Abstract
Recent studies point to the potential role of the (pituitary) adenylate cyclase activating polypeptide receptor 1 (ADCYAP1R1) gene, which has been implicated in stress response, in posttraumatic stress disorder (PTSD). Multiple genetic association studies have examined potential PTSD risk related to this gene, with mixed results. We conducted a meta-analysis of rs2267735 in ADCYAP1R1 in PTSD. A literature search was conducted using PubMed and PsycINFO, resulting in nine studies that met criteria for inclusion in analysis. Biostat’s Comprehensive Meta-Analysis was used to conduct the main meta-analysis on the combined sex sample, as well as two subanalyses examining effects separately in female and male participants. Results indicated that the C allele of rs2267735 conferred significant risk for PTSD in the combined sex data, OR = 1.210, 95% CI [1.007, 1.454], p = .042, and in the subsample of women and girls, OR = 1.328, 95% CI [1.026, 1.719], p = .031; but not in the subsample of men and boys, OR = 0.964, 95% CI [0.733, 1.269], p = .796. These results provide evidence for an association between ADCYAP1R1 and PTSD and indicate that there may indeed be sex differences. Implications of these findings, including the role of rs2267735 as one modulator of the stress system, are discussed.
Exposure to traumatic events is a common experience, with recent worldwide surveys documenting that around 70% of individuals have experienced at least one trauma in their lifetime (Benjet et al., 2016; Liu et al., 2017), although the range varies across countries, likely associated with income, development, and conflict status. Of those who experience a traumatic event, between 5 and 31% meet lifetime criteria for posttraumatic stress disorder (PTSD; Adams & Boscarino, 2006; Breslau et al., 1998), with the number of traumas experienced increasing this risk in a dose-dependent manner (e.g., Kolassa et al., 2010; Neuner et al., 2004). Those who develop PTSD are at increased risk for outcomes such as major depression (Breslau, Davis, Peterson, & Schultz, 2000), substance misuse (Breslau, Davis, & Schultz, 2003), physical health problems (Zayfert, Dums, Ferguson, & Hegel, 2002) and unemployment and marital instability (Kessler, 2000).
In an effort to inform our understanding of the biological underpinnings of PTSD, researchers have examined candidate genes influencing the stress response system, including pituitary adenylate cyclase-activating polypeptide (PACAP) receptor (PAC1), encoded by the gene ADCYAP1R1 and specifically the single nucleotide polymorphism (SNP) rs2267735. Chronic and/or unpredictable stress leads to an increase in PACAP in the bed nucleus of stria terminalis (BNST; the “extended amygdala”), which spurs heightened physiological responses to anxiety-provoking stimuli (e.g., Walker, Toufexis, & Davis, 2003). The BNST is also implicated in communication between the limbic cognitive centers and nuclei involved in stress processing (Crestani et al., 2013). Sustained or unpredictable stress also induces PACAP in the hypothalamic paraventricular nucleus (PVN; Legradi, Hannibal, & Lechan, 1998). This process spurs the release of corticotrophin-releasing hormone (CRH; Legradi et al., 1998) and adrenocorticotrophic hormone (Sapolsky, Romero, & Munck, 2000), thus influencing an individual’s ability to regulate stress response. Recent work has found a link between rs2267735 in ADCYAP1R1 and decreased hippocampal activity (implicated in assigning emotional valences to events; Brady & Sinha, 2005) during contextual fear conditioning tasks among female participants, but not male participants (Pohlack et al., 2015). Therefore, following contact with feared stimuli, some individuals, and in particular female participants at risk in terms of ADCYAP1R1, may experience more pronounced physiological symptoms of anxiety, problems extinguishing fear-related cognitions, and difficulty modulating these stress responses, placing them at increased risk for PTSD.
These basic stress response processes are thought to have multiple etiologic influences, both genetic and environmental in nature, that are mutually impactful. Preclinical and clinical evidence suggests that although heritable influences exist for the basic stress response, environmental events such as early life stressors may also contribute to subsequent stress responding, perhaps via epigenetic mechanisms that may also increase risk for distress-related phenotypes, including PTSD, following trauma exposure (Wilker & Kolassa, 2013). Given that PTSD is moderately heritable (Sartor et al., 2012), an investigation of the PACAP system in predicting PTSD is critically important. In a landmark study by Ressler et al. (2011), which examined 44 SNPs spanning PACAP and PAC1, the SNP rs2267735 (in ADCYAP1R1) predicted PTSD among female participants only. Since this study, numerous replication attempts have led to mixed results, with some finding significant associations and others failing to find an effect (e.g., Chang et al., 2012; Rothbaum et al., 2014).
Meta-analytic approaches have recently been applied to candidate gene studies of PTSD as a systematic method for reconciling inconsistent findings. For example, research examining the impact of genetic variants in the dopaminergic system and PTSD has yielded mixed results (e.g., Gelernter et al., 1999; Voisey et al., 2009). However, meta-analyses suggest that the SNP rs1800498 in DRD2 and the 3’-UTR variable number tandem repeat (VNTR) in SLC6A3 are significantly associated with PTSD (Li et al., 2016), whereas the association between rs4680 in COMT and PTSD was nonsignificant. To our knowledge, a meta-analysis of the association between variants in ADCYAP1R1 and PTSD has not been conducted. Thus, we sought to perform meta-analyses to elucidate the associations among rs2267735 (the most commonly studied ADCYAP1R1 polymorphism in the context of risk for PTSD), traumatic event exposure, and the development of PTSD. Given the extant literature, we hypothesized that the meta-analysis would show a significant effect of rs2267735 on PTSD, but that when analyzed separately by sex, there would be a significant effect for female, but not male, participants.
Method
Procedure
Search and selection of studies for inclusion
The present study aimed to identify existing studies examining the main effects of the ADCYAP1R1 gene and PTSD. Any variants of the gene ADCYAP1R1 with sufficient data to calculate a minimum of 3 effect sizes were included in the final selection (note that only rs2267735 met this criteria). Potential studies were identified through the PubMed and PsycINFO databases (as of September 2015). To ensure that the meta-analysis was up-to-date, an additional date-restricted search was conducted to identify any new studies that came out between September 2015 (initial search) and November 2016. Search terms were as follows: [posttraumatic stress disorder OR PTSD OR traumatic stress] AND [gene OR genetic] AND [ADCYAP1R1 OR PAC1 OR pituitary adenylate cyclase-activating polypeptide type 1 receptor]. Additionally, the reference sections of two recent PTSD genetics review articles (Almli, Fani, Smith, & Ressler, 2014; Voisey, Young, Lawford, & Morris, 2014) were examined to identify any articles that may have been missed through the above search strategy.
Screening of search results
Two review authors (M.J.L. and C.M.S.) independently screened search results to select studies for possible inclusion. The following inclusion criteria were applied to titles and abstracts: (a) original research; (b) use of human subjects; (c) association study including the ADCYAP1R1 gene; (d) PTSD as an outcome; and (e) trauma-exposed control group, with one exception. Specifically, for participants within one dataset (i.e., the Grady Trauma Project), some individuals were not trauma exposed. However, the extremely high level of trauma and very rare exceptions in exposure warranted retention of studies using this sample. Only effect sizes resulting from main effects of ADCYAP1R1 and PTSD were included (effect sizes resulting from G × E analyses were excluded). In cases where the criteria were unclear, articles were more thoroughly examined and a consensus determination was made. Supplemental material was also reviewed for identification and extraction of relevant data. Further, an existing PTSD genome-wide association study (Nievergelt et al., 2015) included additional examination of the ADCYAP1R1 gene. This was excluded in our meta-analysis given that limited information on the specific SNP was available.
Data extraction and coding
Two review authors (M.J.L. and C.M.S.) coded each of the included articles based on a predetermined coding manual that included relevant variables (e.g., lifetime vs. current PTSD, diagnosis vs. severity, gender, race/ethnicity) and specific variables concerning the quality of studies (e.g., inclusion/exclusion criteria, statistical corrections) developed and agreed upon by all authors. Following data extraction and entry, two independent reviewers separately checked the information for agreement across coders. There was 93.0% agreement between M.J.L. and C.M.S. Discrepancies were resolved through discussion until consensus was reached.
Effect Size Calculation
An odds ratio (OR) and confidence interval (CI) were calculated for each study to examine whether the C risk allele was associated with increased likelihood of PTSD diagnoses and symptoms. Two studies (Ressler et al., 2011; Solovieff et al., 2014), modeled the number of copies (i.e., 0, 1, 2) of the G allele as the risk allele, but were transformed here to represent the inverse direction of effects, that is accounting for risk in relation to the C allele. In addition to OR data, descriptive data, including means and standard deviations or frequency of occurrence, were used to calculate effect size. When studies reported results from a multiple regression analysis, OR was calculated using the natural exponential of the unstandardized regression coefficient. In cases where no estimate of standard error was reported, the confidence interval was obtained using equations provided by Altman and Bland (2011). We contacted the authors to request further information in cases of missing data necessary for effect size computation and all studies that met inclusionary criteria were included in the analyses.
Data Analysis
Three separate meta-analyses were conducted using Biostat’s Comprehensive Meta-analysis (www.meta-analysis.com; Borenstein, Hedges, Higgins, & Rothstein, 2015). First, the main meta-analysis was conducted on effect size data with combined sexes (κ = 10 samples from 9 total studies). Next, two additional subanalyses were conducted using studies with sufficient data to calculate effect sizes separately for women (κ = 9) and men (κ = 4). Models employed random effects, which account for sampling error and random effects variance (Lipsey & Wilson, 2001). For completeness, results from a fixed effects model are also presented here. For the main meta-analysis, we conducted statistical tests of two moderators (percent African American and percent female) selected a priori with weighted regression analysis (meta-regression) using a mixed effects model. Percent African American was examined as a moderator via meta-regression for subanalyses that included a sufficient number of studies.
Homogeneity of the effect size distribution was examined with visual inspection, forest plots, and the Q statistic and I2(95% CI) index. The I2 statistic quantifies the amount of statistical impact of heterogeneity on the total observed variation (Borenstein, Hedges, Higgins, & Rothstein, 2009). Interpretations of low (I2 = 25.0%), moderate (I2 = 50.0%), or high (I2 = 75.0%) have been recommended by Higgins, Thompson, Deeks, and Altman (2003). To assess publication bias, we utilized Egger’s regression index (Egger, Smith, Schneider, & Minder, 1997), the funnel plot, Duval and Tweedie’s (2000) trim and fill, and Rosenthal’s (1979) failsafe N.
Many studies exploring genetic associations with PTSD reported findings from multiple outcomes and we prioritized those with sufficient data to calculate OR. For example, some studies reported effect sizes for both PTSD severity and diagnosis or reported PTSD outcomes across lifetime and isolated to specific time periods. Shown in Table S1, we implemented a protocol to handle studies with multiple effect sizes to adhere to the assumption of independence, which refers to the assumption that each measure of effect is representative of independent studies. When data were presented separately for subgroups within an individual study, we conducted a meta-analysis to compute the combined effect size across subgroups under a fixed effects model as recommended by Borenstein, Hedges, Higgins, & Rothstein, (2009).
Finally, because some studies used an additive model, examining the effect of having 0, 1, or 2 risk alleles (C) and others compared high-risk genotype status (CC) with low-risk genotype status (CG/GG), a sensitivity analysis was performed to examine mean effect sizes separately for each type of model. An additional sensitivity analysis to account for study outcome, separately examining the mean effect sizes of studies measuring PTSD diagnosis and studies measuring PTSD severity, was also conducted.
Results
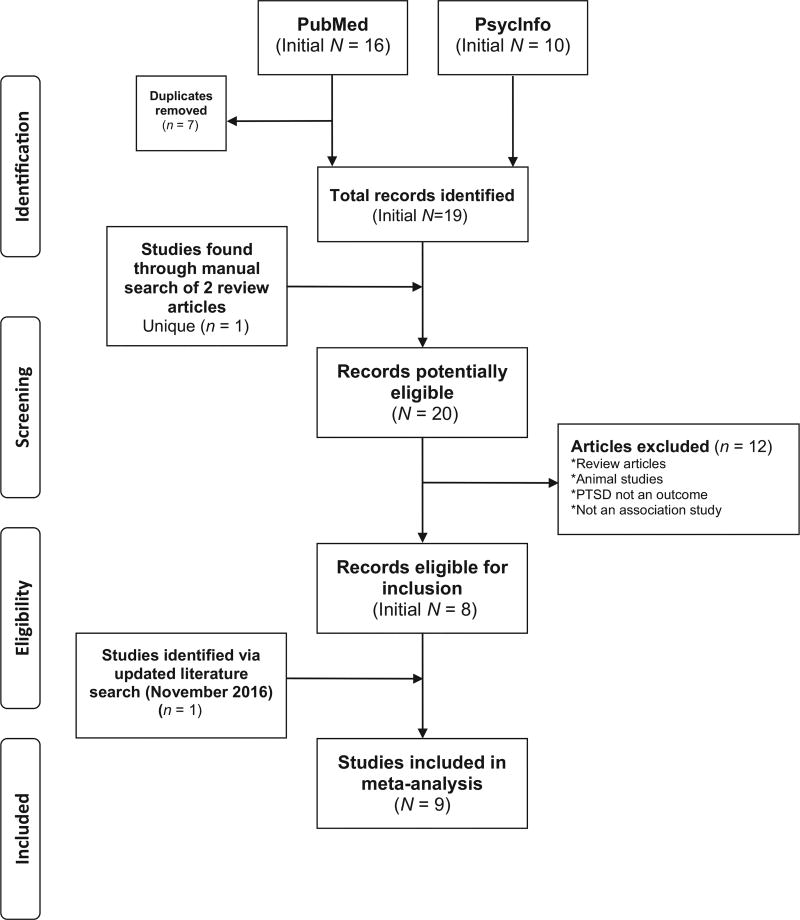
Figure 1 details findings from the search steps, which resulted in 20 unique articles. The only ADCYAP1R1 polymorphism assessed by a minimum of three studies was rs2267735, so this SNP was chosen for meta-analysis. Several manuscripts conducted analyses on multiple samples (Almli et al., 2013; Chang et al., 2012; Ressler et al., 2011; two samples each), bringing the total number of potential samples included for analysis to 12. Two samples were excluded, one from Chang (due to the use of nontrauma-exposed controls) and one from Almli (due to identical data to that used in Ressler et al., 2011). Thus, a total of 10 samples, from 9 different manuscripts (7 from the initial search results, 1 from the date-restricted literature search, and 1 from review papers), met criteria for inclusion in analysis (see Tables 1 and 2 for a summary of each study, combined and separately by sex).
Figure 1.
PRISMA-style flow chart showing selection of studies for meta-analysis of rs2267735 (ADCYAP1R1 gene) and posttraumatic stress disorder (PTSD). Figure is consistent with guidelines provided by Moher, Liberati, Tetzlaff, Altman, and the PRISMA Group (2009).
Table 1.
Included Studies and Outcome Measures (Combined Sexes)
| Study | Outcome | Sample recruitment |
PTSD measure |
Trauma assessment |
N | %AA | % F | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| General | Child | |||||||||
| Almli et al., 2013 | Current PTSD severity | Outpatient clinic-baseda | PSS | TEId | CTQ | 1,163 | 100.0 | 73.8 | 0.84 | [0.28, 2.48] |
| Chang et al., 2012 | Current PTSD diagnosis | Population-based | Structured interviewc | Othere | CTQ | 2,528 | 0 | 100.0 | 0.94 | [0.64, 1.39] |
| Lowe et al., 2015 | Current PTSD severity | Outpatient clinic-baseda | PSS | TEI | NA | 1,361 | 94.1 | 100.0 | 2.10 | [1.04, 4.24] |
| Ressler et al., 2011a | Current PTSD diagnosis | Outpatient clinic-baseda | PSS | TEI | CTQ | 798 | 92.3 | 63.0 | 1.43 | [1.14, 1.80] |
| Ressler et al., 2011b | Current PTSD diagnosis | Outpatient clinic-baseda | PSS | TEI | CTQ | 439 | 92.4 | 59.2 | 1.20 | [0.89, 1.63] |
| Rothbaum et al., 2014 | Current PTSD severity | Outpatient clinic-baseda | PSS | TEI | CTQ | 34 | 82.4 | 61.5 | 2.45 | [0.57, 10.53] |
| Solovieff et al., 2014 | Lifetime PTSD diagnosis | Population-based | Structured interviewc | Other | NA | 2,538 | 0 | 100.0 | 0.96 | [0.85, 1.08] |
| Stevens et al., 2014 | Current PTSD severity | Hospital admission-basedb | PSS | TEI | CTQ | 49 | 100.0 | 100.0 | 1.29 | [0.46, 3.58] |
| Uddin et al., 2013 | Current PTSD diagnosis | Population-based | Structured interviewc | Other | Othere | 401 | 83.1 | 100.0 | 1.65 | [0.71, 3.84] |
| Wang et al., 2013 | Current PTSD severity | Epidemiologic exposure-based | PCL | NR | NR | 319 | 0 | 70.5 | 1.39 | [0.85, 2.26] |
Note. All samples were primarily civilian and most did not examine a specific traumatic event (with the exception of Wang et al., 2013, which used an earthquake sample). PTSD = posttramautic stress disorder; AA = African American; PSS = Posttraumatic Symptom Scale; PCL = Posttraumatic Stress Disorder Checklist; CTQ = Childhood Trauma Questionnaire; NA = not applicable; NR = not reported; TEI = Traumatic Events Inventory.
Medical or nonmedical, includes Veteran Affairs.
Intensive care unit/emergency department/inpatient.
Diagnostic measure used was unspecified structured diagnostic interview, based on criteria according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM-IV; American Psychiatric Association, 1994).
Presented results both adjusted and unadjusted for trauma, unadjusted used in meta-analysis.
Only presented trauma-adjusted results.
Quality Assessment
Studies included in the final analysis either clearly described recruitment processes and inclusion/exclusion criteria in published manuscripts, or provided details to the current study authors in separate correspondence. All included studies identified a psychometrically sound instrument or clinical interview used to measure PTSD and seven studies (Almli et al., 2013; Chang et al., 2012; Lowe et al., 2015; Ressler et al., 2011a,b; Stevens et al., 2014; Uddin et al., 2013) assessed comorbidities. All included studies assessed deviation based on the Hardy-Weinberg equilibrium. Only one study reported a deviation for rs2267735 (Rothbaum et al., 2014), but chose to include it given that it was a trend after multiple testing correction. Finally, of the studies testing multiple comparisons (multiple outcomes or multiple SNPs), five applied statistical corrections (Lowe et al., 2015; Ressler et al., 2011a,b; Solovieff et al., 2014; Uddin et al., 2013) whereas four did not report implementing corrections to address false-positive results (Chang et al., 2012; Rothbaum et al., 2014; Stevens et al., 2014; Wang et al., 2013).
Primary Analyses
Main meta-analysis (combined sexes)
A total of 9,630 participants from 10 samples were included in the meta-analysis examining associations between variation within the ADCYAP1R1 gene (PACAP receptor) SNP rs2267735 and PTSD with effect sizes combined across sexes. Note that five samples only included female participants and were also included in this analysis. Participants with the high-risk allele or genotype (C or CC) were more likely than participants with the low-risk allele or genotype (G or CG/GG) to demonstrate/report PTSD symptoms or diagnosis, OR = 1.210, 95% CI [1.007, 1.454], p = .042 (see Table 3). Effect sizes ranged from OR = 0.840 to 2.446. The heterogeneity of variance analysis was significant, indicating significant between-study variance. A random-effects multiple meta-regression model was significant overall, Q(2) = 11.13, p = .004, R2 analog = 1.0; however, the individual moderator variables (percent female and percent African American) did not emerge as significant (Table S2). This finding appears to be limited by low variability, with the majority of studies including predominantly female samples (κ = 8, > 70.0%) and African American samples (κ = 7, ≥ 80.0%). Trim and fill analysis indicated the imputation of three studies to reduce bias (see Figure S2a), resulting in only a slight reduction in the overall effect size, OR = 1.139, 95% CI [0.955, 1.358]. Egger’s regression was not significant, B = 1.167, SE = 0.577, t(8) = 2.024, p = .078, and Rosenthal’s N indicated a minimum of 12 null hypothesis studies to lead to a p value at or above .05. Taken together, these findings indicate minimal risk for publication bias.
Table 3.
Summary of All Meta-Analytic Results
| Heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Sample | n | k | Model | OR | 95% CI | p | Q | I2 |
| Combined sexes | 9,630 | 10 | Fixed | 1.085 | [0.990, 1.190] | .082 | 17.279* | 47.913 |
| Random | 1.210 | [1.007, 1.454] | .042 | |||||
| Female participants | 8,723 | 9 | Fixed | 1.098 | [0.995, 1.211] | .062 | 24.168* | 66.898 |
| Random | 1.328 | [1.026, 1.719] | .031 | |||||
| Male participants | 873 | 4 | Fixed | 0.964 | [0.733, 1.269] | .796 | 0.610 | 0 |
| Random | 0.964 | [0.733, 1.269] | .796 | |||||
Note. NR = not reported.
p < .05.
Subanalysis a (female subsample)
When the meta-analysis was conducted separately in the female subsample 8,723 participants from nine samples were included. As shown in Table 3, participants with the high-risk allele or genotype (C or CC) were more likely than participants with the low-risk allele or genotype (G or CG/GG) to demonstrate/report PTSD symptoms or diagnosis, OR = 1.328, 95% CI [1.026, 1.719], p = .031. Effect sizes ranged from OR = 0.716 to 2.096. The heterogeneity of variance analysis was significant, indicating significant between-study variance. Meta-regression revealed a trend of increasing effect sizes, κ = 7, B = 0.0046, 95% CI [−0.0006, 0.0098], p = .081, R2 analog = .82, for higher percent African American. Analysis of publication bias indicated minimal risk with a Rosenthal’s N of 16 null hypothesis studies to lead to p ≥ .05. As displayed in Figure S2b, Trim and Fill analysis did not recommend the imputation of any studies. Egger’s regression was not significant, B = 1.410, SE = 0.775, t(7) = 1.818, p = .112, indicating minimal publication bias.
Subanalysis b (male subsample)
Finally, four samples, comprised of 873 participants, were included in the meta-analysis examining rs2267735 and PTSD in male participants only. The effect was not significant, OR = 0.964, 95% CI [0.733, 1.269], p = .796 (see Table 3). Effect sizes ranged from OR = 0.836 to 1.347. The heterogeneity of variance analysis was not significant, indicating minimal between-study variance. Due to the low number of studies, meta-regression was not conducted. The imputation of one study to reduce bias (see Figure S2c) was recommended by Trim and Fill analysis resulting in a slightly reduced effect size of OR = 0.959, 95% CI [0.730, 1.260]. Egger’s regression was not significant, B = 0.235, SE = 0.574, t(2) = 0.410, p = .721, indicating minimal publication bias. Because the meta-analysis was not statistically significant, Rosenthal’s N was not calculated.
Sensitivity Analyses (Combined Sample)
Five samples comprised of 6,299 participants used an additive model, examining the effect of having 0, 1, or 2 risk alleles (C; see Table S3). When examined separately, the studies resulted in a mean effect size of OR = 1.215 that was not statistically significant, 95% CI [0.937, 1.574], p = .142. Results were similar for the five samples that compared high-risk genotype status (CC) to low-risk genotype status (CG/GG; n = 3,331), OR = 1.186, 95% CI [0.905, 1.554], p = .216. Analysis of variance (ANOVA) analog did not reveal significant differences between effect sizes from studies using an additive model (number of C alleles) and studies comparing CC to CG/GG, Q(1) = 0.016, p = .900.
Regarding PTSD outcome, five samples (n = 6,704) provided sufficient data to calculate effect sizes for PTSD diagnosis and five samples (n = 2,926) provided sufficient data to calculate effect sizes for PTSD severity (see Table S4). Results were statistically significant when separately analyzed for severity, OR = 1.488, 95% CI [1.056, 2.096], p = .023, but not for diagnosis, OR = 1.141, 95% CI [0.923, 1.410], p = .221. Note that this discrepancy is likely due to differences in power considering that dichotomization of quantitative data typically results in reduced power and effect sizes in bivariate correlations (MacCallum, Zhang, Preacher, & Rucker, 2002). Further, ANOVA analog did not reveal significant differences between the two types of studies, Q(1) = 1.667, p = .197.
Discussion
This is, to our knowledge, the first meta-analysis of the ADCYAP1R1 gene and PTSD, filling an important gap in the literature, as findings are mixed with regard to positive associations and nonreplications. Although numerous variants of ADCYAP1R1 have been included in individual studies across multiple phenotypes, the only polymorphism that was consistently included in PTSD investigations and thus able to be meta-analyzed was rs2267735. Findings from the present investigation provide support for an association between rs2267735 within the ADCYAP1R1 gene (PACAP receptor) and PTSD, with minimal risk of publication bias observed. Specifically, our findings suggest that the “C” allele is associated with increased risk for PTSD. Effect sizes did not differ between studies that examined the additive effect of the C risk allele (i.e., 0, 1, or 2 copies) and for those that compared individuals who were CC homozygous relative with those who were G carriers. Note that the overall effect size for the combined sample (OR = 1.21) was small, but in line with what would be expected based on the genetics literature (e.g., Gibson, 2012). The effect sizes for PTSD severity and PTSD diagnostic status did not differ. Additionally, percent female and percent African American did not moderate the main effect of rs2267735 on PTSD, likely due to low variability across studies. However, consistent with existing frameworks for understanding the biological influence of ADCYAP1R1, some of our analyses suggest that the effect may be especially strong in women. However, the lack of effect seen for male participants should be considered preliminary, given the smaller sample size (~ 10.0% of the overall sample) and lower power to detect the effect size from the full sample within the male subsample.
The biological implication of these findings is substantial. PACAP is a critical component of the regulation of both central and peripheral stress responses, modulating the hypothalamic–pituitary–adrenal axis through activation of CRH as well as influence on catecholamine transmission and biosynthesis (Mustafa, 2013; Stroth, Holighaus, Ait-Ali, & Eiden, 2011; Vaudry et al., 2009). PACAP is highly preserved across species, permitting the use of transgenic animal models to inform our understanding of PACAP as related to fear, stress, and anxiety. Animal models examining PACAP and PAC1 receptor function using transgenic models have implicated PACAP/PAC1R in anxiety responses, startle and fear behavior, memory, pain, and hypothalamic–pituitary–adrenal function/responsiveness (including normal and stress-evoked corticosterone responses (Hammack & May, 2015; Meloni, Venkataraman, Donahue, & Carlezon, 2016). Consistent with evidence for the effects of PACAP/PAC1R on regulation of expression of CRH activity, a critical role of PACAP expression and signaling has been observed in the bed nucleus of the stria terminalis, the central nucleus of the amygdala, and the basolateral amygdala (Hammack & May, 2015; Lezak et al., 2014; Mustafa, 2013). Importantly, the role of PACAP is shown not only during acute, but also chronic stress (Mustafa et al., 2015), with some evidence that stress effects on anxious and depressive behaviors were contingent on PACAP (Lehmann, Mustafa, Eiden, Herkenham, & Eiden, 2013). Thus, elucidating the role of genetic variation in this locus in relation to PTSD is a critical step forward in understanding the pathogenesis of this key stress-related disorder.
Our results suggest that “C” is the risk allele. However, of some importance, there is considerable variability across studies with respect to how alleles are represented in analyses. Specifically, whereas some studies have examined the effect of the high-risk genotype (CC) vs. low-risk genotypes (GC and GG groups are combined), others have assumed a dose effect (i.e., analyses assume that having 2 C alleles is “worse” than having 1 allele, which in turn is worse than having none). Interestingly, the effect sizes resulting from studies that coded the number of C alleles in an additive fashion (i.e., 0, 1, 2) compared with those who examined the effect of having two risk alleles (CC vs. CG/GG) did not differ. However, careful attention to coding of alleles in future studies, as well as testing for differential effects for type of genetic model is needed.
An important caveat to our sex-specific pattern of findings is that the number of male participants in the investigations included in the meta-analysis is modest; therefore, the male-only analyses should be considered preliminary given that we were underpowered to detect an effect size of 1.21 (from the full sample) within the male subsample (power ~50.0%). However, overall, our analyses supported a particularly strong effect of rs2267735 on PTSD in the female subsample, consistent with evidence that estrogen may regulate PACAP/PAC1R expression. Indeed, animal models have shown estradiol-evoked upregulation of PACAP and PAC1R transcripts in the BNST of ovariectomized female rats (Ressler et al., 2011). Functional hippocampal activity during cued and contextual fear conditioning has also been examined in healthy controls, with PAC1R-dependent hippocampal activation observed during late contextual fear acquisition in female participants (Pohlack et al., 2015). Importantly, however, sex effects have not been observed in research examining dark-enhanced startle in prepubertal children (6–13 years of age), with ADCYAP1R1 rs2267735 found to be associated with startle reactivity across both girls and boys (Jovanovic et al., 2013), suggesting perhaps a later developmental onset of the sex-dependent findings of this locus.
Moderation analyses aimed at understanding the potential for race or ancestry effects were limited by the fact that only African American self-reported race could be tested in published samples. Further, the studies with greater proportions of African American participants were also comprised of greater proportions of female participants, making it difficult to draw strong conclusions about whether there may be unique ancestry effects. HapMap data does point to slightly increased prevalence of the C allele in Yoruban (African) samples relative to European samples (International HapMap Consortium, 2005; www.hapmap.org). Research using samples in which diversity of ancestry and gender are not confounded is needed to better understand whether or how the relation between ADCYAP1R1 and PTSD may be affected by ancestry or sex.
Although our analyses did not support the presence of a “file drawer” problem, it is impossible to completely exclude the possibility that publication bias may exist and that studies that have not detected a significant association between ADCYAP1R1 and PTSD are unpublished. Another important limitation is that, due to characteristics of individuals within included study samples, the effects of key moderators may have been confounded with one another. For example, there is reason to believe that there may be differences as a function of sex, ancestry, and trauma type. However, the samples that are disproportionately female also report African American ancestry and report high levels of exposures to early life and more recent urban traumas whereas the samples with greater numbers of male participants have fewer African Americans and are more likely to report military trauma. There are also fewer studies that have examined men and boys, emphasizing the need for additional work to parse out sex differences. Further, one limitation of meta-analysis is reduced power for detecting variability across moderators (Hedges & Pigott, 2004), particularly for analyses including a small number of studies (e.g., κ = 4). Given this, we were unable to test ancestry as a moderator within the male-only subsample. Thus, this line of research would benefit from replication in larger samples of varied trauma exposures and ancestries, which may allow for disentanglement of potential moderating effects. The focus on current PTSD (vs. lifetime, given the use of current by the majority of studies) represents an additional limitation, given that individuals at genetic risk may not have PTSD when assessed (Koenen et al., 2009). However, current PTSD was more commonly assessed in the extant literature, and thus, analyses of lifetime PTSD were not possible.
Another consideration and direction for future PACAP research is the potential that expression differences may be related not only to genotype, but also to epigenetic changes such as DNA methylation (Dias & Ressler, 2013). Prior research has shown differential methylation patterns in the promoter region of ADCYAP1R1 (cg11218385) as a function of violence exposure (Chen et al., 2013). Interestingly, in Chen et al.’s (2013) sample of children, both ADCYAP1R1 rs2267735 genotype and methylation status were associated with increased odds of asthma. In addition, violence exposure was associated with methylation among children over 9 years of age. Research has also implicated PACAP in immune modulation and inhibition of inflammation (Abad & Var Tan, 2016). Indeed, there is evidence for PACAP’s influence on Th2 cells, leading some to suggest PACAP as a possible therapeutic agent with respect to Th1:Th2 T-cell phenotypes (Abad & Var Tan, 2016; Delgado & Ganea, 2001; Delgado, Leceta, & Ganea, 2002). These findings are relevant to PTSD, as research has repeatedly shown evidence for immune alterations in individuals with PTSD (Gill, Saligan, Woods, & Page, 2009).
We present, to our knowledge, results of the first meta-analysis examining the role of the ADCYAP1R1 gene, specifically rs2267735, in PTSD. Although not without limitations, our results indicate that the ‘C’ allele of rs2267735 may increase PTSD risk, particularly for female participants. This provides support for closer examination of the PACAP genes and their relevance to stress response in the aftermath of trauma on risk for PTSD.
Supplementary Material
Table 2.
Included Studies and Outcome Measures (Separated by Sex)
| Female participants | Male participants | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Study | N | % AA | OR | 95% CI | N | % AA | OR | 95% CI |
| Almli et al., 2013 | 858 | 100.0 | 0.72 | [0.20, 2.50] | 305 | 100.0 | 1.35 | [0.16, 11.60] |
| Chang et al., 2012 | 2,528 | 0 | 0.94 | [0.64, 1.39] | – | – | – | – |
| Lowe et al., 2015 | 1,361 | 94.1 | 2.10 | [1.04, 4.24] | – | – | – | – |
| Ressler et al., 2011a | 503 | NR | 1.72 | [1.29, 2.30] | 295 | NR | 1.04 | [0.71, 1.52] |
| Ressler et al., 2011b | 260 | NR | 1.57 | [1.05, 2.34] | 179 | NR | 0.84 | [0.52, 1.33] |
| Rothbaum et al., 2014 | – | – | – | – | – | – | – | – |
| Solovieff et al., 2014 | 2,538 | 0 | 0.96 | [0.85, 1.08] | – | – | – | – |
| Stevens et al., 2014 | 49 | 100.0 | 1.29 | [0.46, 3.58] | – | – | – | – |
| Uddin et al., 2013 | 401 | 83.1 | 1.65 | [0.71, 3.84] | – | – | – | – |
| Wang et al., 2013 | 225 | 0 | 1.63 | [0.80, 2.98] | 94 | 0 | 1.01 | [0.44, 2.32] |
Note. AA = African American.
Acknowledgments
Ms. Lind and Dr. Sheerin are supported by grant #T32 MH020030 from the National Institute of Mental Health (NIMH). Dr. Bountress is supported by grant #T32 MH18869 from the NIMH. Dr. Amstadter’s time is partially funded by grant #K02 AA023239 from the National Institute on Alcohol Abuse and Alcoholism. Drs. Nugent and Marraccini’s time is funded by grants R01MH105379 and R01MH108641 from the NIMH.
We thank all authors of included studies who contributed their data for use in our analysis and responded to our e-mail requests for additional information concerning their samples. We also thank the undergraduate research assistants in the Amstadter lab, particularly Katie Hummel and Laurel Kovalchick for their help with reliability coding.
References
- Abad C, Var Tan Y. VIP and PACAP as regulators of immunity: New perspectives from a receptor point of view. Journal of Clinical&Experimental Neuroimmunology. 2016;1(104) https://doi.org/10.4172/jceni.1000104. [Google Scholar]
- Adams RE, Boscarino JA. Predictors of PTSD and delayed PTSD after disaster: The impact of exposure and psychosocial resources. Journal of Nervous and Mental Disease. 2006;194:485–493. doi: 10.1097/01.nmd.0000228503.95503.e9. https://doi.org/10.1097/01.nmd.0000228503.95503.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Fani N, Smith AK, Ressler KJ. Genetic approaches to understanding post-traumatic stress disorder. International Journal of Neuropsychopharmacology. 2014;17:355–370. doi: 10.1017/S1461145713001090. https://doi.org/10.1017/S1461145713001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, Ressler KJ. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2013;162B:262–272. doi: 10.1002/ajmg.b.32145. https://doi.org/10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ: British Medical Journal. 2011;343:d2090. doi: 10.1136/bmj.d2090. https://doi.org/10.1136/bmj.d2090. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Benjet C, Bromet E, Karam EG, Kessler RC, McLaughlin KA, Ruscio AM, Koenen KC. The epidemiology of traumatic event exposure worldwide: Results from the World Mental Health Survey Consortium. Psychological Medicine. 2016;46:327–343. doi: 10.1017/S0033291715001981. https://doi.org/10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester, UK: Wiley; 2009. https://doi.org/10.1002/9780470743386. [Google Scholar]
- Borenstein M, Hedges LV, Higgins J, Rothstein HR. Biostat: Comprehensive meta-analysis (Version 3) [Computer software] 2015 Retrieved from http://www.meta-analysis.com.
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. American Journal of Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. https://doi.org/10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. https://doi.org/10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: The posttraumatic stress disorder–major depression connection. Biological Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. https://doi.org/10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. https://doi.org/10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Chang SC, Xie P, Anton RF, De Vivo I, Farrer LA, Kranzler HR, Koenen KC. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Molecular Psychiatry. 2012;17:239–241. doi: 10.1038/mp.2011.118. https://doi.org/10.1038/mp.2011.118. [DOI] [PubMed] [Google Scholar]
- Chen W, Boutaoui N, Brehm JM, Han YY, Schmitz C, Cressley A, Celedon JC. ADCYAP1R1 and asthma in Puerto Rican children. American Journal of Respiratory and Critical Care Medicine. 2013;187:584–588. doi: 10.1164/rccm.201210-1789OC. https://doi.org/10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: A review. Current Neuropharmacology. 2013;11:141–159. doi: 10.2174/1570159X11311020002. https://doi.org/10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Ganea D. VIP and PACAP enhance the in vivo generation of memory TH2 cells by inhibiting peripheral deletion of antigen-specific effectors. Archives of Physiology and Biochemistry. 2001;109:372–376. doi: 10.1076/apab.109.4.372.4240. https://doi.org/10.1076/apab.109.4.372.4240. [DOI] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide promote in vivo generation of memory Th2 cells. FASEB Journal. 2002;16:1844–1846. doi: 10.1096/fj.02-0248fje. https://doi.org/10.1096/fj.02-0248fje. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. PACAP and the PAC1 receptor in post-traumatic stress disorder. Neuropsychopharmacology. 2013;38:245–246. doi: 10.1038/npp.2012.147. https://doi.org/10.1038/npp.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. https://doi.org/10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ: British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. https://doi.org/10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Southwick S, Goodson S, Morgan A, Nagy L, Charney DS. No association between D2 dopamine receptor (DRD2) "A" system alleles, or DRD2 haplotypes, and posttraumatic stress disorder. Biological Psychiatry. 1999;45:620–625. doi: 10.1016/s0006-3223(98)00087-0. https://doi.org/10.1016/S0006-3223(98)00087-0. [DOI] [PubMed] [Google Scholar]
- Gibson G. Rare and common variants: Twenty arguments. Nature Reviews. Genetics. 2012;13:135–145. doi: 10.1038/nrg3118. https://doi.org/10.1038/nrg3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspectives in Psychiatric Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. https://doi.org/10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Hammack SE, May V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: Data convergence from animal and human studies. Biological Psychiatry. 2015;78:167–177. doi: 10.1016/j.biopsych.2014.12.003. https://doi.org/10.1016/j.biopsych.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Pigott TD. The power of statistical tests for moderators in meta-analysis. Psychological Methods. 2004;9:426–445. doi: 10.1037/1082-989X.9.4.426. https://doi.org/10.1037/1082-989X.9.4.426. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. https://doi.org/10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. https://doi.org/10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Molecular Psychiatry. 2013;18:742–743. doi: 10.1038/mp.2012.98. https://doi.org/10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61:4–12. [PubMed] [Google Scholar]
- Koenen KC, De Vivo I, Rich-Edwards J, Smoller JW, Wright RJ, Purcell SM. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses’ Health Study II. BMC Psychiatry. 2009;9:29. doi: 10.1186/1471-244X-9-29. https://doi.org/10.1186/1471-244X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I-T, Ertl V, Eckart C, Kolassa S, Onyut LP, Elbert T. Spontaneous remission from PTSD depends on the number of traumatic event types experienced. Psychological Trauma: Theory, Research, Practice, and Policy. 2010;2:169–174. https://doi.org/10.1037/a0019362. [Google Scholar]
- Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neuroscience Letters. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. https://doi.org/10.1016/S0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE. PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology. 2013;38:702–715. doi: 10.1016/j.psyneuen.2012.09.006. https://doi.org/10.1016/j.psyneuen.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Roman CW, Braas KM, Schutz KC, Falls WA, Schulkin J, Hammack SE. Regulation of bed nucleus of the stria terminalis PACAP expression by stress and corticosterone. Journal of Molecular Neuroscience. 2014;54:477–484. doi: 10.1007/s12031-014-0269-8. https://doi.org/10.1007/s12031-014-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bao Y, He S, Wang G, Guan Y, Ma D, Yang J. The association between genetic variants in the dopaminergic system and posttraumatic stress disorder: A meta-analysis. Medicine. 2016;95(11):e3074. doi: 10.1097/MD.0000000000003074. https://doi.org/10.1097/MD.0000000000003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Liu H, Petukhova MV, Sampson NA, Aguilar-Gaxiola S, Alonso J, Andrade LH World Health Organization World Mental Health Survey Collaborators. Association of DSM-IV posttraumatic stress disorder with traumatic experience type and history in the World Health Organization World Mental Health Surveys. JAMA Psychiatry. 2017;74:270–281. doi: 10.1001/jamapsychiatry.2016.3783. https://doi.org/10.1001/jamapsychiatry.2016.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SR, Pothen J, Quinn JW, Rundle A, Bradley B, Galea S, Koenen KC. Gene-by-social-environment interaction (GxSE) between ADCYAP1R1 genotype and neighborhood crime predicts major depression symptoms in trauma-exposed women. Journal of Affective Disorders. 2015;187:147–150. doi: 10.1016/j.jad.2015.08.002. https://doi.org/10.1016/j.jad.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. https://doi.org/10.1037//1082-989X.7.1.19. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Venkataraman A, Donahue RJ, Carlezon WA., Jr Bi-directional effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on fear-related behavior and c-Fos expression after fear conditioning in rats. Psychoneuroendocrinology. 2016;64:12–21. doi: 10.1016/j.psyneuen.2015.11.003. https://doi.org/10.1016/j.psyneuen.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. https://doi.org/10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Mustafa T. Pituitary adenylate cyclase-activating polypeptide (PACAP): A master regulator in central and peripheral stress responses. Advances in Pharmacology. 2013;68:445–457. doi: 10.1016/B978-0-12-411512-5.00021-X. https://doi.org/10.1016/B978-0-12-411512-5.00021-X. [DOI] [PubMed] [Google Scholar]
- Mustafa T, Jiang SZ, Eiden AM, Weihe E, Thistlethwaite I, Eiden LE. Impact of PACAP and PAC1 receptor deficiency on the neurochemical and behavioral effects of acute and chronic restraint stress in male C57BL/6 mice. Stress. 2015;18:408–418. doi: 10.3109/10253890.2015.1025044. https://doi.org/10.3109/10253890.2015.1025044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T. Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry. 2004;4:34. doi: 10.1186/1471-244X-4-34. https://doi.org/10.1186/1471-244X-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Mustapic M, Yurgil KA, Schork NJ, Miller MW, Baker DG. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. https://doi.org/10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Cacciaglia R, Winkelmann T, Schad LR, Flor H. Neural mechanism of a sex-specific risk variant for posttraumatic stress disorder in the type I receptor of the pituitary adenylate cyclase activating polypeptide. Biological Psychiatry. 2015;78:840–847. doi: 10.1016/j.biopsych.2014.12.018. https://doi.org/10.1016/j.biopsych.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. https://doi.org/10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. The "file drawer problem" and tolerance for null results. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, Ressler KJ. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: A pilot prospective emergency department study. Journal of Clinical Psychiatry. 2014;75:1380–1387. doi: 10.4088/JCP.13m08715. https://doi.org/10.4088/JCP.13m08715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. https://doi.org/10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Nelson EC. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Archives of General Psychiatry. 2012;69:293–299. doi: 10.1001/archgenpsychiatry.2011.1385. https://doi.org/10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieff N, Roberts AL, Ratanatharathorn A, Haloosim M, De Vivo I, King AP, Koenen KC. Genetic association analysis of 300 genes identifies a risk haplotype in SLC18A2 for post-traumatic stress disorder in two independent samples. Neuropsychopharmacology. 2014;39:1872–1879. doi: 10.1038/npp.2014.34. https://doi.org/10.1038/npp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, Ressler KJ. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3158–3163. doi: 10.1073/pnas.1318954111. https://doi.org/10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: A master regulator of neuroendocrine stress circuits and the cellular stress response. Annals of the New York Academy of Sciences. 2011;1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x. https://doi.org/10.1111/j.1749-6632.2011.05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Koenen KC. ADCYAP1R1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depression and Anxiety. 2013;30:251–258. doi: 10.1002/da.22037. https://doi.org/10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological Reviews. 2009;61:283–357. doi: 10.1124/pr.109.001370. https://doi.org/10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Voisey J, Swagell CD, Hughes IP, Morris CP, van Daal A, Noble EP, Lawford BR. The DRD2 gene 957C>T polymorphism is associated with posttraumatic stress disorder in war veterans. Depression and Anxiety. 2009;26:28–33. doi: 10.1002/da.20517. https://doi.org/10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- Voisey J, Young RM, Lawford BR, Morris CP. Progress towards understanding the genetics of posttraumatic stress disorder. Journal of Anxiety Disorders. 2014;28:873–883. doi: 10.1016/j.janxdis.2014.09.014. https://doi.org/10.1016/j.janxdis.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. https://doi.org/10.1016/S0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. Journal of Affective Disorders. 2013;150:156–159. doi: 10.1016/j.jad.2013.01.010. https://doi.org/10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Wilker S, Kolassa I-T. The formation of a neural fear network in posttraumatic stress disorder: Insights from molecular genetics. Clinical Psychological Science. 2013;1:452–469. https://doi.org/10.1177/2167702613479583. [Google Scholar]
- Zayfert C, Dums AR, Ferguson RJ, Hegel MT. Health functioning impairments associated with posttraumatic stress disorder, anxiety disorders, and depression. Journal of Nervous and Mental Disease. 2002;190:233–240. doi: 10.1097/00005053-200204000-00004. https://doi.org/10.1097/00005053-200204000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.