Abstract
Objective
To describe the epidemiology of Neonatal Abstinence Syndrome (NAS) in a 16-county Appalachian area of eastern Tennessee.
Methods
The Tennessee Surveillance System for NAS provided data on maternal sources of opioids. Data linking hospital discharge diagnosis for NAS to birth certificate data allowed us to compare maternal, delivery, and infant characteristics for NAS births with those for non-NAS births.
Results
There were 339 cases of NAS in 2013 and 367 in 2014, for NAS rates of 25.5 and 28.5 per 1,000 live births, respectively. When compared with the state overall, mothers of NAS infants in eastern Tennessee were more likely to use opioids that had been prescribed to another person. There were numerous maternal, infant, and delivery characteristics that were significantly different for NAS births compared with non-NAS births.
Conclusion
NAS is epidemic in the eastern Tennessee area of Appalachia, with unique maternal and infant characteristics that have important implications for primary, secondary, and tertiary prevention.
Keywords: Neonatal abstinence syndrome, opioids, substance abuse, drug abuse
Neonatal abstinence syndrome (NAS) is a withdrawal syndrome that occurs in infants exposed to opioids in utero.1 The increasing number of infants with NAS is an epidemic within an epidemic: while emergency department visits for nonprescription use of opioids more than doubled between 2004 and 2011,2 and deaths attributable to opioids have more than tripled between 1999 and 2013,3 the national rate of NAS has increased almost five-fold between 2000 and 2012 (from 1.20 to 5.8 per 1,000 live births).4 Concomitant with the rise in NAS has been a fourfold rise in admissions to neonatal intensive care units for NAS infants between 2000 and 2012,5 with hospital-related charges doubling between 2009 and 2012 to reach 1.5 billion dollars.4
Four states at least partially in southern Appalachia—Tennessee, Kentucky, Mississippi, and Alabama—have NAS incidence rates almost three times above the national average.4 Several Appalachian counties of east Tennessee have NAS rates more than 10 times the national average, with rates exceeding 60 per 1,000 live births.6 Even though these states are experiencing the heaviest burden of NAS, there have been relatively few studies conducted to determine potentially unique characteristics, complications, and risk factors associated with NAS births in Appalachia. Bunn et al. found elevated rates of congenital malformations in NAS infants in Kentucky compared with non-NAS infants, including circulatory, cardiac, and genito-urinary malformations.7 In a study of 1,974 NAS infants in West Virginia, Stabler et al. found that 85% of NAS births were to mothers receiving Medicaid, compared with 54% of non-NAS births.8 Beyond Appalachia, but still relevant, Lind et al. compared characteristics of 242 NAS births to non-NAS births in Florida, finding that NAS births were more likely to be White, low birthweight, and preterm.9 It is not clear from these and other studies on NAS whether the strikingly higher rates of NAS in the southern Appalachian region can be explained by maternal characteristics unique to this geographic region; it is clear, however, that a better understanding of maternal and infant characteristics and risk factors for NAS is urgently needed. Thus, the purpose of this present study was to identify characteristics of NAS infants and mothers in the eastern, Appalachian region of Tennessee, as a springboard for establishing public health efforts to develop primary, secondary, and tertiary prevention services to address this epidemic.
Setting and background
The University of Tennessee, Department of Public Health contracted with the Tennessee Department of Health to participate in its state health planning efforts in June 2015. This was part of a larger project on payment and health care delivery systems reform overseen by the TennCare Bureau (Tennessee’s Medicaid) of the Tennessee Department of Finance and Administration, and funded as a State Innovation Model by the Center for Medicare and Medicaid Innovation.10 The area of emphasis for the University of Tennessee was perinatal health, and after local consultation with local public health leaders we chose to focus specifically on NAS in the 16-county region surrounding and including Knox County, in eastern Tennessee. Opioid use in general, and NAS in particular, had been identified by the Knox County Health Department11 and the East Tennessee Regional Health Office as a major topic of concern in recent community-based health assessment activities. (Personal communication from Dr. Martha Buchanan and Ms. Janet Ridley to Paul Erwin, July 1, 2015.)
The demographic characteristics of the 16-county area, stratified by urban Knox County, the rural surrounding 15 counties, and the state of Tennessee, are provided in Table 1. For clarity, “eastern Tennessee” will refer to all 16 counties in the study, while the “East Tennessee Region” refers to the 15-county health department region that is predominately rural, which surrounds Knox County, the major urban center in the 16-county area.
Table 1.
Population characteristics, Knox County, East Tennessee Region (15 counties), and Tennessee, 2012–2013 estimates.12,13
| Jurisdiction | Knox County | East Tennessee Region (15 counties) | Tennessee |
|---|---|---|---|
| Total Population | 429,161 | 755,912 | 6,361,0707 |
| Percent White | 88.6% | 96.8% | 81.0% |
| Percent African American | 8.9% | 2.1% | 16.8% |
| Percent with some college education 1 | 70.4% | 31.9–56.1% (40.2%) | 58.2% |
| Percent unemployed 1 | 5.5% | 6.0–11.8% (7.7%) | 6.7% |
| Percent of children in poverty 1 | 23% | 22–40% (30%) | 26% |
Data for East Tennessee Region shown as range of county-level figures for 15 counties, with median noted in parenthesis
The rural (15 county) East Tennessee Region is predominantly White, while the Knox County area alone has more than twice the number of African Americans (38,277) as the East Tennessee Region (15,976). These different demographic characteristics have an important impact on birth-related data because many health behaviors, risk factors, and health outcomes differ significantly by race (including substance use [tobacco and alcohol among the substances], pregnancy rates, low birth weight rates, and infant mortality).14 A second distinction of the rural counties in eastern Tennessee relates to geography, and the transportation challenges this imposes in accessing care, even if care is available. A confounding element of the effect of geography is poverty, particularly for the rural White population: 11 of the 15 counties of the East Tennessee Region have poverty rates higher than Tennessee overall (17.6%), with poverty rates approaching or exceeding 25% in four counties.15 Poverty appears to be particularly relevant to women who give birth to infants with NAS. The Bureau of TennCare reported that during 2008–2014, 87% of all NAS births were born to mothers on TennCare (Medicaid) at the time of delivery.16 Given these distinctions, when possible, analyses for the 16-county area were conducted separately for Knox County and the 15-county East Tennessee Region.
Methods
Data sources
Data for this study came from two sources. The first source was the Tennessee Surveillance System for Neonatal Abstinence Syndrome.15 In 2013, Tennessee made NAS a mandatory reportable condition and established the Tennessee Surveillance System for Neonatal Abstinence Syndrome.17 Weekly and monthly surveillance summaries and annual reports are available to the public at http://tn.gov/health/topic/nas. The Tennessee Department of Health provided a de-identified data file of all reported cases of NAS in the 16-county area for 2013 and 2014.
A second source of data was provided from the Tennessee Bureau of TennCare and the Tennessee Department of Health, consisting of a de-identified dataset linking hospital discharge diagnosis for NAS (ICD-9-CM code 779.5) to birth certificate data. These data were restricted to mothers enrolled in, or infants eligible for, Medicaid at the time of hospital discharge.
Sample and design
We used a retrospective case-control design to compare infants with and without NAS in the 16-county area. Cases were infants diagnosed with NAS, born in 2013 or 2014, with maternal residence in the 16-county area, and with the mother enrolled in or infant eligible for TennCare by the time of hospital discharge following birth. Controls were randomly selected from all other TennCare enrolled or eligible births in 2013 and 2014, not diagnosed with NAS, with frequency matching based on year of birth and maternal county of residence. The linked data file included a total of 1198 records—599 infants with NAS in 2013–2014 across the 16-county area, and 599 randomly selected infants without NAS during the same time period.
Measures
The Tennessee Surveillance System for Neonatal Abstinence Syndrome (data source 1), variables of interest included source(s) of maternal opioid exposure. Hospitals are required to report source(s) of maternal exposure in the following categories: non-prescription substance (illegal drugs), supervised pain therapy, therapy for psychiatric or neurological condition, prescription substance obtained without a prescription, or supervised replacement therapy. The opioid source is then coded in two fashions. The first is non-mutually exclusive, as any one person may have one or multiple sources of exposure. The Tennessee Department of Health then further classifies these exposure sources into mutually exclusive categories as follows:
Prescription drugs only (all exposures were legal prescription to that person, including exposures to doses in excess of the prescribed amount)
Illicit/diverted drugs only (all exposures were illegal drugs or prescription drugs diverted to someone else, i.e., a prescription drug but prescribed to another person)
Prescription and illicit/diverted drugs (exposures reflected categories a plus b)
Unknown (no exposure was identified)
From the hospital discharge/birth certificate linked dataset (data source 2), the variables of interest included all infant and maternal-related variables on the standard birth certificate. Sixteen variables on maternal characteristics were included and reflected demographics, infections, and perinatal events. Delivery characteristics included 10 measures that described various aspects of the birth process. Infant characteristics were captured in 10 measures that included health conditions, TennCare status, birthweight, gestational age, and NICU admission.
There are two NICUs in the 16-county area that are responsible for the weaning of infants with NAS in the region. When NAS is diagnosed after delivery, such infants are automatically admitted to the NICU to begin the weaning process; if delivered at other hospitals without a NICU, the infants are transferred to one of the two NICUs for weaning.
Statistical analysis
Data from the Tennessee Surveillance System for Neonatal Abstinence Syndrome were evaluated using descriptive statistics. To highlight geographic differences, analyses were stratified by Knox County (an urban county), the East Tennessee Region (15 rural counties), and the state of Tennessee when feasible. The linked hospital discharge and birth certificate dataset was analyzed by comparing NAS births to non-NAS births, with tests of statistical significance between these groups using chi square for categorical variables (Fisher’s Exact for cells with n ≤ 5) and Kruskal-Wallis for continuous variables. Unconditional logistic regression was performed to provide an additional measure of association pertaining to maternal characteristics, with frequency matching variables (year of birth, maternal county of residence) included as covariates, plus age as a covariate. All analyses were conducted using SAS software, version SAS 9.3 (copyright 2002–2010, SAS Institute, Inc.). Institutional Review Board approval was provided by the University of Tennessee, Knoxville and the Tennessee Department of Health.
Results
For the 16-county eastern Tennessee area, the Tennessee Surveillance System for Neonatal Abstinence Syndrome confirmed reports of 339 cases of NAS in 2013 and 367 in 2014, for NAS rates of 25.5 per 1,000 live births and 28.5 per 1,000 live births, respectively (Table 2). The 2014 NAS rate of the rural East Tennessee Region was over 50% higher than that of the urban Knox County area.
Table 2.
Neonatal Abstinence Syndrome, Knox County, East Tennessee Region (15 counties), and Tennessee, 2013–201418
| Jurisdiction | 2013 | 2014 | ||
|---|---|---|---|---|
| No. Cases | Rate per 1,000 live births | No. Cases | Rate per 1,000 live births | |
| Knox County | 99 | 18.7 | 103 | 20.2 |
| East Tennessee Region (15 counties) | 240 | 30.1 | 264 | 33.9 |
| Total 16-county eastern Tennessee | 339 | 25.5 | 367 | 28.5 |
| Tennessee | 936 | 11.7 | 1018 | 12.7 |
Table 3 provides information on non-mutually exclusive sources of opioids (any one user may have multiple sources). These data indicate that mothers of NAS infants in eastern Tennessee are more likely to use opioids that have been prescribed to another person (Prescription substance obtained without a prescription) than they are to use illegal drugs. Mothers of NAS infants in eastern Tennessee were also less likely than their counterparts for the state in general to be using opioids as a part of a prescribed treatment or replacement therapy.
Table 3.
Non-Mutually Exclusive Source of Exposure for Neonatal Abstinence Syndrome, if known, Knox County, East Tennessee Region, and Tennessee, 20146
| Percent Use (Not Mutually Exclusive) | |||
|---|---|---|---|
| Source | Knox County (N= 108) (%) |
East Tennessee Region (N= 296) (%) |
Tennessee (N=973) (%) |
| Non-prescription substance (illegal drugs) | 26.0% | 14.0% | 21.6% |
| Supervised pain therapy | 5.0% | 10.0% | 13.5% |
| Therapy for psychiatric or neurological condition | 11.0% | 4.0% | 7.0% |
| Prescription substance obtained without a prescription | 69.0% | 58.0% | 38.0% |
| Supervised replacement therapy | 50.0% | 51.0% | 55.5% |
| No known exposure but clinical signs consistent with NAS | 0 | <.5% | 0.3% |
Figure 1 indicates that mutually exclusive sources of opioids differed between Knox County, the East Tennessee Region, and the state, with a much higher reported use of illicit drugs (which includes illegal drugs and prescription drugs diverted to someone else) in eastern Tennessee.
Figure 1. Mutually Exclusive Source of Exposure for Neonatal Abstinence Syndrome, Knox County, East Tennessee Region (15 counties), and Tennessee, 20146.
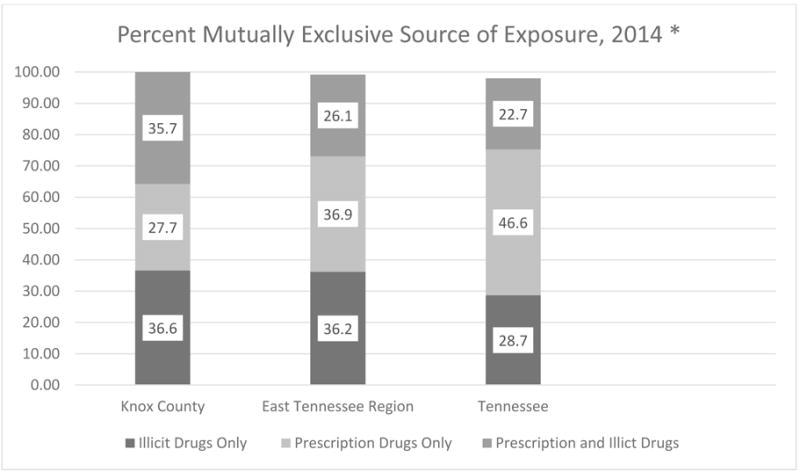
*“Illicit” includes illegal drug or prescription drug diverted to someone else; “Prescription drugs only” include all legal prescriptions to that person, including medically assisted therapy.
In the linked hospital discharge/birth certificate dataset, 326 (54.4%) of the 599 records were from 2013 and 273 (45.6%) were from 2014. Table 4 shows that NAS births were significantly different from non-NAS births regarding several maternal, delivery, and infant characteristics. Compared with mothers of non-NAS births, mothers with NAS infants tended to be older, not married (72.7% vs. 58.4%, p<.0001), and non-Hispanic White (95.5% vs. 80.3%, p<.0001); to have smoked cigarettes during pregnancy (62.3% vs. 25.2%, p<.0001; n= 1,051); and to have a history of hepatitis C (26.5% vs. 1.8%, p<.0001) and herpes simplex (17.4% vs. 8.0%, p<.0001). Mothers with NAS infants received less pre-natal care, with a median of 10 prenatal visits compared with a median of 13 prenatal visits for mothers of non-NAS births (p<.0001). Mothers with NAS infants were less likely to have a Cesarean birth (27.7% vs. 33.9%, p=.02), to have been induced (29.9% vs. 37.1%, p=.009), and to have augmentation of labor (19.9% vs. 24.7%, p=.04). Mothers with NAS infants were more likely to have indications of infection (42.7% vs. 13.9%, p<.0001), while indications of an abnormal condition at time of delivery were more than twice as likely in NAS infants compared with non-NAS infants (19.5% vs. 5.7%, p<.0001). Infants with NAS were more likely to be low birth weight (15.4 % vs. 6.4% at 1501–2500 grams, p<.0001) and to be admitted to the Neonatal Intensive Care Unit (17.4% vs. 4.7%, p<.0001) than non-NAS infants.
Table 4.
Maternal and infant characteristics, for infants with and without a hospital discharge diagnosis of Neonatal Abstinence Syndrome, eastern Tennessee, 2013–2014.
| NAS n (%) |
Non-NAS n (%) |
P* | |
|---|---|---|---|
| N= 599 | N= 599 | ||
| Year | |||
| 2013 | 326 (54.4%) | 326 (54.4%) | N/A |
| 2014 | 273 (45.6%) | 273 (45.6%) | |
| Maternal Characteristic | |||
| Maternal Age (years) | <.0001 | ||
| <20 | 23 (3.8) | 92 (15.4) | |
| 20–24 | 193 (32.2) | 234 (39.1) | |
| 25–29 | 224 (37.4) | 159 (26.5) | |
| 30–34 | 120 (20.0) | 83 (13.9) | |
| 35+ | 39 (6.5) | 31 (5.2) | |
| Marital status | <.0001 | ||
| Not married | 435 (72.7) | 349 (58.4) | |
| Married | 163 (27.3) | 249 (41.6) | |
| Race (n= 1,197) | <.0001 | ||
| Non-Hispanic White | 572 (95.5) | 480 (80.3) | |
| Non-Hispanic Black | 12 (2.0) | 43 (7.2) | |
| Hispanic | 8 (1.3) | 54 (9.0) | |
| Other | 7 (1.2) | 21 (3.5) | |
| Chlamydia** | 42 (7.0) | 27 (4.5) | .06 |
| Hepatitis B** | 3 (.50) | 3 (.50) | 1.00 |
| Hepatitis C** | 159 (26.5) | 11 (1.8) | <.0001 |
| Herpes Simplex** | 104 (17.4) | 48 (8.0) | <.0001 |
| Prenatal care indicated | 543 (92.5) | 594 (99.3) | <.0001 |
| Any smoking pre-pregnancy (n=1,051) | 314 (62.3) | 138 (25.2) | <.0001 |
| Smoking anytime during pregnancy (n=1,053) | 298 (59.7) | 104 (18.8) | <.0001 |
| Median (IQR) | Median (IQR) | P* | |
| Number of previous pregnancies (n= 1,185) | 2.0 (2.0) | 1.0 (2.0) | <.0001 |
| Time since last delivery (months, n= 1,069) | 29.0 (49.0) | 18.0 (43.0) | <.0001 |
| Pre-pregnancy smoking (cigarettes smoked per day in the 3 months before pregnancy; n= 1,051) | 10.0 (20.0) | 0 (1.0) | <.0001 |
| 1st Trimester Smoking (Cigarettes smoked per day; n= 1,080) | 8.0 (15.0) | 0 (0) | <.0001 |
| 2nd Trimester Smoking (Cigarettes smoked per day; n= 1,099) | 6.0 (10.0) | 0 (0) | <.0001 |
| 3rd Trimester Smoking (Cigarettes smoked per day; n= 1,111) | 5.0 (10.0) | 0 (0) | <.0001 |
| Total number of prenatal visits (n= 926) | 10.0 (9.0) | 13.0 (5.0) | <.0001 |
| Delivery Characteristics | |||
|
NAS n (%) |
Non-NAS n (%) |
P* | |
| Cesarean delivery | 166 (27.7) | 203 (33.9) | .02 |
| Vaginal delivery | 411 (68.6) | 362 (60.4) | .003 |
| Induction | 179 (29.9) | 222 (37.1) | .009 |
| Augmentation of labor | 119 (19.9) | 148 (24.7) | .04 |
| Breech | 20 (3.3) | 19 (3.2) | .87 |
| Antibiotics received during labor | 225 (37.6) | 233 (38.9) | .63 |
| Medical risk factors indicated | 238 (39.7) | 208 (34.7) | .07 |
| Morbidity indicated (complications associated with labor and delivery) | 9 (1.5) | 8 (1.3) | .81 |
| Obstetric procedures indicated | 9 (1.5) | 5 (0.8) | .28 |
| Infection indicated | 256 (42.7) | 83 (13.9) | <.0001 |
| Infant Characteristics | |||
| Cephalic | 546 (91.2) | 551 (92.0) | .60 |
| Other fetal presentation | 33 (5.5) | 29 (4.8) | .60 |
| Abnormal conditions indicated*** | 117 (19.5) | 34 (5.7) | <.0001 |
| Congenital abnormalities indicated | 3 (0.5) | 3 (0.5) | 1.00 |
| TennCare status | .0005 | ||
| Eligible child | 33 (5.5) | 70 (11.7) | |
| Enrolled | 566 (94.5) | 529 (88.3) | |
| Infant birthweight (n= 1,195) | .0001 | ||
| ≤ 1500g | 5 (0.8) | 5 (0.8) | |
| 1501–2500g | 92 (15.4) | 38 (6.4) | |
| ≥ 2500g | 500 (83.8) | 555 (92.8) | |
| NICU admission | 104 (17.4) | 28 (4.7) | <.0001 |
| Infant breastfed (n= 1,160) | 227 (39.2) | 371 (63.9) | <.0001 |
| Infant birthweight (n= 1,195) | 2,960 (707) | 3,277 (633) | <.0001 |
| Median (IQR) | Median (IQR) | P* | |
| Gestational age at birth (n= 1,192) | 39.0 (2.0) | 39.0 (2.0) | .06 |
Chi square for categorical variables (Fisher’s Exact for cells with n ≤ 5) and Kruskal-Wallis for continuous variables
Present and/or treated during this pregnancy
- Assisted ventilation required immediately following delivery
- Assisted ventilation required for more than six hours
- NICU admission
- Newborn given surfactant replacement therapy
- Antibiotics received by the newborn for suspected neonatal sepsis
- Seizure or serious neurologic dysfunction
- Significant birth injury (skeletal fracture(s), peripheral nerve injury, and/or soft tissue/solid organ hemorrhage which requires intervention)
Source: TennCare Bureau, Tennessee Department of Finance and Administration, and the Tennessee Department of Health, Division of Vital Statistics
Unconditional logistic regression was used to further explore the association between NAS births and maternal characteristics (Table 5). Controlling for year of birth, maternal county of residence, and maternal age, mothers of NAS births were more likely to have smoked cigarettes before (OR=5.19, 95% CI 3.91, 6.89) and during pregnancy (OR=6.98, 95% CI 5.18, 9.42), and were highly more likely to have indications of infection with HCV (OR=19.51, 95% CI 10.34, 36.81). Additional significant findings are noted in Table 5.
Table 5.
Odds ratioa (OR) estimates for the association of delivering an infant with Neonatal Abstinence Syndrome (NAS) with maternal characteristics, eastern Tennessee, 2013–2014 (n=1,198).
| Maternal characteristic | ORa | 95% Confidence Interval |
|---|---|---|
| Married, No vs. Yes | 2.43* | (1.87, 3.14) |
| Race, African Americans vs. whites | 0.22* | (0.11, 0.44) |
| Prenatal Care, No vs. Yes | 12.26* | (4.79, 31.35) |
| Chlamydia infection, Yes vs. No** | 1.91* | (1.13, 3.23) |
| Hepatitis B infection, Yes vs. No** | 0.69 | (0.12, 4.02) |
| Hepatitis C infection, Yes vs. No** | 19.51* | (10.34, 36.81) |
| Herpes simplex infection, Yes vs. No** | 2.23* | (1.53, 3.25) |
| Pre-pregnancy cigarette use, Yes vs. No | 5.19* | (3.91, 6.89) |
| Cigarettes during pregnancy, Yes vs. No | 6.98* | (5.18, 9.42) |
ORs obtained from unconditional logistic regression models adjusted for maternal age at delivery, maternal county of residence, and year.
P < .05
present and/or treated during this pregnancy
Discussion
This study is one of the first to report on NAS in the Appalachian region of Tennessee and has important policy and clinical implications. Our findings highlight that this Appalachian region of Tennessee experiences a significantly higher burden of NAS than the rest of Tennessee. Women who give birth to NAS infants differ in their prenatal and pregnancy characteristics from their non-NAS counterparts, and differ also in their sources of opioid use compared with Tennessee as a whole. Beyond NAS itself, co-morbidities related to prenatal opioid use and NAS can have serious implications for the long-term health of the mother and child. These data suggest the opportunity to develop more comprehensive prevention approaches for mothers (e.g., STIs and HCV) and infants (e.g., consequences of low birth weight and abnormal conditions) to promote health more efficiently and effectively.
Appalachia is ground zero for the NAS epidemic, thus this study provides rich data to help researchers understand the epidemiology of NAS, including trends, and an adequate sample size that allows subgroups to be considered (i.e., rural vs. urban areas). Appalachia has long been associated with health disparities and widespread health care issues. In 2004, the Appalachian Regional Commission highlighted these disparities, stating that “the region as a whole suffers considerable excess in mortality from leading causes of death when compared with the non-Appalachian U.S.”19 Subsequent studies have highlighted additional disparities within the region, including deficiencies in access to health care, particularly for substance abuse and behavioral health prevention and treatment services. A 2008 Appalachian Region Commission study attributed these disparities to a number of factors, most notably the Appalachian residents’ high rates of poverty and lack of insurance.20 While individual proximal (e.g., smoking/tobacco use) and distal (e.g., lower education and income) risk factors for poor health outcomes explain some of the disparities, it is likely that the interplay of these various levels strongly influences health outcomes. This appears particularly evident for substance abuse and the relative paucity of behavioral health prevention and treatment services. The epidemic of NAS falls along an addiction continuum in Appalachia, from moonshine to amphetamines to opioids, and this epidemic follows the historical path of heavier use of opioids in Appalachia compared with non-Appalachian areas.21
The maternal characteristics of opioid use in Appalachia are different from those in the state, and appear different compared with the nation, and are thus important for developing prevention and treatment policies and programs that are responsive to the Appalachian context. As shown in Figure 1, illicit drugs were a much more common source of (mutually exclusive) exposure for women with NAS infants in both urban (Knox County) and rural (East Tennessee Region) Appalachia compared with Tennessee overall. The greatest opioid source for women outside of eastern Tennessee was prescription drugs only. When Figure 1 is examined in light of Table 3, however, a different interpretation becomes apparent: the most prominent non-mutually exclusive source in the 16-county eastern Tennessee area comes from prescribed substances that were obtained through a prescription to another person (i.e., diverted use). The overall picture that emerges is that mothers of NAS infants in eastern Tennessee are more likely than mothers of NAS infants in the state overall to use prescription medications obtained without a prescription. The heavier use of diverted prescriptions reflects in part the large number of opioid prescriptions written in Appalachia. McDonald et al. recently reported that providers in several states within the Appalachian region write prescriptions at 1.5–2 times the morphine dose equivalent average for all states.22 The eastern Tennessee opiate sources reported (Table 3) also contrast with those reported in previous research. Tolia et al. reported 2012–2013 national estimates for maternal (for mothers of NAS infants) opioid use, with 31% indicating methadone, 15% buprenoprphine, and 24% using opioid pain relievers.5 In Florida, Lind et al. reported that among mothers with NAS births, 59.9% were on methadone, 3.7% were using buprenorphine, while less than 1% of mothers reported using heroin during pregnancy; however, 55.5% of mothers with NAS births also reported that they were using opioids illicitly.9 Notable, however, is the fact that the Tennessee Surveillance System for Neonatal Abstinence Syndrome does not distinguish between correct use of a prescribed substance by the person to whom the prescription is written, and use by that person of doses in excess of the prescribed dose or for reasons other than the prescribed indication. Without consistent definitions and methods for categorizing sources of opioids across these studies (e.g., in using the term “illicit”), direct comparisons between opioid use in eastern Tennessee and outside of Tennessee provide only general impressions.
Mothers of NAS infants in eastern Tennessee are at substantially higher risk of having HCV compared with mothers of non-NAS infants (26.5% vs. 1.80%, p<.0001); overall, 42.7% of mothers of NAS infants had one or more infections, compared with 13.9% for mothers of non-NAS infants (p<.0001). In general, predominantly rural Appalachian states including Tennessee, Kentucky, Virginia, and West Virginia have seen a dramatic increase in HCV.23 In relation to differences between mothers with and without NAS infants, HCV may reflect the increasing use of heroin and other injected drugs among women with NAS births. Rural Appalachian injection drug users rapidly progress from their first illicit drug use to the first injection drug use, with a median time of just three years, and synthetic opioids such as oxycodone are prominent drugs of choice for injection.24 Data from the 2012 National Survey on Drug Use and Health indicate that about 669,000 Americans reported using heroin in the past year, and this number has been increasing since 2007.25 The increasing availability and use of heroin is in part both a cause and an effect of price differentials: the street price of heroin has been widely reported to be less than the street price of prescription opioids.26 With no formal needle-exchange programs in place, heroin users in eastern Tennessee, as in many places across the United States, will face on-going risks of infection. While HCV infection is the nation’s leading cause of cirrhosis, liver failure, hepatocellular carcinoma, and liver transplantation, women with HCV face additional challenges, including the problems of antiviral treatment during pregnancy and breast feeding, and the potential for vertical transmission to their offspring.27 This compounds the health challenges for NAS infants, who already have significantly higher morbidity than non-NAS infants.
Limitations
There are several limitations to this study. First, the 16-county area of focus for this study does not include all counties in Tennessee officially recognized by the Appalachian Regional Commission as being in the Appalachian Region (which includes 52 of Tennessee’s 95 counties). Tennessee’s NAS Surveillance System data do not indicate apparent differences between these 16 counties and the other Appalachian counties, but we did not fully assess NAS across all 52 counties. The study’s 16-county East Tennessee area is completely surrounded by other Appalachian counties and reflects both urban and rural areas within Appalachia. The area may or may not be generally representative of the larger group of Appalachian counties in Tennessee.
Second, for the matched dataset using hospital discharge diagnosis of NAS and birth certificate data, we only included NAS births to women who were enrolled, or infants who were eligible for, TennCare (Medicaid). Although between 70–85% of NAS births in Tennessee have been to women enrolled in TennCare, there may be important differences between Medicaid-eligible mothers compared with those with private or no insurance.
Third, during the years covered by this study (2013–2014) there were new laws enacted in Tennessee that may have affected both the occurrence and reporting of NAS. In 2012, the Tennessee General Assembly enacted the Tennessee Prescription Safety Act,28 establishing the Controlled Substance Monitoring Database Program. This law requires providers to check the database before prescribing controlled substances and to register people to whom such substances are dispensed. In 2013, the Tennessee General Assembly enacted the Safe Harbor Act,29 which prioritizes the treatment of pregnant women for drug addiction at publicly funded treatment facilities, requires physicians to encourage treatment before the end of the 20th week of pregnancy if drug use is confirmed, and grants the Tennessee Department of Children’s Services authorization to file a petition to end the mother’s parental rights if she is not considered fit to take care of her child. In 2014, the Tennessee General Assembly enacted Public Chapter 820, which stipulated that a woman could be charged with a misdemeanor if she illegally used narcotics during pregnancy and if the baby was harmed as a result.30 There was no requirement, however, for providers to report to law enforcement, and women were not automatically prosecuted if referred to the Department of Children’s Services. This law was allowed to “sunset” in July 2016 and is no longer in effect. Based on East Tennessee Children’s Hospital data, we are aware that during the two years of enforcement of Public Chapter 820, mothers with NAS births were much less likely to have received prenatal care than prior to this law (personal communication, Carla Saunders, East Tennessee Children’s Hospital, to Laurie Meschke, March 11, 2016). It is quite possible, therefore, that these laws may have had independent and/or additive effects on NAS during the time period of this study. On-going monitoring of NAS in this region following the sunset of Public Chapter 820 is thus warranted.
Conclusions
Mothers of NAS births in the Appalachian region of Tennessee are likely to use diverted prescription medications, i.e., medications obtained without a personal prescription to the user. While there is no direct evidence that women may have shifted from prescribed drugs to cheaper illegal drugs following the enactment of the Tennessee Prescription Safety Act, this remains a potential unintended consequence that warrants further study. Trend data will be critical for assessing this potential shift in source of opioids. The higher prevalence of HCV infection in mothers of NAS infants not only reflects the type of drug exposure, it poses serious long-term health challenges for both mothers and infants. Harm reduction approaches to preventing disease transmission among injection drug users have shown success in other geographic areas;31 however, improving the availability of substance abuse prevention and treatment services throughout Appalachia remains a first-order challenge. Infants with NAS face additional health challenges given the higher burden of co-morbidities, and the long-term health consequences of NAS vis-à-vis child development remain unexplored. Finally, as with many health and social issues in Appalachia, public health practitioners can play an important role in improving understanding among lawmakers and policymakers of the larger social determinants of health that affect women’s lives and influence and sometimes determine the paths they choose.
Abbreviations
- NAS
Neonatal Abstinence Syndrome
- STIs
Sexually Transmitted Infections
- HCV
Hepatitis C Virus
References
- 1.Hudak ML, Tan RC, Frattarelli DA, et al. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–e560. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Agency. Drug abuse warning network, 2011: National estimates of drug-related emergency department visits. 2011 Available at: http://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Accessed 05/10, 2016.
- 3.Kolodny A, Courtwright DT, Hwang CS, et al. The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction. Annual Review of Public Health. 2015;36(1):559–574. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 4.Patrick S, Davis M, Lehman C, Cooper W. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. Journal of Perinatology. 2015 doi: 10.1038/jp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolia VN, Patrick SW, Bennett MM, et al. Increasing Incidence of the Neonatal Abstinence Syndrome in U.S. Neonatal ICUs. New England Journal of Medicine. 2015;372(22):2118–2126. doi: 10.1056/NEJMsa1500439. [DOI] [PubMed] [Google Scholar]
- 6.Miller AM, Warren MD, Tennessee Department of Health Neonatal Abstinence Syndrome Surveillance Annual Report. 2014–2015 Available at: https://tn.gov/assets/entities/health/attachments/NAS_Annual_report_2014_FINAL.pdf. Accessed 05/10, 2016.
- 7.Bunn TL, Ouyang BJ, Slavova S. Newborn Congenital Malformations Associated With Prenatal Exposure to Drugs in Kentucky, 2009–2013. South Med J. 2016 Feb;109(2):124–129. doi: 10.14423/SMJ.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 8.Stabler ME, Long DL, Chertok IR, Giacobbi PR, Pilkerton C, Lander LR. Neonatal Abstinence Syndrome in West Virginia Substate Regions, 2007–2013. The Journal of Rural Health. 2016 doi: 10.1111/jrh.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lind JN, Petersen EE, Lederer PA, et al. Infant and maternal characteristics in neonatal abstinence syndrome–selected hospitals in Florida, 2010–2011. MMWR Morbidity and Mortality Weekly Report. 2015 Mar 6;64(8):213–216. [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Medicare and Medicaid Innovation. State Innovation Models Initiative: Round Two. 2014 Available at: https://innovation.cms.gov/initiatives/State-Innovations-Round-Two/. Accessed 05/11, 2016.
- 11.Community Health Council of Knoxville/Knox County Tennessee. Community Health Improvement Plan. 2016 Available at: http://healthyknox.org/CHIP%202016.pdf Accessed 07/13, 2016.
- 12.Tennessee Department of Health. Health Information Tennessee. 2015 Available at: http://hit.state.tn.us/index.shtml. Accessed 12/09, 2015.
- 13.Robert Wood Johnson Foundation. County Health Rankings. 2015 Available at: http://www.countyhealthrankings.org/. Accessed 04/28, 2015.
- 14.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. American Journal of Obstetrics & Gynecology. 202(4):335–343. doi: 10.1016/j.ajog.2009.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Census Bureau. Quick Facts. 2013 Available at: http://www.indexmundi.com/facts/united-states/quick-facts/tennessee/percent-of-people-of-all-ages-in-poverty. Accessed 12/09, 2015.
- 16.Tennessee Department of Health Care Finance & Administration. Neonatal Abstinence Syndrome Among TennCare Enrollees – 2014 data. 2015 Available at: https://www.tn.gov/assets/entities/tenncare/attachments/TennCareNASData2014.pdf. Accessed 05/11, 2016.
- 17.Warren MD, Miller AM, Traylor J, Bauer A, Patrick SW. Implementation of a Statewide Surveillance System for Neonatal Abstinence Syndrome—Tennessee, 2013. MMWR Morbidity and Mortality Weekly Report. 2015;64(5):125–128. [PMC free article] [PubMed] [Google Scholar]
- 18.Tennessee Department of Health. NAS Update Archive. 2015 Available at: http://tn.gov/health/article/nas-update-archive. Accessed 12/09, 2015.
- 19.Halverson JA, Harner J, Appalachian Regional Commission An Analysis of Disparities in Health Status and Access to Health Care in the Appalachian Region. 2004 Available at: http://www.arc.gov/research/researchreportdetails.asp?REPORT_ID=82.
- 20.Halverson JA, Bischak G, Appalachian Regional Commission Underlying Socioeconomic Factors Influencing Health Disparities in the Appalachian Region. 2008 Available at: http://www.arc.gov/assets/research_reports/SocioeconomicFactorsInfluencingHealthDisparitiesinAppalachianRegion5.pdf.
- 21.Havens JR, Talbert JC, Walker R, Leedham C, Leukefeld CG. Trends in controlled-release oxycodone (OxyContin) prescribing among Medicaid recipients in Kentucky, 1998–2002. J Rural Health Summer. 2006;22(3):276–278. doi: 10.1111/j.1748-0361.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 22.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. The Journal of Pain: Official journal of the American Pain Society. 2012 Oct;13(10):988–996. doi: 10.1016/j.jpain.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zibbell JE, Iqbal K, Patel R, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged≤ 30 years-Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morbidity and Mortality Weekly Report. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 24.Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: a cross-sectional comparison and retrospective survival analysis. Addiction. 2012;107(3):587–596. doi: 10.1111/j.1360-0443.2011.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute on Drug Abuse, U.S. National Institutes of Health. What is the scope of heroin use in the United States? 2016 Available at: https://www.drugabuse.gov/publications/research-reports/heroin/scope-heroin-use-in-united-states. Accessed 06/22, 2016.
- 26.Ohio Substance Abuse Monitoring Network. Drug Abuse Trends in the Cincinnati Region. 2013 Available at: http://mha.ohio.gov/Portals/0/assets/Research/OSAM-TRI/Cincinnati.pdf. Accessed 06/28, 2016.
- 27.Beste LA, Bondurant HC, Ioannou GN. Prevalence and management of chronic hepatitis C virus infection in women. The Medical Clinics of North America. 2015 May;99(3):575–586. doi: 10.1016/j.mcna.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Tennessee General Assembly. Controlled Substance Monitoring Database Program. 2012 Available at: https://www.tn.gov/health/article/CSMD-faq.
- 29.Tennessee General Assembly. Safe Harbor Act of 2013. Available at: https://legiscan.com/TN/research/SB0459/2013. Accessed 12/12, 2015.
- 30.Tennessee Department of Health. Neonatal Abstinence Syndrome. 2015 Available at: http://tn.gov/health/topic/nas. Accessed 12/09, 2015.
- 31.Clarke K, Harris D, Zweifler JA, Lasher M, Mortimer RB, Hughes S. The Significance of Harm Reduction as a Social and Health Care Intervention for Injecting Drug Users: An Exploratory Study of a Needle Exchange Program in Fresno, California. Social Work in Public Health. 2016 May 11;:1–10. doi: 10.1080/19371918.2015.1137522. [DOI] [PMC free article] [PubMed] [Google Scholar]


