholar]
- 182.Gong S, Zhou Q, LeDoux MS. Blink-related sensorimotor anatomy in the rat. Anat Embryol. 2003;207(3):193–208. doi: 10.1007/s00429-003-0341-6. [DOI] [PubMed] [Google Scholar]
- 183.Simons E, Smith PG. Sensory and autonomic innervation of the rat eyelid: neuronal origins and peptide phenotypes. J Chem Neuroanat. 1994;7(1–2):35–47. doi: 10.1016/0891-0618(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 184.Baljet B, VanderWerf F. Connections between the lacrimal gland and sensory trigeminal neurons: a WGA/HRP study in the cynomolgous monkey. J Anat. 2005;206(3):257–263. doi: 10.1111/j.1469-7580.2005.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kirch W, Horneber M, Tamm ER. Characterization of Meibomian gland innervation in the cynomolgus monkey (Macaca fascicularis) Anat Embryol. 1996;193(4):365–375. doi: 10.1007/BF00186693. [DOI] [PubMed] [Google Scholar]
- 186.Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88(3):221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- 187.Meng ID, Hu JW, Benetti AP, Bereiter DA. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. J Neurophysiol. 1997;77(1):43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- 188.Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO(2) pulses in the rat. J Neurophysiol. 1999;82(5):2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- 189.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149(2):235–242. doi: 10.1016/j.pain.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci Off J Soc Neurosci. 2004;24(17):4224–4232. doi: 10.1523/JNEUROSCI.0381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pozo MA, Cervero F. Neurons in the rat spinal trigeminal complex driven by corneal nociceptors: receptive-field properties and effects of noxious stimulation of the cornea. J Neurophysiol. 1993;70(6):2370–2378. doi: 10.1152/jn.1993.70.6.2370. [DOI] [PubMed] [Google Scholar]
- 192.Meng ID, Hu JW, Bereiter DA. Differential effects of morphine on corneal-responsive neurons in rostral versus caudal regions of spinal trigeminal nucleus in the rat. J Neurophysiol. 1998;79(5):2593–2602. doi: 10.1152/jn.1998.79.5.2593. [DOI] [PubMed] [Google Scholar]
- 193.Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res. 1995;107(2):166–180. doi: 10.1007/BF00230039. [DOI] [PubMed] [Google Scholar]
- 194.Hirata H, Takeshita S, Hu JW, Bereiter DA. Cornea-responsive medullary dorsal horn neurons: modulation by local opioids and projections to thalamus and brain stem. J Neurophysiol. 2000;84(2):1050–1061. doi: 10.1152/jn.2000.84.2.1050. [DOI] [PubMed] [Google Scholar]
- 195.Okamoto K, Tashiro A, Thompson R, Nishida Y, Bereiter DA. Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci. 2012;36(11):3492–3499. doi: 10.1111/j.1460-9568.2012.08272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rahman M, Okamoto K, Thompson R, Bereiter DA. Trigeminal pathways for hypertonic saline- and light-evoked corneal reflexes. Neuroscience. 2014;277:716–723. doi: 10.1016/j.neuroscience.2014.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80(2):465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 198.Bereiter DA, Okamoto K, Tashiro A, Hirata H. Endotoxin-induced uveitis causes long-term changes in trigeminal subnucleus caudalis neurons. J Neurophysiol. 2005;94(6):3815–3825. doi: 10.1152/jn.00616.2005. [DOI] [PubMed] [Google Scholar]
- 199.Ebersberger A, Ringkamp M, Reeh PW, Handwerker HO. Recordings from brain stem neurons responding to chemical stimulation of the subarachnoid space. J Neurophysiol. 1997;77(6):3122–3133. doi: 10.1152/jn.1997.77.6.3122. [DOI] [PubMed] [Google Scholar]
- 200.Schepelmann K, Ebersberger A, Pawlak M, Oppmann M, Messlinger K. Response properties of trigeminal brain stem neurons with input from dura mater encephali in the rat. Neuroscience. 1999;90(2):543–554. doi: 10.1016/s0306-4522(98)00423-0. [DOI] [PubMed] [Google Scholar]
- 201.Malick A, Strassman RM, Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J Neurophysiol. 2000;84(4):2078–2112. doi: 10.1152/jn.2000.84.4.2078. [DOI] [PubMed] [Google Scholar]
- 202.Kerr FW, Kruger L, Schwassmann HO, Stern R. Somatotopic organization of mechanoreceptor units in the trigeminal nuclear complex of the macaque. J Comp Neurol. 1968;134(2):127–144. doi: 10.1002/cne.901340202. [DOI] [PubMed] [Google Scholar]
- 203.Greenwood LF, Sessle BJ. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: II. Role of trigeminal nucleus caudalis in modulating responses to innocuous and noxious stimuli. Brain Res. 1976;117(2):227–238. doi: 10.1016/0006-8993(76)90732-0. [DOI] [PubMed] [Google Scholar]
- 204.Davis KD, Dostrovsky JO. Responses of feline trigeminal spinal tract nucleus neurons to stimulation of the middle meningeal artery and sagittal sinus. J Neurophysiol. 1988;59(2):648–666. doi: 10.1152/jn.1988.59.2.648. [DOI] [PubMed] [Google Scholar]
- 205.Chen WH, Chui C, Lin HS, Yin HL. Salt-and-pepper eye pain and brainstem stroke. Clin Neurol Neurosurg. 2012;114(7):972–975. doi: 10.1016/j.clineuro.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 206.Jacquin MF, Chiaia NL, Haring JH, Rhoades RW. Intersubnuclear connections within the rat trigeminal brainstem complex. Somatosens Mot Res. 1990;7(4):399–420. doi: 10.3109/08990229009144716. [DOI] [PubMed] [Google Scholar]
- 207.Voisin DL, Doméjean-Orliaguet S, Chalus M, Dallel R, Woda A. Ascending connections from the caudal part to the oral part of the spinal trigeminal nucleus in the rat. Neuroscience. 2002;109(1):183–193. doi: 10.1016/s0306-4522(01)00456-0. [DOI] [PubMed] [Google Scholar]
- 208.Hirata H, Okamoto K, Bereiter DA. GABA(A) receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis. J Neurophysiol. 2003;90(5):2837–2849. doi: 10.1152/jn.00544.2003. [DOI] [PubMed] [Google Scholar]
- 209.Warren S, May PJ. Morphology and connections of intratrigeminal cells and axons in the macaque monkey. Front Neuroanat. 2013;7:11. doi: 10.3389/fnana.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chiang CY, Hu B, Hu JW, Dostrovsky JO, Sessle BJ. Central sensitization of nociceptive neurons in trigeminal subnucleus oralis depends on integrity of subnucleus caudalis. J Neurophysiol. 2002;88(1):256–264. doi: 10.1152/jn.00944.2001. [DOI] [PubMed] [Google Scholar]
- 211.Davis KD, Dostrovsky JO. Effect of trigeminal subnucleus caudalis cold block on the cerebrovascular-evoked responses of rostral trigeminal complex neurons. Neurosci Lett. 1988;94(3):303–308. doi: 10.1016/0304-3940(88)90035-3. [DOI] [PubMed] [Google Scholar]
- 212.Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007;179(4):691–702. doi: 10.1007/s00221-006-0826-7. [DOI] [PubMed] [Google Scholar]
- 213.Kaas JH, Nelson RJ, Sur M, Dykes RW, Merzenich MM. The somatotopic organization of the ventroposterior thalamus of the squirrel monkey, Saimiri sciureus. J Comp Neurol. 1984;226(1):111–140. doi: 10.1002/cne.902260109. [DOI] [PubMed] [Google Scholar]
- 214.Rausell E, Jones EG. Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. J Neurosci Off J Soc Neurosci. 1991;11(1):226–237. doi: 10.1523/JNEUROSCI.11-01-00226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Noseda R, Monconduit L, Constandil L, Chalus M, Villanueva L. Central nervous system networks involved in the processing of meningeal and cutaneous inputs from the ophthalmic branch of the trigeminal nerve in the rat. Cephalalgia Int J Headache. 2008;28(8):813–824. doi: 10.1111/j.1468-2982.2008.01588.x. [DOI] [PubMed] [Google Scholar]
- 216.Aicher SA, Hermes SM, Hegarty DM. Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis. Neuroscience. 2013;232:182–193. doi: 10.1016/j.neuroscience.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- 218.Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468(1):24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- 219.Mazzola L, Isnard J, Mauguière F. Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex New York NY 1991. 2006;16(7):960–968. doi: 10.1093/cercor/bhj038. [DOI] [PubMed] [Google Scholar]
- 220.Penfield W, Boldrey E. Somatic motor and snsory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 221.Dreyer DA, Loe PR, Metz CB, Whitsel BL. Representation of head and face in postcentral gyrus of the macaque. J Neurophysiol. 1975;38(3):714–733. doi: 10.1152/jn.1975.38.3.714. [DOI] [PubMed] [Google Scholar]
- 222.Nelson RJ, Sur M, Felleman DJ, Kaas JH. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol. 1980;192(4):611–643. doi: 10.1002/cne.901920402. [DOI] [PubMed] [Google Scholar]
- 223.Moulton EA, Becerra L, Rosenthal P, Borsook D. An approach to localizing corneal pain representation in human primary somatosensory cortex. PloS One. 2012;7:e44643. doi: 10.1371/journal.pone.0044643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Morrison M, Allen PZ. Lactoperoxidase: identification and isolation from Harderian and lacrimal glands. Sci (New York, NY) 1966;152(3729):1626–1628. doi: 10.1126/science.152.3729.1626. [DOI] [PubMed] [Google Scholar]
- 225.Allen PZ, Morrison M. Lactoperoxidase. VI. Immunochemical studies on lactoperoxidase from the milk of several species. Arch Biochem Biophys. 1966;113(3):540–547. doi: 10.1016/0003-9861(66)90231-1. [DOI] [PubMed] [Google Scholar]
- 226.Broekhuyse RM. Lactoferrin and the protective function of the lacrimal fluid. Ophthalmol J Int d’ophtalmologie Int J Ophthalmol Z fur Augenheilkd. 1976;173(3–4):268–270. doi: 10.1159/000307908. [DOI] [PubMed] [Google Scholar]
- 227.Franklin RM, Prendergast RA, Silverstein AM. Secretory immune system of rabbit ocular adnexa. Investig Ophthalmol Vis Sci. 1979;18(10):1093–1096. [PubMed] [Google Scholar]
- 228.Rennie IG, Parsons MA. Lysozyme distribution in human lacrimal glands and other ocular adnexa. Arch Ophthalmol Chic Ill 1960) 1981;99(10):1850–1853. doi: 10.1001/archopht.1981.03930020724020. [DOI] [PubMed] [Google Scholar]
- 229.Delaire A, Lassagne H, Gachon AM. New members of the lipocalin family in human tear fluid. Exp eye Res. 1992;55(4):645–647. doi: 10.1016/s0014-4835(05)80178-2. [DOI] [PubMed] [Google Scholar]
- 230.Putney JW, Jr, VandeWalle CM, Leslie BA. Stimulus-secretion coupling in the rat lacrimal gland. Am J Physiol. 1978;235(5):C188–C198. doi: 10.1152/ajpcell.1978.235.5.C188. [DOI] [PubMed] [Google Scholar]
- 231.Marty A, Tan YP, Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Trautmann A, Marty A. Activation of Ca-dependent K channels by car-bamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A. 1984;81(2):611–615. doi: 10.1073/pnas.81.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wood RL, Mircheff AK. Apical and basal-lateral Na/K-ATPase in rat lacrimal gland acinar cells. Investig Ophthalmol Vis Sci. 1986;27(8):1293–1296. [PubMed] [Google Scholar]
- 234.Alexander JH, van Lennep EW, Young JA. Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization techniques. Pflugers Archiv Eur J Physiol. 1972;337(4):299–309. doi: 10.1007/BF00586647. [DOI] [PubMed] [Google Scholar]
- 235.Alexander JH, Young JA, van Lennep EW. The ultrastructure of the duct system in the rat extraorbital lacrimal gland. Z fur Zellforsch Mikrosk Anat Viennaa, Austria 1948) 1973;144(4):453–466. doi: 10.1007/BF00307373. [DOI] [PubMed] [Google Scholar]
- 236.Nikkinen A, Lehtosalo JI, Uusitalo H, Palkama A, Panula P. The lacrimal glands of the rat and the Guinea pig are innervated by nerve fibers containing immunoreactivities for substance P and vasoactive intestinal polypeptide. Histochemistry. 1984;81(1):23–27. doi: 10.1007/BF00495396. [DOI] [PubMed] [Google Scholar]
- 237.Dartt DA, Baker AK, Vaillant C, Rose PE. Vasoactive intestinal polypeptide stimulation of protein secretion from rat lacrimal gland acini. Am J Physiol. 1984;247(5 Pt 1):G502–G509. doi: 10.1152/ajpgi.1984.247.5.G502. [DOI] [PubMed] [Google Scholar]
- 238.Sibony PA, Walcott B, McKeon C, Jakobiec FA. Vasoactive intestinal polypeptide and the innervation of the human lacrimal gland. Arch Ophthalmol Chic Ill 1960) 1988;106(8):1085–1088. doi: 10.1001/archopht.1988.01060140241033. [DOI] [PubMed] [Google Scholar]
- 239.Matsumoto Y, Tanabe T, Ueda S, Kawata M. Immunohistochemical and en-zymehistochemical studies of peptidergic, aminergic and cholinergic innervation of the lacrimal gland of the monkey (Macaca fuscata) J Aut Nerv Syst. 1992;37(3):207–214. doi: 10.1016/0165-1838(92)90042-f. [DOI] [PubMed] [Google Scholar]
- 240.Seifert P, Spitznas M. Vasoactive intestinal polypeptide (VIP) innervation of the human eyelid glands. Exp eye Res. 1999;68(6):685–692. doi: 10.1006/exer.1999.0652. [DOI] [PubMed] [Google Scholar]
- 241.Ríos JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, et al. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp eye Res. 2005;80(4):477–491. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ding C, Walcott B, Keyser KT. Neuronal nitric oxide synthase and the autonomic innervation of the mouse lacrimal gland. Investig Ophthalmol Vis Sci. 2001;42(12):2789–2794. [PubMed] [Google Scholar]
- 243.Ding C, Walcott B, Keyser KT. Sympathetic neural control of the mouse lacrimal gland. Investig Ophthalmol Vis Sci. 2003;44(4):1513–1520. doi: 10.1167/iovs.02-0406. [DOI] [PubMed] [Google Scholar]
- 244.Dartt DA, Botelho SY. Protein in rabbit lacrimal gland fluid. Investig Ophthalmol Vis Sci. 1979;18(11):1207–1209. [PubMed] [Google Scholar]
- 245.Dartt DA, Knox I, Palau A, Botelho SY. Proteins in fluids from individual orbital glands and in tears. Investig Ophthalmol Vis Sci. 1980;19(11):1342–1347. [PubMed] [Google Scholar]
- 246.Dartt DA, Møller M, Poulsen JH. Lacrimal gland electrolyte and water secretion in the rabbit: localization and role of (Na+ + K+)-activated ATPase. J Physiol. 1981;321:557–569. doi: 10.1113/jphysiol.1981.sp014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ubels JL, Foley KM, Rismondo V. Retinol secretion by the lacrimal gland. Investig Ophthalmol Vis Sci. 1986;27(8):1261–1268. [PubMed] [Google Scholar]
- 248.Rismondo V, Ubels JL. Isotretinoin in lacrimal gland fluid and tears. Arch Ophthalmol Chic Ill 1960) 1987;105(3):416–420. doi: 10.1001/archopht.1987.01060030136044. [DOI] [PubMed] [Google Scholar]
- 249.Ruskell GL. Changes in nerve terminals and acini of the lacrimal gland and changes in secretion induced by autonomic denervation, Z fur Zellforsch Mikrosk Anat Viennaa. Austria1948) 1969;94(2):261–281. doi: 10.1007/BF00339361. [DOI] [PubMed] [Google Scholar]
- 250.Toshida H, Nguyen DH, Beuerman RW, Murakami A. Evaluation of novel dry eye model: preganglionic parasympathetic denervation in rabbit. Investig Ophthalmol Vis Sci. 2007;48(10):4468–4475. doi: 10.1167/iovs.06-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Thörig L, van Haeringen NJ, Wijngaards G. Comparison of enzymes of tears, lacrimal gland fluid and lacrimal gland tissue in the rat. Exp eye Res. 1984;38(6):605–609. doi: 10.1016/0014-4835(84)90178-7. [DOI] [PubMed] [Google Scholar]
- 252.Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, et al. A mouse model of keratoconjunctivitis sicca. Investig Ophthalmol Vis Sci. 2002;43(3):632–638. [PubMed] [Google Scholar]
- 253.Mauduit P, Jammes H, Rossignol B. M3 muscarinic acetylcholine receptor coupling to PLC in rat exorbital lacrimal acinar cells. Am J Physiol. 1993;264(6 Pt 1):C1550–C1560. doi: 10.1152/ajpcell.1993.264.6.C1550. [DOI] [PubMed] [Google Scholar]
- 254.Nakamura M, Tada Y, Akaishi T, Nakata K. M3 muscarinic receptor mediates regulation of protein secretion in rabbit lacrimal gland. Curr eye Res. 1997;16(6):614–619. doi: 10.1076/ceyr.16.6.614.5077. [DOI] [PubMed] [Google Scholar]
- 255.Lemullois Michel, Rossignol Bernard, Mauduit Philippe. Immunolocalization of myoepithelial cells in isolated acini of rat exorbital lacrimal gland: cellular distribution of muscarinic receptors. Biol Cell. 1996;86(2–3):175–181. doi: 10.1016/0248-4900(96)84782-4. [DOI] [PubMed] [Google Scholar]
- 256.Ubels JL, Hoffman HM, Srikanth S, Resau JH, Webb CP. Gene expression in rat lacrimal gland duct cells collected using laser capture microdissection: evidence for K+ secretion by duct cells. Investig Ophthalmol Vis Sci. 2006;47:1876–1885. doi: 10.1167/iovs.05-0363. [DOI] [PubMed] [Google Scholar]
- 257.Kelleher RS, Hann LE, Edwards JA, Sullivan DA. Endocrine, neural, and immune control of secretory component output by lacrimal gland acinar cells. J Immunol. 1991;146:3405–3412. [PubMed] [Google Scholar]
- 258.Lambert RW, Kelleher RS, Wickham LA, Vaerman JP, Sullivan DA. Neuroendocrinimmune modulation of secretory component production by rat lacrimal, salivary, and intestinal epithelial cells. Investig Ophthalmol Vis Sci. 1994;35(3):1192–1201. [PubMed] [Google Scholar]
- 259.Qian L, Wang Y, Xie J, Rose CM, Yang T, Nakamura T, et al. Biochemical changes contributing to functional quiescence in lacrimal gland acinar cells after chronic ex vivo exposure to a muscarinic agonist. Scand J Immunol. 2003;58(5):550–565. doi: 10.1046/j.1365-3083.2003.01343.x. [DOI] [PubMed] [Google Scholar]
- 260.Gierow JP, Lambert RW, Mircheff AK. Fluid phase endocytosis by isolated rabbit lacrimal gland acinar cells. Exp eye Res. 1995;60(5):511–525. doi: 10.1016/s0014-4835(05)80066-1. [DOI] [PubMed] [Google Scholar]
- 261.Adeghate EA, Singh J, Howarth FC, Burrows S. Control of porcine lacrimal gland secretion by non-cholinergic, non-adrenergic nerves: effects of electrical field stimulation, VIP and NPY. Brain Res. 1997;758(1–2):127–135. doi: 10.1016/s0006-8993(97)00215-1. [DOI] [PubMed] [Google Scholar]
- 262.Hodges RR, Zoukhri D, Sergheraert C, Zieske JD, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Investig Ophthalmol Vis Sci. 1997;38(3):610–619. [PubMed] [Google Scholar]
- 263.Edman MC, Andersson SV, Delbro D, Gierow JP. Functional expression of the adenosine A1 receptor in rabbit lacrimal gland. Exp eye Res. 2008;86(1):110–117. doi: 10.1016/j.exer.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 264.Gonsalvez DG, Kerman IA, McAllen RM, Anderson CR. Chemical coding for cardiovascular sympathetic preganglionic neurons in rats. J Neurosci Off J Soc Neurosci. 2010;30(35):11781–11791. doi: 10.1523/JNEUROSCI.0796-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Botelho SY, Martinez EV, Pholpramool C, Prooyen HC, Janssen JT, De Palau A. Modification of stimulated lacrimal gland flow by sympathetic nerve impulses in rabbit. Am J Physiol. 1976;230(1):80–84. doi: 10.1152/ajplegacy.1976.230.1.80. [DOI] [PubMed] [Google Scholar]
- 266.Parod RJ, Putney JW., Jr An alpha-adrenergic receptor mechanism controlling potassium permeability in the rat lacrimal gland acinar cell. J Physiol. 1978;281:359–369. doi: 10.1113/jphysiol.1978.sp012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Parod RJ, Putney JW., Jr Stimulus-permeability coupling in rat lacrimal gland. Am J Physiol. 1980;239(2):G106–G113. doi: 10.1152/ajpgi.1980.239.2.G106. [DOI] [PubMed] [Google Scholar]
- 268.Hodges RR, Shatos MA, Tarko RS, Vrouvlianis J, Gu J, Dartt DA. Nitric oxide and cGMP mediate alpha1D-adrenergic receptor-Stimulated protein secretion and p42/p44 MAPK activation in rat lacrimal gland. Investig Ophthalmol Vis Sci. 2005;46(8):2781–2789. doi: 10.1167/iovs.05-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ding C, Walcott B, Keyser KT. The alpha1- and beta1-adrenergic modulation of lacrimal gland function in the mouse. Investig Ophthalmol Vis Sci. 2007;48(4):1504–1510. doi: 10.1167/iovs.05-1634. [DOI] [PubMed] [Google Scholar]
- 270.Meneray MA, Bennett DJ, Nguyen DH, Beuerman RW. Effect of sensory denervation on the structure and physiologic responsiveness of rabbit lacrimal gland. Cornea. 1998;17(1):99–107. doi: 10.1097/00003226-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 271.Hodges RR, Vrouvlianis J, Shatos MA, Dartt DA. Characterization of P2X7 purinergic receptors and their function in rat lacrimal gland. Investig Ophthalmol Vis Sci. 2009;50(12):5681–5689. doi: 10.1167/iovs.09-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hodges RR, Vrouvlianis J, Scott R, Dartt DA. Identification of P2X? and P2X? purinergic receptors activated by ATP in rat lacrimal gland. Investig Ophthalmol Vis Sci. 2011;52(6):3254–3263. doi: 10.1167/iovs.10-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Dartt DA, Hodges RR. Cholinergic agonists activate P2X7 receptors to stimulate protein secretion by the rat lacrimal gland. Investig Ophthalmol Vis Sci. 2011;52(6):3381–3390. doi: 10.1167/iovs.11-7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dartt DA, Hodges RR. Interaction of alpha1D-adrenergic and P2X(7) receptors in the rat lacrimal gland and the effect on intracellular [Ca2+] and protein secretion. Investig Ophthalmol Vis Sci. 2011;52:5720–5729. doi: 10.1167/iovs.11-7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ubels JL, Gipson IK, Spurr-Michaud SJ, Tisdale AS, Van Dyken RE, Hatton MP. Gene expression in human accessory lacrimal glands of Wolfring. Investig Ophthalmol Vis Sci. 2012;53(11):6738–6747. doi: 10.1167/iovs.12-10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Seifert P, Spitznas M, Koch F, Cusumano A. Light and electron microscopic morphology of accessory lacrimal glands. Adv Exp Med Biol. 1994;350:19–23. doi: 10.1007/978-1-4615-2417-5_3. [DOI] [PubMed] [Google Scholar]
- 277.Seifert P, Spitznas M. Immunocytochemical and ultrastructural evaluation of the distribution of nervous tissue and neuropeptides in the meibomian gland. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur Klin und Exp Ophthalmol. 1996;234(10):648–656. doi: 10.1007/BF00185300. [DOI] [PubMed] [Google Scholar]
- 278.Montagna William, Ellis Richard A. Cholinergic innervation of the Meibomian glands. Anat Rec. 1959;135(2):121–127. doi: 10.1002/ar.1091350207. [DOI] [PubMed] [Google Scholar]
- 279.Leeson TS. Tarsal (MEIGOMIAN) glands of the rat. Br J Ophthalmol. 1963;47:222–231. doi: 10.1136/bjo.47.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Uddman R, Alumets J, Ehinger B, Håkanson R, Lorén I, Sundler F. Vasoactive intestinal peptide nerves in ocular and orbital structures of the cat. Investig Ophthalmol Vis Sci. 1980;19(8):878–885. [PubMed] [Google Scholar]
- 281.Aisa J, Lahoz M, Serrano P, Pérez-Castejón MC, Junquera C, Martínez-Ciriano MC, et al. Acetylcholinesterase-positive and paraformaldehyde-induced-fluorescence-positive innervation in the upper eyelid of the sheep (Ovis aries) Histol Histopathol. 2001;16(2):487–496. doi: 10.14670/HH-16.487. [DOI] [PubMed] [Google Scholar]
- 282.Miraglia T, Gomes NF. The meibomian glands of the marmoset (Callithrix jacchus) Acta anat. 1969;74(1):104–113. [PubMed] [Google Scholar]
- 283.Perra MT, Serra A, Sirigu P, Turno F. Histochemical demonstration of acetylcholinesterase activity in human Meibomian glands. Eur J Histochem EJH. 1996;40(1):39–44. [PubMed] [Google Scholar]
- 284.Hartschuh W, Reinecke M, Weihe E, Yanaihara N. VIP-immunoreactivity in the skin of various mammals: immunohistochemical, radioimmunological and experimental evidence for a dual localization in cutaneous nerves and merkel cells. Peptides. 1984 Mar-Apr;5(2):239–245. doi: 10.1016/0196-9781(84)90213-4. [DOI] [PubMed] [Google Scholar]
- 285.Hartschuh W, Weihe E, Reinecke M. Peptidergic (neurotensin, VIP, substance P) nerve fibres in the skin. Immunohistochemical evidence of an involvement of neuropeptides in nociception, pruritus and inflammation. Br J Dermatol. 1983;109(Suppl 25):14–17. doi: 10.1111/j.1365-2133.1983.tb06811.x. [DOI] [PubMed] [Google Scholar]
- 286.Liu S, Li J, Tan DT, Beuerman RW. The eyelid margin: a transitional zone for 2 epithelial phenotypes. Arch Ophthalmol Chic Ill 1960. 2007;125(4):523–532. doi: 10.1001/archopht.125.4.523. [DOI] [PubMed] [Google Scholar]
- 287.Zhu HY, Riau AK, Barathi VA, Chew J, Beuerman RW. Expression of neural receptors in mouse meibomian gland. Cornea. 2010;29(7):794–801. doi: 10.1097/ICO.0b013e3181ca3262. [DOI] [PubMed] [Google Scholar]
- 288.Kam WR, Sullivan DA. Neurotransmitter influence on human meibomian gland epithelial cells. Investig Ophthalmol Vis Sci. 2011;52(12):8543–8548. doi: 10.1167/iovs.11-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chanthaphavong RS, Murphy SM, Anderson CR. Chemical coding of sympathetic neurons controlling the tarsal muscle of the rat. Aut Neurosci basic & Clin. 2003;105(2):77–89. doi: 10.1016/S1566-0702(03)00045-6. [DOI] [PubMed] [Google Scholar]
- 290.Candia OA, Shi XP, Alvarez LJ. Reduction in water permeability of the rabbit conjunctival epithelium by hypotonicity. Exp eye Res. 1998;66(5):615–624. doi: 10.1006/exer.1998.0462. [DOI] [PubMed] [Google Scholar]
- 291.Jumblatt MM, McKenzie RW, Jumblatt JE. MUC5AC mucin is a component of the human precorneal tear film. Investig Ophthalmol Vis Sci. 1999;40(1):43–49. [PubMed] [Google Scholar]
- 292.Ellingham RB, Berry M, Stevenson D, Corfield AP. Secreted human conjunctival mucus contains MUC5AC glycoforms. Glycobiology. 1999;9(11):1181–1189. doi: 10.1093/glycob/9.11.1181. [DOI] [PubMed] [Google Scholar]
- 293.Kessler TL, Mercer HJ, Zieske JD, McCarthy DM, Dartt DA. Stimulation of goblet cell mucous secretion by activation of nerves in rat conjunctiva. Curr eye Res. 1995;14(11):985–992. doi: 10.3109/02713689508998519. [DOI] [PubMed] [Google Scholar]
- 294.Diebold Y, Ríos JD, Hodges RR, Rawe I, Dartt DA. Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Investig Ophthalmol Vis Sci. 2001;42(10):2270–2282. [PubMed] [Google Scholar]
- 295.Ríos JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Investig Ophthalmol Vis Sci. 1999;40(6):1102–1111. [PubMed] [Google Scholar]
- 296.Li D, Jiao J, Shatos MA, Hodges RR, Dartt DA. Effect of VIP on intracellular [Ca2+], extracellular regulated kinase 1/2, and secretion in cultured rat conjunctival goblet cells. Investig Ophthalmol Vis Sci. 2013;54:2872–2884. doi: 10.1167/iovs.12-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Jumblatt JE, Jumblatt MM. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp eye Res. 1998;67(3):341–346. doi: 10.1006/exer.1998.0520. [DOI] [PubMed] [Google Scholar]
- 298.Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Investig Ophthalmol Vis Sci. 1995;36(9):1818–1827. [PubMed] [Google Scholar]
- 299.Tei M, Moccia R, Gipson IK. Developmental expression of mucin genes ASGP (rMuc4) and rMuc5ac by the rat ocular surface epithelium. Investig Ophthalmol Vis Sci. 1999;40(9):1944–1951. [PubMed] [Google Scholar]
- 300.Argüeso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investig Ophthalmol Vis Sci. 2003;44(6):2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 301.Spurr-Michaud S, Argüeso P, Gipson I. Assay of mucins in human tear fluid. Exp eye Res. 2007;84(5):939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pflugfelder SC, Liu Z, Monroy D, Li DQ, Carvajal ME, Price-Schiavi SA, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Investig Ophthalmol Vis Sci. 2000;41(6):1316–1326. [PubMed] [Google Scholar]
- 303.Shi XP, Candia OA. Active sodium and chloride transport across the isolated rabbit conjunctiva. Curr eye Res. 1995;14(10):927–935. doi: 10.3109/02713689508995132. [DOI] [PubMed] [Google Scholar]
- 304.Kompella UB, Kim KJ, Shiue MH, Lee VH. Cyclic AMP modulation of active ion transport in the pigmented rabbit conjunctiva. J Ocular Pharmacol Ther Off J Assoc Ocular Pharmacol Ther. 1996;12(3):281–287. doi: 10.1089/jop.1996.12.281. [DOI] [PubMed] [Google Scholar]
- 305.Marfurt CF, Jones MA, Thrasher K. Parasympathetic innervation of the rat cornea. Exp eye Res. 1998;66(4):437–448. doi: 10.1006/exer.1997.0445. [DOI] [PubMed] [Google Scholar]
- 306.Mertaniemi P, Ylätupa S, Partanen P, Tervo T. Increased release of immunoreactive calcitonin gene-related peptide (CGRP) in tears after excimer laser keratectomy. Exp eye Res. 1995;60(6):659–665. doi: 10.1016/s0014-4835(05)80007-7. [DOI] [PubMed] [Google Scholar]
- 307.Klyce SD, Beuerman RW, Crosson CE. Alteration of corneal epithelial ion transport by sympathectomy. Investig Ophthalmol Vis Sci. 1985;26(4):434–442. [PubMed] [Google Scholar]
- 308.Hiraoka M, Shimamura M. Neural mechanisms of the corneal blinking reflex in cats. Brain Res. 1977;125(2):265–275. doi: 10.1016/0006-8993(77)90620-5. [DOI] [PubMed] [Google Scholar]
- 309.Manning KA, Evinger C. Different forms of blinks and their two-stage control. Exp Brain Res. 1986;64(3):579–588. doi: 10.1007/BF00340495. [DOI] [PubMed] [Google Scholar]
- 310.Pellegrini JJ, Evinger C. The trigeminally evoked blink reflex. II. Mechanisms of paired-stimulus suppression. Exp Brain Res. 1995;107(2):181–196. doi: 10.1007/BF00230040. [DOI] [PubMed] [Google Scholar]
- 311.Ongerboer de Visser BW. The corneal reflex: electrophysiological and anatomical data in man. Prog Neurobiol. 1980;15(1):71–83. doi: 10.1016/0301-0082(80)90016-7. [DOI] [PubMed] [Google Scholar]
- 312.Henriquez VM, Evinger C. Modification of cornea-evoked reflex blinks in rats. Exp Brain Res. 2005;163(4):445–456. doi: 10.1007/s00221-004-2200-y. [DOI] [PubMed] [Google Scholar]
- 313.Evinger C. A brain stem reflex in the blink of an eye. News Physiol Sci. 1995;10:147–153. [Google Scholar]
- 314.Evinger C, Manning KA, Sibony PA. Eyelid movements. Mechanisms and normal data. Investig Ophthalmol Vis Sci. 1991;32(2):387–400. [PubMed] [Google Scholar]
- 315.Nakamori K, Odawara M, Nakajima T, Mizutani T, Tsubota K. Blinking is controlled primarily by ocular surface conditions. Am J Ophthalmol. 1997;124(1):24–30. doi: 10.1016/s0002-9394(14)71639-3. [DOI] [PubMed] [Google Scholar]
- 316.Acosta MC, Gallar J, Belmonte C. The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals. Exp eye Res. 1999;68(6):663–669. doi: 10.1006/exer.1998.0656. [DOI] [PubMed] [Google Scholar]
- 317.Wu Z, Begley CG, Situ P, Simpson T, Liu H. The effects of mild ocular surface stimulation and concentration on spontaneous blink parameters. Curr eye Res. 2014;39(1):9–20. doi: 10.3109/02713683.2013.822896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wu Z, Begley CG, Situ P, Simpson T. The effects of increasing ocular surface stimulation on blinking and sensation. Investig Ophthalmol Vis Sci. 2014;55(3):1555–1563. doi: 10.1167/iovs.13-13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Naase T, Doughty MJ, Button NF. An assessment of the pattern of spontaneous eyeblink activity under the influence of topical ocular anaesthesia. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur Klin und Exp Ophthalmol. 2005;243(4):306–312. doi: 10.1007/s00417-004-0990-z. [DOI] [PubMed] [Google Scholar]
- 320.Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur Klin und Exp Ophthalmol. 2004;242(4):306–312. doi: 10.1007/s00417-003-0845-z. [DOI] [PubMed] [Google Scholar]
- 321.Himebaugh NL, Begley CG, Bradley A, Wilkinson JA. Blinking and tear break-up during four visual tasks. Optom Vis Sci Off Publ Am Acad Optom. 2009;86(2):E106–E114. doi: 10.1097/OPX.0b013e318194e962. [DOI] [PubMed] [Google Scholar]
- 322.Rahman M, Okamoto K, Thompson R, Katagiri A, Bereiter DA. Sensitization of trigeminal brainstem pathways in a model for tear deficient dry eye. Pain. 2015;156(5):942–950. doi: 10.1097/j.pain.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.York M, Ong J, Robbins JC. Variation in blink rate associated with contact lens wear and task difficulty. Am J Optom Arch Am Acad Optom. 1971;48(6):461–467. doi: 10.1097/00006324-197106000-00001. [DOI] [PubMed] [Google Scholar]
- 324.Jansen ME, Begley CG, Himebaugh NH, Port NL. Effect of contact lens wear and a near task on tear film breakup. Optom Vis Sci Off Publ Am Acad Optom. 2010;87(5):350–357. doi: 10.1097/OPX.0b013e3181d951df. [DOI] [PubMed] [Google Scholar]
- 325.Kleinman JE, Karson CN, Weinberger DR, Freed WJ, Berman KF, Wyatt RJ. Eye-blinking and cerebral ventricular size in chronic schizophrenic patients. Am J psychiatry. 1984;141(11):1430–1432. doi: 10.1176/ajp.141.11.1430. [DOI] [PubMed] [Google Scholar]
- 326.Karson CN. Physiology of normal and abnormal blinking. Adv Neurol. 1988;49:25–37. [PubMed] [Google Scholar]
- 327.Mackert A, Flechtner KM, Woyth C, Frick K. Increased blink rates in schizophrenics. Influences of neuroleptics and psychopathology. Schizophr Res. 1991 Jan-Feb;4(1):41–47. doi: 10.1016/0920-9964(91)90008-f. [DOI] [PubMed] [Google Scholar]
- 328.Bentivoglio AR, Bressman SB, Cassetta E, Carretta D, Tonali P, Albanese A. Analysis of blink rate patterns in normal subjects. Mov Disord Off J Mov Disord Soc. 1997;12(6):1028–1034. doi: 10.1002/mds.870120629. [DOI] [PubMed] [Google Scholar]
- 329.Doughty MJ. Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optom Vis Sci Off Publ Am Acad Optom. 2001;78(10):712–725. doi: 10.1097/00006324-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 330.Hirokawa K, Yagi A, Miyata Y. Comparison of blinking behavior during listening to and speaking in Japanese and English. Percept Mot Ski. 2004;98(2):463–472. doi: 10.2466/pms.98.2.463-472. [DOI] [PubMed] [Google Scholar]
- 331.Patel S, Henderson R, Bradley L, Galloway B, Hunter L. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci Off Publ Am Acad Optom. 1991;68(11):888–892. doi: 10.1097/00006324-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 332.Cardona G, Garcia C, Seres C, Vilaseca M, Gispets J. Blink rate, blink amplitude, and tear film integrity during dynamic visual display terminal tasks. Curr Eye Res. 2011 doi: 10.3109/02713683.2010.544442. [DOI] [PubMed] [Google Scholar]
- 333.Kaminer J, Powers AS, Horn KG, Hui C, Evinger C. Characterizing the spontaneous blink generator: an animal model. J Neurosci Off J Soc Neurosci. 2011;31(31):11256–11267. doi: 10.1523/JNEUROSCI.6218-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Doughty MJ, Naase T. Further analysis of the human spontaneous eye blink rate by a cluster analysis-based approach to categorize individuals with ‘normal’ versus ‘frequent’ eye blink activity. Eye contact Lens. 2006;32(6):294–299. doi: 10.1097/01.icl.0000224359.32709.4d. [DOI] [PubMed] [Google Scholar]
- 335.Peshori KR, Schicatano EJ, Gopalaswamy R, Sahay E, Evinger C. Aging of the trigeminal blink system. Exp Brain Res. 2001;136(3):351–363. doi: 10.1007/s002210000585. [DOI] [PubMed] [Google Scholar]
- 336.Agostino R, Bologna M, Dinapoli L, Gregori B, Fabbrini G, Accornero N, et al. Voluntary, spontaneous, and reflex blinking in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2008;23(5):669–675. doi: 10.1002/mds.21887. [DOI] [PubMed] [Google Scholar]
- 337.Evinger C, Bao JB, Powers AS, Kassem IS, Schicatano EJ, Henriquez VM, et al. Dry eye, blinking, and blepharospasm. Mov Disord. 2002;17(Suppl 2):S75–S78. doi: 10.1002/mds.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137(2):337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 340.Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24(6):577–583. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- 341.Devor M. Neuropathic pain: pathophysiological response of nerves to injury. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Wall and Melzack’s Text Book of Pain. 6. Elsevier; Philadelhia, PA: 2013. pp. 861–888. [Google Scholar]
- 342.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198e1–205e1. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.VanDerMeid KR, Su SP, Ward KW, Zhang JZ. Correlation of tear inflammatory cytokines and matrix metalloproteinases with four dry eye diagnostic tests. Investig Ophthalmol Vis Sci. 2012;53(3):1512–1518. doi: 10.1167/iovs.11-7627. [DOI] [PubMed] [Google Scholar]
- 344.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci Off J Soc Neurosci. 2008;28(52):14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Schweizerhof M, Stösser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15(7):802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 346.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain. 2010;149(2):305–315. doi: 10.1016/j.pain.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 347.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699–1775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- 349.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol Chic Ill 1960) 2012;130(1):90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Gandhi NB, Su Z, Zhang X, Volpe EA, Pelegrino FS, Rahman SA, et al. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. J Leukoc Biol. 2013;94(6):1293–1301. doi: 10.1189/jlb.1012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, et al. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am J Ophthalmol. 2013;156(2):247–253. e1. doi: 10.1016/j.ajo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 352.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55(3):365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 353.Guenther S, Reeh PW, Kress M. Rises in [Ca2+]imediate capsaicin- and proton-induced heat sensitization of rat primary nociceptive neurons. Eur J Neurosci. 1999;11(9):3143–3150. doi: 10.1046/j.1460-9568.1999.00734.x. [DOI] [PubMed] [Google Scholar]
- 354.Huang J, Zhang X, McNaughton PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr Neuropharmacol. 2006;4(3):197–206. doi: 10.2174/157015906778019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chen WC, Davis RL. Voltage-gated and two-pore-domain potassium channels in murine spiral ganglion neurons. Hear Res. 2006;222(1–2):89–99. doi: 10.1016/j.heares.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 356.Tappe-Theodor A, Constantin CE, Tegeder I, Lechner SG, Langeslag M, Lepcynzsky P, et al. Gα(q/11) signaling tonically modulates nociceptor function and contributes to activity-dependent sensitization. Pain. 2012;153(1):184–196. doi: 10.1016/j.pain.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 357.Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci. 2014;17(2):164–174. doi: 10.1038/nn.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Sci (New York, NY) 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 359.Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, et al. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96(16):9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Mizumura K, Murase S. Role of nerve growth factor in pain. Handb Exp Pharmacol. 2015;227:57–77. doi: 10.1007/978-3-662-46450-2_4. [DOI] [PubMed] [Google Scholar]
- 361.Kusuda R, Cadetti F, Ravanelli MI, Sousa TA, Zanon S, De Lucca FL, et al. Differential expression of microRNAs in mouse pain models. Mol pain. 2011;7:17. doi: 10.1186/1744-8069-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Dong Y, Li P, Ni Y, Zhao J, Liu Z. Decreased microRNA-125a-3p contributes to upregulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PloS One. 2014;9:e111594. doi: 10.1371/journal.pone.0111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Belmonte C, Gallar J, López-Briones LG, Pozo MA. Polymodality in noci-ceptive neurons: experimental models of chemotransduction. In: Urban L, editor. Cellular Mechanisms of Sensory Processing. Springer-Verlag; London: 1994. pp. 87–117. [Google Scholar]
- 364.Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, et al. Direct inhibition of the cold-activated TRPM8 ion channel by Gαq. Nat Cell Biol. 2012;14(8):851–858. doi: 10.1038/ncb2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Guthoff Rudolf F, Wienss Holger, Hahnel Christian, Wree Andreas. Epithelial innervation of human cornea. Cornea. 2005;24(5):608–613. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- 366.Gallar J, Acosta MC, Gutiérrez AR, Belmonte C. Impulse activity in corneal sensory nerve fibers after photorefractive keratectomy. Investig Ophthalmol Vis Sci. 2007;48(9):4033–4037. doi: 10.1167/iovs.07-0012. [DOI] [PubMed] [Google Scholar]
- 367.Al-Aqaba Mouhamed Ali, Faraj Lana, Fares Usama, Otri Ahmad Muneer, Dua Harminder S. The morphologic characteristics of corneal nerves in advanced keratoconus as evaluated by acetylcholinesterase technique. Am J Ophthalmol. 2011;152(3):364–376. doi: 10.1016/j.ajo.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 368.Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helintö M, Tervo TM. Corneal innervation and morphology in primary Sjögren’s syndrome. Investig Ophthalmol Vis Sci. 2003;44(6):2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 369.Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Investig Ophthalmol Vis Sci. 2004;45(9):3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 370.Erdélyi B, Kraak R, Zhivov A, Guthoff R, Németh J. In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur Klin und Exp Ophthalmol. 2007;245(1):39–44. doi: 10.1007/s00417-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 371.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome. Exp eye Res. 2008;86(6):879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 372.Labbé A, Liang Q, Wang Z, Zhang Y, Xu L, Baudouin C, et al. Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Investig Ophthalmol Vis Sci. 2013;54(8):5144–5150. doi: 10.1167/iovs.13-12370. [DOI] [PubMed] [Google Scholar]
- 373.Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009;144(1–2):187–199. doi: 10.1016/j.pain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 374.Waxman SG, Merkies IS, Gerrits MM, Dib-Hajj SD, Lauria G, Cox JJ, et al. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. Lancet Neurol. 2014;13(11):1152–1160. doi: 10.1016/S1474-4422(14)70150-4. [DOI] [PubMed] [Google Scholar]
- 375.Habib AM, Wood JN, Cox JJ. Sodium channels and pain. Handb Exp Pharmacol. 2015;227:39–56. doi: 10.1007/978-3-662-46450-2_3. [DOI] [PubMed] [Google Scholar]
- 376.Tibbs GR, Posson DJ, Goldstein PA. Voltage-Gated Ion Channels in the PNS: Novel Therapies for Neuropathic Pain? Trends Pharmacol Sci. 2016;37(7):522–542. doi: 10.1016/j.tips.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 377.Kurose M, Meng ID. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. J Neurophysiol. 2013;110(2):495–504. doi: 10.1152/jn.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Meng ID, Barton ST, Mecum NE, Kurose M. Corneal sensitivity following lacrimal gland excision in the rat. Investig Ophthalmol Vis Sci. 2015;56(5):3347–3354. doi: 10.1167/iovs.15-16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol. 2013;74(5):630–636. doi: 10.1002/ana.24017. [DOI] [PubMed] [Google Scholar]
- 380.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci Off J Soc Neurosci. 2006;26(4):1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Xiao WH, Bennett GJ. Persistent low-frequency spontaneous discharge in A-fiber and C-fiber primary afferent neurons during an inflammatory pain condition. Anesthesiology. 2007;107(5):813–821. doi: 10.1097/01.anes.0000286983.33184.9c. [DOI] [PubMed] [Google Scholar]
- 382.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32(12):611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Meng ID, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp eye Res. 2013;117:79–87. doi: 10.1016/j.exer.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 385.Tashiro A, Okamoto K, Chang Z, Bereiter DA. Behavioral and neurophysiological correlates of nociception in an animal model of photokeratitis. Neuroscience. 2010;169(1):455–462. doi: 10.1016/j.neuroscience.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Hylden JL, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37(2):229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- 387.Bereiter DA, Bereiter DF. N-methyl-D-aspartate and non-N-methyl-D-as-partate receptor antagonism reduces Fos-like immunoreactivity in central trigeminal neurons after corneal stimulation in the rat. Neuroscience. 1996;73(1):249–258. doi: 10.1016/0306-4522(96)00038-3. [DOI] [PubMed] [Google Scholar]
- 388.Bereiter DA, Bereiter DF, Tonnessen BH, Maclean DB. Selective blockade of substance P or neurokinin A receptors reduces the expression of c-fos in trigeminal subnucleus caudalis after corneal stimulation in the rat. Neuroscience. 1998;83(2):525–534. doi: 10.1016/s0306-4522(97)00433-8. [DOI] [PubMed] [Google Scholar]
- 389.Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144(1–2):110–118. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Woolf CJ, Chong MS. Preemptive analgesia-treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77(2):362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 391.Koltzenburg Martin, Erik Torebjörk H, Wahren Lis Karin. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117(3):579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- 392.Meng ID, Bereiter DA. Differential distribution of Fos-like immunoreactivity in the spinal trigeminal nucleus after noxious and innocuous thermal and chemical stimulation of rat cornea. Neuroscience. 1996;72(1):243–254. doi: 10.1016/0306-4522(95)00541-2. [DOI] [PubMed] [Google Scholar]
- 393.Okamoto K, Bereiter DF, Tashiro A, Bereiter DA. Ocular surface-evoked Fos-like immunoreactivity is enhanced in trigeminal subnucleus caudalis by prior exposure to endotoxin. Neuroscience. 2009;159(2):787–794. doi: 10.1016/j.neuroscience.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 394.Ro LS, Chang KH. Neuropathic pain: mechanisms and treatments. Chang Gung Med J. 2005;28(9):597–605. [PubMed] [Google Scholar]
- 395.Oswald DJ, Lee A, Trinidad M, Chi C, Ren R, Rich CB, et al. Communication between corneal epithelial cells and trigeminal neurons is facilitated by purinergic (P2) and glutamatergic receptors. PloS One. 2012;7:e44574. doi: 10.1371/journal.pone.0044574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ren K, Dubner R. Neuronglia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21(5):570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain a J Neurol. 2015;138(Pt 3):604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain, Behav Immun. 2007;21(2):131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13(6):501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 401.Khasabov SG, Malecha P, Noack J, Tabakov J, Okamoto K, Bereiter DA, et al. Activation of rostral ventromedial medulla neurons by noxious stimulation of cutaneous and deep craniofacial tissues. J Neurophysiol. 2015;113(1):14–22. doi: 10.1152/jn.00125.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Barr MS, Farzan F, Davis KD, Fitzgerald PB, Daskalakis ZJ. Measuring GABAergic inhibitory activity with TMS-EEG and its potential clinical application for chronic pain. J Neuroimmun Pharmacol Off J Soc Neuroimmune Pharmacol. 2013;8(3):535–546. doi: 10.1007/s11481-012-9383-y. [DOI] [PubMed] [Google Scholar]
- 403.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 404.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocular Surf. 2009;7(1):28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 405.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J pain Off J Am Pain Soc. 2011;12(11 Suppl):T61–T74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Neziri AY, Curatolo M, Limacher A, Nüesch E, Radanov B, Andersen OK, et al. Ranking of parameters of pain hypersensitivity according to their discriminative ability in chronic low back pain. Pain. 2012;153(10):2083–2091. doi: 10.1016/j.pain.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 407.Granovsky Y. Conditioned pain modulation: a predictor for development and treatment of neuropathic pain. Curr pain headache Rep. 2013;17(9):361. doi: 10.1007/s11916-013-0361-8. [DOI] [PubMed] [Google Scholar]
- 408.Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye Lond Engl. 2015;29(3):301–312. doi: 10.1038/eye.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Galor A, Felix ER, Feuer W, Shalabi N, Martin ER, Margolis TP, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015;99(8):1126–1129. doi: 10.1136/bjophthalmol-2014-306481. [DOI] [PubMed] [Google Scholar]
- 410.Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye contact Lens. 2010;36(1):33–38. doi: 10.1097/ICL.0b013e3181c739ad. [DOI] [PubMed] [Google Scholar]
- 411.Williamson JF, Huynh K, Weaver MA, Davis RM. Perceptions of dry eye disease management in current clinical practice. Eye contact Lens. 2014;40(2):111–115. doi: 10.1097/ICL.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Varikooty J, Simpson TL. The interblink interval I: the relationship between sensation intensity and tear film disruption. Investig Ophthalmol Vis Sci. 2009;50(3):1087–1092. doi: 10.1167/iovs.08-1843. [DOI] [PubMed] [Google Scholar]
- 413.Begley CG, Himebaugh N, Renner D, Liu H, Chalmers R, Simpson T, et al. Tear breakup dynamics: a technique for quantifying tear film instability. Optom Vis Sci Off Publ Am Acad Optom. 2006;83(1):15–21. doi: 10.1097/01.opx.0000195569.36185.fd. [DOI] [PubMed] [Google Scholar]
- 414.Vehof J, Kozareva D, Hysi PG, Harris J, Nessa A, Williams FK, et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol. 2013;131(10):1304–1308. doi: 10.1001/jamaophthalmol.2013.4399. [DOI] [PubMed] [Google Scholar]
- 415.Merskey H, Bogduk N. Part III: Pain terms, a current list with definitions and notes on usage. Classif chronic pain. 1994;2:209–214. [Google Scholar]
- 416.Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocular Surf. 2009;7(4):199–211. doi: 10.1016/s1542-0124(12)70187-8. [DOI] [PubMed] [Google Scholar]
- 417.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 418.Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161–166. doi: 10.1111/aos.12012. [DOI] [PubMed] [Google Scholar]
- 419.Goyal S, Hamrah P. Understanding neuropathic corneal pain-gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31:59–70. doi: 10.3109/08820538.2015.1114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol Chic Ill 1960) 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 422.Begley CG, Chalmers RL, Mitchell GL, Nichols KK, Caffery B, Simpson T, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea. 2001;20(6):610–618. doi: 10.1097/00003226-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 423.Johnson ME, Murphy PJ. Measurement of ocular surface irritation on a linear interval scale with the ocular comfort index. Investig Ophthalmol Vis Sci. 2007;48(10):4451–4458. doi: 10.1167/iovs.06-1253. [DOI] [PubMed] [Google Scholar]
- 424.Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204–1210. doi: 10.1097/ICO.0b013e318294b0c0. [DOI] [PubMed] [Google Scholar]
- 425.Uchino M, Dogru M, Yagi Y, Goto E, Tomita M, Kon T, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci Off Publ Am Acad Optom. 2006;83(11):797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]
- 426.Abetz L, Rajagopalan K, Mertzanis P, Begley C, Barnes R, Chalmers R, et al. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual life outcomes. 2011;9:111. doi: 10.1186/1477-7525-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol Chic Ill 1960) 1998;116(11):1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 428.Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21(6):578–583. doi: 10.1097/00003226-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 429.Caudle LE, Williams KA, Pesudovs K. The Eye Sensation Scale: an ophthalmic pain severity measure. Optom Vis Sci Off Publ Am Acad Optom. 2007;84(8):752–762. doi: 10.1097/OPX.0b013e31812f7690. [DOI] [PubMed] [Google Scholar]
- 430.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology. 2016;123(7):1458–1468. doi: 10.1016/j.ophtha.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Vehof J, Sillevis Smitt-Kamminga N, Kozareva D, Nibourg SA, Hammond CJ. Clinical Characteristics of Dry Eye Patients With Chronic Pain Syndromes. Am J Ophthalmol. 2016;162:59–65. e2. doi: 10.1016/j.ajo.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 432.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 433.Cochet P, Bonnet R. L’esthesiometrie corneenne. Clin Ophthalmol. 1960;4 [Google Scholar]
- 434.Vega JA, Simpson TL, Fonn D. A noncontact pneumatic esthesiometer for measurement of ocular sensitivity: a preliminary report. Cornea. 1999;18(6):675–681. doi: 10.1097/00003226-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 435.Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Investig Ophthalmol Vis Sci. 1999;40(2):513–519. [PubMed] [Google Scholar]
- 436.Corneal sensitivity: measurement and clinical importance. Springer Science & Business Media; 2012. [Google Scholar]
- 437.Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocular Surf. 2014;12(1):32–45. doi: 10.1016/j.jtos.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 438.Golebiowski B, Papas E, Stapleton F. Corneal mechanical sensitivity measurement using a staircase technique. Ophthalmic & Physiol Opt J Br Coll Ophthalmic Opt Optom. 2005;25(3):246–253. doi: 10.1111/j.1475-1313.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 439.Millodot Michel. Psychophysical scaling of corneal sensitivity. Psychon Sci. 1968;12(8):401–402. [Google Scholar]
- 440.Feng Y, Simpson TL. Nociceptive sensation and sensitivity evoked from human cornea and conjunctiva stimulated by CO2. Investig Ophthalmol Vis Sci. 2003;44(2):529–532. doi: 10.1167/iovs.02-0003. [DOI] [PubMed] [Google Scholar]
- 441.Murphy Paul J, Patel Sudi, Marshall John. A new non-contact corneal aesthesiometer (NCCA) Ophthalmic Physiol Opt. 1996;16(2):101–107. [PubMed] [Google Scholar]
- 442.Papas EB, Keay L, Golebiowski B. Estimating a just-noticeable difference for ocular comfort in contact lens wearers. Investig Ophthalmol Vis Sci. 2011;52(7):4390–4394. doi: 10.1167/iovs.10-7051. [DOI] [PubMed] [Google Scholar]
- 443.Basuthkar Sundar Rao S, Simpson TL. Measurement of difference thresholds on the ocular surface. Investig Ophthalmol Vis Sci. 2014;55(2):1095–1100. doi: 10.1167/iovs.13-12874. [DOI] [PubMed] [Google Scholar]
- 444.Norn MS. Conjunctival sensitivity in normal eyes. Acta Ophthalmol. 1973;51(1):58–66. doi: 10.1111/j.1755-3768.1973.tb08246.x. [DOI] [PubMed] [Google Scholar]
- 445.McGowan DP, Lawrenson JG, Ruskell GL. Touch sensitivity of the eyelid margin and palpebral conjunctiva. Acta Ophthalmol. 1994;72(1):57–60. doi: 10.1111/j.1755-3768.1994.tb02738.x. [DOI] [PubMed] [Google Scholar]
- 446.Golebiowski B, Chim K, So J, Jalbert I. Lid margins: sensitivity, staining, meibomian gland dysfunction, and symptoms. Optom Vis Sci Off Publ Am Acad Optom. 2012;89(10):1443–1449. doi: 10.1097/OPX.0b013e3182693cef. [DOI] [PubMed] [Google Scholar]
- 447.Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996;15(3):235–239. doi: 10.1097/00003226-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 448.Versura P, Frigato M, Cellini M, Mulè R, Malavolta N, Campos EC. Diagnostic performance of tear function tests in Sjogren’s syndrome patients. Eye Lond Engl. 2007;21(2):229–237. doi: 10.1038/sj.eye.6702204. [DOI] [PubMed] [Google Scholar]
- 449.Barboza MN, Barboza GN, de Melo GM, Sato E, Dantas MC, Dantas PE, et al. Correlação entre sinais e sintomas de olho seco em pacientes portadores da síndrome de Sjögren. Arq Bras Oftalmol. 2008 Jul-Aug;71(4):547–552. doi: 10.1590/s0004-27492008000400015. [DOI] [PubMed] [Google Scholar]
- 450.Toker E, Asfuroğlu E. Corneal and conjunctival sensitivity in patients with dry eye: the effect of topical cyclosporine therapy. Cornea. 2010;29(2):133–140. doi: 10.1097/ICO.0b013e3181acf68d. [DOI] [PubMed] [Google Scholar]
- 451.Kim IG, Lee JH, Kim SS. Reduced corneal sensitivity in patients with rheumatoid arthritis. Cornea. 2012;31(12):1381–1385. doi: 10.1097/ICO.0b013e31824d0e22. [DOI] [PubMed] [Google Scholar]
- 452.Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Investig Ophthalmol Vis Sci. 2012;53(8):4926–4931. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 453.Nepp J, Wirth M. Fluctuations of Corneal Sensitivity in Dry Eye Syndromes-A Longitudinal Pilot Study. Cornea. 2015;34(10):1221–1226. doi: 10.1097/ICO.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 454.Rahman EZ, Lam PK, Chu CK, Moore Q, Pflugfelder SC. Corneal Sensitivity in Tear Dysfunction and its Correlation With Clinical Parameters and Blink Rate. Am J Ophthalmol. 2015;160(5):858–866. e5. doi: 10.1016/j.ajo.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Adatia FA, Michaeli-Cohen A, Naor J, Caffery B, Bookman A, Slomovic A. Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjögren’s syndrome. Can J Ophthalmol J Can d’ophtalmologie. 2004;39(7):767–771. doi: 10.1016/s0008-4182(04)80071-1. [DOI] [PubMed] [Google Scholar]
- 456.Situ P, Simpson TL, Fonn D, Jones LW. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Investig Ophthalmol Vis Sci. 2008;49(7):2971–2976. doi: 10.1167/iovs.08-1734. [DOI] [PubMed] [Google Scholar]
- 457.De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137(1):109–115. doi: 10.1016/s0002-9394(03)00897-3. [DOI] [PubMed] [Google Scholar]
- 458.Spierer O, Felix ER, McClellan AL, Parel JM, Gonzalez A, Feuer WJ, et al. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Investig Ophthalmol Vis Sci. 2016;57(2):617–625. doi: 10.1167/iovs.15-18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Bourcier T, Acosta MC, Borderie V, Borrás F, Gallar J, Bury T, et al. Decreased corneal sensitivity in patients with dry eye. Investig Ophthalmol Vis Sci. 2005;46(7):2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 460.Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Investig Ophthalmol Vis Sci. 2007;48(1):173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 461.Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: Implications of use of a non-contact air jet aesthesiometer versus the Cochet–Bonnet aesthesiometer. Exp Eye Res. 2011;92(5):408–413. doi: 10.1016/j.exer.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 462.Kaido M, Kawashima M, Ishida R, Tsubota K. Relationship of Corneal Pain Sensitivity With Dry Eye Symptoms in Dry Eye With Short Tear Break-Up Time. Investig Ophthalmol Vis Sci. 2016;57(3):914–919. doi: 10.1167/iovs.15-18447. [DOI] [PubMed] [Google Scholar]
- 463.Bron AJ, Yokoi N, Gafney E, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocular Surf. 2009;7(2):78–92. doi: 10.1016/s1542-0124(12)70299-9. [DOI] [PubMed] [Google Scholar]
- 464.Sullivan B. Challenges in using signs and symptoms to evaluate new biomarkers of dry eye disease. Ocular Surf. 2014;12(1):2–9. doi: 10.1016/j.jtos.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 465.Chao C, Stapleton F, Badarudin E, Golebiowski B. Ocular surface sensitivity repeatability with Cochet-Bonnet esthesiometer. Optom Vis Sci Off Publ Am Acad Optom. 2015;92(2):183–189. doi: 10.1097/OPX.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 466.Bourcier T, Acosta MC, Borderie V, Borrás F, Gallar J, Bury T, et al. Decreased corneal sensitivity in patients with dry eye. Investig Ophthalmol Vis Sci. 2005;46(7):2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 467.Geber C, Baumgärtner U, Schwab R, Müller H, Stoeter P, Dieterich M, et al. Revised definition of neuropathic pain and its grading system: an open case series illustrating its use in clinical practice. Am J Med. 2009;122(10 Suppl):S3–S12. doi: 10.1016/j.amjmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 468.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocular Surf. 2012;10(1):2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 469.Carney LG, Hill RM. The nature of normal blinking patterns. Acta Ophthalmol. 1982;60(3):427–433. doi: 10.1111/j.1755-3768.1982.tb03034.x. [DOI] [PubMed] [Google Scholar]
- 470.The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf. 2007;2007(5):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 471.Ousler GW, 3rd, Hagberg KW, Schindelar M, Welch D, Abelson MB. The Ocular Protection Index. Cornea. 2008;27(5):509–513. doi: 10.1097/ICO.0b013e31816583f6. [DOI] [PubMed] [Google Scholar]
- 472.Orchard LN, Stern JA. Blinks as an index of cognitive activity during reading. Integr Physiol Behav Sci Off J Pavlov Soc. 1991 Apr-Jun;26(2):108–116. doi: 10.1007/BF02691032. [DOI] [PubMed] [Google Scholar]
- 473.Stave J, Zinser G, Grummer G, Guthoff R. Modified Heidelberg Retinal Tomograph HRT. Initial results of in vivo presentation of corneal structures. Ophthalmologe. 2002;99:276–280. doi: 10.1007/s003470100535. [DOI] [PubMed] [Google Scholar]
- 474.Kheirkhah A, Dohlman TH, Amparo F, Arnoldner MA, Jamali A, Hamrah P, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122(4):662–668. doi: 10.1016/j.ophtha.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Aggarwal S, Colon CM, Kheirkhah A, Hamrah P. Efficacy of Autologous Serum Tears for Treatment of Severe Corneal Pain in Patients with Corneal Neuropathy: An In Vivo Confocal Microscopy Study. Investig Ophthalmol Vis Sci. 2014;55:1468. [Google Scholar]
- 476.Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocular Surf. 2015;13(3):250–262. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012 Sep-Nov;27(5–6):138–148. doi: 10.3109/08820538.2012.711416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Villani E, Baudouin C, Efron N, Hamrah P, Kojima T, Patel SV, et al. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr eye Res. 2014;39(3):213–231. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Villani E, Galimberti D, Viola F, Mapelli C, Del Papa N, Ratiglia R. Corneal involvement in rheumatoid arthritis: an in vivo confocal study. Investig Ophthalmol Vis Sci. 2008;49(2):560–564. doi: 10.1167/iovs.07-0893. [DOI] [PubMed] [Google Scholar]
- 480.Villani Edoardo, Magnani Fabrizio, Viola Francesco, Santaniello Alessandro, Scorza Raffaella, Nucci Paolo, et al. In Vivo Confocal Evaluation of the Ocular Surface Morpho-Functional Unit in Dry Eye. Optom Vis Sci. 2013;90(6):576–586. doi: 10.1097/OPX.0b013e318294c184. [DOI] [PubMed] [Google Scholar]
- 481.Hosal BM, Ornek N, Zilelioglu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye. 2005;19:1276–1279. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 482.Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011;152(1):140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137(1):109–115. doi: 10.1016/s0002-9394(03)00897-3. [DOI] [PubMed] [Google Scholar]
- 484.Zhang M, Chen J, Luo L, Xiao Q, Sun M, Liu Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005;24(7):818–824. doi: 10.1097/01.ico.0000154402.01710.95. [DOI] [PubMed] [Google Scholar]
- 485.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren’s syndrome: an in vivo confocal study. Investig Ophthalmol Vis Sci. 2007;48(5):2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 486.Theophanous C, Jacobs DS, Hamrah P. Corneal Neuralgia after LASIK. Optom Vis Sci Off Publ Am Acad Optom. 2015;92(9):e233–e240. doi: 10.1097/OPX.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 487.Begley CG, Chalmers RL, Abetz L, Venkataraman K, Mertzanis P, Caffery BA, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Investig Ophthalmol Vis Sci. 2003;44(11):4753–4761. doi: 10.1167/iovs.03-0270. [DOI] [PubMed] [Google Scholar]
- 488.Cuevas M, González-García MJ, Castellanos E, Quispaya R, de Parra PL, Fernández I, et al. Correlations among symptoms, signs, and clinical tests in evaporative-type dry eye disease caused by Meibomian gland dysfunction (MGD) Curr eye Res. 2012;37(10):855–863. doi: 10.3109/02713683.2012.683508. [DOI] [PubMed] [Google Scholar]
- 489.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest Ophthalmol Vis Sci. 2004;45:722–727. 1. doi: 10.1167/iovs.03-0803. [DOI] [PubMed] [Google Scholar]
- 490.Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 491.Schaumburg CS, Siemasko KF, De Paiva CS, Wheeler LA, Niederkorn JY, Pflugfelder SC, et al. Ocular Surface APCs Are Necessary for Autoreactive T Cell-Mediated Experimental Autoimmune Lacrimal Kerato-conjunctivitis. J Immunol. 2011;187(7):3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 492.Pflugfelder SC, Stern ME. Mucosal environmental sensors in the pathogenesis of dry eye. Expert Rev Clin Immunol. 2014;10(9):1137–1140. doi: 10.1586/1744666X.2014.944163. [DOI] [PubMed] [Google Scholar]
- 493.Mayer WJ, Mackert MJ, Kranebitter N, Messmer EM, Grüterich M, Kampik A, et al. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr eye Res. 2012;37(11):1012–1018. doi: 10.3109/02713683.2012.696172. [DOI] [PubMed] [Google Scholar]
- 494.Knickelbein JE, Buela KA, Hendricks RL. Antigen-presenting cells are stratified within normal human corneas and are rapidly mobilized during ex vivo viral infection. Investig Ophthalmol Vis Sci. 2014;55(2):1118–1123. doi: 10.1167/iovs.13-13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Lin H, Li W, Dong N, Chen W, Liu J, Chen L, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Investig Ophthalmol Vis Sci. 2010;51(1):122–128. doi: 10.1167/iovs.09-3629. [DOI] [PubMed] [Google Scholar]
- 496.Marsovszky L, Resch MD, Németh J, Toldi G, Medgyesi E, Kovács L, et al. In vivo confocal microscopic evaluation of corneal Langerhans cell density, and distribution and evaluation of dry eye in rheumatoid arthritis. Innate Immun. 2013;19(4):348–354. doi: 10.1177/1753425912461677. [DOI] [PubMed] [Google Scholar]
- 497.Marsovszky L, Németh J, Resch MD, Toldi G, Legány N, Kovács L, et al. Corneal Langerhans cell and dry eye examinations in ankylosing spondylitis. Innate Immun. 2014;20(5):471–477. doi: 10.1177/1753425913498912. [DOI] [PubMed] [Google Scholar]
- 498.Marsovszky L, Resch MD, Visontai Z, Németh J. Confocal microscopy of epithelial and langerhans cells of the cornea in patients using travoprost drops containing two different preservatives. Pathol Oncol Res POR. 2014;20(3):741–746. doi: 10.1007/s12253-014-9755-0. [DOI] [PubMed] [Google Scholar]
- 499.Villani E, Garoli E, Termine V, Pichi F, Ratiglia R, Nucci P. Corneal Confocal Microscopy in Dry Eye Treated with Corticosteroids. Optom Vis Sci Off Publ Am Acad Optom. 2015;92(9):e290–e295. doi: 10.1097/OPX.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 500.Kheirkhah A, Rahimi Darabad R, Cruzat A, Hajrasouliha AR, Witkin D, Wong N, et al. Corneal Epithelial Immune Dendritic Cell Alterations in Sub types of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Investig Ophthalmol Vis Sci. 2015;56(12):7179–7185. doi: 10.1167/iovs.15-17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Qazi Y, Kheirkhah A, Blackie C, Cruzat A, Trinidad M, Williams C, et al. In vivo detection of clinically non-apparent ocular surface inflammation in patients with meibomian gland dysfunction-associated refractory dry eye symptoms: a pilot study. Eye Lond Engl. 2015;29(8):1099–1110. doi: 10.1038/eye.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Sornelli F, Lambiase A, Mantelli F, Aloe L. NGF and NGF-receptor expression of cultured immortalized human corneal endothelial cells. Mol Vis. 2010;16:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 503.He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostagl Leukot Essent Fat acids. 2010 Apr-Jun;82(4–6):319–325. doi: 10.1016/j.plefa.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Wu Ying, Chu Renyuan, Zhou Xingtao, Dai Jinhui, Qu Xiaomei. Determination of the Nerve Growth Factor Level in the Central Cornea After LASIK and Epi-LASIK Treatment in a Rabbit Model System. Cornea. 2009;28(10):1144–1148. doi: 10.1097/ICO.0b013e3181a2a7e3. [DOI] [PubMed] [Google Scholar]
- 505.Lee HK, Lee KS, Kim HC, Lee SH, Kim EK. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am J Ophthalmol. 2005;139(6):965–971. doi: 10.1016/j.ajo.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 506.Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol Chic Ill 1960) 2011;129(8):981–986. doi: 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- 507.Liu Q, McDermott AM, Miller WL. Elevated nerve growth factor in dry eye associated with established contact lens wear. Eye contact Lens. 2009;35(5):232–237. doi: 10.1097/ICL.0b013e3181b3e87f. [DOI] [PubMed] [Google Scholar]
- 508.Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp eye Res. 1998;66(4):421–435. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- 509.De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392(6674):394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 510.Mikulec AA, Tanelian DL. CGRP increases the rate of corneal re-epithelialization in an in vitro whole mount preparation. J Ocular Pharmacol Ther Off J Assoc Ocular Pharmacol Ther. 1996;12(4):417–423. doi: 10.1089/jop.1996.12.417. [DOI] [PubMed] [Google Scholar]
- 511.Marfurt CF, Murphy CJ, Florczak JL. Morphology and neurochemistry of canine corneal innervation. Investig Ophthalmol Vis Sci. 2001;42(10):2242–2251. [PubMed] [Google Scholar]
- 512.Yamada M, Ogata M, Kawai M, Mashima Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am J Ophthalmol. 2000;129(5):671–672. doi: 10.1016/s0002-9394(00)00415-3. [DOI] [PubMed] [Google Scholar]
- 513.Yamada M, Ogata M, Kawai M, Mashima Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am J Ophthalmol. 2000;129(5):671–672. doi: 10.1016/s0002-9394(00)00415-3. [DOI] [PubMed] [Google Scholar]
- 514.Yamada M, Ogata M, Kawai M, Mashima Y, Nishida T. Substance P and its metabolites in normal human tears. Investig Ophthalmol Vis Sci. 2002;43(8):2622–2625. [PubMed] [Google Scholar]
- 515.Macia L, Rao PT, Wheway J, Sierro F, Mackay F, Herzog H. Y1 signalling has a critical role in allergic airway inflammation. Immunol Cell Biol. 2011;89(8):882–888. doi: 10.1038/icb.2011.6. [DOI] [PubMed] [Google Scholar]
- 516.Chao Cecilia, Stapleton Fiona, Zhou Xiangtian, Chen Shihao, Zhou Shi, Golebiowski Blanka. Structural and functional changes in corneal innervation after laser in situ keratomileusis and their relationship with dry eye. Graefe’s Arch Clin Exp Ophthalmol. 2015;253(11):2029–2039. doi: 10.1007/s00417-015-3120-1. [DOI] [PubMed] [Google Scholar]
- 517.Mertaniemi P, Ylätupa S, Partanen P, Tervo T. Increased release of immunoreactive calcitonin gene-related peptide (CGRP) in tears after excimer laser keratectomy. Exp Eye Res. 1995;60(6):659–665. doi: 10.1016/s0014-4835(05)80007-7. [DOI] [PubMed] [Google Scholar]
- 518.Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol Chic Ill 1960) 2011;129(8):981–986. doi: 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- 519.Tervo T, Vesaluoma M, Bennett GL, Schwall R, Helena M, Liang Q, et al. Tear hepatocyte growth factor (HGF) availability increases markedly after excimer laser surface ablation. Exp Eye Res. 1997;64(4):501–504. doi: 10.1006/exer.1996.0226. [DOI] [PubMed] [Google Scholar]
- 520.Vesaluoma M, Teppo AM, Grönhagen-Riska C, Tervo T. Increased release of tumour necrosis factor-alpha in human tear fluid after excimer laser induced corneal wound. Br J Ophthalmol. 1997;81(2):145–149. doi: 10.1136/bjo.81.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Vesaluoma M, Teppo AM, Grönhagen-Riska C, Tervo T. Release of TGF-beta 1 and VEGF in tears following photorefractive keratectomy. Curr eye Res. 1997;16(1):19–25. doi: 10.1076/ceyr.16.1.19.5119. [DOI] [PubMed] [Google Scholar]
- 522.Yu CQ, Zhang M, Matis KI, Kim C, Rosenblatt MI. Vascular endothelial growth factor mediates corneal nerve repair. Investig Ophthalmol Vis Sci. 2008;49(9):3870–3878. doi: 10.1167/iovs.07-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Nichols KK, Nichols JJ, Zadnik K. Frequency of dry eye diagnostic test procedures used in various modes of ophthalmic practice. Cornea. 2000;19(4):477–482. doi: 10.1097/00003226-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 524.Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158:261–272. doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Onysko M, Legerski P, Potthoff J, Erlandson M. Targeting neuropathic pain: consider these alternatives. J Fam Pract. 2015;64(8):470–475. [PubMed] [Google Scholar]
- 526.Hsu DZ, Chu PY, Jou IM. Daily sesame oil supplement attenuates joint pain by inhibiting muscular oxidative stress in osteoarthritis rat model. J Nutr Biochem. 2016;29:36–40. doi: 10.1016/j.jnutbio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 527.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22(5):467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 528.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 529.Levitt AE, Galor A, Weiss JS, Felix ER, Martin ER, Patin DJ, et al. Chronic dry eye symptoms after LASIK: parallels and lessons to be learned from other persistent post-operative pain disorders. Mol Pain. 2015;11:21. doi: 10.1186/s12990-015-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209. doi: 10.1002/14651858.CD011209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Acosta MC, Berenguer-Ruiz L, García-Gálvez A, Perea-Tortosa D, Gallar J, Belmonte C. Changes in mechanical, chemical, and thermal sensitivity of the cornea after topical application of nonsteroidal anti-inflammatory drugs. Investig Ophthalmol Vis Sci. 2005;46(1):282–286. doi: 10.1167/iovs.04-0884. [DOI] [PubMed] [Google Scholar]
- 532.Ortiz MI, Castañeda-Hernández G, Izquierdo-Vega JA, Sánchez-Gutiérrez M, Ponce-Monter HA, Granados-Soto V. Role of ATP-sensitive K+ channels in the antinociception induced by non-steroidal anti-inflammatory drugs in streptozotocin-diabetic and non-diabetic rats. Pharmacol Biochem Behav. 2012;102(1):163–169. doi: 10.1016/j.pbb.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 533.Huang CW, Hung TY, Liao YK, Hsu MC, Wu SN. Underlying mechanism of regulatory actions of diclofenac, a nonsteroidal anti-inflammatory agent, on neuronal potassium channels and firing: an experimental and theoretical study. J Physiol Pharmacol Off J Pol Physiol Soc. 2013;64(3):269–280. [PubMed] [Google Scholar]
- 534.Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;27:CD007938. doi: 10.1002/14651858.CD007938.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Attal N, Bouhassira D. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms? Pain. 2015;156(Suppl 1):S104–S114. doi: 10.1097/01.j.pain.0000460358.01998.15. [DOI] [PubMed] [Google Scholar]
- 536.Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90(4):532–545. doi: 10.1016/j.mayocp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 537.Wang TX, Yin D, Guo W, Liu YY, Li YD, Qu WM, et al. Antinociceptive and hypnotic activities of pregabalin in a neuropathic pain-like model in mice. Pharmacol Biochem Behav. 2015;135:31–39. doi: 10.1016/j.pbb.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 538.Lichtinger Alejandro, Purcell Tracy L, Schanzlin David J, Chayet Arturo S. Gabapentin for Postoperative Pain After Photorefractive Keratectomy: A Prospective, Randomized, Double-blind, Placebo-controlled Trial. J Refract Surg. 2011;27(8):613–617. doi: 10.3928/1081597X-20110210-01. [DOI] [PubMed] [Google Scholar]
- 539.Pakravan M, Roshani M, Yazdani S, Faramazi A, Yaseri M. Pregabalin and gabapentin for post-photorefractive keratectomy pain: a randomized controlled trial. Eur J Ophthalmol. 2012;22(Suppl 7):S106–S113. doi: 10.5301/ejo.5000143. [DOI] [PubMed] [Google Scholar]
- 540.Faktorovich EG, Melwani K. Efficacy and safety of pain relief medications after photorefractive keratectomy: review of prospective randomized trials. J Cataract Refract Surg. 2014;40(10):1716–1730. doi: 10.1016/j.jcrs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 541.Knotkova H, Cruciani RA. Non-invasive transcranial direct current stimulation for the study and treatment of neuropathic pain. Methods Mol Biol Clift NJ) 2010;617:505–515. doi: 10.1007/978-1-60327-323-7_37. [DOI] [PubMed] [Google Scholar]
- 542.Castelnuovo G, Giusti EM, Manzoni GM, Saviola D, Gatti A, Gabrielli S, et al. Psychological Treatments and Psychotherapies in the Neurorehabilitation of Pain: Evidences and Recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Front Psychol. 2016;7:115. doi: 10.3389/fpsyg.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Majithia N, Smith TJ, Coyne PJ, Abdi S, Pachman DR, Lachance D, et al. Scrambler Therapy for the management of chronic pain, Support care cancer Off. J Multinatl Assoc Support Care Cancer. 2016;24(6):2807–2814. doi: 10.1007/s00520-016-3177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]