Abstract
Instructions, suggestions, and other types of social information can have powerful effects on pain and emotion. Prominent examples include observational learning, social influence, placebo, and hypnosis. These different phenomena and their underlying brain mechanisms have been studied in partially separate literatures, which we discuss, compare, and integrate in this review. Converging findings from these literatures suggest that 1) instructions and social information affect brain systems associated with the generation of pain and emotion, and with reinforcement learning, and that 2) these changes are mediated by alterations in prefrontal systems responsible for top-down control and the generation of affective meaning. We argue that changes in expectation and appraisal, a process of assessing personal meaning and implications for wellbeing, are two potential key mediators of the effects of instructions and social information on affective experience. Finally, we propose a tentative model of how prefrontal regions, especially dorsolateral and ventromedial prefrontal cortex may regulate affective processing based on instructions and socially transmitted expectations more broadly.
Keywords: Placebo, Social conformity, Social Influence, Observational learning, Hypnosis, fMRI, Fear, Emotion Regulation, dlPFC, vmPFC, Appraisal, Expectation
Introduction
Instructions and suggestions are quintessential forms of everyday social influence. Using language, complex relationships in the environment can be described in an incredibly precise and nuanced way. With verbal instructions, humans can direct other humans’ behavior much faster and more efficiently than any reinforcement schedule would allow in other species (Cole et al., 2013; Roepstorff and Frith, 2004). Other people’s words can “plant” a very specific idea in someone’s mind and—together with the right receptivity and mindset—they can constitute powerful influence on subjective experience. These ideas form the basis of multiple theories of conceptual learning (e.g. De Houwer, 2009; Kirsch, 2004) and related therapeutic approaches like acceptance and commitment therapy (e.g., Hayes, 2004). They go back to early theories on expectations and conceptual processes such as the ‘New Look’ (Bruner and Postman, 1948) and resonate with related contemporary ones emphasizing the role of expectations and conceptual thought in experience, such as levels of construal theory (Liberman and Trope, 2008), evaluative priming (Bargh et al., 2012), hierarchical Bayesian models of learning and decision-making (Tenenbaum et al., 2006), and predictive coding accounts (Friston, 2010; Rao and Ballard, 1999).
A dramatic case of how suggestions can affect experience and behavior is voodoo death: the observation that in primitive tribal societies people subjected to ritualistic spells and black magic can die within a couple of days or weeks, despite having previously been without any symptoms of disease (Cannon, 1942). In modern societies, people might be less susceptible to die from pure suggestion. Yet, there is accumulating evidence that different forms of instructions, suggestions, social observation, and other socially transmitted information can influence what we experience, how we interpret the world around us, and what we value. In this review, we focus on the “socially instructed” modulation of emotion and pain, which has been studied across different fields and using different paradigms.
We focus on four areas: First, learning by observing others (‘observational learning’) has long been studied in developmental psychology, including the development of aggression (Bandura et al., 1963), fear, and pain (Colloca and Benedetti, 2009; Goubert et al., 2011; Vögtle et al., 2013), and might be an important phylo- and ontogenetic precursor of more abstract forms of instructions and social influence effects. Recent brain imaging studies have addressed how adults learn about fear and reward by observing others (Olsson and Phelps, 2007). Second, social influence and conformity effects have been described extensively in social psychology, starting with the experiment of Solomon Asch (1951). Those influences can be based on observation of others’ behavior or symbolic information about others’ behaviors and preferences. During the last few years, social influence has been addressed in cognitive and social neuroscience using brain imaging methods, with a novel focus on affective processes such as valuation and emotion (Campbell-Meiklejohn et al., 2010; Klucharev et al., 2009; Zaki et al., 2011). Third, instructions about task goals and the structure of what participants should experience, usually given by an experimenter, are integral to most psychological experiments. Yet, cognitive psychology and cognitive neuroscience have often taken them for granted, and only recently have researchers turned their attention to how human brains learn about pain and emotion from direct verbal instructions (e.g., Atlas et al., 2016; Grings et al., 1973; Phelps et al., 2001). As specific examples of instruction effects, a literature on the brain correlates of placebo effects has been emerging (e.g., Petrovic et al., 2002; Wager et al., 2004), as well as a growing interest in understanding the neurophysiological processes underlying hypnosis (Rainville et al., 1999)—both of which are fascinating examples of how social instructions and suggestions can have deep impact on the experience of emotion and pain. Finally, recently, researchers have started to study how top-down instructions and social information may interact with bottom-up experience-based learning (e.g., Atlas et al., 2016; Biele et al., 2011; Jepma and Wager, 2015; Li et al., 2011; Staudinger and Büchel, 2013).
These topics are studied in different literatures, which in many cases make surprisingly little contact with each other. Here, we argue for a common basis, in socially transmitted conceptual thought. With socially transmitted, we mean that the source of the information is usually one or more other agents. Thus, transmission occurs via direct observation, but also via symbolic communication (e.g., language, signs). Conceptual thought refers to the activation and manipulation of interrelated concepts or patterns of concepts. They are typically conscious and relationships among different concepts can change depending on the context. Expectations are an example—thoughts about future outcomes. The topics discussed here are not the only instances in which socially transmitted conceptual thought may be important, but particularly informative for our understanding of emotion and pain. The aim of this review is therefore to describe these phenomena from an integrative perspective.
Across these topics, several key questions emerge. First, to what degree are the experience and physiology of pain and emotion influenced by instruction or social information alone? Some suggestion-related effects could be partially due to more experience-based associative learning such as classical conditioning. As we will discuss below, this point may be especially (but not only) important for placebo effects, during which instructions are often reinforced using conditioning procedures. Second, how deeply does socially transmitted information influence the fundamental processes that give rise to pain and emotion? Instructions may just lead to compliance, i.e. responding in line with the instructor without deeper changes in attitudes and experience (Asch, 1951; Cialdini and Goldstein, 2004; Milgram and Gudehus, 1978), or they may also change deeper levels of affective processing. This question has elicited considerable debate in the research on social conformity, but also in placebo and other social suggestion effects. Third, what are the underlying brain mechanisms and is there any common basis in brain systems across these different effects? We will argue that despite their differences, various types of social information effects may share some common mediators, especially changes in expectations and appraisal processes, which may be implemented in prefrontal brain systems and their interactions with limbic and subcortical brain circuits.
We will start by reviewing brain imaging studies regarding social observational learning of emotion, and then discuss more abstract forms of information transmission, such as verbal and symbolic instructions and suggestions. We further review how conceptual and social information can interact to varying degrees with bottom-up, experience-based learning. In the last sections, we summarize commonalities and differences across different phenomena and types of social influence, and end by presenting a preliminary framework of putative brain mechanisms and open questions for future research.
1. Social information effects on pain and emotion
1.1. Social observation
Observational learning—learning from direct observation of other agents—is common across numerous species, from honeybees to humans (Gariépy et al., 2014; Olsson and Phelps, 2007). In the following sections, we focus on three main areas of research: Observational threat conditioning, social observation effects on pain and placebo analgesia, and social learning of appetitive responses to reward.
1.1.1. Vicarious threat and fear learning
A large body of research suggests that fear can be learned by observation of others (see reviews by Bandura and Walters, 1977; Mineka et al., 1984; Olsson and Phelps, 2007). Seeing another monkey being bitten by a snake will increase behavioral threat responses to snakes in the observing monkey (Mineka et al., 1984). In reaction to novel objects or unexpected events, both infants and older children will look at their caregiver in order to know how they are supposed to react, and then express emotions consistent with those of the caregiver—a process called social referencing (Campos et al., 1981; Feinman and Lewis, 1983). For example, children can develop fears by observing their parents expressing fearful emotional expression to previously neutral stimuli, such as novel animals (e.g., Muris et al., 2010). Social observation learning has also been demonstrated in rodents. A recent study found that male mice displayed freezing behavior both during observation of another mouse receiving foot shocks, as well as when placed in the same context again 24h later (Jeon et al., 2010). The strength of this observational threat response was increased for familiar conspecifics, including siblings and mating partners, compared to unfamiliar mice (Jeon et al., 2010).
Despite the enormous body of work on social fear learning using behavioral methods in humans and other animals, only a few studies have addressed the underlying brain mechanisms. In the study by Jeon et al. (2010), lesions of the anterior cingulate cortex (ACC) before observing a cagemate receiving foot shocks disrupted observation-induced freezing. Lesions after observation had no effect, suggesting that ACC is critical for the encoding of the cagemate’s distress, but not memory storage or expression. Standard classical shock conditioning also remained intact after ACC lesions. In contrast, lesions of the lateral amygdala disrupted both the acquisition and expression of observational threat learning (Jeon et al., 2010), consistent with a well-established role of the amygdala in encoding cue-threat associations (Davis et al., 2001; LeDoux, 2003).
Functional brain imaging studies in humans confirm the involvement of the dorsal ACC and amygdala in observational threat and fear learning (Mechias et al., 2010). In one of these studies (Olsson and Phelps, 2007), participants first observed a movie of another person expressing distress when experiencing painful electric shocks to the wrist, which were paired with a conditioned stimulus (CS). In a separate test phase, participants then underwent the same conditioning task themselves and expected to receive shocks paired with the same CS. The results showed increased skin conductance responses and increased amygdala activation to the CS during both observation and test, despite the fact that the participants never received shocks themselves. This demonstrates that social observation influences physiological response. Additional activations were observed in anterior insula (AI), ACC, and dorsolateral prefrontal cortex (dlPFC) (Olsson et al., 2007)—regions involved in the processing of pain, empathy, and other emotions. The authors concluded that the observation of a distressed conspecific can act like an unconditioned stimulus—especially for highly empathic individuals (Olsson et al., 2015)—, and thereby engages similar pathways as direct classical conditioning (Olsson et al., 2015; Olsson and Phelps, 2007). Conversely, both direct and vicarious fear extinction involve vmPFC-amygdala interactions (Golkar et al., 2016).
Several other studies have investigated observational fear learning using social referencing paradigms, in which previously neutral objects are paired with fearful (and sometimes also other emotional) facial expressions (Blair et al., 2016; Hooker et al., 2006; Hooker et al., 2008; Meffert et al., 2015). Paralleling the findings for observational fear conditioning in humans and mice, the results show that when objects are referenced by fearful expressions, participants show increased amygdala activity both during learning as well as recognition of these objects (Hooker et al., 2008; Meffert et al., 2015). For example, a particular novel shape may be paired with an image of a fearful face, and another shape paired with a neutral face. However, just the association with fearful faces is not enough—amygdala responses were stronger when the fearful face was shown gazing at the novel shape rather than looking away. This effect was enhanced in individuals high in neuroticism (Hooker et al., 2008) or diagnosed with social anxiety disorder (Blair et al., 2016). These studies provide an experimental model of the type of social referencing that occurs throughout the lifespan, but is particularly important during development.
Together, these studies suggest the operation of potentially similar associative mechanisms in the amygdala during both observational and direct fear learning (Olsson and Phelps, 2007). They further point at an important role of amygdala-prefrontal interactions during social learning of fear (Jeon et al., 2010; Olsson and Phelps, 2007). Such interactions may be important in anxiety disorders and regulation of fear responses more broadly (Etkin et al., 2010; Kim and Whalen, 2009; Urry et al., 2006). To our knowledge, no fMRI study so far has directly compared emotional learning induced by observation and direct conditioning in the same subjects.
1.1.2. Observational learning of pain and placebo
Social learning also shapes expectations and attitudes about pain, as well as the experience of pain and its expression in front of others (Goubert et al., 2011). One seminal study (Craig and Prkachin, 1978) showed that observing a “pain-tolerant” confederate led to reduced verbal pain reports and physiological responses to pain when the observer subsequently underwent the same painful stimulation. Similarly, facial expressions of fear increase pain reports in the observer in adults (Reicherts et al., 2013), as does observation of someone else suffering (Loggia et al., 2008).
As in fear learning, social referencing and modeling might be very important to shape pain and pain regulation (Goubert et al., 2011). For instance, children show lower pain tolerance in a cold pressor task if their mothers previously expressed high pain during the same task (Goodman and McGrath, 2003). Similarly, adults rated higher pain-related fear after observing another person displaying high pain during a cold pressor task (Helsen et al., 2011). Conversely, observing others can also help to reduce pain or to induce placebo effects. For example, seeing another person, either live or in a video, experiencing relief during one experimental condition (such as a green light), will evoke placebo analgesia in the observer, when they subsequently receive the same placebo treatment (Colloca and Benedetti, 2009; Hunter et al., 2014; for nocebo see Świder and Bąbel, 2013; Vögtle et al., 2013).
No studies, to our knowledge, have investigated the neural correlates of direct observational learning on pain experience or placebo analgesia. Candidate brain regions include AI and ACC, given their involvement in processing both one’s own and observed pain (e.g. Corradi-Dell’Acqua et al., 2011; Decety and Jackson, 2004; Krishnan et al., 2016; Lamm et al., 2011; Singer et al., 2004), in pain prediction and anticipation (Atlas et al., 2010), and in observational learning of fear (Olsson et al., 2007).
1.1.3. Social reward learning
Similarly, the experience of appetitive stimuli may be shaped by social observation and learning (Gariépy et al., 2014). For instance, monkeys prefer the types of food eaten by other members of their group, but switch to another food when migrating to a new group with different preferences (van de Waal et al., 2013).
In terms of potential brain mechanisms, it has been shown that positive social referencing, i.e. pairing novel objects with happy facial expressions gazing at the objects, activates the hippocampus and amygdala (Hooker et al., 2008; Meffert et al., 2015), suggesting that these regions are important for associative learning of pleasant stimuli as well. This work is consistent with a large literature on the importance of the amygdala for reward as well as punishment learning (Baxter and Murray, 2002; Gallagher and Chiba, 1996).
Several fMRI studies have addressed the question of how the human brain represents vicarious rewards during observational learning. An accumulating body of research indicates that during joint action and observation, people represent others’ actions (Sebanz et al., 2006; Vesper et al., 2010) and their associated outcomes (e.g. correct or incorrect, gain or loss) (Koban and Pourtois, 2014). Partially similar brain mechanisms are involved during the monitoring of others’ and one’s own actions (de Bruijn et al., 2009; Koban et al., 2012; for a review see Koban and Pourtois, 2014). For example, ACC and AI activated both during observation and commission of errors (Koban et al., 2013; Shane et al., 2008). When observing another person performing a probabilistic choice task, vmPFC and ventral striatum are thought to encode vicarious reward prediction errors (Burke et al., 2010). Damage to vmPFC, on the other hand, impairs observational learning (Kumaran et al., 2015). A more dorsal portion of the rostral anterior cingulate gyrus has been shown to track others’ net rewards (Apps and Ramnani, 2014), and more generally others’ motivational states (Apps et al., 2016). Together, these studies outline the brain networks involved in social action monitoring (Apps et al., 2016; Koban and Pourtois, 2014) and vicarious reward learning (Burke et al., 2010), and suggest that portions of the dorsal anterior cingulate cortex may play a common role in the effect of social observation on negative and positive emotion. An open question is whether such influences are limited to effects on decision-making, or change core valuation and experience in the observer. This question has been addressed in the context of social influence and conformity, to which we will turn next.
1.2. Social influence and conformity
Humans learn not only from direct observation of others’ behavior or emotional states, but are also highly susceptible to the influences of social norms (Cialdini and Goldstein, 2004). Social conformity has typically been studied in the context of changes in overt behavior (c.f. Asch, 1951), but it may influence deeper layers of attitudes and beliefs as well (Deutsch and Gerard, 1955)—in which case the term “social influence” may be more appropriate. Social influence can be seen as occupying a middle ground between observational learning, on one hand, and social instruction or suggestion on the other.
A growing number of recent brain imaging and psychophysiological studies have addressed social influence across different cognitive and affective processes (Wu et al., 2016), including value and preferences (Berns et al., 2010; Campbell-Meiklejohn et al., 2010; Klucharev et al., 2009; Klucharev et al., 2011; Zaki et al., 2011), visual perception (Berns et al., 2005), memory (Edelson et al., 2011; Edelson et al., 2014), pain (Koban and Wager, 2016; Yoshida et al., 2013), and even self-perception (Koban et al., in press; Korn et al., 2012). All these studies have shown that presenting participants with the opinions or ratings of a group of other individuals shifts the participant’s evaluation or behavior towards the group rating. In keeping with the topic of this review, we focus our discussion on studies investigating social influence or conformity on affective processes (valuation, emotion, and pain). Broadly speaking, these studies on social influence have tested three key questions: First, what are the brain mechanisms that detect mismatch or alignment with group norms? Second, how do those mechanisms predict adjustments in behavior or affective valuation? And third, do social influence effects indeed change affective and evaluative processes—or are they mere decision or reporting biases?
In order to address the first two questions, one of the first fMRI studies on social influence (Klucharev et al., 2009) presented male participants with pictures of attractive female faces, asked them to rate their attractiveness, and then gave them feedback about how other people rated the attractiveness. Other people’s ratings could be the same as, higher, or lower than participants’ own ratings. When participants rated attractiveness the same as their peer group, they showed increased activity in the ventral striatum—suggesting that being in line with the group might be rewarding (Klucharev et al., 2009; Wu et al., 2016). In contrast, when participants’ ratings were incongruent with those of the group (e.g. participants’ ratings were higher or lower), Klucharev and colleagues observed increased activity in dMFC, AI, and lateral PFC, regions often associated with conflict processing, negative outcomes, and need for cognitive and behavioral adjustments (Botvinick et al., 2001; Shackman et al., 2011; Shenhav et al., 2013). Moreover, activity in dMFC to incongruent ratings predicted subsequent behavioral adjustments towards the group norm (Klucharev et al., 2009). In line with the interpretation that this brain region is causally involved in signaling a “need to adjust”, transient down-regulation of dMFC using rTMS abolishes the behavioral conformity effect (Klucharev et al., 2011).
Importantly, behavioral adjustments and brain activity to a mismatch with the group depends on the social context (Izuma, 2013; Wu et al., 2016). People adjust their preferences to the norms of the in-group, but not to preference norms of an undesirable out-group such as sex-offenders—in contrast, they even adjust their preferences away from those of the out-group (Izuma and Adolphs, 2013). At the brain level, dorsomedial prefrontal cortex was more strongly activated by being in agreement with sex-offenders than when being in disagreement with them (Izuma and Adolphs, 2013). These findings point to an important role of other social-cognitive processes in social influence.
To answer the second question, regarding the impact level of social influence, recent studies have tested how markers of affective processing in the brain change as a function of social information. For instance, Zaki and colleagues (see also Campbell-Meiklejohn et al., 2010; Nook and Zaki, 2015; Zaki et al., 2011) first identified value- and reward-related brain areas (including ventral striatum, vmPFC, and OFC) using a monetary-incentive delay task. Similar to the Klucharev et al. (2009) study, they then had male participants rate the attractiveness of female faces, and subsequently provided feedback about other people’s ratings. When they presented the same faces again later, Zaki and colleagues measured how activity in value-related regions changed as a function of the others’ ratings. In line with the idea that social influence would change not only self-reported attractiveness, but also value-related processing in the human brain, their results demonstrated increases in value-related brain areas for faces that had been rated as more attractive by others, and decreases for faces that had been rated as less attractive by others (Zaki et al., 2011).
Extending these findings to aversive processing, a recent study (Koban and Wager, 2016; see also Yoshida et al., 2013) investigated whether social information can also alter the experience of pain. At the beginning of each trial, participants would see the ratings of other people, which could be either high or low on average, and were then stimulated with brief painful heat on their forearm. Koban and Wager measured both self-reported pain intensity as well as physiological (skin conductance) responses to the painful heat. If social influences on pain reflect mere conformity, one would expect changes in self-report, but not in autonomic physiology such as skin conductance responses. Interestingly, the authors found that participants rated pain as more intense and had higher skin conductance responses when the same heat stimulus was preceded by high social pain ratings compared to low social pain ratings (Koban and Wager, 2016). These results suggest deep and strong effects of social information on the affective processing of pain and associated autonomic responses.
In sum, social influence is a powerful way to nudge people’s preferences and to change emotional experiences, including affective responses to both appetitive and painful events. On the neurophysiological level, these changes seem to be mediated by lateral prefrontal areas together with dACC and anterior insula, which may detect mismatch with desirable group norms and trigger changes in value- or affective processing areas.
1.3. Social instruction and suggestion
Even without evoking group norms, suggestions and instructions can have powerful effects on experience and behavior (Halligan and Oakley, 2014; Michael et al., 2012). Effects of instructions and suggestions on emotion and pain have been investigated using brain imaging in several domains, including instructed fear, placebo and nocebo effects, and hypnosis.
1.3.1. Instructed threat
Next to experience-based learning (i.e. direct classical conditioning) and observational learning, fear can be both evoked and reduced by simple instructions (Bridger and Mandel, 1964; Costa et al., 2015; Grings et al., 1973; Olsson and Phelps, 2004; Raes et al., 2014). For instance, seeing a warning sign about rattlesnakes is probably sufficient to alert a hiker and to be more startled when she sees something moving in the high grass next to the trail.
One of the first fMRI studies on this topic compared brain activity to instructed threat stimuli with instructed safe stimuli. The instructed threat condition elicited higher amygdala activity compared to the safe condition—despite the fact that participants never actually received any shocks (Phelps et al., 2001). These activations are similar to what has been shown for direct classical conditioning and observational learning, suggesting partially similar mechanisms (Delgado et al., 2006; Mechias et al., 2010; Olsson and Phelps, 2007). Patients with left amygdala lesions failed to show any startle response to instructed threat (Funayama et al., 2001). In addition to the amygdala, activations for instructed threat have been observed in the insula, ACC, and premotor cortex, leading to the suggestion that these areas might be involved in the conscious anticipation of threat and transmission of its representation to the amygdala (Olsson and Phelps, 2007; Phelps et al., 2001) or the representation of threat contingencies in the dACC (Mechias et al., 2010).
1.3.2. Placebo and nocebo effects
Placebo effects are improvements in symptoms caused by a sham treatment compared to a no treatment control. By contrast, nocebo effects describe the appearance or worsening of symptoms based on sham treatments, such as the experience of side effects after consuming an actually inert substance. Placebo and nocebo effects have been studied across a large number of domains, including pain, Parkinson, depression, emotion, and motor performance (Benedetti, 2014; Bingel and Tracey, 2008; Büchel et al., 2014; Colloca and Benedetti, 2005; Wager and Atlas, 2015; Weimer et al., 2015). They are classic examples of how suggestions and instructions can alter experience. Yet, it is important to keep in mind that experimental placebo studies, which have compared placebo treatment to no-treatment controls, often use a combination of suggestion and conditioning to elicit maximal expectations and treatment effects. Both expectations and learning are thought to be important for placebo effects (Benedetti, 2014; Enck et al., 2013; Wager and Atlas, 2015), and it is unclear how much of the effects are due to purely conceptual expectations, and are thus created or modifiable by suggestions. For example, in placebo studies on pain, participants are often given a cream or pill with the instruction that this was a powerful analgesic. Following this placebo administration, this instruction is often reinforced by lowering stimulus intensity (unbeknown to the participant), leading the participant to experience actual relief and attribute it to the placebo (e.g. Atlas et al., 2010; Colloca et al., 2010; Geuter et al., 2013; Koban et al., 2012; Morton et al., 2010; Price et al., 1999; Wager et al., 2004). Despite an important involvement of learning in placebo (Colloca and Benedetti, 2009; Schafer et al., 2015), suggestions are central to placebo and nocebo effects, and can be effective even alone, i.e. without conditioning (e.g., Aslaksen and Flaten, 2008; Aslaksen et al., 2015; Benedetti et al., 1999; Johansen et al., 2003; Lorber et al., 2007; Meissner, 2009; Schenk et al., 2014; van Laarhoven et al., 2011).
The brain mechanisms of placebo effects on pain are becoming increasingly better understood, (for recent reviews see Benedetti, 2014; Büchel et al., 2014; Enck et al., 2013; Wager and Atlas, 2015). Placebo effects on pain are typically accompanied by reduced activity in pain-processing brain regions, such as thalamus, ACC, and insula (Amanzio et al., 2011; Meissner et al., 2011; Wager and Atlas, 2015; Wager and Fields, 2013)—however, some studies have also reported increased anterior insula and ACC activity in anticipation of or during placebo analgesia (Geuter et al., 2013; Kong et al., 2006; Wager et al., 2004). Further, placebo effects are paralleled by increased activity in another set of brain regions, including dlPFC, OFC, vmPFC, and PAG (Amanzio et al., 2011; Bingel et al., 2006; Eippert et al., 2009; Meissner et al., 2011; Petrovic et al., 2010; Petrovic et al., 2002; Wager and Atlas, 2015). These areas are thought to be involved in the maintenance of instructed beliefs, the representation of value, and—more generally during pain processing—the descending regulation of nociceptive processing (Fields, 2004; Loggia et al., 2008; Tracey, 2010).
While the brain mechanisms of nocebo hyperalgesia are far less studied than those of placebo analgesia, some parallels emerge from the studies published so far. For example, the thalamus, insula, ACC, somatosensory cortices, and frontal as well as parietal operculi show increased activation to painful stimuli following nocebo treatment (Ellerbrock et al., 2015; Jensen et al., 2014; Kong et al., 2008; Rodriguez-Raecke et al., 2010; Schmid et al., 2015). The operculum seems to particularly critical since it tracks behavioral hyperalgesia following mere instructions (Rodriguez-Raecke et al., 2010) and increases its functional coupling with the basal ganglia and thalamus over time (Ellerbrock et al., 2015). By contrast, rACC shows increased activation following a nocebo instruction compared to control, which hints at a regulatory role of the rACC as similar to that in placebo analgesia (Ellerbrock et al., 2015).
While still less studied, progress has been made for placebo effects on other modalities such as depression (Leuchter et al., 2002; Mayberg et al., 2002), negative (Koban et al., in revision; Meyer et al., 2015; Petrovic et al., 2005; Rütgen et al., 2015; Schienle et al., 2014), and positive emotions (Ellingsen et al., 2013) as well. Parallel to placebo studies on pain, these studies suggest reductions in areas associated with emotional experience such as ACC and AI (Petrovic et al., 2005; Rütgen et al., 2015; Schienle et al., 2014), and often associated increased activations in prefrontal regions including OFC and dlPFC (Benedetti, 2014; Koban et al., in revision; Mayberg et al., 2002; Petrovic et al., 2005).
Across different types of pain and emotion, administration of placebos is thought to evoke expectations, represented in prefrontal areas (especially dlPFC and vmPFC) that trigger activation of regulatory pathways in limbic and brainstem areas (e.g. Büchel et al., 2014; Wager and Atlas, 2015). These regulatory pathways may in turn reduce activation in areas associated with pain, arousal, or negative affect, such as ACC and AI.
1.3.3. Hypnosis
Hypnosis can be seen as a state of focused attention and altered consciousness, in which people respond to imaginative suggestions (for recent reviews see Lynn et al., 2008; Oakley and Halligan, 2009; Raz, 2011). During hypnosis, the participants are suggested sensations and experiences without being suggested a change in the actual environment (Lynn et al., 2008), such as “you are now beginning to feel your arms getting heavier, as if they were pulled down by some invisible force”. To some degree similar to placebo effects (Lynn et al., 2008; Raz, 2007), hypnotic suggestibility is thought to be driven by response expectancies and automatic activation of response sets (Lynn et al., 2008) and thus offers an fascinating case to study the influence of top-down over bottom-up processes (Raz, 2011).
Hypnotic suggestion can have profound effects on emotional experience and pain, and is clinically employed even for relief during surgeries (Faymonville et al., 2006) and the management of chronic pain (e.g. Patterson and Jensen, 2003). Several brain-imaging studies have investigated the neurophysiological processes underlying the effects of hypnosis and hypnotic suggestion on pain. During suggestion of reduced or no pain, participants report lower pain and show reduced activations of pain-processing brain regions such as insula, thalamus, and ACC (e.g., Abrahamsen et al., 2010; Derbyshire et al., 2009; Faymonville et al., 2000; Rainville et al., 1997; Rainville et al., 1999; Vanhaudenhuyse et al., 2009). Conversely, suggesting painfulness in the absence of actual noxious stimulation activates brain areas associated with pain processing, and more so than just imagination of pain (e.g., Derbyshire et al., 2004; Raij et al., 2005). Less is know about how suggestions modulate pain experience and pain processing in the brain. Using PET, Rainville et al. (1999) addressed this question by comparing hypnotic state with suggestion to hypnotic state without any further pain-related suggestions. They found increased regional blood flow in the left inferior frontal gyrus, the left and right dlPFC, as well as lateral parietal cortex and precuneus (Rainville et al., 1999), suggesting that these regions—in line with their role in top-down control of attention (Corbetta and Shulman, 2002; Miller and Cohen, 2001)—might be involved in the actualization of “suggestions involving alteration of the meanings of perceptual experiences” (Rainville et al., 1999, p. 199). In line with these earlier findings, dlPFC activity during verbal initiation of hypnotic pain suggestion has been shown to predict subsequently experienced pain and pain-related activation of SII (Hoeft et al., 2012; Raij et al., 2009).
Interestingly, few studies have investigated the brain bases of hypnotic suggestion on other affective processes than pain. One recent study tested the effects of hypnotic suggestion on disgust for different salty and sweet snacks (Ludwig et al., 2014). The results showed a significant modulation of snack preferences based on hypnotic, and to a slightly smaller extent, non-hypnotic suggestion. These behavioral findings were paralleled by changes in vmPFC activation, in line with this region’s role in value-based decision-making (Ludwig et al., 2014). Precuneus activity was higher during hypnotic compared to non-hypnotic suggestion (Ludwig et al., 2014), in line with other evidence of a role of this region in hypnosis (Cojan et al., 2009; Rainville et al., 1999) and for the sensory and “imaginal self” more broadly (Vuilleumier, 2014).
In sum, hypnosis is a powerful tool harnessing suggestions for pain relief and emotion modulation (Lynn et al., 2000; Raz, 2011). Yet, the brain bases of how hypnotic and other suggestions alter affective experience are just beginning to be understood. Converging findings suggest a direct modulation of affective processing by hypnosis, which might be implemented by top-down modulation via lateral prefrontal areas and altered processing of self-related processing and consciousness in precuneus. More studies are needed to investigate how emotions and affective processes are altered during hypnosis, and how these processes relate to other types of instructions and suggestions.
1.4. Interactions of social information and experience-based learning
Besides directly influencing people’s affective experiences, recent studies have shown that instructions and suggestions also influence bottom-up experience-based learning. Reinforcement learning is the process through which agents learn associations by directly experiencing rewarding or punishing outcomes related to stimuli and actions. Studies in psychology and neuroscience have provided a wealth of evidence regarding the computational and neural mechanisms underlying reinforcement learning, especially in the appetitive domain (Dayan and Daw, 2008; Niv, 2009).
Luckily, in many situations, appropriate behavior does not have to be learnt through trial-and-error, but can be achieved by following instructions, rules, or advice from others. That is, we can learn which behaviors/decisions are advantageous, and which should be avoided, without having to personally experience the negative consequences of suboptimal courses of action. It has been proposed that decisions based on instructed knowledge and decisions based on experience-based learning are subserved by distinct systems (e.g., Ashby et al., 1998; Hertwig et al., 2004). Yet, when instructions are added to an experience-based learning situation, how does this shape learning, and via which neural and computational mechanisms? We will discuss the emerging literature on interactions between instructions and reinforcement learning separately for appetitive and aversive learning.
1.4.1. Instruction effects on appetitive learning
The dopaminergic system is thought to have a key role in reward-driven learning. Specifically, the phasic activity of midbrain dopamine (DA) neurons, and their striatal projection areas, encode the difference between expected and obtained rewards—so-called reward prediction errors—which drive learning and future approach or avoidance behavior (Schultz, 1998; Schultz et al., 1997).
Several recent studies have addressed how instructions and suggestions influence instrumental reward learning. These studies have shown that both correct and incorrect instructions or advice can bias people’s choice behavior towards the instructed best options (Biele et al., 2011; Doll et al., 2009). Neuroimaging studies have revealed that instructions also influence the brain systems underlying experience-based learning. One fMRI study found that the presence of explicit (correct) information about reinforcement probabilities during a probabilistic learning task was associated with reduced prediction-error signaling in the striatum, as well as reduced discrimination between win and loss outcomes in the vmPFC, ventral striatum, and hippocampus (Li et al., 2011). A similar blunting of outcome-evoked responses in the vmPFC and ventral striatum has been found when participants were following advised choices, compared to when they did not follow instructed advice (Biele et al., 2011). A related study has shown that bad advice can diminish learning, especially if the advice is reinforced in a few initial trials (Staudinger and Büchel, 2013). Together, these results suggest that the presence of prior information—in the form of either experimental instructions or advice from others—suppresses the brain systems involved in stimulus evaluation and experience-based learning.
In contrast to the suppressing effect of instructions on outcome-evoked activation in the vmPFC and ventral striatum, instructions about reinforcement probabilities were associated with increased activation to gains in the left dlPFC (Li et al., 2011). Moreover, the strength of this effect was functionally correlated with the suppression of value-sensitive (vmPFC and ventral striatum) activations by instructions, suggesting that the dlPFC may suppress the experience-based learning system when humans can rely on instructed information. In addition, in the advice-following study (Biele et al., 2011), activations to both gains and losses were stronger following advised than non-advised choices in a region comprising the septal area and left caudate head. Since the septal area has been linked to social behavior and trust (Harbaugh et al., 2007; Kirk et al., 2016; Krueger et al., 2007), this may reflect the reinforcing aspect of trusting others and following their advice.
Computationally, there are at least two different ways in which instructions could shape learning. First, instructions may increase the subjective value of outcomes from recommended (vs. non-recommended) choices, possibly because instruction following is intrinsically rewarding. That is, instruction following may boost the positive experience of subsequent rewards, whereas it reduces the impact of negative (e.g., loss or punishment) outcomes. These effects can be captured computationally by implementing an outcome bonus in reinforcement learning models, which augments the value of outcomes resulting from advised or instructed choices (Biele et al., 2009; Biele et al., 2011). The increased activation in the septal area/caudate head for outcomes following advised vs. non-advised choices possibly reflected such an outcome bonus signal (Biele et al., 2011).
Alternatively, instructions may influence the learning process itself, by modulating the impact that new outcomes have on preexisting beliefs. In reinforcement learning models, the degree to which beliefs are updated based on observed prediction errors is determined by the learning rate, such that higher learning rates result in stronger belief updating towards the most recent outcome. Instructions may bias learning by enhancing learning rates for instruction-confirming relative to instruction-disconfirming outcomes. It has been proposed that such a confirmation bias may result from the modification of striatal dopaminergic prediction-error signaling by instruction representations in the prefrontal cortex and hippocampus, in such a way that striatal responses to instruction-confirming outcomes are amplified whereas those to instruction-disconfirming outcomes are inhibited (Doll et al., 2011; Doll et al., 2009). This top-down modulation of striatal reinforcement-learning mechanisms can cause learned value representations in the striatum to adhere to the instructed information, even when instructions do not affect outcome valuation. A possible psychological mechanism underlying the confirmation bias on learning rate involves the attribution of prediction errors: prediction errors that disconfirm instructions may not trigger belief updating (low learning rate) because they are attributed to coincidence (bad luck) or random fluctuations in outcomes that are not representative of the average situation. In contrast, observed outcomes that confirm instructions may enhance learning because they are attributed to the actual state of the world. Finally, note that although the outcome-bonus and confirmation-bias accounts differ conceptually, they are difficult to dissociate definitively as they make highly similar predictions about the effects of instructions on choice behavior and value representations over time.
1.4.2. Instruction effects on aversive learning
Compared to reward learning, the mechanisms underlying aversive learning, and their modulation by instructions are less well understood. According to an influential theory, the dopamine system oppositely affects reward- and punishment-based learning through phasic increases (‘bursts’) and decreases (‘dips’) in DA release, respectively (Frank, 2005). Several studies using pharmacological manipulation of the dopamine system in healthy and Parkinson’s populations have supported this idea (e.g., Bödi et al., 2009; Cools et al., 2006; Cools et al., 2009; Frank et al., 2004; Palminteri et al., 2009), although other studies have found that rewards and punishments both elicit dopamine bursts (e.g., Brischoux et al., 2009; Horvitz, 2000; Jensen et al., 2007; Matsumoto and Hikosaka, 2009).
A recent fMRI study on pain-avoidance learning (Roy et al., 2014) suggests that learning from primary aversive outcomes may be subserved by a brain system that is largely separate from that involved in reward learning. In contrast to findings of reward-learning studies (Rutledge et al., 2010), this pain-avoidance learning study found no evidence for the encoding of pain prediction errors in the ventral striatum, but instead found robust aversive prediction error activation in the periaqueductal grey (PAG) (Roy et al., 2014), consistent with prior work in animals (Fendt and Fanselow, 1999; McNally et al., 2011). Thus, a key difference between reward and (primary aversive) punishment learning may be that prediction errors (the teaching signals) are represented in the ventral striatum vs. PAG, respectively. The vmPFC, on the other hand, seems to encode value in a universal way across the reward and punishment domains: vmPFC activation increases with increasing reward values and decreases with increasing punishment positive “better” outcomes and decreases with increasing punishment values (Kim et al., 2006; Roy et al., 2014).
As describe above, instructions modulate learning-related activation of the ventral striatum and vmPFC in non-aversive learning situations. In the aversive domain, the PAG seems to respond to instructions and suggestions as well. Roy et al. (2014, Exp. 3) further showed that the aversive prediction error response in the PAG was stronger in a placebo than in a control condition. Thus, there was a larger PAG response when cognitive expectations were violated, as participants expected less pain during placebo analgesia. Note that this effect is in the opposite direction of the (suppressing) effects of instructions on ventral striatal/vmPFC activation reported above.
Finally, a recent study examined instruction effects on fear conditioning (Atlas et al., 2016). Similar to the instructed reward-learning study reported above (Li et al., 2011), this study found that instructions about cue-pain associations activated the left dlPFC (Atlas et al., 2016). In addition, striatal and OFC/vmPFC activation encoded the instructed value of predictive cues, and correlated with instruction-related activation in dlPFC. In contrast, amygdala responses were insensitive to instructions and were based on experienced cue-outcome associations alone (Atlas et al., 2016).
In sum, instructions seem to modify prediction-error encoding teaching signals in the ventral striatum and PAG in various ways. Reward prediction errors can be blunted by instructions and advice (Biele et al., 2011; Li et al., 2011), whereas aversive PEs may be enhanced by placebo-induced low pain expectations (Roy et al., 2014). Amygdala signals may be less sensitive to instructions, and hence may be part of a “slower” (model-free) learning system (Atlas et al., 2016). Together, these studies suggest that instructions and social suggestion can impact not only immediate experience of rewards or punishments, but also learning about those experiences. Initial evidence points at an important role of the dlPFC in instruction effects on learning, by modulating or suppressing reinforcement signaling (Atlas et al., 2016; Doll et al., 2011; Li et al., 2011). Future studies should elicit interactions between instructions and associative learning in more detail in order to identify commonalities and differences between aversive and appetitive domains.
2. Discussion
Above, we have illustrated how different types of socially transmitted, conceptual information can have powerful effects on affective experience, across different types of evaluative processes, emotions, and pain.
On the one hand, there are substantial differences in the types of experimental paradigms used, and how information is transmitted to participants across paradigms. For instance, social observational learning can be seen as much less abstract and as more grounded in experiential learning than instruction effects. Further, while hypnosis and placebo both influence emotional experiences via suggestions and may share some common characteristics (Raz, 2007), they also differ: Placebo typically involves some kind of deception, whereas hypnosis is based on suggestion of altered experiences without implying changes in the actual physical environment (Lynn et al., 2008).
On the other hand, the literature reviewed above also reveals some interesting parallels in the psychological and neurophysiological pathways underlying different types of suggestions. Together, the studies point to two broad conclusions. First, influences of social observation, instructions, and suggestions do not only induce superficial changes in reporting or compliance. Instead, these processes seem to change affect-related physiological responses, learning about aversive and appetitive events, and activity in associated brain areas. Prominent among these are the ventral striatum and vmPFC, amygdala, AI, and ACC. Second, these changes seem to be paralleled by increased activity in several other areas, most prominently the dlPFC and the vmPFC. As illustrated in Fig. 1, the dlPFC in particular shows increased activations for instruction versus no instruction, placebo versus control, social influence versus no social influence, and similar contrasts. It is also significantly activated in an automated Neurosynth meta-analysis (Yarkoni et al., 2011) on the term “instructions” (see Fig. 1A). In the following, we will discuss two neurocognitive processes, which may be associated with these brain imaging findings, namely changes in expectations and in affective appraisal. These processes are not mutually exclusive mechanisms, but complement and interact with each other.
Figure 1. Brain correlates of instructions and the dlPFC.
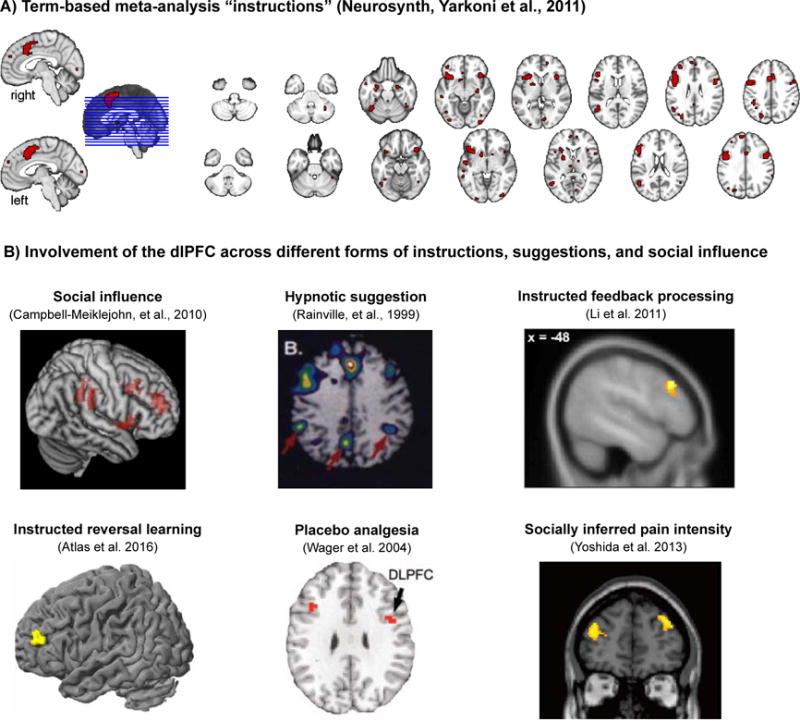
A) An automated large-scale meta-analysis (NeuroSynth, Yarkoni et al., 2011) on the term “instructions” (FDR-corrected P < 0.01) shows significant activity in several regions discussed in this review, including the dACC, AI, and dlPFC. B) Example studies of instruction and suggestion effects across different experimental paradigms and functional domains, highlighting an important role for dlPFC in instruction-related changes in affective processing.
2.1. Changes in expectations
One mechanism through which socially transmitted information can alter cognitive and affective processing is by changing expectations, i.e. representations of likely future external and internal states. Expectations are often driven by previous experiences, including social observational learning. For example, observing someone else showing painful expression while getting a flu shot might change the expectations regarding one’s own next vaccination. In social influence and conformity effects on affective processing, expectations might be formed based on what is perceived as the social norm. In line with this idea, self-reported expectations about upcoming pain intensity have been shown to mediate social influence effects on pain reports and physiology (Koban and Wager, 2016). Further, expectations have an important role in instruction and suggestion effects. They are important mediators of placebo and nocebo effects (Büchel et al., 2014; Geers et al., 2005; Kirsch, 2004; Petrovic et al., 2005; Tracey, 2010; Wager et al., 2007) and may determine the efficacy of hypnotic suggestion as well (e.g., Kirsch, 1985).
Once induced, expectations are powerful in shaping perception and cognition (Kirsch, 1985). One possible implementation on the neuronal or algorithmic level could be via hierarchical Bayesian decision making as put forward in predictive coding theories. These theories assume that the neural processing of sensory input is a combination of top-down sensory predictions and prediction errors—or in other words, a combination of top-down expectation and sensory bottom-up input (Bar, 2009; Friston, 2010; Summerfield and Egner, 2009). The top-down predictions are thought to be generated by an internal model of the world optimized by the statistics of natural stimuli. In a noisy sensory environment, these internal predictions help the brain to form efficient and robust representations of the world and incoming stimuli. By including socially transmitted information from conspecifics, these predictions can be optimized. This framework assumes descending effects of expectations across all processing hierarchies and distributed across brain areas, depending partially on the sensory or cognitive modality involved (Büchel et al., 2014; Den Ouden et al., 2012; Friston, 2010).
Complementing this distributed implementation of predictive coding, more explicit and consciously accessible expectations (compared to no expectations), have been shown to increase activation and functional connectivity of the medial PFC (Bar, 2007; Summerfield et al., 2006) and the dlPFC (Rachnev, 2013}—which may bias ascending connections via feedback projections (e.g., Rahnev et al., 2011; Summerfield et al., 2006). Other potentially important regions for generating expectations include the medial temporal lobe, especially hippocampus and parahippocampal areas, which are crucial not only for retrospective memory but also for predictions about the future (Schacter and Addis, 2007). Hence, these regions may also be involved in mediating expectations effects on pain and emotion induced by learning, instructions, and social information.
2.2. Changes in appraisals and meaning
A second mechanism through which instructions and social information can modulate affective experience, is by changing affective appraisal. Rather than changes in the sensation of the stimulus itself, changes in conscious and unconscious cognitive evaluations regarding the stimulus can alter the emotional meaning of the event (Ellsworth and Scherer, 2003; Scherer, 2005). For example, placebo treatments may alter the experience of a painful event not only by changing expectations, but also by changing the meaning of the pain, for instance by making the person feel less anxious about the pain and more in control. Similarly, observational learning and social influence might induce powerful changes in appraisals. According to social appraisal theory, emotions rarely happen in a social vacuum. Instead, events are appraised as a function of others’ emotional reactions to it (Fischer et al., 2003)—for example, we might find a joke more funny when others are laughing too. Thus, social appraisals might be a key mechanism of how the opinions and feelings of others, especially socially desirable and close others, shape our own emotional states (Willroth et al., in press).
Changes in appraisal are likely reflected in a variety of brain regions, depending on what appraisal processes are involved (Brosch and Sander, 2013; Sander et al., 2005). Yet, different appraisal processes might converge in areas associated with the generation of affective meaning, especially the vmPFC (Roy et al., 2012). Integrating information from different cortical and subcortical areas, this hub is well positioned to in turn influence processing in descending brain stem systems as well as cortical regions involved in the processing of affective information (Roy et al., 2012).
2.3. A tentative model of how instructions change affective experience
How does the brain transform instructions and social information into changes in affective states? And how can top-down information alter the processing of bottom-up nociceptive or sensory input? In the following, we outline a tentative model of the underlying cognitive and neurophysiological processes (see Figure 2).
Figure 2. A tentative model of key regions mediating instruction-related changes in affective processing.
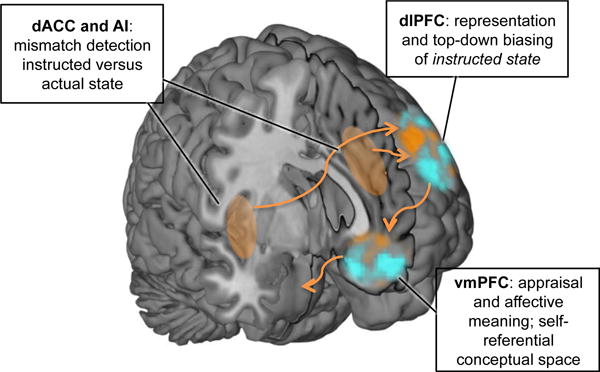
In this model, dlPFC represent the instructed state— a prediction of the organism’s state given the instructions. This instructed state representation could influence activity across the whole brain by top-down biasing affective processing in line with the instructed state. vmPFC integrates the input from dlPFC with ongoing conceptual and appraisal processes, thereby generating affective meaning in a self-referential conceptual space. Multidimensional predictions from vmPFC are transmitted to brainstem systems in order to regulate physiological responses, nociception, and arousal. dACC and AI are involved in detecting mismatch between social information and experienced affective state, thereby signalling need for enhanced control to dlPFC.
An affective or nociceptive stimulus, such as having a fresh cut in the feet, will activate nociceptive pathways and cause the experience of pain. At the same time, social information and instructions may be transmitted in different modalities, e.g. using spoken or written language, signs, or observation of others. Depending on the modality, the context, and the agent of the instruction, this information may be processed by a combination of language processing and semantic association areas, in concert with brain areas involved in social cognition and mentalizing. For example, when treating the painful wound on the foot, a doctor might administer a painkiller with the verbal instruction that this will alleviate the pain very soon.
We argue that this social suggestion leads to a representation of the instructed state in dlPFC (see Fig. 2), and potentially in functionally tightly coupled parietal regions. With instructed state representation, we mean the representation of the internal and external environment, as if the instruction was true. In other words, the instructed state representation is a prediction about one’s experiences given the instruction. For instance, during placebo treatment, this representation might reflect an abstract and multi-dimensional prediction of “I will feel better soon”. In line with its broader role in cognitive control for top-down attention selection and strengthening task-relevant features and processing (Banich, 2009; Botvinick et al., 2001; Miller and Cohen, 2001), dlPFC may use this instructed state representation to bias processing across the whole brain to “match” this prediction. This top-down biasing may involve up- and down-regulation of different aspects of stimulus processing and affective appraisal processes across the brain. For instance, in order to match the instructed state during placebo analgesia, dlPFC might down-regulate attention to nociceptive processes in pain-related brain areas and increase appraisals of safety and control, thereby changing the meaning or the valuation of the pain, in affective-meaning related areas, especially the vmPFC.
We propose that the vmPFC (see Fig. 2) has another key role in instruction-driven changes in affect, namely linking affective events to contextual and semantic proceses (Constantinescu et al., 2016; Roy et al., 2012). This region is also important in value (Daw et al., 2006; Rushworth et al., 2011), internal thought (Andrews-Hanna, 2012; Andrews-Hanna et al., 2014), representation of state space (Schuck et al., 2016), and self-related processing (Jenkins and Mitchell, 2011; Kelley et al., 2002). Thus, we speculate that it might be crucial to translate more abstract instructed state representations into a self-referential space of expectations and conceptual meaning. The vmPFC may also be important for linking those expectations to changes in descending autonomic and physiological systems (Bingel et al., 2006; Geuter et al., in press; Roy et al., 2012).
Finally, the dACC and AI seem to play an important role in instruction-based changes in affect and pain. These regions are associated with many diverse functions associated to alerting and switching, including conflict and error detection (Botvinick et al., 2001; Holroyd and Coles, 2002) and the value of decision and control processes (Kolling et al., 2016; Shenhav et al., 2013). In line with other recent findings of cognitive dissonance (Van Veen et al., 2009), social prediction error (Apps et al., 2016; Behrens et al., 2008; Klucharev et al., 2009), and social conflict processing (Koban et al., 2014), we speculate that dACC and AI may also detect mismatch between instructed state, self-referential state, and bottom-up sensory or nociceptive input, and thereby trigger adjustments in top-down control (in dlPFC, see Fig. 2) (Botvinick et al., 2001; in context of placebo effects, see Koban et al., 2012; Shenhav et al., 2013).
In sum, social observation, instructions, and other related types of social suggestion may evoke their powerful influences on emotional experience and behavior by harnessing top-down attentional biasing systems, to transform expectations into changes in emotional appraisal and meaning. This account is consistent with recent proposals of emotional and interoceptive experiences as a constructive process that is biased on prior expectations and beliefs (e.g., Barrett and Simmons, 2015). It is also in line with views that propose that social information and instruction is crucial for top-down control of experience and behavior (Roepstorff and Frith, 2004) and for the development of executive functions (e.g., Monfardini et al., 2016; Yu and Smith, 2016). While we acknowledge that different forms of social information and instructions reviewed here (especially social observational learning and hypnosis) will have slightly different underlying neurophysiological mechanisms and influence instructed state representation in prefrontal areas by different means, we hope that some of the ideas outlined in this model can be used to further develop our understanding of social instructions and suggestions more broadly.
3. Conclusions and future directions
Social instructions and suggestions, in various forms ranging from social observation, peer influence, to hypnosis and placebo, can have profound influences on the experience of emotion and pain, on appetitive and aversive learning, and their neurophysiological correlates. While these phenomena differ from each other in some aspects, they also share common features and may rely on partially overlapping cognitive processes and brain mechanisms. We propose that changes in expectations and in affective appraisals are two key mechanisms by which instructions and suggestions alter affective experience and we suggest a tentative model of how those changes might be implemented in functional brain circuits.
More work is needed to test and further characterize these mechanisms, and to identify commonalities and differences between different types of socially learned and instructed emotion and pain modulation. Here, we suggest several important directions for future research. First, behavioral and brain imaging studies should systematically compare different types of social information and contextual regulatory effects. For example, studies could directly compare hypnosis, placebo effects, observational learning and/or social information effects in the same subjects. Future studies could also investigate transfer and interactions between those different types of effects, and between different affective target states (see e.g., Rütgen et al., 2015). Another important aspect of comparison across modulatory effects will be to test similarity of brain pathways using fine-grained brain imaging methods such as multivariate pattern analysis (MVPA) and connectivity analyses.
Second, more experimental studies are needed to test the hypothesis that changes in expectations and appraisals are key mechanisms across diverse instruction and social information effects and to identify the brain correlates of these crucial processes. This could be assessed by studies that use self-reported or behaviorally revealed expectations as mediators, and by studies that identify physiological and neural markers of expectations and appraisals, particularly using MVPA. Creative and novel approaches are needed to characterize the representation of conceptual processing (c.f., Constantinescu et al., 2016) and of specific expectations and appraisals in prefrontal brain regions, such as dlPFC and vmPFC. Once such neurophysiological markers of conceptual processes and state representations are identified, future work could test how they are modulated by social information and in turn influence the experience and neurophysiological processing of emotion and pain.
Third, large-scale studies could address the open question of individual differences in different types of instruction and social learning effects. What neurophysiological, personality, or situational factors (or interactions between them; Koban et al., 2013) make individuals susceptible to social instruction effects? Are those the same across different types of social instructions, observational learning, and other socially transmitted expectations? Relatedly, future studies could investigate how instruction and social information effects develop over the lifespan, how they interact with psychopathology, and how they could be harnessed in the prevention and treatment of mental health problems (e.g., placebo and hypnosis).
Finally, more work is needed to fully understand how instructions and social information interact with experience-based, bottom-up learning, especially in the aversive domain (Atlas et al., 2016; Roy et al., 2014). Such studies may also test the boundary conditions of social information effects, i.e. in what cases instructed information does not have an effect or may even lead to contrast effects (that are in the opposite direction of the instructed information, Crombez and Wiech, 2011).
Highlights.
Instructions and social information have powerful effects on emotion and pain
Physiological and brain responses mirror experienced and behavioral effects
Changes in expectations and appraisal are key mediators of instruction effects
Prefrontal systems may bias affective processing based on instructions
Acknowledgments
This work was funded by NIH grants R01DA035484 and R01MH076136 (TDW) and by the DFG (GE 2774-1/1 to SG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsen R, Dietz M, Lodahl S, Roepstorff A, Zachariae R, Østergaard L, Svensson P. Effect of hypnotic pain modulation on brain activity in patients with temporomandibular disorder pain. PAIN. 2010;151:825–833. doi: 10.1016/j.pain.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ, Ramnani N. The anterior cingulate gyrus signals the net value of others’ rewards. The Journal of Neuroscience. 2014;34:6190–6200. doi: 10.1523/JNEUROSCI.2701-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps Matthew AJ, Rushworth Matthew FS, Chang Steve WC. The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron. 2016;90:692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch SE. Effects of group pressure upon the modification and distortion of judgments. Groups, Leadership, and Men S. 1951:222–236. [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychol Rev. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Aslaksen PM, Flaten MA. The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosomatic medicine. 2008;70:811–818. doi: 10.1097/PSY.0b013e31818105ed. [DOI] [PubMed] [Google Scholar]
- Aslaksen PM, Zwarg ML, Eilertsen HIH, Gorecka MM, Bjørkedal E. Opposite effects of the same drug: reversal of topical analgesia by nocebo information. PAIN. 2015;156:39–46. doi: 10.1016/j.pain.0000000000000004. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. The Journal of neuroscience. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Doll BB, Li J, Daw ND, Phelps EA. Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala. eLife. 2016;5:e15192. doi: 10.7554/eLife.15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A, Ross D, Ross SA. Imitation of film-mediated aggressive models. The Journal of Abnormal and Social Psychology. 1963;66:3. doi: 10.1037/h0048687. [DOI] [PubMed] [Google Scholar]
- Bandura A, Walters RH. Social learning theory 1977 [Google Scholar]
- Banich MT. Executive function the search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci (Regul Ed) 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bar M. Predictions: a universal principle in the operation of the human brain. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2009;364:1181–1182. doi: 10.1098/rstb.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh JA, Schwader KL, Hailey SE, Dyer RL, Boothby EJ. Automaticity in social-cognitive processes. Trends Cogn Sci (Regul Ed) 2012;16:593–605. doi: 10.1016/j.tics.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nature Reviews Neuroscience. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature reviews neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456:245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. 2014;84:623–637. doi: 10.1016/j.neuron.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Baldi S, Casadio C, Maggi G. Inducing placebo respiratory depressant responses in humans via opioid receptors. European Journal of Neuroscience. 1999;11:625–631. doi: 10.1046/j.1460-9568.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- Berns GS, Capra CM, Moore S, Noussair C. Neural mechanisms of the influence of popularity on adolescent ratings of music. Neuroimage. 2010;49:2687–2696. doi: 10.1016/j.neuroimage.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Chappelow J, Zink CF, Pagnoni G, Martin-Skurski ME, Richards J. Neurobiological correlates of social conformity and independence during mental rotation. Biological psychiatry. 2005;58:245–253. doi: 10.1016/j.biopsych.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Biele G, Rieskamp J, Gonzalez R. Computational models for the combination of advice and individual learning. Cogn Sci. 2009;33:206–242. doi: 10.1111/j.1551-6709.2009.01010.x. [DOI] [PubMed] [Google Scholar]
- Biele G, Rieskamp J, Krugel LK, Heekeren HR. The Neural Basis of Following Advice PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. PAIN. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology. 2008;23:371–380. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- Blair KS, Otero M, Teng C, Geraci M, Lewis E, Hollon N, Blair RJR, Ernst M, Grillon C, Pine DS. Learning from other people’s fear: amygdala-based social reference learning in social anxiety disorder. Psychol Med. 2016:1–11. doi: 10.1017/S0033291716001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bödi N, Kéri S, Nagy H, Moustafa A, Myers CE, Daw N, Dibó G, Takats A, Bereczki D, Gluck MA. Reward-learning and the novelty-seeking personality: a between-and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009:awp094. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bridger WH, Mandel IJ. A comparison of GSR fear responses produced by threat and electric shock. Journal of Psychiatric Research. 1964;2:31–40. doi: 10.1016/0022-3956(64)90027-5. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proceedings of the National Academy of Sciences. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch T, Sander D. Comment: the appraising brain: towards a neurocognitive model of appraisal processes in emotion. Emotion Review. 2013;5:163–168. [Google Scholar]
- Bruner JS, Postman L. Symbolic Value as an Organizing Factor in Perception. The Journal of Social Psychology. 1948;27:203–208. doi: 10.1080/00224545.1948.9918925. [DOI] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81:1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Tobler PN, Baddeley M, Schultz W. Neural mechanisms of observational learning. Proceedings of the National Academy of Sciences. 2010;107:14431–14436. doi: 10.1073/pnas.1003111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, Frith CD. How the opinion of others affects our valuation of objects. Current Biology. 2010;20:1165–1170. doi: 10.1016/j.cub.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JJ, Stenberg C, et al. Perception, appraisal, and emotion: The onset of social referencing. Infant social cognition: Empirical and theoretical considerations. 1981;273:314. [Google Scholar]
- Cannon WB. “voodoo” Death. American Anthropologist. 1942;44:169–181. doi: 10.2105/ajph.92.10.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini RB, Goldstein NJ. Social influence: Compliance and conformity. Annu Rev Psychol. 2004;55:591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- Cojan Y, Waber L, Schwartz S, Rossier L, Forster A, Vuilleumier P. The brain under self-control: modulation of inhibitory and monitoring cortical networks during hypnotic paralysis. Neuron. 2009;62:862–875. doi: 10.1016/j.neuron.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Cole MW, Laurent P, Stocco A. Rapid instructed task learning: A new window into the human brain’s unique capacity for flexible cognitive control. Cognitive, Affective, & Behavioral Neuroscience. 2013;13:1–22. doi: 10.3758/s13415-012-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nature Reviews Neuroscience. 2005;6:545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. PAIN. 2009;144:28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. PAIN. 2010;151:430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu AO, O’Reilly JX, Behrens TEJ. Organizing conceptual knowledge in humans with a gridlike code. Science. 2016;352:1464–1468. doi: 10.1126/science.aaf0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D’Esposito M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. The Journal of Neuroscience. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. The Journal of Neuroscience. 2011;31:17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Bradley MM, Lang PJ. From threat to safety: Instructed reversal of defensive reactions. Psychophysiology. 2015;52:325–332. doi: 10.1111/psyp.12359. [DOI] [PubMed] [Google Scholar]
- Craig KD, Prkachin KM. Social modeling influences on sensory decision theory and psychophysiological indexes of pain. J Pers Soc Psychol. 1978;36:805–815. doi: 10.1037//0022-3514.36.8.805. [DOI] [PubMed] [Google Scholar]
- Crombez G, Wiech K. You may (not always) experience what you expect: in search for the limits of the placebo and nocebo effect. PAIN. 2011;152:1449–1450. doi: 10.1016/j.pain.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ, et al. The amygdala: vigilance and emotion. Molecular psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Daw ND. Decision theory, reinforcement learning, and the brain. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:429–453. doi: 10.3758/CABN.8.4.429. [DOI] [PubMed] [Google Scholar]
- de Bruijn ERA, de Lange FP, von Cramon DY, Ullsperger M. When Errors Are Rewarding. J Neurosci. 2009;29:12183–12186. doi: 10.1523/JNEUROSCI.1751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J. The propositional approach to associative learning as an alternative for association formation models. Learning & Behavior. 2009;37:1–20. doi: 10.3758/LB.37.1.1. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The Functional Architecture of Human Empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Den Ouden HEM, Kok P, De Lange FP. How prediction errors shape perception, attention, and motivation. Frontiers in psychology. 2012;3:548. doi: 10.3389/fpsyg.2012.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SWG, Whalley MG, Oakley DA. Fibromyalgia pain and its modulation by hypnotic and non-hypnotic suggestion: an fMRI analysis. Eur J Pain. 2009;13:542–550. doi: 10.1016/j.ejpain.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Derbyshire SWG, Whalley MG, Stenger VA, Oakley DA. Cerebral activation during hypnotically induced and imagined pain. Neuroimage. 2004;23:392–401. doi: 10.1016/j.neuroimage.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Gerard HB. A study of normative and informational social influences upon individual judgment. The journal of abnormal and social psychology. 1955;51:629. doi: 10.1037/h0046408. [DOI] [PubMed] [Google Scholar]
- Doll BB, Hutchison KE, Frank MJ. Dopaminergic genes predict individual differences in susceptibility to confirmation bias. J Neurosci. 2011;31:6188–6198. doi: 10.1523/JNEUROSCI.6486-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Jacobs WJ, Sanfey AG, Frank MJ. Instructional control of reinforcement learning: a behavioral and neurocomputational investigation. Brain research. 2009;1299:74–94. doi: 10.1016/j.brainres.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson M, Sharot T, Dolan RJ, Dudai Y. Following the crowd: Brain Substrates of Long-Term Memory Conformity. Science. 2011;333:108–111. doi: 10.1126/science.1203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson MG, Dudai Y, Dolan RJ, Sharot T. Brain Substrates of Recovery from Misleading Influence. The Journal of Neuroscience. 2014;34:7744–7753. doi: 10.1523/JNEUROSCI.4720-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Ellerbrock I, Wiehler A, Arndt M, May A. Nocebo context modulates long-term habituation to heat pain and influences functional connectivity of the operculum. PAIN. 2015;156:2222–2233. doi: 10.1097/j.pain.0000000000000297. [DOI] [PubMed] [Google Scholar]
- Ellingsen DM, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, Leknes S. Placebo improves pleasure and pain through opposite modulation of sensory processing. PNAS. 2013;110:17993–17998. doi: 10.1073/pnas.1305050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PC, Scherer KR. Appraisal processes in emotion. Handbook of affective sciences. 2003;572:V595. [Google Scholar]
- Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nature Reviews Drug Discovery. 2013;12:191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of Anterior Cingulate Activation and Connectivity With the Amygdala During Implicit Regulation of Emotional Processing in Generalized Anxiety Disorder. American Journal of Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faymonville ME, Boly M, Laureys S. Functional neuroanatomy of the hypnotic state. J Physiol Paris. 2006;99:463–469. doi: 10.1016/j.jphysparis.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Lamy M, Maquet P. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 2000;92:1257–1267. doi: 10.1097/00000542-200005000-00013. [DOI] [PubMed] [Google Scholar]
- Feinman S, Lewis M. Social referencing at ten months: A second-order effect on infants’ responses to strangers. Child development. 1983:878–887. doi: 10.1111/j.1467-8624.1983.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience & Biobehavioral Reviews. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nature Reviews Neuroscience. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fischer AH, Manstead ASR, Zaalberg R. Social influences on the emotion process. European Review of Social Psychology. 2003;14:171–201. [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of cognitive neuroscience. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a unified brain theory? Nature Reviews Neuroscience. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. Journal of Cognitive Neuroscience. 2001;13:721–729. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Chiba AA. The amygdala and emotion. Current opinion in neurobiology. 1996;6:221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Gariépy J-F, Watson KK, Du E, Xie DL, Erb J, Amasino D, Platt ML. Social learning in humans and other animals. Front Neurosci. 2014;8 doi: 10.3389/fnins.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AL, Helfer SG, Kosbab K, Weiland PE, Landry SJ. Reconsidering the role of personality in placebo effects: dispositional optimism, situational expectations, and the placebo response. Journal of psychosomatic research. 2005;58:121–127. doi: 10.1016/j.jpsychores.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Geuter S, Eippert F, Hindi Attar C, Büchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67:227–236. doi: 10.1016/j.neuroimage.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Koban L, Wager TD. The cognitive neuroscience of placebo effects: Concepts, predictions, and physiology. Annual Reviews in Neuroscience. doi: 10.1146/annurev-neuro-072116-031132. in press. [DOI] [PubMed] [Google Scholar]
- Golkar A, Haaker J, Selbing I, Olsson A. Neural signals of vicarious extinction learning. Soc Cogn Affect Neurosci. 2016:nsw068. doi: 10.1093/scan/nsw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JE, McGrath PJ. Mothers’ modeling influences children’s pain during a cold pressor task. PAIN. 2003;104:559–565. doi: 10.1016/S0304-3959(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Goubert L, Vlaeyen JWS, Crombez G, Craig KD. Learning About Pain From Others: An Observational Learning Account. The Journal of Pain. 2011;12:167–174. doi: 10.1016/j.jpain.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Grings WW, Schell AM, Carey CA. Verbal control of an autonomic response in a cue reversal situation. Journal of Experimental Psychology. 1973;99:215. [Google Scholar]
- Halligan PW, Oakley DA. Hypnosis and beyond: Exploring the broader domain of suggestion. Psychology of Consciousness: Theory, Research, and Practice. 2014;1:105. [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hayes SC. Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies. Behavior therapy. 2004;35:639–665. doi: 10.1016/j.beth.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Helsen K, Goubert L, Peters ML, Vlaeyen JWS. Observational learning and pain-related fear: An experimental study with colored cold pressor tasks. The Journal of Pain. 2011;12:1230–1239. doi: 10.1016/j.jpain.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Hertwig R, Barron G, Weber EU, Erev I. Decisions from experience and the effect of rare events in risky choice. Psychol Sci. 2004;15:534–539. doi: 10.1111/j.0956-7976.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Gabrieli JDE, Whitfield-Gabrieli S, Haas BW, Bammer R, Menon V, Spiegel D. Functional brain basis of hypnotizability. Arch Gen Psychiatry. 2012;69:1064–1072. doi: 10.1001/archgenpsychiatry.2011.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Germine LT, Knight RT, D’Esposito M. Amygdala response to facial expressions reflects emotional learning. The Journal of Neuroscience. 2006;26:8915–8922. doi: 10.1523/JNEUROSCI.3048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Miyakawa A, Knight RT, D’Esposito M. The influence of personality on neural mechanisms of observational fear and reward learning. Neuropsychologia. 2008;46:2709–2724. doi: 10.1016/j.neuropsychologia.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hunter T, Siess F, Colloca L. Socially-induced placebo analgesia: a comparison of a pre-recorded versus live face-to-face observation. Eur J Pain. 2014;18:914–922. doi: 10.1002/j.1532-2149.2013.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K. The neural basis of social influence and attitude change. Current opinion in neurobiology. 2013;23:456–462. doi: 10.1016/j.conb.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Izuma K, Adolphs R. Social Manipulation of Preference in the Human Brain. Neuron. 2013;78:563–573. doi: 10.1016/j.neuron.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social neuroscience. 2011;6:211–218. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J. A neural mechanism for nonconscious activation of conditioned placebo and nocebo responses. Cerebral Cortex. 2014:bhu275. doi: 10.1093/cercor/bhu275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepma M, Wager TD. Conceptual Conditioning Mechanisms Mediating Conditioning Effects on Pain. Psychological science. 2015;26:1728–1739. doi: 10.1177/0956797615597658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen O, Brox J, Flaten MA. Placebo and nocebo responses, cortisol, and circulating beta-endorphin. Psychosomatic Medicine. 2003;65:786–790. doi: 10.1097/01.psy.0000082626.56217.cf. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of cognitive neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol. 2006;4:e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. The Journal of Neuroscience. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Gu X, Sharp C, Hula A, Fonagy P, Montague PR. Mindfulness training increases cooperative decision making in economic exchanges: Evidence from fMRI. NeuroImage. 2016;138:274–283. doi: 10.1016/j.neuroimage.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985;40:1189. [Google Scholar]
- Kirsch I. Conditioning, expectancy, and the placebo effect: comment on Stewart-Williams and Podd (2004) Psychol Bull. 2004;130:341–343. doi: 10.1037/0033-2909.130.2.341. discussion 344–345. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Munneke MAM, Smidts A, Fernández G. Downregulation of the posterior medial frontal cortex prevents social conformity. The Journal of Neuroscience. 2011;31:11934–11940. doi: 10.1523/JNEUROSCI.1869-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Brass M, Lynn MT, Pourtois G. Placebo analgesia affects brain correlates of error processing. PloS one. 2012;7:e49784. doi: 10.1371/journal.pone.0049784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Corradi-Dell’Acqua C, Vuilleumier P. Integration of error agency and representation of others’ pain in the anterior insula. Journal of Cognitive Neuroscience. 2013;25:258–272. doi: 10.1162/jocn_a_00324. [DOI] [PubMed] [Google Scholar]
- Koban L, Kross E, Woo CW, Ruzic L, Wager TD. Frontal-brainstem pathways mediating placebo effects on social rejection. doi: 10.1523/JNEUROSCI.2658-16.2017. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Pichon S, Vuilleumier P. Responses of medial and ventrolateral prefrontal cortex to interpersonal conflict for resources. Soc Cogn Affect Neurosci. 2014;9:561–569. doi: 10.1093/scan/nst020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Pourtois G. Brain systems underlying the affective and social monitoring of actions: An integrative review. Neuroscience & Biobehavioral Reviews. 2014;46(Part 1):71–84. doi: 10.1016/j.neubiorev.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Koban L, Schneider R, Ashar YK, Andrews-Hanna JR, Landy L, Wager TD, Arch JJ. Social anxiety is characterized by negatively biased learning about performance and the self. Emotion. doi: 10.1037/emo0000296. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Wager TD. Beyond conformity: Social influences on pain reports and physiology. Emotion. 2016;16:24. doi: 10.1037/emo0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth MFS. Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci. 2016;19:1280–1285. doi: 10.1038/nn.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, LaViolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. The Journal of Neuroscience. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. The Journal of neuroscience. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn CW, Prehn K, Park SQ, Walter H, Heekeren HR. Positively Biased Processing of Self-Relevant Social Feedback. The Journal of Neuroscience. 2012;32:16832–16844. doi: 10.1523/JNEUROSCI.3016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Woo CW, Chang LJ, Ruzic L, Gu X, Lopez-Solà M, Jackson PL, Pujol J, Fan J, Wager TD. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife. 2016;5:e15166. doi: 10.7554/eLife.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, McCabe K, Moll J, Kriegeskorte N, Zahn R, Strenziok M, Heinecke A, Grafman J. Neural correlates of trust. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20084–20089. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Warren DE, Tranel D. Damage to the Ventromedial Prefrontal Cortex Impairs Learning from Observed Outcomes. Cereb Cortex. 2015;25:4504–4518. doi: 10.1093/cercor/bhv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M. Changes in brain function of depressed subjects during treatment with placebo. American Journal of Psychiatry. 2002;159:122–129. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- Li J, Delgado MR, Phelps EA. How instructed knowledge modulates the neural systems of reward learning. PNAS. 2011;108:55–60. doi: 10.1073/pnas.1014938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman N, Trope Y. The psychology of transcending the here and now. Science. 2008;322:1201–1205. doi: 10.1126/science.1161958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Mogil JS, Catherine Bushnell M. Empathy hurts: compassion for another increases both sensory and affective components of pain perception. PAIN. 2008;136:168–176. doi: 10.1016/j.pain.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Lorber W, Mazzoni G, Kirsch I. Illness by suggestion: expectancy, modeling, and gender in the production of psychosomatic symptoms. Annals of Behavioral Medicine. 2007;33:112–116. doi: 10.1207/s15324796abm3301_13. [DOI] [PubMed] [Google Scholar]
- Ludwig VU, Stelzel C, Krutiak H, Magrabi A, Steimke R, Paschke LM, Kathmann N, Walter H. The suggestible brain: posthypnotic effects on value-based decision-making. Soc Cogn Affect Neurosci. 2014;9:1281–1288. doi: 10.1093/scan/nst110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn SJ, Kirsch I, Barabasz A, Cardeña E, Patterson D. Hypnosis as an empirically supported clinical intervention: The state of the evidence and a look to the future. International Journal of Clinical and Experimental Hypnosis. 2000;48:239–259. doi: 10.1080/00207140008410050. [DOI] [PubMed] [Google Scholar]
- Lynn SJ, Kirsch I, Hallquist MN. The Oxford handbook of hypnosis: Theory, research, and practice. 2008. Social cognitive theories of hypnosis; pp. 111–139. [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. American Journal of Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends in neurosciences. 2011;34:283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Meffert H, Brislin SJ, White SF, Blair JR. Prediction errors to emotional expressions: the roles of the amygdala in social referencing. Soc Cogn Affect Neurosci. 2015;10:537–544. doi: 10.1093/scan/nsu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K. Effects of placebo interventions on gastric motility and general autonomic activity. Journal of psychosomatic research. 2009;66:391–398. doi: 10.1016/j.jpsychores.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Meissner K, Bingel U, Colloca L, Wager TD, Watson A, Flaten MA. The placebo effect: advances from different methodological approaches. J Neurosci. 2011;31:16117–16124. doi: 10.1523/JNEUROSCI.4099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Yuen KSL, Ertl M, Polomac N, Mulert C, Büchel C, Kalisch R. Neural Mechanisms of Placebo Anxiolysis. The Journal of Neuroscience. 2015;35:7365–7373. doi: 10.1523/JNEUROSCI.4793-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael RB, Garry M, Kirsch I. Suggestion, Cognition, and Behavior. Current Directions in Psychological Science. 2012;21:151–156. [Google Scholar]
- Milgram S, Gudehus C. Obedience to authority. Ziff-Davis Publishing Company; 1978. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mineka S, Davidson M, Cook M, Keir R. Observational conditioning of snake fear in rhesus monkeys. Journal of Abnormal Psychology. 1984;93:355–372. doi: 10.1037//0021-843x.93.4.355. [DOI] [PubMed] [Google Scholar]
- Monfardini E, Redouté J, Hadj-Bouziane F, Hynaux C, Fradin J, Huguet P, Costes N, Meunier M. Others’ sheer presence boosts brain activity in the attention (but not the motivation) network. Cerebral Cortex. 2016;26:2427–2439. doi: 10.1093/cercor/bhv067. [DOI] [PubMed] [Google Scholar]
- Morton DL, Brown CA, Watson A, El-Deredy W, Jones AKP. Cognitive changes as a result of a single exposure to placebo. Neuropsychologia. 2010;48:1958–1964. doi: 10.1016/j.neuropsychologia.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Muris P, van Zwol L, Huijding J, Mayer B. Mom told me scary things about this animal: Parents installing fear beliefs in their children via the verbal information pathway. Behaviour Research and Therapy. 2010;48:341–346. doi: 10.1016/j.brat.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Niv Y. Reinforcement learning in the brain. Journal of Mathematical Psychology. 2009;53:139–154. [Google Scholar]
- Nook EC, Zaki J. Social Norms Shift Behavioral and Neural Responses to Foods. Journal of Cognitive Neuroscience. 2015;27:1412–1426. doi: 10.1162/jocn_a_00795. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Halligan PW. Hypnotic suggestion and cognitive neuroscience. Trends Cogn Sci (Regul Ed) 2009;13:264–270. doi: 10.1016/j.tics.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Olsson A, McMahon K, Papenberg G, Zaki J, Bolger N, Ochsner KN. Vicarious fear learning depends on empathic appraisals and trait empathy. Psychological science. 2015 doi: 10.1177/0956797615604124. 0956797615604124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychological Science. 2004;15:822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Palminteri S, Lebreton M, Worbe Y, Grabli D, Hartmann A, Pessiglione M. Pharmacological modulation of subliminal learning in Parkinson’s and Tourette’s syndromes. Proceedings of the National Academy of Sciences. 2009;106:19179–19184. doi: 10.1073/pnas.0904035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull. 2003;129:495. doi: 10.1037/0033-2909.129.4.495. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in Emotional Processing— Induced Expectations of Anxiety Relief Activate a Generalized Modulatory Network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. PAIN. 2010;150:59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. PAIN. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Raes AK, De Houwer J, De Schryver M, Brass M, Kalisch R. Do CS-US pairings actually matter? A within-subject comparison of instructed fear conditioning with and without actual CS-US pairings. PloS one. 2014;9:e84888. doi: 10.1371/journal.pone.0084888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnev D, Lau H, de Lange FP. Prior expectation modulates the interaction between sensory and prefrontal regions in the human brain. The Journal of Neuroscience. 2011;31:10741–10748. doi: 10.1523/JNEUROSCI.1478-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij TT, Numminen J, Närvänen S, Hiltunen J, Hari R. Brain correlates of subjective reality of physically and psychologically induced pain. PNAS. 2005;102:2147–2151. doi: 10.1073/pnas.0409542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij TT, Numminen J, Närvänen S, Hiltunen J, Hari R. Strength of prefrontal activation predicts intensity of suggestion-induced pain. Hum Brain Mapp. 2009;30:2890–2897. doi: 10.1002/hbm.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. Journal of Cognitive Neuroscience. 1999;11:110–125. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- Rao RPN, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Raz A. Hypnobo: Perspectives on Hypnosis and Placebo. American Journal of Clinical Hypnosis. 2007;50:29–36. doi: 10.1080/00029157.2007.10401595. [DOI] [PubMed] [Google Scholar]
- Raz A. Hypnosis: a twilight zone of the top-down variety Few have never heard of hypnosis but most know little about the potential of this mind-body regulation technique for advancing science. Trends Cogn Sci (Regul Ed) 2011;15:555–557. doi: 10.1016/j.tics.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Reicherts P, Gerdes ABM, Pauli P, Wieser MJ. On the mutual effects of pain and emotion: facial pain expressions enhance pain perception and vice versa are perceived as more arousing when feeling pain. PAIN. 2013;154:793–800. doi: 10.1016/j.pain.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Doganci B, Breimhorst M, Stankewitz A, Büchel C, Birklein F, May A. Insular cortex activity is associated with effects of negative expectation on nociceptive long-term habituation. The Journal of Neuroscience. 2010;30:11363–11368. doi: 10.1523/JNEUROSCI.2197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff A, Frith C. What’s at the top in the top-down control of action? Script-sharing and ‘top-top’control of action in cognitive experiments. Psychological Research. 2004;68:189–198. doi: 10.1007/s00426-003-0155-4. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014;17:1607–1612. doi: 10.1038/nn.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci (Regul Ed) 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Rütgen M, Seidel EM, Silani G, Riečanskỳ I, Hummer A, Windischberger C, Petrovic P, Lamm C. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proceedings of the National Academy of Sciences. 2015;112:E5638–E5646. doi: 10.1073/pnas.1511269112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RB, Dean M, Caplin A, Glimcher PW. Testing the reward prediction error hypothesis with an axiomatic model. The Journal of Neuroscience. 2010;30:13525–13536. doi: 10.1523/JNEUROSCI.1747-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Scherer KR. A systems approach to appraisal mechanisms in emotion. Neural networks. 2005;18:317–352. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer SM, Colloca L, Wager TD. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. The Journal of Pain. 2015;16:412–420. doi: 10.1016/j.jpain.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk LA, Sprenger C, Geuter S, Büchel C. Expectation requires treatment to boost pain relief: An fMRI study. PAIN®. 2014;155:150–157. doi: 10.1016/j.pain.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Scherer KR. What are emotions? And how can they be measured? Social science information. 2005;44:695–729. [Google Scholar]
- Schienle A, Ubel S, Schöngaßner F, Ille R, Scharmüller W. Disgust regulation via placebo: an fMRI study. Soc Cogn Affect Neurosci. 2014;9:985–990. doi: 10.1093/scan/nst072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J, Bingel U, Ritter C, Benson S, Schedlowski M, Gramsch C, Forsting M, Elsenbruch S. Neural underpinnings of nocebo hyperalgesia in visceral pain: A fMRI study in healthy volunteers. Neuroimage. 2015;120:114–122. doi: 10.1016/j.neuroimage.2015.06.060. [DOI] [PubMed] [Google Scholar]
- Schuck NW, Cai MB, Wilson RC, Niv Y. Human orbitofrontal cortex represents a cognitive map of state space. Neuron. 2016;91:1402–1412. doi: 10.1016/j.neuron.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Bekkering H, Knoblich G. Joint action: bodies and minds moving together. Trends Cogn Sci (Regul Ed) 2006;10:70–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MS, Stevens M, Harenski CL, Kiehl KA. Neural correlates of the processing of another’s mistakes: a possible underpinning for social and observational learning. Neuroimage. 2008;42:450–459. doi: 10.1016/j.neuroimage.2007.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick Matthew M, Cohen Jonathan D. The Expected Value of Control: An Integrative Theory of Anterior Cingulate Cortex Function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Büchel C. How initial confirmatory experience potentiates the detrimental influence of bad advice. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.02.074. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Egner T. Expectation (and attention) in visual cognition. Trends Cogn Sci (Regul Ed) 2009;13:403–409. doi: 10.1016/j.tics.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive Codes for Forthcoming Perception in the Frontal Cortex. Science. 2006;314:1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- Świder K, Bąbel Pl. The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. PAIN®. 2013;154:1312–1317. doi: 10.1016/j.pain.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Tenenbaum JB, Griffiths TL, Kemp C. Theory-based Bayesian models of inductive learning and reasoning. Trends Cogn Sci (Regul Ed) 2006;10:309–318. doi: 10.1016/j.tics.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate’s foraging decisions. Science. 2013;340:483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- van Laarhoven AIM, Vogelaar ML, Wilder-Smith OH, van Riel PLCM, van de Kerkhof PCM, Kraaimaat FW, Evers AWM. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. PAIN®. 2011;152:1486–1494. doi: 10.1016/j.pain.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Krug MK, Schooler JW, Carter CS. Neural activity predicts attitude change in cognitive dissonance. Nat Neurosci. 2009;12:1469–1474. doi: 10.1038/nn.2413. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Boly M, Balteau E, Schnakers C, Moonen G, Luxen A, Lamy M, Degueldre C, Brichant JF, Maquet P, Laureys S, Faymonville ME. Pain and non-pain processing during hypnosis: a thulium-YAG event-related fMRI study. Neuroimage. 2009;47:1047–1054. doi: 10.1016/j.neuroimage.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Vesper C, Butterfill S, Knoblich G, Sebanz N. A minimal architecture for joint action. Neural Networks. 2010;23:998–1003. doi: 10.1016/j.neunet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Vögtle E, Barke A, Kröner-Herwig B. Nocebo hyperalgesia induced by social observational learning. PAIN®. 2013;154:1427–1433. doi: 10.1016/j.pain.2013.04.041. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323–337. doi: 10.1016/j.neucli.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nature Reviews Neuroscience. 2015;16:403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Fields H. Placebo analgesia. Textbook of Pain. 2013:362–373. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human μ-opioid activity during pain. Proceedings of the National Academy of Sciences. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: mediators and moderators. The Lancet Psychiatry. 2015;2:246–257. doi: 10.1016/S2215-0366(14)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willroth EC, Koban L, Hilimire MR. Social information influences emotional experience and late positive potential response to affective pictures. Emotion. doi: 10.1037/emo0000289. in press. [DOI] [PubMed] [Google Scholar]
- Wu H, Luo Y, Feng C. Neural signatures of social conformity: A coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews. 2016;71:101–111. doi: 10.1016/j.neubiorev.2016.08.038. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Koltzenburg M, Dolan RJ. Uncertainty increases pain: evidence for a novel mechanism of pain modulation involving the periaqueductal grey. J Neurosci. 2013;33:5638–5646. doi: 10.1523/JNEUROSCI.4984-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Smith LB. The social origins of sustained attention in one-year-old human infants. Current Biology. 2016;26:1235–1240. doi: 10.1016/j.cub.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychological Science. 2011;22:894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]


