Abstract
Pancreatic ductal adenocarcinoma (PDAC) is highly aggressive disease and current treatment regimens fail to effectively cure PDAC. Development of resistance to current therapy is one of the key reasons for this outcome. Nimbolide (NL), a triterpenoid obtained from Azadirachta indica, exhibits anticancer properties in various cancer including PDAC cells. However, the underlying mechanism of this anticancer agent in PDAC cells remains undefined. We show that NL exerts a higher level of apoptotic cell death compared to the first-line agent gemcitabine for PDAC, as well as other anticancer agents including sorafenib and curcumin. The anticancer efficacy of NL was further evidenced by a reduction in the CD44+ as well as cancer stem-like cell (CSC) population, as it causes decreased sphere formation. Mechanistically, the anticancer efficacy of NL associates with reduced mutant p53 as well as increased mitochondrial activity in the form of increased mitochondrial reactive oxygen species and mitochondrial mass. Together, this study highlights the therapeutic potential of NL in mutant p53 expressing pancreatic cancer.
Keywords: Pancreatic cancer, Nimbolide, Apoptosis, Mutant p53, Cancer stem cells, Mitochondria
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) stands as the third most common cause of cancer-related deaths in the United States [1]. It is a highly aggressive disease with a dismal five-year survival rate of ~9%. It is expected that approximately 48,860 new cases of PDAC will be diagnosed and 40,560 individuals will die of this disease in the United States alone in 2017 [2]. Surgical resection with radical intent in conjunction with adjuvant chemotherapy is currently the best form of treatment, raising the five-year survival rate to around 20% [3]. Unfortunately, PDAC develops with unspecific symptoms and thus, we often fail to catch the disease early at a time when tumor is localized at the primary side and have not spread to distant sites [4, 5]. In addition, it is important to note that even patients that have undergone radical pancreatoduodenectomy carry a high risk of disease recurrence, possibly due to micrometastasis at the time of detection, and will most often require further care in the form of chemotherapy [4].
Gemcitabine, a deoxycytidine analog, remained the first-line treatment as a single agent for locally advanced and metastatic PDAC [6], but it provides only a modest survival benefit [7, 8]. Recently, other combination drugs (gemcitabine+folfirinox, and erlotinib+gemcitabine) were also approved, which were successful in improving the patient’s survival from a couple of weeks to a few months, compared to gemcitabine alone [9, 10]. Regardless, no current treatment has been extremely effective due to underlying chemoresistance mechanisms. Many patients have been found to either intrinsically possess or subsequently develop resistance to gemcitabine during the course of treatment [11]. Resistance is found in those with reduced levels of human equilibrative nucleoside transporter-1 [12, 13] or deoxycytidine kinase [14, 15], or elevated levels of ribonucleoside reductase [16]. Therefore, there is a demand for novel therapeutics for the treatment of PDAC.
Sorafenib (SRF) is a multikinase inhibitor and targets the mitogen-activated protein kinase (MAPK) pathway, vascular endothelial growth factor receptor-2 (VEGFR-2) and -3 (VEGFR-3), platelet-derived growth factor receptor-β (PDGFRβ), Fms-Related Tyrosine Kinase 3 (FLT3), and mast/stem cell growth factor receptor Kit (c-KIT) [17, 18]. It is approved for the treatment of renal cell carcinoma, hepatocellular carcinoma, papillary thyroid cancer, and follicular thyroid cancer [19]. Data suggests that sorafenib has potential therapeutic benefit for pancreatic cancer due to its ability to target various pathways. However, results from combination therapy utilizing sorafenib plus gemcitabine indicate that sorafenib is not able to enhance chemotherapy in advanced pancreatic cancer [20]. Therefore, it will be interesting to know whether sorafenib enhances the sensitivity of other anticancer agent to pancreatic cancer cells.
Numerous studies indicate the beneficial use of herbs and spices in the treatment of diseases including cancer. The recent popularity of these natural substances stems from their general safety, affordability, and availability. For example, neem (Azadirachta indica) is a medicinal plant that has been extensively used in India for over the past 2,000 years due to its versatility in acting as an anti-inflammatory, antimalarial, antifungal, antibacterial, and hypoglycemic agent [21]. Nimbolide (NL) [22], one of the bioactive compounds derived from neem, displays toxicity in a large number of human cancer cell lines including lung carcinoma [23] [24], lymphoma [19, 25], leukemia [26, 27], melanoma [21, 26], breast carcinoma [28, 29], prostate carcinoma [19, 30], colon carcinoma [19, 21, 31], hepatic carcinoma [28, 32], osteosarcoma [33], and glioblastoma [34]. In addition, NL has ability to inhibit tumor growth in several animal studies [35–37]. Although the multiple mechanisms by which NL exerts cytotoxic effects against various cancers has been studied [38, 39], there is little information regarding the mechanism of action of NL in pancreatic cancer. Recent studies have shown that NL reduces cell viability as well migration and invasion, which involves increased levels of reactive oxygen species (ROS) and inhibition of epithelial–mesenchymal transition [40]. Our findings further explore the underlying mechanism of NL-induced caspase activation and its comparison to other therapeutic agents. We highlight the effects of NL on cancer stem-like cell population and various parameters of mitochondrial function, which will indicate therapeutic potential of NL for the treatment of PDAC via targeting of mutant p53.
2. Material & Methods
2. 1 Cell lines, reagents and antibodies
MIA PaCa-2 and BX-PC3 pancreatic cancer cell lines were used in this study. MIA PaCa-2 cells were maintained using RPMI-1640, 10% FBS, 5% penicillin-streptomycin and 2.5% horse serum. BXPC-3 cells were maintained using RPMI-1640, 10% FBS and 5% penicillin-streptomycin. RWPE-1, a normal prostatic cell line, was grown in Keratonocyte-SFM supplied with human recombinant Epidermal Growth Factor and Bovine Pituitary Extract. All reagents used in this study were of the highest grade of purity. NL, curcumin and sorafenib were purchased from BioVision Inc., USA, Sabinsa, East Windsor, NJ, USA, respectively. Gemcitabine was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Pifithrin-α and pifithrin-μ were obtained from Cayman Chemical Company (Ann Arbor, MI, USA). Primary antibodies for MDM2, p53, and actin were procured from Santa Cruz Biotechnology (Santa Cruz, TX, USA). Antibodies for HSP60 and APAF-1 were respectively from EMD Millipore (Billerica, MA, USA) and BD Biosciences (San Jose, CA, USA). Antibodies for caspase 3 were purchased from Enzo Life Sciences (East Farmingdale, NY, USA). Secondary antibodies were from Amersham Pharmacia Biotech (Piscataway, NJ, USA).
2.2 Treatment and isolation of cell lysates
Cells were seeded in a 6-well cell culture plate and incubated for 24 h followed by treatment with different drugs for including NL, gemcitabine, SRF, and curcumin, respectively for various time periods. Whole cell lysates were prepared according to a protocol described earlier [41].
2.3 Western blotting
Whole cell lysates (WCL) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) procured from Bio-Rad (Hercules, CA, USA) and transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA). Membranes were blocked in 5% nonfat milk for 30 min and washed with PBS-T (1× PBS and 0.05% Tween 20) and further incubated with respective primary antibodies. Anti-mouse or anti-rabbit horseradish peroxidase-conjugated antibodies were used as secondary antibodies.
2.4 Mito-ROS, Mito-MP, and Mito-Mass
Flow cytometry analysis was performed to quantify mitochondrialreactive oxygen species (Mito-ROS), mitochondrial membrane potential (Mito-MP), and mitochondrial mass (Mito-Mass) in control and treated groups. The Mito-ROS, Mito-MP, and Mito-Mass were estimated in the control and treated groups using MitoSox red, MitoTracker® Orange, and MitoTracker® Green probes (Life Technologies, USA), respectively. Cells were analyzed using flow cytometry (LSR IIA, BD Biosciences). The results were calculated by WinList 3D 7.1 software and presented in fold changes of the geometry mean in comparison to untreated control.
2.5 Caspase-3, -8 and -9 activities
The caspase-3, -8 and -9 activities were determined as described earlier [42]. Briefly, DEVD-AFC, IETD-AFC, and LEHD-AFC from Enzo Life Sciences (East Farmingdale, NY, USA) were used as substrates for caspase-3, -8 and -9, respectively. Fluorescence was read at excitation 400/30 nm and emission 508/20 nm using a plate reader (BioTek Microplate Readers, USA). Protein concentration of whole cell lysates were determined by a micro BCA kit (Thermo Fisher Scientific, Grand Island, NY, USA) where bovine serum albumin (BSA) was taken as a standard. The fluorescence obtained for caspase-3, -8 and -9 were normalized by their respective protein concentrations. The results were presented in fold change compared to control.
2.6 Annexin-V/PI staining
Control and treated cells were stained with Annexin-V Alexafluor 488/PI kit (Invitrogen, USA) for cell death, according to the manufacturer’s instructions. The stained cells were captured by flow cytometry (LSRIIA, BD Biosciences), collecting 10,000 events. Data were analyzed using Win List 3D 7.1 software.
2.7 Immunofluorescence microscopy and flow cytometry
The expression of cell surface marker CD44 was analyzed by immunofluorescence microscopy and flow cytometry as described earlier [43]. The expression of CD44 was analyzed by immunofluorescence using FITC tagged CD44 antibody and was used to detect CD44 expressions followed by counterstaining with DAPI.
2.8 Sphere formation assays
To investigate the impact of NL on cancer stem cells, sphere formation assays were performed. At log phase, adherent MIA PACa-2 cells were dissociated into single cells using accumax. 1 × 104 cells were seeded in ultra-low attachment 6-well plates (Corning Inc, Corning, NY, USA) containing serum-free DMEM/F-12 (1:1 ratio) media supplemented with 1% penicillin-streptomycin, B27 and N2 supplements (Gibco), 20 ng/ml rhEGF and 20 ng/ml fibroblast growth factor (Invitrogen, Carlsbad CA, USA). After seeding, cells were observed on a daily basis to ensure that spheres were forming as a result of cell multiplication and not due to adherence of nearby cells. Cells received treatment either on the same day of cell seeding or 3 days after. Cells were treated with 5 µM NL for 72 h and imaged.
2.9 Clonogenic assays
MIA PaCa-2 (5.0 × 102 cells) were seeded in a 6-well, flat bottom tissue culture plate. Following 24 h incubation, cells were treated with 0.5, 1, or 5 µM NL for 6 days. Cells were then rinsed with 1× PBS, fixed with chilled 95% ethanol at room temperature for 10 mins, and stained with 0.5% crystal violet for 40 mins and bright field images were taken.
2.10 Transwell migration assays
MIA PaCa and BX-PC3 cells (5.0 × 104 cells for each cell line) suspended in media containing 0.5% FBS, in the absence or presence of 5 µM NL, were placed into the upper chamber of a transwell insert (8 µm pore size; Corning). Media containing 7% FBS was placed in the lower chamber. After a 16 h incubation period, non-migrated cells in the upper membrane were removed with cotton wool. Cells which migrated through the membrane were fixed with chilled methanol and stained with 0.5% crystal violet. Using a light microscope at 20× magnification, the number of stained cells were counted in 3 random fields of each transwell insert.
2.11 Analysis from publicly available datasets
cBioportal [44, 45] was used to download the PAAD TCGA (provisional) dataset for mutational and survivorship analyses pertaining to p53 status.
2.12 Inhibition of p53
The inhibition of p53 functions were carried out using p53 inhibitors, PFT-α and PFT-μ. Briefly, MIA PaCa-2 and BX-PC3 cells (200×103/well) were seeded in a 6-well plate. Following 24 h incubation, cells were treated with PFT-α, or PFT-μ (5 µM each). After 24 h, cells were treated with NL (5 µM) for 24 h.
2.13 Statistical analysis
All experiments were carried out in triplicates. Significant differences between means were assessed via analysis of variance using GraphPad Prism Version 6. A *p < 0.05 value was accepted as significant. Significance is denoted as compared to control, unless otherwise indicated.
3. Results
3.1 NL induces higher levels of caspase activation compared to anticancer agents in pancreatic cancer
We first determined the levels of apoptotic cell death in pancreatic cancer upon treatment with NL as well as gemcitabine (GMC), the leading anticancer single agent for the treatment of pancreatic cancer [6, 7, 39]. Sorafenib (SRF) was also used to test the possibility of using this drug in combination with NL. Anticancer effects of NL were also compared to curcumin, another plant-derived compound, which induces apoptosis in various cancer cells [46]. Two pancreatic cancer cell lines, MIA PaCa-2 and BXPC-3, were used to explore caspase activity after drug treatments.
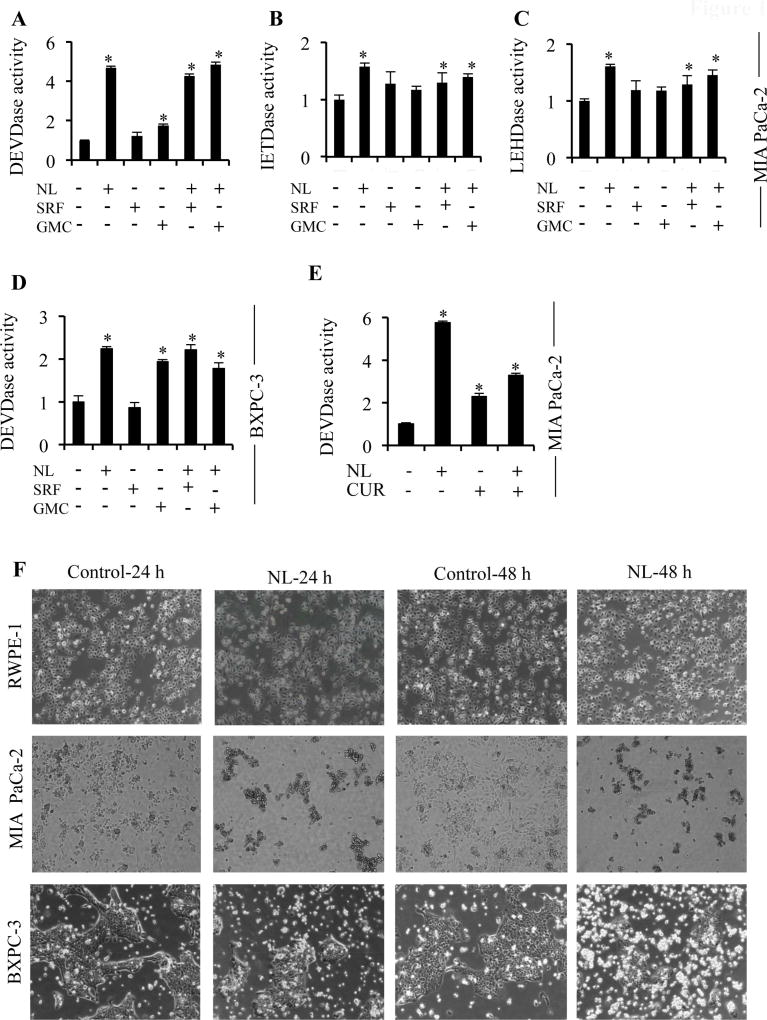
A 5µM concentration of NL was utilized here, based on studies by Subramani et al. [40]. We observed that in MIA PaCa-2 cells, NL exposure for 24 h induced higher levels of caspase-3 activation compared to gemcitabine (Figure 1A) and as expected, treatment with sorafenib did not induce caspase-3 activation at this time point. In addition, NL activated both initiator caspases, caspase-8 and caspase-9 (Figure 1B–C). Similarly in BXPC-3 cells, elevated caspase-3 activity was observed upon NL exposure (Figure 1D) compared to gemcitabine, and sorafenib again had no effect on caspase-3 activation. Combination of NL with either sorafenib or gemcitabine did not enhance NL-induced caspase-3 activity in both types of cells. Next, we compared NL-induced caspase activation with curcumin, a well-known phytochemical that induces apoptosis (Figure 1E). NL induced higher levels of caspase-3 activity compared to curcumin alone or NL plus curcumin. Importantly, NL did not induce cell death in RWPE-1 cells, normal human prostate epithelial cells, compared to MIA Paca-2 and BXPC-3 (Figure 1F). Altogether, NL demonstrates a higher potential for inducing apoptosis in pancreatic cancer cells compared to gemcitabine, sorafenib, and curcumin. Additionally, this treatment appears to be non-toxic towards normal cells.
Figure 1. NL induces higher levels of caspase activity compared to current PDAC therapeutic agents.
MIA PaCa-2 and BXPC-3 cells were treated with 5 µM NL, 5 µM Sorafenib (SRF), 5 µM Gemcitabine (GMC), or 5 µM Curcumin (CUR). (A–C) Caspase-3, -8 and -9 activities in MIA PaCa-2 cells after 24 h of NL, SRF, and GMC treatment. (D) Caspase-3 activity in BXPC-3 cells after 24 h of NL, SRF, and GMC treatment. (E) Caspase-3 activity in MIA PaCa-2 cells after 24 h of NL and CUR treatment. (F) Images of RWPE-1, MIA PaCa-2, and BXPC-3 cells treated with NL for 24 h and 48 h. Data are mean ± SD, n = 3 and presented as fold-change compared to respective controls. Statistical significance was determined by a t-test: *p<0.05.
3.2 NL induces apoptosis in pancreatic cancer cells
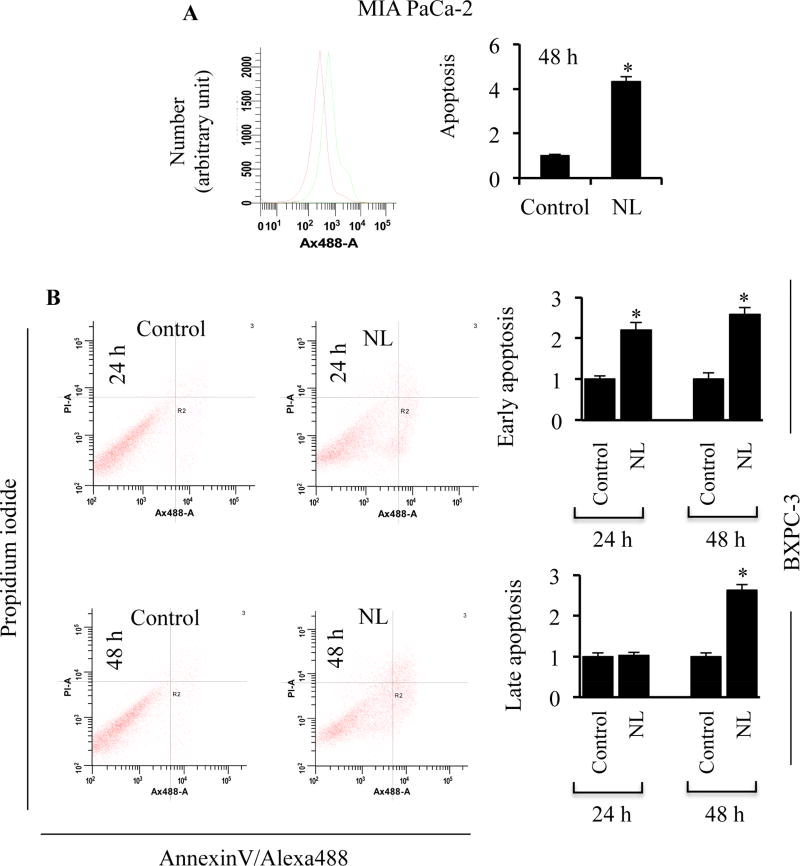
To determine whether increased caspase activation associates with apoptotic cell death in pancreatic cancer, we quantified apoptosis via Annexin-V-Alexafluor 488 and propidium iodide (PI) staining upon exposure to NL. We observed a four-fold increase in apoptosis upon NL treatment compared to untreated cells (Figure 2A). We further quantified early and late apoptotic activity upon NL exposure. Annexin-V+PI− staining signifies early apoptosis and Annexin-V+PI+ staining is regarded as late apoptosis. Early apoptotic cell populations were detected after 24 and 48 h treatments and late apoptotic cell populations were not detected until the 48 h time point (Figure 2B). Together, NL induces early as well as late apoptosis in pancreatic cancer cells.
Figure 2. NL induces apoptosis in pancreatic cancer cells.
(A) Fold change in Annexin-V staining in MIA PaCa-2 cells following 5 µM NL exposure for 48 h. (B) Fold change in Annexin-V/PI staining in BXPC-3 cells following 5 µM NL exposure for 24 and 48 h. Data are mean ± SD, n = 3. Statistical significance was determined by a t-test: *p<0.05.
3.3 NL reduces stemness in pancreatic cancer
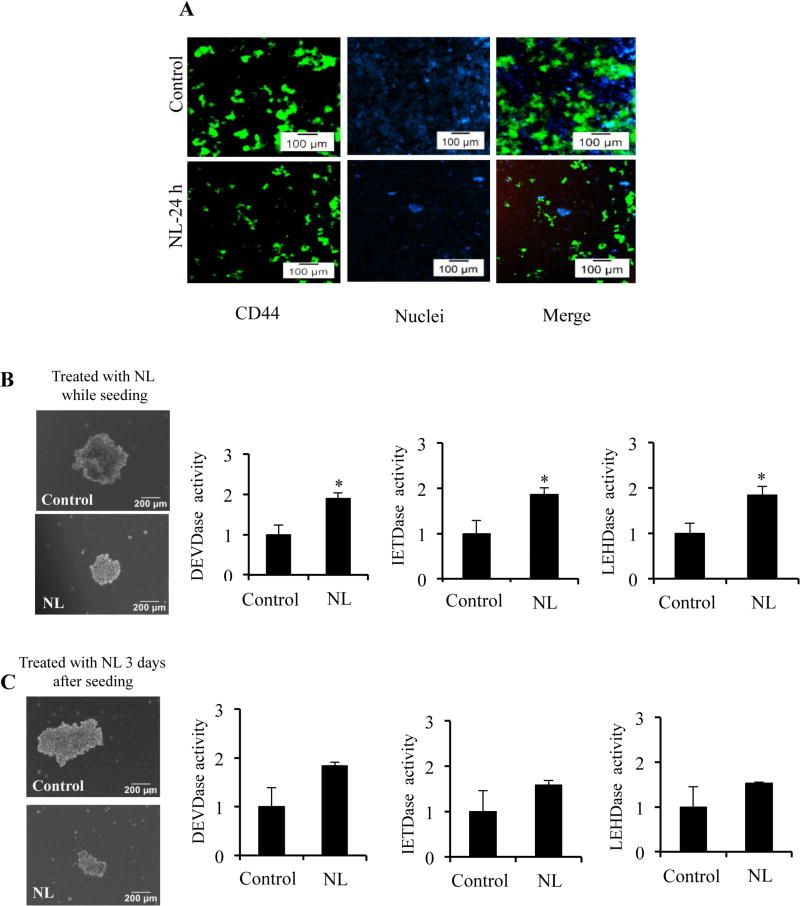
Cancer cell populations are highly heterogeneous and are known to contain CSCs, which aid in therapeutic resistance and tumor recurrence [47]. We next studied the effects of NL on pancreatic cancer stem-like cells (CSCs), which can be identified by various cell surface markers. Specifically, MIA PaCa-2 cells have been reported to contain CSCs, which express CD44 but lack CD24 [48]. To understand how NL affects these populations, cells were stained with fluorescently tagged antibodies to visualize the CD44+ population in treated and control cells. The CD44+ population of MIA PaCa-2 cells decreased with NL exposure (Figure 3A). To further analyze CSCs, we studied the impact of NL on MIA PaCa-2-derived spheres. Reduced sphere size was observed in the NL treated groups compared to controls, as shown in two different NL treatment conditions, treatment of cells while seeding and treatment of cells three days after seeding (Figure 3B and 3C). Decreases in sphere size can be attributed to NL-induced apoptotic activity, as upregulated caspase-3, -8 and -9 activities were observed in both treatment conditions (Figure 3B and 3C). Thus, NL exposure decreases the population of pancreatic cancer cells with the typical CSC phenotype and reduces the growth of these cells.
Figure 3. NL exposure reduces enrichment of cancer stem-like cells and inhibits sphere formation.
(A) MIA PaCa-2 cells were treated with 5 µM NL and stained with FITC-tagged CD44 for 30 min. DAPI was used for nuclear staining. Expression of CD44+ populations at 24 h exposure of 5 µM NL was analyzed using fluorescence microscopy.
For sphere formation, single cell suspensions of MIA PaCa-2 were seeded (1×103 cells) into ultra-low attachment plates. Representative bright field images of spheres and caspase-3, -8 and -9 activities of spheres which were (B) treated on the day of seeding cells or (C) treated 3 days after seeding cells with 5 µM NL for 72 h. Data are mean ± SD, n = 3 and presented as fold-change compared to respective controls.. Statistical significance was determined by a t-test: *p<0.05.
3.4 NL enhances mitochondrial mass and ROS
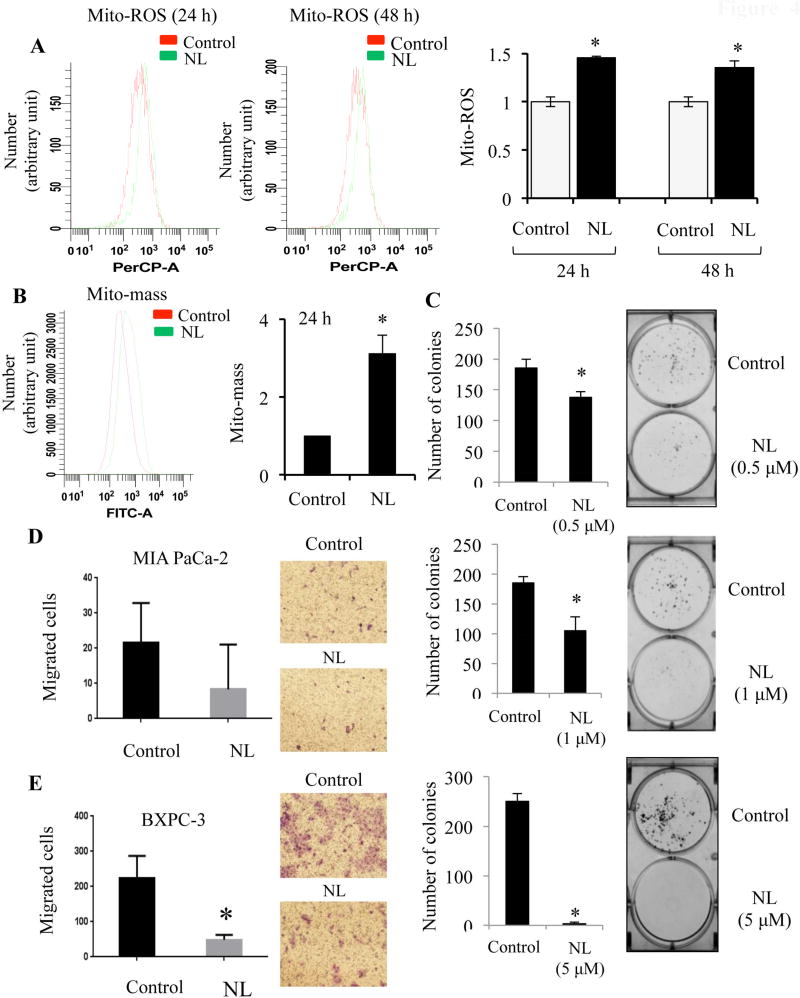
Cancer cells commonly possess elevations in metabolic activity, oxidative stress, and reactive oxygen species (ROS) generation [49, 50]. Many antitumor drugs further exacerbate this generation of ROS, thereby promoting DNA-damage and cell death [51]. ROS can originate from endogenous sources such as the mitochondria and peroxisomes [52]. Specifically, mitochondria-generated ROS (Mito-ROS) plays an important role in the release of cytochrome c and other pro-apoptotic proteins, which in turn trigger caspase activation and apoptosis [53]. Physiological functions of the mitochondria, such as ATP generation through the respiratory chain, are maintained by the mitochondrial membrane potential (Mito-MP) [54]. Cells respond to cellular stress by altering mitochondrial mass (Mito-mass) [55]. For example, cells may increase mitochondrial mass to meet increased ATP demands [56]. Therefore, NL-induced changes in Mito-ROS, Mito-MP, and Mito-mass were analyzed to elucidate the physiological status of the mitochondria within cells. Mito-ROS levels, as indicated by MitoSox Red staining, were up-regulated after NL exposure for 24 h and this increase was sustained after 48 h (Figure 4A). Although there was no change in Mito-MP (data not shown), Mito-mass was upregulated after 24 h (Figure 4B). These results suggest that NL induces mitochondrial dysfunctions that ultimately contribute to caspase-dependent apoptosis in pancreatic cancer cells.
Figure 4. NL enhances mitochondrial ROS and mass, inhibits colony formation, and impairs cell motility.
BXPC-3 and MIA PaCa-2 cells were treated with NL (5 µM) for 24 and 48 h. Flow cytometry was carried out to estimate mitochondrial ROS (using MitoSOX red), and mitochondrial mass (using MitoTracker green). (A) Mitochondrial ROS (Mito-ROS) in BXPC-3 cells after 24 and 48 h of NL, and presented in fold-change. (B) Mitochondrial mass (Mito-mass) in MIA PaCa-2 cells after 24 h of NL, and presented in fold-change. (C) For clonogenic assays, single cell suspensions of MIA PaCa-2 cells were plated in six well plate (500 cells/well). After a 24 h incubation, cells were treated with 0.5 µM or 1 µM or 5 µM NL for 6 days. Following this, cells were fixed, stained with 0.5% crystal violet, and the number of colonies were quantified. For a transwell migration assay, MIA PaCa-2 and BXPC-3 cells were suspended in the upper chamber of a transwell insert (8 µm pore size) with media containing 0.5% FBS, while the lower chamber held media containing 7% FBS. Cells of the upper chamber received 5 µM NL and a 16 h incubation period was allowed. Non-migrated cells were removed. Cells which migrated through the insert were fixed with methanol and stained with 0.5% crystal violet. Using a light microscope at 20× magnification, the number of migrated cells per field were visualized and counted for (D) MIA PaCa-2 and (E) BX-PC3 cells. Data are mean ± SD, n = 3. Statistical significance was determined by a t-test: *p<0.05.
3.5 NL suppresses clonogenicity and cell migration
Next, we investigated the impact of NL on colony formation and migratory potential in pancreatic cancer cells. A clonogenic assay tests the survival capacity of single cells according to their ability to form colonies after drug treatment [57]. NL at reduced concentrations of 0.5 µM and 1 µM inhibited colony formation (Figure 4C). The previously used concentration of 5 µM NL prevented colony formation almost entirely. Metastasis is the cause of approximately 90% of cancer-related deaths [58]. Migratory activity of cancer cells is the initial event of the metastatic cascade. Using a transwell migration assay, migration was observed to decrease in both MIA PaCa-2 and BXPC-3 cells after NL treatment (Figure 4D–E). Overall, NL impairs the long-term survival and metastatic potential of pancreatic cancer cells.
3.6 Mutant tumor suppressor p53 plays a key role in NL-induced cell death
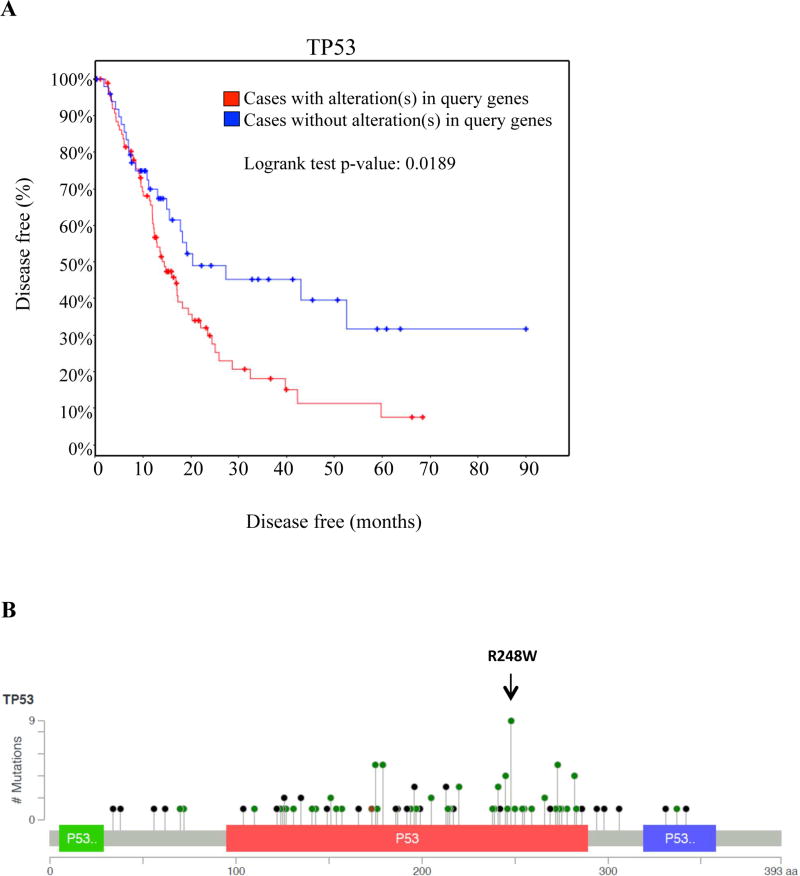
Mutations in tumor suppressor p53 are correlated with poor patient prognosis [59]. In PDAC, p53 mutations occur in up to 76% of patients [60] and these alterations are also associated with decreased time to recurrence (Figure 5A). Many of these mutations are mapped on the DNA-binding domain of p53. Based on TCGA analysis, the most common modification in PDAC is the R248W mutation (Figure 5B).
Figure 5. Mutations in p53 are prevalent in pancreatic adenocarcinoma and predict time to recurrence.
(A) Time to recurrence between patients with no mutations (blue lines) and patients with p53 mutations (red line), displayed as a Kaplan-Meier graph. Censored values are presented as dots. (B) The distribution of frequently occurring p53 mutations along the amino acid sequence of p53. Note that the R248W alteration is the most prevalent in patients. Data was generated via cBioportal.
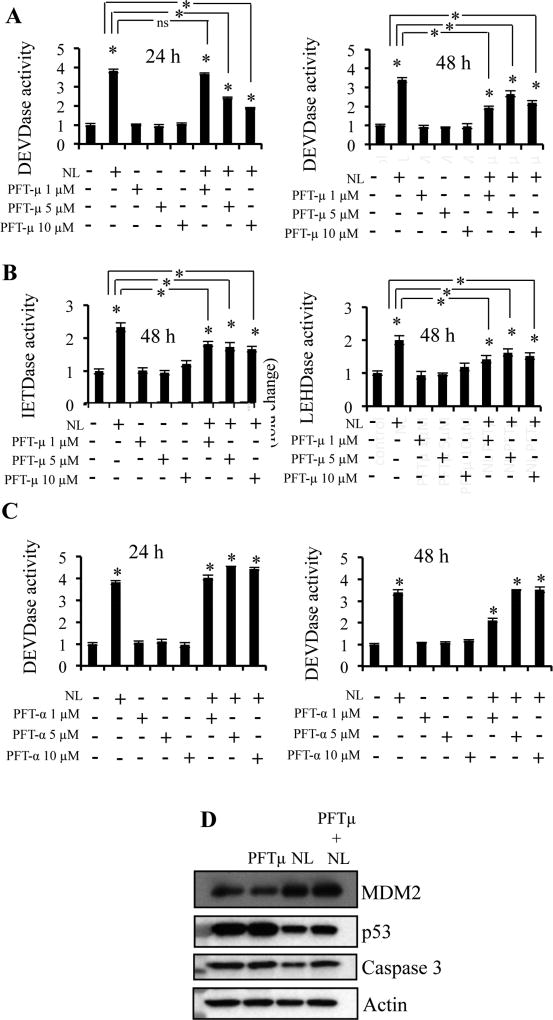
Therefore, we explored the role of mutant p53 status in NL-induced apoptosis. MIA PaCa-2 contains mutant p53 (p53R248W) [61], which displays a reduced affinity for Bcl-2 and Bcl-xL [62]. Pifithrin-μ (PFTμ), a small molecule that inhibits p53-dependent apoptosis via abrogating p53 translocation to mitochondria [63], was used to diminish the anti-apoptotic activity of the mutant p53. Cells were pre-treated with PFT-μ for 24 h prior to NL treatments. We used PFT-μ doses that alone had no effect on caspase-3, -8, or -9 activities (Figure 6A–B). However, increasing concentrations of PFT-μ inhibited NL-induced caspase-3 activity after 24 h and 48 h (Figure 6A) as well as NL-induced caspase-8 and -9 activities after 48 h (Figure 6B). We also utilized pifithrin-α (PFTα), a small molecule, which inhibits p53 transcriptional activity [64]. PFTα did not reduce NL-induced caspase-3 activity (Figure 6C). Western blot (Figure 6D) analysis demonstrated increased MDM2 expression and decreased mutant p53 expression upon NL exposure while PFT-μ had no effect on these proteins. NL decreased procaspase-3 levels, i.e. elevated the cleavage of proscaspase-3 and this decrease was prevented by PFT-μ.
Figure 6. Reduction of mutant p53 associates with increased mitochondrial apoptosis.
MIA PaCa-2 cells were plated. 24 h before NL exposure, cells were pretreated with 1, 5, or 10 µM Pifithrin-μ (PFT-μ) or Pifithrin-α (PFT-α) for p53 inhibition. Cells were then treated with 5 µM NL for 24 h. (A–B) Caspase-3, -8 and -9 activities in control and NL-treated MIA PaCa-2 cells previously treated with PFT-μ. (C) Caspase-3 activities in control and NL-treated MIA PaCa-2 cells previously treated with PFT-α. (D) Western Blot analysis of lysates from MIA PaCa-2 which were pretreated with 5 µM PFT-μ for 24 h, and then treated with 5 µM NL for 24 h. Data are mean ± SD, n = 3 and presented as fold-change compared to respective controls. Statistical significance was determined by a t-test: *p<0.05.
Overall, our data support the idea that NL-induced apoptosis in pancreatic cancer is mediated by the reduction of mutant p53. However, the remaining mutant p53 can also continue to function through mitochondrial apoptosis.
4. Discussion
Studies show that the United States will see 53,670 new cases of pancreatic adenocarcinoma (PDAC) and as many as 43,090 afflicted individuals will die in 2017 [2]. To manage the disease, many patients will receive gemcitabine, the first-line chemotherapeutic agent for PDAC [6]. However, resistance to this treatment is well known [11] and patient survival will be extended by only six months at most [7, 8]. In addition, gemcitabine is highly toxic to any dividing cells and therefore can cause adverse effects in patients including nausea and vomiting, peripheral edema [65], cutaneous erythema [66], and kidney malfunction [67]. So there remains a great need for the discovery of alternative agents that can combat PDAC without significant adverse effects. In this regard, various plant-derived compounds have been identified which seem to possess anticancer activity with a lack of toxicity towards normal cells [17]. Our current study offers insight into the potential use of one such compound, NL [22], in the context of PDAC. Originating from the neem plant [18], NL has been reported to be cytotoxic towards numerous cancer cell lines [19, 21, 24–28] [30, 35], inhibits tumor growth in several animal studies [31–33], and potentially considered as a chemopreventive agent as evidenced by a human colorectal cancer xenograft model [33]. However, the mechanism utilized by NL for the treatment of PDAC is relatively unknown.
In the present study, NL has been compared to other chemotherapeutic agents against PDAC. Our findings suggest that NL has the potential to induce apoptosis in pancreatic cancer cells in a greater manner than the current first-line agent for pancreatic cancer, gemcitabine. Our study showed that NL induced higher caspase-3 activity compared to both gemcitabine and sorafenib. Sorafenib treatment was ineffective against both MIA PaCa-2 and BXPC-3 cells, and generally unable to potentiate the caspase-activating effects of NL, as is the case during the treatment of patients [20]. Curcumin, another plant-derived compound known to exert anticancer effects [46, 68], showed lower level of caspase activation relative to NL. With these observations, we postulated that NL alone could impart excellent death-inducing activity in pancreatic cancer. Another finding that compelled us to commence treatment with NL was that although NL induced apoptosis in MIA PaCa-2 and BXPC-3 pancreatic cancer cell lines, NL at the tested doses was unable to induce death in RWPE-1, a non-tumorigenic human cell line. This infers that NL likely possesses limited toxicity against normal human cells.
Perhaps the greatest barrier in cancer therapy is the development of drug resistance. Tumor populations harbor a mixed phenotype and contain cancer stem cells (CSCs), a subpopulation which contains high proliferative potential and confers resistance to apoptosis [47]. A xenograft model of primary human PDAC tumor has been shown to house cells populations which express stem cell markers namely CD44, CD24, and EpCAM, and possess high tumorigenic and self-renewal properties [69]. An analysis of PDAC cell lines commonly used in culture indicates that their CSCs express CD44 but lack CD24 expression [48]. Our data demonstrate that NL reduces the typical CSCs population by decreasing the CD44+ population in pancreatic cancer cells. We then developed and analyzed pancreatospheres, which are reported to contain self-renewal and self-differentiation properties [70]. MIA PaCa-2-derived spheres decreased in size due to NL and these spheres displayed enhanced caspase-3, -8 and -9 activities. Collectively, NL appears to differentially target CSC subpopulations in pancreatic cancer in a manner that subverts the CSC landscape and fashions it to be less conducive to CSC proliferation. This ability of NL to decrease the expression of CD44 is highly attractive considering that CSCs, namely those which express CD44, may impart chemo- as well as radiation- resistance in patients with PDAC [71] and that expression of CD44 by pancreatic tumor cells is associated with increased tumor formation abilities [72].
The role of the mitochondria as a key player in the regulation of apoptosis is a well-accepted phenomenon [73]. Mitochondrial dysfunction is often associated with increased ROS production [74]. Upregulated mitochondrial ROS and mitochondrial mass in response to NL suggests that NL induces mitochondrial activity in pancreatic cancer cells. Interestingly, literature also suggests that NL can suppress tumor cell migration and invasion via down-regulation of metalloproteinase-2/9 [75]. NL reduces the migratory abilities of both MIA Paca-2 and BXPC-3cells. The potential for NL to inhibit metastasis in pancreatic cancer is encouraging, given that metastasis is prevalent in PDAC and that efforts to increase overall survival in patients with metastatic disease over the past two decades have been highly disappointing [76].
Increased ROS in cancer cells induces cell death by oxidizing cellular macromolecules including DNA. Oxidation of DNA leads to DNA damage which results in activation of p53, a major tumor suppressor protein in the mammalian cells [77]. Not surprisingly, mutations in p53 are very common in almost all cancers including PDAC (75% of all cases) and one of the major reasons behind the poor prognosis, high metastatic rate, and ineffective PDAC treatment [60]. Therefore, pancreatic cancer cell lines we chose to use in this study each contain a unique mutated p53 allele and loss of the wild type allele. MIA PaCa-2 carries an R248W p53 mutation and BXPC3 contains a Y220C p53 mutation, individually located on the DNA binding domain [61]. Mutant p53R248W is associated with a gain-of-function and impairs cellular response to DNA damage, leading to genetic instability and promotion of tumorigenesis [78]. As a result, cancer patients displaying this mutation experience decreased survival times [79]. However, p53Y220C mutant is associated with a loss-of-function, in which the p53 core domain is largely destabilized [80]. These variations may explain the discrepancies in the ability of NL to induce caspase-3 activity in both cell lines, i.e. NL more potently induces caspases in MIA PaCa-2 compared to BXPC-3 cells. However, it should be noted that BXPC-3 lacks KRAS mutation commonly associated with pancreatic cancer [61], and this may also contribute to the efficacy of NL. Due to the prevalence of p53R248W in PDAC, we studied the role of this mutant p53 in MIA PaCa-2 during NL treatment. NL increased MDM2 levels that, in turn, reduced the expression of p53R248W. Expression of p53 mutants [p53R172H (p53R175H in humans); and p53R273H] in murine pancreas leads to highly aggressive PDAC [81–83]. In addition, p53 exerts tumor suppressive function by binding to the promoter region of CD44 causing its downregulation [84]. Mutation in DNA binding domain of p53 as commonly observed in PDAC enhances CD44 expression, which contributes even higher metastatic potential and drug resistance in pancreatic cancer [85, 86]. Therefore, decreased CD44+ population, sphere-forming ability, and migratory potential of PDAC cells in response to NL treatment can be attributed to downregulation of mutant p53 [81, 87]. Utilizing the p53 inhibitor PFT-μ, we observed that despite the mutant state of p53R248W, it continues to serve a role in inducing caspase 3 activity and thus apoptosis. However, the transactivational activities of p53R248W appear to play no part in NL-induced apoptosis as we found that PFT-α failed to inhibit the NL-induced caspase-3 activation in MIA PaCa2 cells.
In summary, exposure of pancreatic cancer cells to NL stimulated caspase activation, apoptosis, and mitochondrial dysregulation, as well as inhibition of the cancer stem cell population and cell migration. Since no treatment strategy is available for patients with PDAC harboring mutant p53, the mortality rate is very high. Furthermore, enhanced CD44 positive population leads to development of resistance against the most commonly used drug, gemcitabine. Thus downregulation of both mutant p53 and CD44 positive cells by NL will have significance in treating patients with PDAC.
Highlights.
Nimbolide is a more effective caspase activator compared to gemcitabine.
Nimbolide treatment depletes CD44+ population in pancreatic cancer cells.
Nimbolide-induced apoptosis associates with increased mitochondrial activity.
Reduced levels of mutant p53 may contribute to anticancer effects of nimbolide.
Acknowledgments
This work was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number RO1CA160685, the American Cancer Society Research Scholar Grant RSG-12-214-01 – CCG, and the National Cancer Institute Center Support Grant P30 CA016056 to the Roswell Park Cancer Institute. We apologize to those colleagues whose publications inadvertently were not cited.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW C. European Study Group for Pancreatic. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 4.Andersson R, Vagianos CE, Williamson RC. Preoperative staging and evaluation of resectability in pancreatic ductal adenocarcinoma. HPB (Oxford) 2004;6(1):5–12. doi: 10.1080/13651820310017093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, Levy MJ, Chak A, Fockens P, Goggins M, Bruno M C. International Cancer of Pancreas Screening. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62(3):339–47. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8–16. doi: 10.1016/j.ejphar.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 8.Ishii H, Furuse J, Nagase M, Yoshino M. Impact of gemcitabine on the treatment of metastatic pancreatic cancer. J Gastroenterol Hepatol. 2005;20(1):62–6. doi: 10.1111/j.1440-1746.2004.03487.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Hu GF, Zhang QQ, Tang N, Guo J, Liu LY, Han X, Wang X, Wang ZH. Efficacy and safety of gemcitabine plus erlotinib for locally advanced or metastatic pancreatic cancer: a systematic review and meta-analysis. Drug design, development and therapy. 2016;10:1961–72. doi: 10.2147/DDDT.S105442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HS, Park SW. Systemic Chemotherapy in Advanced Pancreatic Cancer. Gut and liver. 2016;10(3):340–7. doi: 10.5009/gnl15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, Sandvold ML. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44(7):782–6. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 12.Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, Cass C, Lai R, Mackey JR. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10(20):6956–61. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 13.Giovannetti E, Del Tacca M, Mey V, Funel N, Nannizzi S, Ricci S, Orlandini C, Boggi U, Campani D, Del Chiaro M, Iannopollo M, Bevilacqua G, Mosca F, Danesi R. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66(7):3928–35. doi: 10.1158/0008-5472.CAN-05-4203. [DOI] [PubMed] [Google Scholar]
- 14.Kroep JR, Loves WJ, van der Wilt CL, Alvarez E, Talianidis I, Boven E, Braakhuis BJ, van Groeningen CJ, Pinedo HM, Peters GJ. Pretreatment deoxycytidine kinase levels predict in vivo gemcitabine sensitivity. Mol Cancer Ther. 2002;1(6):371–6. [PubMed] [Google Scholar]
- 15.Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96(3):457–63. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman AM, Eijk PP, Ruiz van Haperen VW, Smid K, Veerman G, Hubeek I, van den Ijssel P, Ylstra B, Peters GJ. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res. 2005;65(20):9510–6. doi: 10.1158/0008-5472.CAN-05-0989. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 18.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 19.Debarbieux L, Pirnay JP, Verbeken G, De Vos D, Merabishvili M, Huys I, Patey O, Schoonjans D, Vaneechoutte M, Zizi M, Rohde C. A bacteriophage journey at the European Medicines Agency. FEMS Microbiol Lett. 2016;363(2):fnv225. doi: 10.1093/femsle/fnv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cascinu S, Berardi R, Sobrero A, Bidoli P, Labianca R, Siena S, Ferrari D, Barni S, Aitini E, Zagonel V, Caprioni F, Villa F, Mosconi S, Faloppi L, Tonini G, Boni C, Conte P, Di Costanzo F, Cinquini M C. Italian Group for the Study of Digestive Tract. Sorafenib does not improve efficacy of chemotherapy in advanced pancreatic cancer: A GISCAD randomized phase II study. Dig Liver Dis. 2014;46(2):182–6. doi: 10.1016/j.dld.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Biswas SK, Milanfar P. One Shot Detection with Laplacian Object and Fast Matrix Cosine Similarity. IEEE Trans Pattern Anal Mach Intell. 2016;38(3):546–62. doi: 10.1109/TPAMI.2015.2453950. [DOI] [PubMed] [Google Scholar]
- 22.McGinley KF, Tay KJ, Moul JW. Prostate cancer in men of African origin. Nat Rev Urol. 2016;13(2):99–107. doi: 10.1038/nrurol.2015.298. [DOI] [PubMed] [Google Scholar]
- 23.Sastry BS, Suresh Babu K, Hari Babu T, Chandrasekhar S, Srinivas PV, Saxena AK, Madhusudana Rao J. Synthesis and biological activity of amide derivatives of nimbolide. Bioorg Med Chem Lett. 2006;16(16):4391–4. doi: 10.1016/j.bmcl.2006.05.105. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Chen J, Sun Y, Yan Y, Kong L, Li Y, Qiu M. Cytotoxic triterpenoids from Azadirachta indica. Planta Med. 2011;77(16):1844–7. doi: 10.1055/s-0030-1271197. [DOI] [PubMed] [Google Scholar]
- 25.Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Isobe S, Tsushida T. Antiproliferative effect on human cancer cell lines after treatment with nimbolide extracted from an edible part of the neem tree (Azadirachta indica) Phytother Res. 2007;21(3):245–50. doi: 10.1002/ptr.2058. [DOI] [PubMed] [Google Scholar]
- 26.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19(6):347–61. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kigodi PG, Blasko G, Thebtaranonth Y, Pezzuto JM, Cordell GA. Spectroscopic and biological investigation of nimbolide and 28-deoxonimbolide from Azadirachta indica. J Nat Prod. 1989;52(6):1246–51. doi: 10.1021/np50066a008. [DOI] [PubMed] [Google Scholar]
- 28.Cardin DB, Goff L, Li CI, Shyr Y, Winkler C, DeVore R, Schlabach L, Holloway M, McClanahan P, Meyer K, Grigorieva J, Berlin J, Chan E. Phase II trial of sorafenib and erlotinib in advanced pancreatic cancer. Cancer Med. 2014;3(3):572–9. doi: 10.1002/cam4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elumalai P, Gunadharini DN, Senthilkumar K, Banudevi S, Arunkumar R, Benson CS, Sharmila G, Arunakaran J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215(2):131–42. doi: 10.1016/j.toxlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Raja S. Neuroleptic malignant syndrome probably induced by asenapine. J Neuropsychiatry Clin Neurosci. 2014;26(4):E36. doi: 10.1176/appi.neuropsych.13090224. [DOI] [PubMed] [Google Scholar]
- 31.Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Tsushida T. Inhibition of colon cancer (HT-29) cell proliferation by a triterpenoid isolated from Azadirachta indica is accompanied by cell cycle arrest and up-regulation of p21. Planta Med. 2006;72(10):917–23. doi: 10.1055/s-2006-946694. [DOI] [PubMed] [Google Scholar]
- 32.Kavitha K, Vidya Priyadarsini R, Anitha P, Ramalingam K, Sakthivel R, Purushothaman G, Singh AK, Karunagaran D, Nagini S. Nimbolide, a neem limonoid abrogates canonical NF-kappaB and Wnt signaling to induce caspase-dependent apoptosis in human hepatocarcinoma (HepG2) cells. Eur J Pharmacol. 2012;681(1–3):6–14. doi: 10.1016/j.ejphar.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Cohen E, Quistad GB, Casida JE. Cytotoxicity of nimbolide, epoxyazadiradione and other limonoids from neem insecticide. Life Sci. 1996;58(13):1075–81. doi: 10.1016/0024-3205(96)00061-6. [DOI] [PubMed] [Google Scholar]
- 34.Karkare S, Chhipa RR, Anderson J, Liu X, Henry H, Gasilina A, Nassar N, Roychoudhury J, Clark JP, Kumar A, Pauletti GM, Ghosh PK, Dasgupta B. Direct inhibition of retinoblastoma phosphorylation by nimbolide causes cell-cycle arrest and suppresses glioblastoma growth. Clin Cancer Res. 2014;20(1):199–212. doi: 10.1158/1078-0432.CCR-13-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priyadarsini RV, Manikandan P, Kumar GH, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic Res. 2009;43(5):492–504. doi: 10.1080/10715760902870637. [DOI] [PubMed] [Google Scholar]
- 36.Harish Kumar G, Vidya Priyadarsini R, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010;28(4):392–401. doi: 10.1007/s10637-009-9263-3. [DOI] [PubMed] [Google Scholar]
- 37.Gupta SC, Prasad S, Sethumadhavan DR, Nair MS, Mo YY, Aggarwal BB. Nimbolide, a limonoid triterpene, inhibits growth of human colorectal cancer xenografts by suppressing the proinflammatory microenvironment. Clin Cancer Res. 2013;19(16):4465–76. doi: 10.1158/1078-0432.CCR-13-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodduluru LN, Kasala ER, Thota N, Barua CC, Sistla R. Chemopreventive and therapeutic effects of nimbolide in cancer: the underlying mechanisms. Toxicol In Vitro. 2014;28(5):1026–35. doi: 10.1016/j.tiv.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Elumalai P, Arunakaran J. Review on molecular and chemopreventive potential of nimbolide in cancer. Genomics Inform. 2014;12(4):156–64. doi: 10.5808/GI.2014.12.4.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramani R, Gonzalez E, Arumugam A, Nandy S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S, Narayan M, Dwivedi A, Lakshmanaswamy R. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci Rep. 2016;6:19819. doi: 10.1038/srep19819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandra D, Liu JW, Tang DG. Early mitochondrial activation and cytochrome c up-regulation during apoptosis. J Biol Chem. 2002;277(52):50842–54. doi: 10.1074/jbc.M207622200. [DOI] [PubMed] [Google Scholar]
- 42.Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24(15):6592–607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar S, Chaudhary AK, Kumar R, O'Malley J, Dubrovska A, Wang X, Yadav N, Goodrich DW, Chandra D. Combination therapy induces unfolded protein response and cytoskeletal rearrangement leading to mitochondrial apoptosis in prostate cancer. Mol Oncol. 2016;10(7):949–65. doi: 10.1016/j.molonc.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldhahn K, Hintersteininger M, Steiner G, Erker T, Kloesch B. Enhanced Antiproliferative and Pro-apoptotic Activities of a Novel Curcumin-related Compound in Jurkat Leukemia T-Cells. Anticancer Res. 2015;35(5):2675–80. [PubMed] [Google Scholar]
- 47.Pietras A. Cancer stem cells in tumor heterogeneity. Adv Cancer Res. 2011;112:255–81. doi: 10.1016/B978-0-12-387688-1.00009-0. [DOI] [PubMed] [Google Scholar]
- 48.Wei HJ, Yin T, Zhu Z, Shi PF, Tian Y, Wang CY. Expression of CD44, CD24 and ESA in pancreatic adenocarcinoma cell lines varies with local microenvironment. Hepatobiliary Pancreat Dis Int. 2011;10(4):428–34. doi: 10.1016/s1499-3872(11)60073-8. [DOI] [PubMed] [Google Scholar]
- 49.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–8. [PubMed] [Google Scholar]
- 50.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–52. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38(1):96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 52.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 53.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913–22. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 54.Joshi DC, Bakowska JC. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp. 2011;(51) doi: 10.3791/2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–59. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 58.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 59.Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1(3):233–40. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 60.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 61.Berrozpe G, Schaeffer J, Peinado MA, Real FX, Perucho M. Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int J Cancer. 1994;58(2):185–91. doi: 10.1002/ijc.2910580207. [DOI] [PubMed] [Google Scholar]
- 62.Hagn F, Klein C, Demmer O, Marchenko N, Vaseva A, Moll UM, Kessler H. BclxL changes conformation upon binding to wild-type but not mutant p53 DNA binding domain. J Biol Chem. 2010;285(5):3439–50. doi: 10.1074/jbc.M109.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arima Y, Nitta M, Kuninaka S, Zhang D, Fujiwara T, Taya Y, Nakao M, Saya H. Transcriptional blockade induces p53-dependent apoptosis associated with translocation of p53 to mitochondria. J Biol Chem. 2005;280(19):19166–76. doi: 10.1074/jbc.M410691200. [DOI] [PubMed] [Google Scholar]
- 64.Sohn D, Graupner V, Neise D, Essmann F, Schulze-Osthoff K, Janicke RU. Pifithrin-alpha protects against DNA damage-induced apoptosis downstream of mitochondria independent of p53. Cell Death Differ. 2009;16(6):869–78. doi: 10.1038/cdd.2009.17. [DOI] [PubMed] [Google Scholar]
- 65.Aapro MS, Martin C, Hatty S. Gemcitabine--a safety review. Anticancer Drugs. 1998;9(3):191–201. doi: 10.1097/00001813-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Ko CJ, Saif MW. Recurrent cutaneous toxic erythema induced by gemcitabine in a patient with pancreatic cancer. Cutan Ocul Toxicol. 2009;28(3):144–8. doi: 10.1080/15569520903046942. [DOI] [PubMed] [Google Scholar]
- 67.Glezerman I, Kris MG, Miller V, Seshan S, Flombaum CD. Gemcitabine nephrotoxicity and hemolytic uremic syndrome: report of 29 cases from a single institution. Clin Nephrol. 2009;71(2):130–9. doi: 10.5414/cnp71130. [DOI] [PubMed] [Google Scholar]
- 68.Jin H, Qiao F, Wang Y, Xu Y, Shang Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol Rep. 2015;34(5):2782–9. doi: 10.3892/or.2015.4258. [DOI] [PubMed] [Google Scholar]
- 69.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 70.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 71.Mizukami T, Kamachi H, Mitsuhashi T, Tsuruga Y, Hatanaka Y, Kamiyama T, Matsuno Y, Taketomi A. Immunohistochemical analysis of cancer stem cell markers in pancreatic adenocarcinoma patients after neoadjuvant chemoradiotherapy. BMC Cancer. 2014;14:687. doi: 10.1186/1471-2407-14-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, Sarkar FH. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72(1):335–45. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orrenius S. Mitochondrial regulation of apoptotic cell death. Toxicol Lett. 2004;149(1–3):19–23. doi: 10.1016/j.toxlet.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 74.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Babykutty S, S PP, J NR, Kumar MA, Nair MS, Srinivas P, Gopala S. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP-2/9 expression via inhibiting ERK1/2 and reducing DNA-binding activity of NF-kappaB in colon cancer cells. Mol Carcinog. 2012;51(6):475–90. doi: 10.1002/mc.20812. [DOI] [PubMed] [Google Scholar]
- 76.Worni M, Guller U, White RR, Castleberry AW, Pietrobon R, Cerny T, Gloor B, Koeberle D. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas. 2013;42(7):1157–63. doi: 10.1097/MPA.0b013e318291fbc5. [DOI] [PubMed] [Google Scholar]
- 77.Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44(8):1529–35. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song H, Hollstein M, Xu Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol. 2007;9(5):573–80. doi: 10.1038/ncb1571. [DOI] [PubMed] [Google Scholar]
- 79.Xu J, Wang J, Hu Y, Qian J, Xu B, Chen H, Zou W, Fang JY. Unequal prognostic potentials of p53 gain-of-function mutations in human cancers associate with drug-metabolizing activity. Cell Death Dis. 2014;5:e1108. doi: 10.1038/cddis.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bullock AN, Henckel J, Fersht AR. Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: definition of mutant states for rescue in cancer therapy. Oncogene. 2000;19(10):1245–56. doi: 10.1038/sj.onc.1203434. [DOI] [PubMed] [Google Scholar]
- 81.Weissmueller S, Manchado E, Saborowski M, Morris JPt, Wagenblast E, Davis CA, Moon SH, Pfister NT, Tschaharganeh DF, Kitzing T, Aust D, Markert EK, Wu J, Grimmond SM, Pilarsky C, Prives C, Biankin AV, Lowe SW. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell. 2014;157(2):382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG, Frame MC, Evans TR, Sansom OJ. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107(1):246–51. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 84.Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, McAllister SS, Jacks T, Weinberg RA. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134(1):62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125(10):2323–31. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 86.Jiang W, Zhang Y, Kane KT, Collins MA, Simeone DM, di Magliano MP, Nguyen KT. CD44 regulates pancreatic cancer invasion through MT1-MMP. Mol Cancer Res. 2015;13(1):9–15. doi: 10.1158/1541-7786.MCR-14-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, Bissell MJ, Osborne TF, Tian B, Lowe SW, Silva JM, Borresen-Dale AL, Levine AJ, Bargonetti J, Prives C. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148(1–2):244–58. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]