Abstract
Prenatal exposure to valproic acid (VPA) alters rodent social interactions in a dose-dependent way: exposure to a high dose of VPA (>500 mg/kg) mid-gestation decreases social interactions whereas a moderate dose of VPA (350 mg/kg) increases peer-directed social behavior. The moderate dose also decreases expression of the mRNA for serine in amygdala and orbitofrontal cortex. In this study, we examined whether D-cycloserine could ameliorate VPA-induced alterations in ultrasonic vocalizations (USVs), social interactions, and locomotor activity. Pregnant Sprague Dawley rats were given intraperintoneal injections of VPA (200 mg/kg each) on gestational days 12, 12.5 and 13; controls were injected with saline. Offspring received a subcutaneous injection of saline or D-cycloserine (32 or 64 mg/kg) either acutely (one hour prior to testing) or repeatedly (once per day for four days). Social interactions were assessed during late adolescence, and USVs were recorded concomitantly. Male and female rats that were exposed to VPA demonstrated more locomotor activity than control animals during habituation to the testing chamber. VPA-exposed males showed increased play fighting. D-cycloserine normalized the VPA-induced increase in play fighting in males and also increased social motivation in females. When the pair contained a VPA-exposed rat, significantly fewer USVs were emitted and 16% of the vocalizations were of a novel waveform. These effects were not seen in pairs containing VPA-exposed animals that were treated with D-cycloserine. Overall, these findings are consistent with data from other laboratories suggesting that D-cycloserine may be a promising pharmacotherapeutic compound for improving social behavior disorders.
Keywords: adolescence, autism, prenatal, rat, social behavior, ultrasonic vocalization
1. INTRODUCTION
Prenatal exposure to valproic acid (VPA) alters rodent social behavior and brain structure in a dose-dependent manner. Exposure to high doses of VPA (500 – 800 mg/kg) decreases social interaction (e.g., Schneider, 2008; Schneider and Przewlocki, 2005; Markram et al., 2008; Dufour-Rainfray et al., 2010). In contrast, a single exposure to 350 mg/kg during mid-gestation in the rat accentuates social behavior and alters gene expression (Cohen et al., 2013). Of note, it decreases expression of the mRNA for serine in the amygdala and orbitofrontal cortex.
Serine plays a critical role in the brain. It binds to the co-agonist, or “glycine” site on the N-methyl-D-aspartate (NMDA) receptor. Binding of a co-agonist plus glutamate is necessary for NMDA receptor activation (Johnson and Ascher, 1987), and receptor characteristics such as affinity for glutamate, internalization, and desensitization can be altered by the co-agonist (e.g., Wolosker et al., 2008). Initially thought to originate in astrocytes, it is now known that D-serine can also be produced by neurons (Wolosker and Radzishevsky, 2013).
Disruption at glutamatergic synapses can contribute to abnormal social behavior (e.g., Kumar and Christian, 2009). Reduced social behavior is reported in mice that either have less binding at the glycine site on the NMDA receptor (Labrie et al., 2009) or have decreased expression of the NR1 subunit of the NMDA receptor (Halene et al., 2009). The SHANK2 knockout mouse also has alterations in receptor type, spine number, synaptic transmission, and behavior (Schmeisser et al., 2012; Won et al., 2012). Behaviorally, SHANK2 knockouts demonstrate a repetitive behavior, decreased social interactions, increased locomotor activity, and decreased USV production. Treatment with D-cycloserine (DCS), a partial agonist of glutamate and glycine sites of glutamatergic receptors (Sheinin et al., 2001), can normalize both the aberrant function of the NMDA receptors and the social interaction phenotype seen in the SHANK2 knockout animals (Won et al., 2012). DCS has also shown promise for ameliorating social behavior deficits in murine models of autism (e.g., Benson et al., 2013; Jacome et al., 2011; Burket et al., 2013; Deutsch et al., 2011; Deutsch et al., 2012).
Given that prenatal exposure to VPA alters social behavior and disrupts glutamatergic balance in the brain, we hypothesized that DCS may ameliorate the VPA-induced behavioral deficits. To test this, we examined social behavior and USVs in rats that were exposed prenatally to a moderate dose of VPA, and tested whether DCS can ameliorate alterations associated with prenatal VPA exposure.
2. MATERIALS AND METHODS
2.1.1 Animals: Prenatal exposure
Timed pregnant Sprague Dawley rats were received on gestational day (G) 6 (Harlan, Fredrick, MD, USA). The first day on which a sperm-positive plug was identified was designated G1. Rats were housed at the University of Maryland School of Medicine in an AAALAC accredited facility. Rooms were temperature-controlled (22°C) and maintained on a 12-hr light (07:00 to 19:00)/12-hr dark cycle. All procedures were performed with approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland, Baltimore and were in accordance with the guidelines for animal care established by the National Institutes of Health.
Pregnant dams were given three intraperitoneal (i.p.) injections (G12, G12.5, and G13) of 200 mg/kg VPA each (25% w/v sodium valproate (152064; MP Biomedicals, Santa Ana CA) in physiological saline; pH 7.5). Control females received i.p. injections of an equivalent volume of physiological saline. Litters were culled to ten pups within 24 hrs of birth on postnatal day (P) 0 and left with their dam. As best as possible, the ratio of male and female pups was maintained as 6:4.
2.1.2 Animals: Postnatal drug exposure
On P21, pups were weaned, and male and female offspring were separated and group housed with same-sex littermates (2–3 rats/cage). Prior to social behavior testing (described below), offspring were injected subcutaneously in the nape with DCS or saline. DCS was given at two doses to males; 32 mg/kg or 64 mg/kg, or at 32 mg/kg to females. The doses chosen were based on published data demonstrating that a dose of 32 mg/kg DCS ameliorated social behavior deficits in 4-week-old mice (Deutsch et al., 2011; Jacome et al., 2011). Subjects were either injected once 1 hr prior to testing (single injection) or once per day for four days (multiple injections) prior to testing. To ensure experimental consistency, animals that received multiple injections were administered their final injection 1 hr prior to testing. Only one male and one female animal from each litter was assigned to any given prenatal/postnatal exposure group (n = 8–12 per group).
2.2 Ultrasonic vocalizations
USVs for pairs of animals (one experimental subject and one non-manipulated social partner) were recorded during a modified social interaction test (see below) using an ultrasound microphone (Condenser Microphone 116H; Avisoft Bioacoustics, Berlin, Germany) placed above the testing box. The sampling rate was 187.5 kHz, and data were recorded in a 16-bit format. Data acquisition (Avisoft-RECORDER Version 4.5; Avisoft Bioacoustics) and assessment software (Avisoft SAS Lab Pro; Avisoft Bioacoustics) were used to assess latency to begin vocalizing, as well as the number of 50 kHz (hedonic calls) and 22 kHz (alarm calls) USVs (for review see Portfors, 2007).
Fifty kHz calls were further assessed by categorizing their waveforms as described elsewhere (Wohr et al., 2008). Waveforms were categorized as: simple (a short flat waveform in which the frequency remains constant; audible playback of such USVs sound like short clear notes), frequency modulated (FM call, a wave form in which the frequency modulates either up or down in frequency, or both; during audible playback FM USVs often sound like birdsong or keyboard trills), harmonic (a waveform in which the frequency changes in a step-like or harmonized fashion; rendered audible these waveforms sound like harmonized notes), and a novel “atypical” waveform (in which the waveform is modulated in frequency characteristic of FM or harmonic waveforms but lacks the distinct form/appearance of typical of either FM or harmonic waveforms. Audible playback of these atypical waveforms reveal them to differ from harmonized notes and trilling song characteristic of harmonic and FM USVs, instead atypical waveform sounds are raspy and discordant). Representative sonograms are shown in Figure 2. The proportion (%) of each waveform was calculated.
Figure 2. 50 kHz Ultrasonic Vocalization Waveforms.
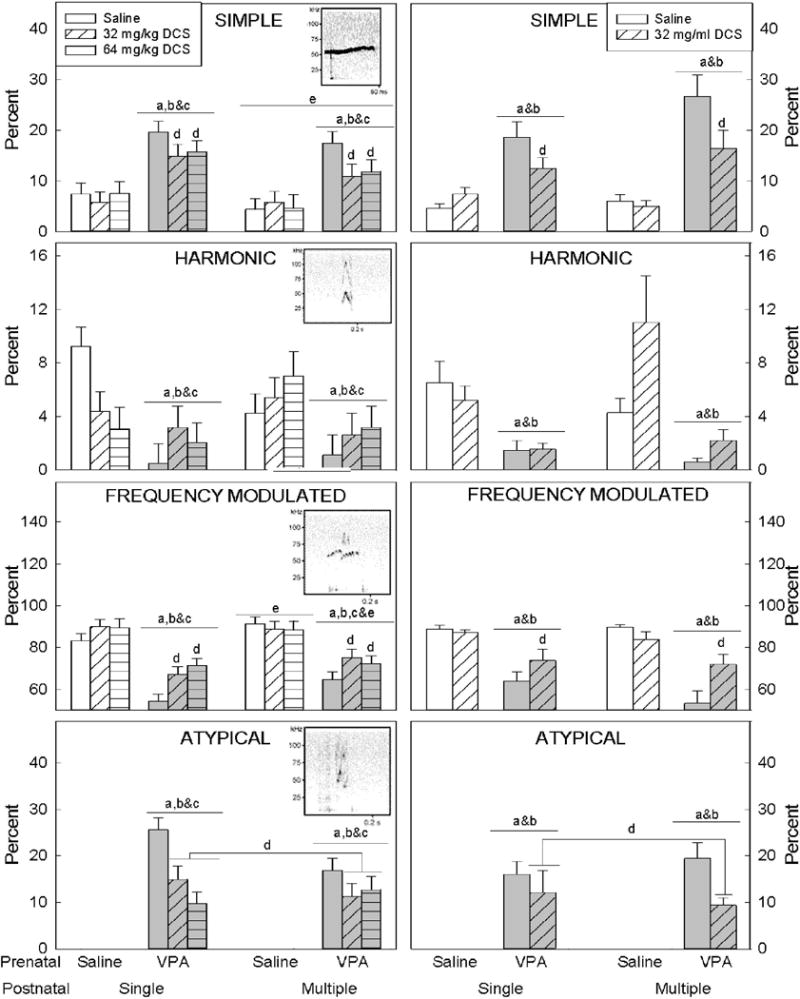
The percentage of simple, harmonic, frequency modulated and atypical USV waveforms in a testing session are presented for males (left) and females (right). A thumbnail of an exemplar wave form is depicted in the upper right quadrant of each of the male graphs.
Multiple injections, regardless of treatment, increased the occurrence of simple and frequency modulated waveforms emitted by male subjects. In both males and females, VPA exposure largely increased the representation of simple and atypical USV waveforms while decreasing the utterances of harmonic and frequency modulated waveforms compared to saline exposed animals; excepting harmonic calls, DCS treatment was able to reduce VPA-induced changes in waveform expression to closer to control levels.
Lowercase letters denote a significant difference to a specific group (or groups when presented in combination) (p<0.05). a) saline/saline; b) saline/DCS 32 mg/kg; c) saline/DCS 64 mg/kg; d) VPA/saline; e) denotes differences between animals that received a single injection and animals that received multiple injections. n = 8–12 per group.
2.3 Social Interaction Test
On one day between P40-P45, animals underwent testing in a modified social interaction test using a Plexiglas box (30 cm × 20 cm × 20 cm) that was divided into two equally sized compartments by a clear partition with a semicircular hole (7 cm × 5 cm), allowing one animal at a time to move between compartments as described previously (Mooney and Varlinskaya, 2011; Middleton et al., 2012; Varlinskaya et al., 1999). Prior to any social interaction, experimental subjects were marked on the back with a black Sharpie™ and isolated for a total of 30 min (20 min alone in a holding cage + 10 min habituation to the testing apparatus) in the dimly lit testing room. During the 10-min habituation period, subjects were allowed to explore the testing box. Following habituation, a novel non-prenatally exposed and drug-naïve play partner (matched for sex, age, and weight +/- 10 g) was introduced, and their social interactions were videotaped for 10 min.
Five measures were scored by an investigator unaware of experimental condition of any animal: play fighting, social investigation, contact behavior, social motivation, and locomotor activity. Play fighting (or social play) was defined as pinning, pouncing or playful nape attack, following and chasing. Social investigation was defined as the sniffing of any part of the body of the partner, and contact behavior was assessed by summing frequencies of crawling over and under the partner and social grooming. Social motivation was determined from the number of crosses between compartments and whether the experimental animal crossed towards or away from the play partner. It was expressed as a coefficient of social preference/avoidance: coefficient (%) = (crossovers to – crossovers from)/(crossovers to + crossovers from). A positive coefficient denotes social preference, while a negative coefficient denotes social avoidance.
Locomotor activity of the experimental animal in the test box was assessed during the habituation phase. Distance (m) and speed (m/s) were determined from the video-tape using a video-tracking system (Any-Maze, Stoelting Co, Wood Dale, IL).
2.4 Statistical Analysis
For each measure, the mean (± standard error of the mean) was calculated for each treatment group. Data from males and females was analyzed separately. A three-way analysis of variance (ANOVA) was applied to determine the overall effect of prenatal exposure and postnatal treatment using Sigma Plot 12.3 software (Systat Software Inc, San Jose CA). For males, separate for each measure 2 (prenatal exposure: VPA or saline) × 2 (injection: single or multiple) × 3 (postnatal DCS dose: 0, 32, or 64 mg/kg) ANOVAs were used. For females, data for each measure were analyzed using 2 (prenatal exposure: VPA or saline) × 2 (injection: single or multiple) × 2 (DCS dose: 0 or 32 mg/kg) ANOVAs. In order to determine the source of any significant interactions or main effects (p value ≤ 0.05), data were probed using Holm-Sidak test or Bonferroni-corrected planned comparisons.
3. RESULTS
3.1 Dam and litter outcomes
Dam and litter outcomes are shown in Table 1. One-way ANOVA did not identify a significant effect of VPA dose on dam body weight gain between G12 and G19 (F2,17 = 2.595, p = 0.108), average pup weight on P0 (F2,17 = 0.0490, p = 0.952), or number of pups per litter (F2,31 = 0.988, p = 0.385). There was a significant effect of prenatal exposure on the percent of male pups (F2,31 = 4.073, p = 0.028); saline-injected dams had a significantly higher percent of male pups (~60%) than VPA-injected dams or non-manipulated dams (~44% for both).
Table 1.
Dam and Litter Outcomes
| Prenatal Exposure | Daily body weight gain dams (g) | Dam body weight on G19 (g) | # pups | % male | Pup weight on P0 (g) |
|---|---|---|---|---|---|
| Non-manipulated | 8.94 ± 0.18 | 308.80 ± 4.12 | 12.82 ± 0.80 | 44.27 ± 5.39 | 6.07 ± 0.10 |
| Saline-exposed | 9.95 ± 0.43 | 314.67 ± 5.96 | 13.50 ± 0.54 | 59.98 ± 3.82* | 6.02 ± 0.12 |
| VPA-exposed | 8.75 ± 0.45 | 304.00 ± 10.56 | 12.00 ± 0.84 | 44.18 ± 3.76 | 6.05 ± 0.13 |
Data shown are mean ± standard error of the mean (n = 5–7 per group).
significantly different (p<0.05) to non-manipulated and VPA-exposed.
Both male and female offspring that were injected acutely with DCS and tested on P40–42 had lower body weights than animals that received multiple injections and were tested on P43–45 (F1,106 = 27.85, p<0.001; Table 2). There was also a significant main effect of prenatal exposure on weight such that VPA-exposed adolescent rats weighted on average 8–12 g less than saline-exposed controls (F1,106=12.16, p<0.001 for male and F1,79=14.13, p<0.001 for female offspring).
Table 2.
Body Weight on Day of Testing
| Prenatal/Postnatal | Male | Female | ||
|---|---|---|---|---|
| 1 injection | 4 injections | 1 injection | 4 injections | |
| Saline/Saline | 143.18 ± 3.96 | 159.64 ± 3.96 | 117.73 ± 4.67 | 132.60 ± 4.90 |
| Saline/DCS 32 mg/kg | 138.90 ± 4.15 | 157.50 ± 4.15 | 123.17 ± 4.48 | 127.18 ± 4.67 |
| Saline/DCS 64 mg/kg | 136.33 ± 4.38 | 148.14 ± 4.96 | ||
| VPA/Saline | 133.36 ± 3.96 | 146.50 ± 4.15 | 110.10 ± 4.90 | 115.33 ± 4.48 |
| VPA/32 mg/kg DCS | 133.00 ± 4.38 | 143.00 ± 4.38 | 109.30 ± 4.90 | 115.82 ± 4.67 |
| VPA/64 mg/kg DCS | 134.82 ± 3.96 | 142.00 ± 4.15 | ||
Data shown are mean ± standard error of the mean (n = 8–12 per group).
3.2 USV outcomes
3.2.1 22 kHz calls
Overall, the number of 22 kHz USVs (alarm calls) was extremely low; only ~10% of all animals tested emitted an alarm call during their social interaction session (19 subjects in total). Among these animals, the greatest number of alarm calls in the 10 min session was four. The alarm calls were distributed across most of the treatment groups, the exception was that control males that received multiple injections of 64 mg/kg DCS showed no 22 kHz calls (data not shown).
3.2.2 Latency to 50 kHz USV
Typically, animals began vocalizing within approximately 20 s of the social partner entering the box. The latency for animals to begin vocalizing was affected by prenatal exposure; when one of the animals in the box was VPA-exposed, there was a longer latency prior to vocalizing compared to pairs containing Sal-exposed rats (F1,106=5.26, p=0.02 for males, F1,78=11.42, p=0.001 for females, Figure 1).
Figure 1. 50 kHz Ultrasonic Vocalizations.
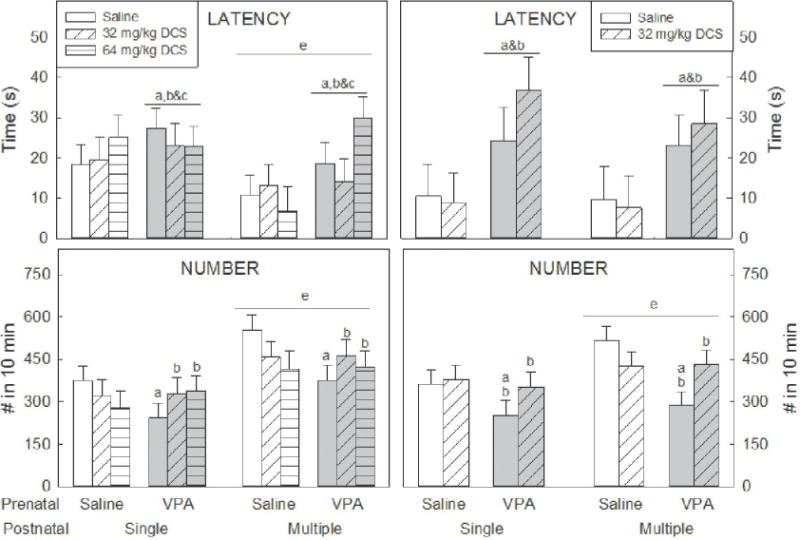
Latency to the first 50 kHz vocalization was recorded in males (top left) and females (top right). When pairs contained a VPA-exposed animal, latency was increased. In males that received four injections, latency was shorter than animals that only received a single injection.
The number of 50 kHz vocalizations during the 10 min social interaction was recorded (bottom). Animals that received multiple injections vocalized more than animals that were only injected once. Pairs containing a VPA-exposed + saline-injected rat vocalized less than pairs containing saline-exposed + saline-injected group (bottom). In VPA-exposed males, DCS mitigated this effect (bottom left).
Lowercase letters denote a significant (p<0.05) difference to a specific group (or groups when presented in combination). a) saline/saline; b) saline/DCS 32 mg/kg; c) saline/DCS 64 mg/kg; d) VPA/saline; e) denotes differences between animals that received a single injection and animals that received multiple injections. n = 8–12 per group.
When pairs contained a male that received multiple injections (saline or DCS), latency to vocalize was shorter than pairs containing an animal that received a single injection of DCS (F1,106=5.37, p=0.02, Figure 1), regardless of prenatal exposure or postnatal DCS dose. There was no effect of postnatal DCS dose or number of injections in females (F1,78=0.027, p=0.605 and F1,78=0.408, p=0.525, respectively).
3.2.3 50 kHz calls
All animal pairs emitted 50 kHz (hedonic) calls during the 10 min social interaction session. A three-way ANOVA showed no effect of prenatal exposure on the number of 50 kHz USV calls made by males (F1,106=1.392, p=0.241), but these were significantly increased in female pairs (F1,78=6.228, p=0.041). There was also no effect of dose of DCS (F1,106=0.273, p=0.761 for males and F1,78=1.503, p=0.224 for females). The ANOVA did identify an effect of the number of injections; the number of 50 kHz USVs was significantly higher when an animal injected multiple times was included in the pair compared to pairs that had an animal that received a single injection. This was apparent in males (F1,106=16.83, p<0.001, Figure 1) and females (F1,78=5.01, p=0.03, Figure 1) regardless of prenatal or postnatal exposure. There was also a significant interaction between prenatal exposure and postnatal DCS dose on the total number of calls in both males (F2,106=3.41, p=0.04, Figure 1) and females (F1,78=6.28, p=0.01, Figure 1). Probing this interaction in the males revealed that pairs containing a VPA-exposed and saline-injected rat vocalized less than pairs containing saline-exposed + saline-injected group (p=0.02). In contrast, a pair containing a VPA-exposed male that had been injected with DCS (regardless of the number of injections or the dose) vocalized at a comparable rate to pairs containing a prenatal saline + postnatal saline animal. In females, pairs containing a prenatal VPA + postnatal saline animal emitted fewer 50 kHz calls compared to pairs containing prenatal saline + postnatal saline (p=0.001) and VPA + 32 mg/kg DCS injected groups (p=0.02).
3.2.4 50 kHz Waveform analysis
3.2.4a Prenatal Exposure to VPA
Fifty kHz USVs were separated into four types: simple, harmonic, frequency modulated, and atypical. Simple, harmonic, and frequency modulated forms were seen across all treatment groups, whereas the atypical form was only seen when VPA-treated animals were present (Figure 2). The proportion of each type of call was significantly altered by prenatal exposure in both males (simple F1,106=51.13, p<0.001; harmonic F1,106=14.84, p<0.001; frequency modulated F1,106=99.45, p<0.001) and females (simple F1,78=49.69, p<0.001; harmonic F1,78=23.96, p<0.001; frequency modulated F1,78=61.47, p<0.001). When the pair contained a control animal prenatally exposed to saline, simple waveforms were relatively rare and accounted for only ~6% of all USVs, as did harmonic calls (~6%), and the remaining calls were frequency modulated (~88%). In contrast, in pairs containing VPA-exposed animals approximately 16% of all USVs were simple forms, ~2% of calls were harmonic, and frequency modulated forms accounted for ~66% of USVs. The remaining 16% of waveforms were classified as a novel atypical form, which was never present in sonograms from pairs containing prenatal saline-exposed animals.
3.2.4b Effects of DCS
DCS did not alter the proportion of each type of USVs waveform in saline-exposed control animals, but did change it in animals exposed to VPA prenatally (Figure 2). In males, DCS (regardless of dose or the number of injections) marginally increased the proportion of frequency modulated USVs in VPA-exposed males from 59% in VPA + saline to 71% in VPA + DCS animals (F1,106=2.74, p=0.069). At the same time, the proportion of simple waveforms decreased in the males (from 18.5% to 13.5%; F2,106=4.71, p=0.011). Atypical waveforms were still seen but DCS significantly (F2,106=3.927, p=0.023) reduced their frequency from 21% to 11%.
In females, DCS (regardless of dose or the number of injections) significantly (F1,78=10.54, p=0.002) increased the proportion of frequency modulated calls in VPA-exposed animals from 59% to 73%, decreased the proportion of simple calls from 22.5% to 14% (F1,78=6.30, p=0.014), and reduced atypical calls from 18% to 11% (F2,78=4.60, p=0.035).
The proportion of harmonic calls remained ~2% in VPA-exposed animals regardless of sex or DCS treatment (F1,106=2.057, p=0.133 for males and F1,78=0.762, p=0.385 for females).
3.3 Social interactions
3.3.1 Play fighting
In males, the three-way ANOVA identified significant main effects of prenatal treatment and injection number on play fighting. VPA-exposed males showed more play fighting than their saline-exposed counterparts (F1,104 = 4.002, p = 0.0481; Figure 3 left). Males that received multiple injections engaged in more play behavior than males given a single injection (F1,104 = 4.359, p = 0.0393), regardless of prenatal exposure condition. There was also a significant interaction between prenatal exposure, DCS dose, and injection number (F2,104=3.478, p= 0.0345). Probing this interaction revealed that VPA-exposed male rats given a single injection of saline played more than saline-exposed controls (p=0.019). A single injection of DCS ameliorated this effect, returning play to control levels regardless of dose. Conversely, control male rats prenatally exposed to saline showed dose-related differences; animals injected with the high dose of DCS (64 mg/kg) played significantly less than those injected with the 32 mg/kg dose (p=0.0365), but only marginally less than the saline-challenged group.
Figure 3. Social behavior.
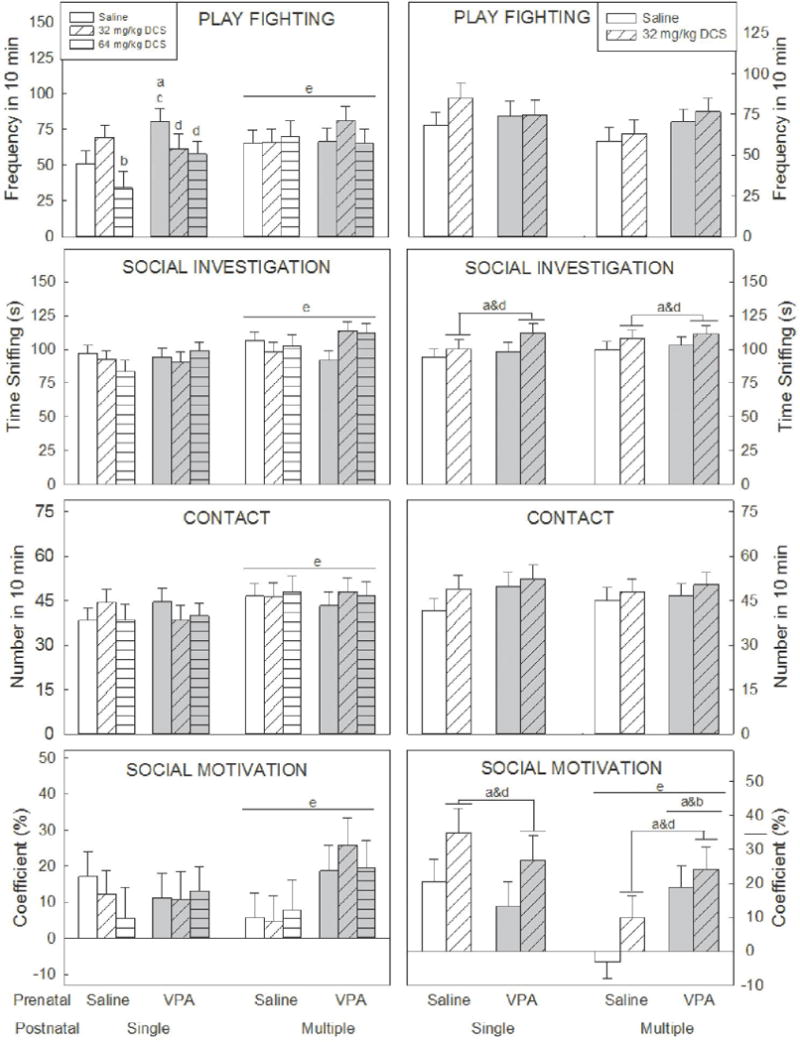
Four dimensions of social behavior were scored: play fighting (pinning, pouncing or playful nape attack, following and chasing), social investigation (sniffing), contact, and social motivation (coefficient that determines whether an animal moves towards or away from the partner).
Play fighting was increased in males that received multiple injections. In males that received a single injection, VPA + saline increased play fighting and DCS ameliorated this change. In prenatal saline animals 32 mg/kg DCS increased play fighting. Females did not show any effects of VPA or DCS on play fighting.
Social investigation was increased in males that received multiple injections, and in females that received DCS.
Contact was increased in males that received multiple injections. This behavior was not affected in females.
Social motivation was increased in males and females that received multiple injections. DCS increased social motivation in females. Females exposed to saline prenatally and multiple saline injections, showed social avoidance (negative coefficient).
Lowercase letters denote a significant difference to a specific group (or groups when presented in combination) (p<0.05). a) saline/saline; b) saline/DCS 32 mg/kg; c) saline/DCS 64 mg/kg; d) VPA/saline; e) denotes differences between animals that received a single injection and animals that received multiple injections. n = 8–12 per group.
Analysis of the female play fighting data revealed that there was no effect of VPA, DCS dose, or number of injections on play fighting (F1,74=0.684, p=0.411, F1,74=1.326, p=0.2.53,and F1,74=1.777, p=0.187, respectively; Figure 3 right).
3.3.2 Social Investigation
There were no specific effects of VPA or DCS in males (F1,104=0.748, p=0..389 for VPA and F1,104=0.074, p=0.928 for DCS, respectively), however, there was an effect of injection number on social investigation (F1,104 =7.710, p= 0.0065; Figure 3 left): animals that received multiple injections exhibited more investigation than animals that received a single injection.
In females, VPA did not alter social investigation (F1,74=1.657, p=0.202), but DCS significantly increased this form of social interaction (main effect of DCS dose (F1,74 = 4.221, p=0.0435; Figure 3 right)).
3.3.3 Contact Behavior
In males, contact behavior did not differ as a function of prenatal exposure (F1,104=0.004, p=0.945) or DCS dose (F1,104=0.073, p=0.930), but it was significantly altered by the number of injections (F1,104= 4.545, p=0.0354; Figure 3 left), with more contact behavior evident in animals given multiple injections.
Contact behavior of females was not affected by prenatal exposure, DCS treatment, or number of injections (F1,74=1.549, p=0.217; F1,74=1.780, p=0.186; and F1,74=0.038, p=0.845, respectively).
3.3.4 Social Motivation
In male rats, the coefficient was positive for all groups, denoting social preference rather than avoidance (Figure 3 left), and there was no significant effect of prenatal exposure to VPA or postnatal treatment with DCS on this measure (F1,104=3.28, p=0.073 and F1,104=0.077, p=0.926, respectively).
In females, prenatal VPA exposure did not alter social motivation (F1,74=1.234, p=0.270), however, DCS increased social preference regardless of other experimental factors (F1,74=5.943, p=0.0172, Figure 3 right). Multiple injections significantly reduced social preference in all animals (F1,74 =5.847, p=0.0181, Figure 2), and the number of injections interacted with prenatal exposure (F1,74=7.417, p=0.0081). Specifically, saline-exposed females given multiple injections had a lower social preference score relative to the VPA-exposed animals (p=0.006), or to animals given a single injection of DCS (p=0.001).
3.4 Locomotor activity
Examination of locomotor activity during habituation to the apparatus revealed that VPA-exposed rats traveled significantly longer distances compared to saline-exposed controls, this was seen in both males (F1,104=4.75, p=0.03, Figure 4 left) and females (F1,74=7.32, p=0.008, Figure 4 right). Neither postnatal treatments, nor injection number altered distance traveled (F1,104=0.247, p=0.782; F1,104=2.100, p=0.150, respectively for males: F1,74=0.161, p=0.689; F1,74=0.768, p=0.384, respectively for females).
Figure 4. Locomotor activity.
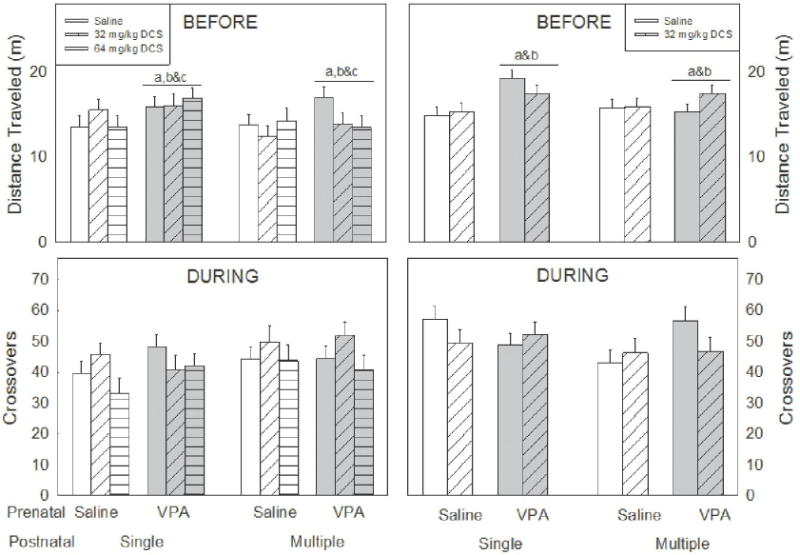
Locomotor activity was recorded during habituation (top), when the experimental animal was alone in the testing chamber, and during social interaction (bottom). During habituation, animals prenatally exposed to VPA showed increased locomotor activity. This was not evident during social interaction.
Lowercase letters denote a significant difference to a specific group (or groups when presented in combination) (p<0.05). a) saline/saline; b) saline/DCS 32 mg/kg; c) saline/DCS 64 mg/kg. n = 8–12 per group.
Speed was also unaffected; neither prenatal exposure, postnatal DCS treatments, or injection number change the velocity of the males (F1,104=1.185, p=0.279; F1,104=0.054, p=0.947; and F1,104=0.236, p=0.628, respectively) or females (F1,74=2.334, p=0.131; F1,74=0.150, p=0.700; and F1,74=0.071, p=0.933, respectively) (data not shown). Overall, speed averaged ~ 0.2–0.3 m/s (± standard error 0.02–0.05 m/s).
4. DISCUSSION
4.1 VPA-induced alterations
When one of the animals present during testing was prenatally exposed to VPA, there was a dramatic reduction in the number of USVs emitted by the pair of animals in the social behavior box. The recorded USVs are the sum of two animals in the box and therefore cannot be attributable to one animal or the other. While we believe that it is most likely that the alterations in USV patterns come from the VPA-exposed animals, we cannot discount the possibility that unusual behavior of the VPA-exposed animals elicits alterations in the USVs emitted by the non-manipulated social partner.
USVs emitted by rats are social signals. Adolescent and adult rats emit two main types of USV: 22 kHz USVs that can be considered to be “social alarm signals”, and 50 kHz USVs also thought of as “hedonic calls” (Portfors, 2007). The 22 kHz call typically occurs between 18 to 32 kHz, has a long duration, is emitted during negative affective circumstances, and is correlated with indices of fear (for review see Portfors, 2007). In contrast, the 50 kHz calls are between 33 and 96 kHz and have a relatively short duration. Different types of the 50 kHz calls have different associations (e.g., Burgdorf et al., 2011). The simple, or flat, vocalization may be associated with non-positive interactions, whereas the frequency modulated and harmonic calls are emitted during positive affective circumstances and correlate with increased locomotor activity, exploration, approach behaviors and play (Portfors, 2007; Knutson et al., 2002; Burgdorf et al., 2008). Alarm calls were relatively rare in this study and were not specifically associated with any of the exposures, suggesting that there was no exposure that caused animals to find social interaction particularly stressful. Assessment of the 50 kHz USV waveforms indicated less-complex USVs emitted by the VPA-exposed rats, with more simple forms present; specifically, when pairs contained males or females that were exposed to VPA prenatally the proportion of simple 50 kHz USVs increased and frequency modulated calls decreased. This may indicate a decrease in the hedonic value of social interactions in these pairs. In these same animals, males showed increased play fighting whereas females showed no significant effects of VPA on any of the social behavioral measures. Thus, the relationship between the USV form and the social behavior may be disrupted. Intriguingly, we also identified a novel atypical waveform, the “atypical” or “disorganized” form that was only seen when VPA-exposed animals were present. The role of this call, or whether it is caused by a change in the morphology of the craniofacial features or vocalization apparatus, is unknown at this time.
Mice exposed to a high dose of VPA during gestation also show reduced USVs, although the calls tested were distress calls of young pups and premating vocalizations (Gandal et al., 2010), and USVs in mice are thought to not be associated with valency of the interaction (Portfors, 2007).
Social behaviors are evident during entire lifespan but they change with age, and in general adolescents show more play fighting than their older counterparts (e.g., Panksepp, 1981), whereas more social investigation is evident in older than younger rats (Varlinskaya and Spear, 2008). Prenatal VPA exposure induced subtle sex-specific alterations in social interactions during mid-adolescence. Specifically, males showed an increase in play behavior. This form of social behavior gradually decreases with age (e.g., Panksepp, 1981), thus, the increased play behavior in males prenatally exposed to VPA may reflect immaturity. Others report delays in early maturation events (Schneider and Przewlocki, 2005), however, at this time it is not known if prenatal exposure to VPA delays puberty.
Outcomes in this paper are in agreement with our findings following a single exposure to a moderate dose of VPA (350 mg/kg) on G12 (Cohen et al., 2013), but are in contrast with other findings following acute exposure to high doses of VPA during gestation and testing at younger ages (P30-P35; Schneider and Przewlocki, 2005). We chose to use a moderate dose of VPA because of its teratogenicity (e.g., Vorhees, 1987); in that report, and in our hands high doses of VPA (500 mg/kg and above) lead to fetal resorption. In contrast, multiple doses of 200 mg/kg were reported to have no effect on fetal weight, maternal weight gain, the sex ratio, or weight of newborn pups, weight of adult offspring, or postnatal mortality, nor does it cause fetal resorption or alter litter size, and only caused few relatively minor defects (Vorhees, 1987). In general, our findings are in agreement with these, with the exception that we observed lower body weights in adolescent animals (which is also seen following exposure to a high dose of VPA (Schneider and Przewlocki, 2005).
Locomotor activity was increased in animals exposed to VPA prenatally. Hyperactivity has previously been reported following prenatal exposure to a high dose of VPA (Schneider and Przewlocki, 2005).
4.2 DCS effects
Prenatal exposure to VPA dramatically reduced the number of USVs, and DCS given during late adolescence ameliorated this VPA-induced alteration. Intriguingly, neither the dose of DCS nor the number of injections was important for this effect. Treatment with DCS also increased the proportion of USVs classified as frequency modulated in both males and females, while reducing simple and atypical forms. Thus, DCS improved communication, although not back to control levels. Improved communication following DCS treatment has been reported in humans (Guastella et al., 2008). And another NMDA receptor glycine site partial agonist, GLYX-13, has been shown to improve deficits in a rat line selectively bred for low USV emission (Moskal et al., 2011).
Treatment with DCS was also able to ameliorate some, although not all, of the VPA-induced effects on social behavior. In males, a single dose of DCS ameliorated the VPA-induced increase in play fighting, and in females, DCS increased social motivation. This could be due to its ability to reduce social anxiety, as is seen in some humans (e.g., (Guastella et al., 2008; Hofmann et al., 2006; Smits et al., 2013; Hofmann et al., 2013). Others also show that DCS improves social deficits in rodents (Benson et al., 2013; Jacome et al., 2011; Deutsch et al., 2011; Burket et al., 2013; Won et al., 2012).
The hyperactivity during habituation is not ameliorated by DCS.
4.3 Multiple injections
Animals that received multiple injections showed some differences in behavior when compared with non-injected animals or those that only received a single injection. For example, animals that received multiple injections had increased numbers of USVs, males showed increased play fighting, and control females given multiple injections had a lower social motivation than females given a single injection or those exposed to VPA prenatally. It is possible that these effects may be a maturation effect as animals that received multiple injections were 2–3 days older on the day of testing than animals injected only once, however, we believe that it is unlikely that 2–3 days can make such a difference. Instead, we suggest that these effects are more likely due to the stress associated with handling and/or multiple injections or the habituation of the dams to handling and injections. For example, the social preference coefficient is very sensitive to anxiogenic manipulations (Doremus-Fitzwater et al., 2009; Varlinskaya et al., 2010), thus the decreased social motivation seen in saline-exposed females given multiple injections suggests they may be very sensitive to the stress of injection, whereas males are not.
4.4 Summary
Overall, these findings are consistent with data from other laboratories suggesting that prenatal exposure to VPA induces alterations in behavior and communication, and that D-cycloserine can ameliorate these changes.
Acknowledgments
The authors thank Celina Tran and Nathan Nguyen for technical assistance and Dr. Jeffery Burgdorf for advice regarding the USV data. This research was supported by Autism Speaks (4946 to SMM) and the National Institute of Alcohol Abuse and Alcoholism (AA018693 and AA0178231 to SMM).
ABBREVIATIONS
- ASD
autistic spectrum disorder
- DCS
D-cycloserine
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- G
gestational day
- i.p
intraperitoneal
- mg/kg
milligrams per kilogram body weight
- P
postnatal day
- PB
0.10 M phosphate buffer, pH 7.4
- USV
ultrasonic vocalization
- VPA
valproic acid
5. LITERATURE CITED
- Benson AD, Burket JA, Deutsch SI. Balb/c mice treated with d-cycloserine arouse increased social interest in conspecifics. Brain Res Bull (United States) 2013;99:95–99. doi: 10.1016/j.brainresbull.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50 kHz ultrasonic vocalizations: A tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev (United States) 2011;35:1831–1836. doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol (United States) 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burket JA, Benson AD, Tang AH, Deutsch SI. D-cycloserine improves sociability in the BTBR T+ Itpr3tf/J mouse model of autism spectrum disorders with altered ras/raf/ERK1/2 signaling. Brain Res Bull (United States) 2013;96:62–70. doi: 10.1016/j.brainresbull.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen OS, Varlinskaya EI, Wilson CA, Glatt SJ, Mooney SM. Acute prenatal exposure to a moderate dose of valproic acid increases social behavior and alters gene expression in rats. Int J Dev Neurosci (England) 2013;31:740–750. doi: 10.1016/j.ijdevneu.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Jacome LF, Cannon WR, Herndon AL. D-cycloserine improves the impaired sociability of the balb/c mouse. Brain Res Bull (United States) 2011;84:8–11. doi: 10.1016/j.brainresbull.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Pepe GJ, Burket JA, Winebarger EE, Herndon AL, Benson AD. D-cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Res (Netherlands) 2012;1439:96–107. doi: 10.1016/j.brainres.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav (United States) 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour-Rainfray D, Vourc’h P, Le Guisquet AM, Garreau L, Ternant D, Bodard S, Jaumain E, Gulhan Z, Belzung C, Andres CR, Chalon S, Guilloteau D. Behavior and serotonergic disorders in rats exposed prenatally to valproate: A model for autism. Neurosci Lett (Ireland) 2010;470:55–59. doi: 10.1016/j.neulet.2009.12.054. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating gamma oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiatry (United States) 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry (United States) 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, Blendy JA, Dow HC, Brodkin ES, Schneider F, Gur RC, Siegel SJ. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav (England) 2009;8:661–675. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry (United States) 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA, Rosenfield D, Simon N, Otto MW, Meuret AE, Marques L, Fang A, Tart C, Pollack MH. D-cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry (United States) 2013;170:751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. D-cycloserine enhances social exploration in the balb/c mouse. Brain Res Bull (United States) 2011;85:141–144. doi: 10.1016/j.brainresbull.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature (ENGLAND) 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull (United States) 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kumar RA, Christian SL. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep (United States) 2009;9:188–197. doi: 10.1007/s11910-009-0029-2. [DOI] [PubMed] [Google Scholar]
- Labrie V, Clapcote SJ, Roder JC. Mutant mice with reduced NMDA-NR1 glycine affinity or lack of D-amino acid oxidase function exhibit altered anxiety-like behaviors. Pharmacol Biochem Behav (United States) 2009;91:610–620. doi: 10.1016/j.pbb.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology (United States) 2008;33:901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Dev Neurosci (Switzerland) 2012;34:115–128. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: Effects of age, sex, and timing of exposure. Behav Brain Res (Netherlands) 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal JR, Burgdorf J, Kroes RA, Brudzynski SM, Panksepp J. A novel NMDA receptor glycine-site partial agonist, GLYX-13, has therapeutic potential for the treatment of autism. Neurosci Biobehav Rev (United States) 2011;35:1982–1988. doi: 10.1016/j.neubiorev.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol (UNITED STATES) 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci (United States) 2007;46:28–34. [PubMed] [Google Scholar]
- Schmeisser MJ, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature (England) 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol (England) 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology (United States) 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology (England) 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- Smits JA, Hofmann SG, Rosenfield D, Deboer LB, Costa PT, Simon NM, O’Cleirigh C, Meuret AE, Marques L, Otto MW, Pollack MH. D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: Prognostic and prescriptive variables. J Consult Clin Psychol (United States) 2013;81:1100–1112. doi: 10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult sprague-dawley rats: Impact of social deprivation and test context familiarity. Behav Brain Res (Netherlands) 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav (United States) 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: Role of housing conditions and partner’s activity. Physiol Behav (UNITED STATES) 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Teratogenicity and developmental toxicity of valproic acid in rats. Teratology (UNITED STATES) 1987;35:195–202. doi: 10.1002/tera.1420350205. [DOI] [PubMed] [Google Scholar]
- Wohr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav (United States) 2008;93:766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Radzishevsky I. The serine shuttle between glia and neurons: Implications for neurotransmission and neurodegeneration. Biochem Soc Trans (England) 2013;41:1546–1550. doi: 10.1042/BST20130220. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J (England) 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park SG, Lee JS, Lee K, Kim D, Bae YC, Kaang BK, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature (England) 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]


