ABSTRACT
A heavily pretreated patient with triple negative breast cancer distinguished by cutaneous metastases received p53MVA vaccine in combination with pembrolizumab. Her cutaneous metastases regressed and after 2 cycles of therapy, a skin biopsy showed a complete pathological response. Systemic response was confirmed with restaging CT and bone scans. Activation of p53-specific T cell responses and elevation of multiple immune response genes in peripheral blood correlated with the rapid clinical response which lasted for 6 months after the initiation of combined therapy.
KEYWORDS: immunotherapy, MVA, p53, PD-1, pembrolizumab, triple negative breast cancer, T cell response, vaccine, vaccinia
Introduction
Triple negative breast cancer (TNBC) represents approximately 15% of all breast cancers, and is associated with poor outcomes with a median survival of only 13.3 months in the metastatic setting.1 Due to the lack of expression of estrogen (ER), progesterone (PR), and epidermal growth factor receptor-2/neu (HER-2/neu) receptors that are targets for most breast cancer therapies, cytotoxic chemotherapy associated with significant systemic toxicity remains the only treatment option.2,3 Hence, effective and less toxic targeted therapy is urgently needed to improve outcomes in TNBC.
A majority of solid tumors, including TNBC, carry p53 gene mutations resulting in the accumulation of p53 protein within tumor cells.4 Most mutations of p53 involve the alteration of a single amino acid, thus, the majority of p53 epitopes processed and presented for T cell recognition on tumor cells are wild-type in sequence. Of notable interest, wild-type p53 is not presented on the surface of normal parenchymal cells in healthy adults making the protein cryptic for the immune system.5 However, humans retain the potential of developing anti-p53 immune responses when p53 becomes available for presentation as an antigen. To take advantage of this scenario, we have developed a genetically engineered Modified Vaccinia Ankara (MVA) viral vector to express wild-type human p53 transgene (p53MVA).6,7 Using p53MVA to deliver full-length p53 has the potential to generate sustained antigen expression and the presentation of numerous antigenic epitopes in the context of various HLA molecules.6–8 In the first-in-human phase I trial, p53MVA vaccination was well tolerated and increased the frequency of p53-reactive T cells that were detected in peripheral blood.9
Cancer evades immune surveillance by maintaining a highly immunosuppressive tumor microenvironment. Multiple solid tumors including TNBC10 have been shown to upregulate PD-1 ligand (PD-L1) surface molecules to modulate immune-regulating checkpoints. PD-1 (CD279), an inhibitory checkpoint receptor expressed on activated T cells, upon interaction with its ligands PD-L1 or PD-L2 transmits a negative control signal that limits T cell activity. This antitumor immune activity can be potentially restored by blocking PD-1/PD-L1 interaction with antibodies directed against PD-1 or PD-L1. One of the anti-PD-1 antibodies – pembrolizumab showed acceptable safety profile and clinical activity in TNBC patients with the overall response rate of 19% and complete response of just 4%.11 Currently, there are ongoing phase II and III clinical trials that evaluate pembrolizumab as a monotherapy or in combination with chemotherapy in TNBC patients.12
We previously reported that p53MVA vaccine single agent trial participants had significantly higher frequencies of PD-1+ T cells in their peripheral blood than healthy controls.9 Furthermore, the percentage of PD-1+ T cells and peak of anti-p53 response showed an inverse correlation in the CD8+ T cell compartment.8 We hypothesized in a previous report that the immunological responses observed in the p53MVA single agent study could be boosted to clinically beneficial levels if the PD-1-mediated immune suppression was inhibited.13 Hence a phase I clinical trial evaluating the combination of p53MVA and pembrolizumab was initiated at City of Hope.
Results
Patient report
A 69-year-old woman was initially diagnosed with right-sided, cT3N1 MO, locally advanced triple negative breast cancer in the Fall of 2008. She received 6 cycles of neoadjuvant docetaxel plus cyclophosphamide therapy, and underwent a mastectomy and axillary lymph node biopsy in June of 2009. Surgical pathology revealed residual ypT1aN1 invasive ductal carcinoma. Approximately 2 months after surgery, there was evidence of dermal recurrence and she received 6 cycles of carboplatin plus gemcitabine followed by adjuvant radiation therapy to the chest wall. Two years later the patient experienced a chest wall recurrence which was biopsy proven. A PET/CT in June 2012 revealed fluorodeoxyglucose (FDG) uptake in the left femur. She was started on capecitabine and denosumab and received radiation therapy to the left-sided femur. Four months later, she suffered a pathological fracture of the left-sided femur which was treated with an intramedullary nailing, while continuing on capecitabine and denosumab. Chemotherapy was switched to second line therapy with paclitaxel upon evidence of dermal progression. In January 2014, she underwent left-sided, proximal femur resection for nonunion and a prosthesis readjustment. Her chemotherapy was changed to third-line therapy with eribulin from February 2014 till January 2015. Due to another episode of dermal metastases progression, chemotherapy was switched to a fourth line therapy with ixabepilone in February 2015. A bone metastases specimen was sent for FoundationOne® genomic analysis which demonstrated a single TP53 D281E mutation. This missense mutation is known to result in a loss of function and overexpression of p53 protein. In July 2015 she was started on fifth line therapy liposomal doxorubicin and completed 3 cycles. Upon clinical examination, worsening dermal lesions were apparent but a PET scan did not show overt visceral disease. In September 2015, she started single-agent, 6th line gemcitabine and completed 3 cycles of treatment. This course of therapy was complicated by thrombocytopenia with continued progression as determined by PET/CT imaging in November 2015, which showed increased FDG uptake and sclerotic osseous lesions in the left side of the ileum and L3 vertebral body. The patient reported increased back pain and was referred to radiation oncology for palliative radiation. Gemcitabine was discontinued. The patient completed palliative radiation to the left ileum and L3 in December 2015, which improved her pain. In January 2016 she developed new dermal lesions on the right upper extremity. The patient enrolled in a clinical trial of PHI-70 (oral FdCyd plus THU) in February 2016. The first cycle was complicated by hospitalizations for grade 3 diarrhea, nausea, vomiting, and abdominal pain. A PET/CT in May 2016 showed increased FDG activity in the left-side iliac bone and a portion of the left iliac wing, and the study drug was held. The patient received her last dose of PHI-70 in June 2016. She was referred to palliative radiation and consideration of hospice care due to no further treatment options. She was referred in August 2016 to our center for a phase I study of p53MVA vaccine in combination with pembrolizumab (Fig. 1 A; NCT02432963), at which time, she had clinically evident macular dermal metastases.
Figure 1.
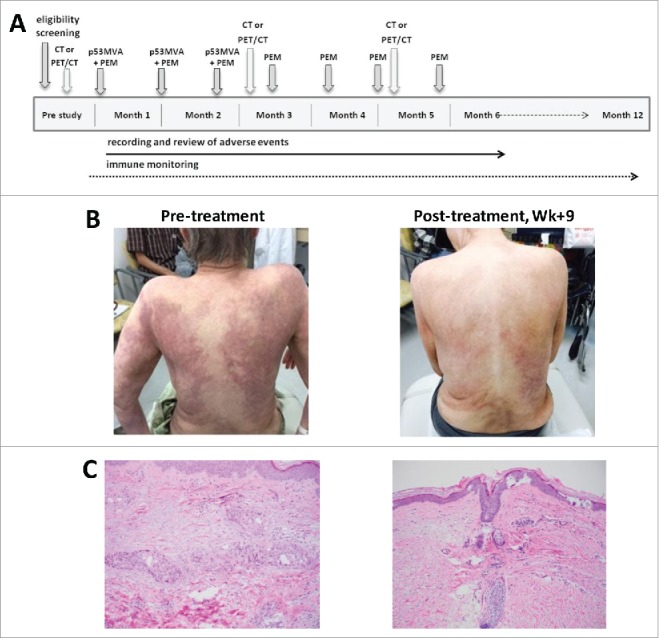
Regression of cutaneous metastases after 2 doses of p53MVA vaccine and pembrolizumab. (A) The treatment schema: p53MVA vaccine and pembrolizumab were given concurrently every 3 weeks for 3 cycles. Pembolizumab alone was then administered every 3 weeks for an additional 4 doses. (B) Patient's skin pre- and 9 weeks post-treatment: before treatment (left) diffuse skin metastases covering 50% of the body area were visible. After 2 cycles of combined therapy (right) significant improvement was noted. (C) Pre- and post-treatment histopathology: pre-treatment skin punch biopsy (left) shows tumor nests composed of pleomorphic cells present predominantly within lymphovascular spaces. Post-treatment biopsy (right) demonstrates mild fibrosis and superficial perivascular lymphocytic infiltrate with no residual malignant cells present.
Clinical response
Prior to treatment the patient had diffuse dermal metastases in the form of erythematous macules distributed on her back, chest wall, bilateral arms and thighs, covering at least 50% of her body surface area, which were associated with severe itchiness and pain (Fig. 1B). Pretreatment skin punch biopsy revealed subcutaneous clusters of tumor cells and lymphovascular invasion (Fig. 1 C). Immunohistochemistry staining confirmed ER−/PR−/HER-2− status. Additional analysis performed by the Foundation Medicine, Inc. (Morrisville, NC) with the use of Ventana PD-L1 (SP142) antibody showed no detectable expression of PD-L1 on tumor cells. While the level of PD-L1 expression has proven to be a useful tool at predicting likelihood of the response to anti-PD-1 treatment, high expression does not guarantee response and low expression does not predict failure. The growing consensus is that patients with PD-L1-negative tumors may still benefit from PD-1/PD-L1 checkpoint inhibitors.14 The first dose of p53MVA and pembrolizumab was given in August 2016. Six weeks later, dermal metastases showed significant improvement with reduced erythematous macules and itchiness (Fig. 1B, right panel). By week 9, her skin lesions had regressed almost entirely. A skin punch biopsy performed on the same area as the pre-treatment biopsy demonstrated no evidence of residual tumor (Fig. 1 C). Restaging CT scan and bone scan in December 2016 showed enhancing nodules in the left low back musculature as being the same or decreased, concomitant with decreased periosteal signal enhancement compared with August 2016. Of the several bilateral subcentimeter pulmonary nodules present at baseline in August 2016, only one 3 mm nodule remained. During clinic follow up in December 2016, the patient remained free of skin metastases. Post treatment FoundationOne® mutational analysis of paraspinal soft tissue mass biopsy revealed metastatic breast cancer with the following genomic alterations: PTEN Y76*, INPP4B K444*, MLL3 E78fs*17, PIK3R1 1571_L573del, RB1 C278fs*1, TP53 D281E and R209fs*6. Additional disease-relevant genes were found not to be altered: ERBB2, BRCA1 and BRCA2. FoundationOne® is a next-generation sequencing based assay that identifies genomic alterations within cancer-related genes. This particular assay analyzed 315 genes as well as introns of 28 genes involved in chromosomal genetic rearrangements. The patient tolerated the combined therapy well, with only low grade adverse events reported. The highest grade adverse events recorded as ‘possibly attributed’ to p53MVA or pembrolizumab were grade 2 nausea and grade 1 vomiting. A transient grade 1 skin rash was reported as ‘possibly related’ to pembrolizumab.
Although complete clearance of cutaneous metastases was seen as early as 9 weeks into the treatment, dermal disease showed evidence of minimal relapse at week 33 which is apparent at the time of writing of this report. Nevertheless, the patient is alive and enjoying a good quality of life. The patient decided to discontinue her treatment at City of Hope and transferred care to her local oncologist to continue receiving pembrolizumab treatment, near her place of residence several hundred miles away from City of Hope.
Immune monitoring studies
Combined therapy activated persistent p53-specific CD8+ T cell responses in the peripheral blood which was associated with lymphocytic infiltration of the resolved dermal metastases (Fig. 2). Upregulation of CD137 expression on the surface of CD8+ and CD4+ T cells upon stimulation with p53 peptides and p53MVA reflects increased frequencies of p53-specific T cells in the circulation after vaccination, particularly between weeks 9 and 24 (Fig. 2 A and 2B). Skin infiltrating CD8+ T cells were visualized and quantified by multiplex immunohistochemistry analysis of tissue sections (Fig. 2 C-F). All the immune cells, including CD8+ T cells (Fig. 2 C), were notably decreased in numbers compared with the pre-treatment tissue but percentages of CD137+ (Fig. 2D) and PD-1+ (Fig. 2E) CD8+ activated T cells as well as tissue resident effector/memory CD8+CD103+ T cells (Fig. 2 F) were increased. The presence of these critical subsets of CD8+ T cells in situ suggests their role during the successful immune effector phase of therapy.
Figure 2.
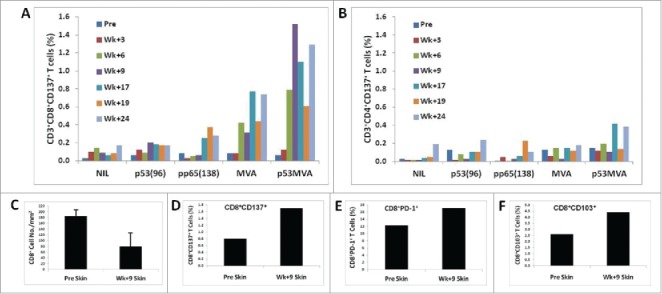
p53MVA/pembrolizumab activate persistent p53-specific CD8+ T cell responses in the blood the timing of which correlates with lymphocytic infiltration of the resolved dermal metastases. The response of CD8+ (A) and CD4+ (B) T cells from PBMCs after 24-h stimulation culture with p53MVA, MVA, p5396, and pp65138, as determined by flow cytometric analysis. The upregulation of CD137 expression on the surface of CD3+CD8+ T cells reflects increased frequencies of p53-specific T cells in the circulation after vaccination, particularly between weeks 9 and 24. Culture conditions: NIL – medium alone; p53(96) – pool of peptides derived from wild type p53 sequence; pp65(138) – control peptides derived from pp65 CMV; MVA – wild type MVA vaccinia virus; p53MVA – recombinant MVA virus. Bar graphs (C-F) show the frequency of CD8+ T cell subsets quantified from multiplexed immunohistochemistry skin biopsy sections before and 9 weeks into the treatment. Total CD8+ cell count decreased in the skin tissue at week 9 into the treatment (C). However, increased proportions of CD8+CD137+ (D) and CD8+PD-1+ (E) activated T cells as well as CD8+CD103+ tissue resident effector/memory T cells (F) in the skin tissue at week 9, compared with pre-treatment, suggest that these cells contribute to the elimination of cutaneous metastases in situ.
Multiplexed gene expression analysis of PBMC samples
Five PBMC samples were assessed for differential expression of immune profile and T cell function genes (Fig. 3). As seen by the dendrograms on heat maps, PBMC samples collected 9 and 24 weeks after initiation of treatment showed visible segregation from the week 6, 3 and pre-treatment samples. The transcriptome of the T cell function data (Fig. 3B) with its peaks of activity at weeks 9 and 24 overlaps the heat map of all PBMC data (Fig. 3 A), indicating a prominent role of T cell populations during the response to p53MVA vaccine and pembrolizumab. T cell gene expression and associated immune response categories such as antigen processing, cytotoxicity, macrophage and NK cell functions peaked at weeks 9 and 24 (Fig. 3 C). Numerous markers of successful T cell stimulation were elevated post treatment, including durable upregulation of T-bet (TBX21), Eomes, OX40 (TNFRSF4), CXCR3, IFN-γ, CXCL10, and KLRG1 (Fig. 3D). At the same time, upregulation of genes associated with T cell inhibition or exhaustion were also seen, such as CTLA-4, LAG-3, TIM-3 (HAVCR2), TIGIT, and MAF (Fig. 3D). These molecules and their signaling properties may play a role in determining the balance between activation/duration and inhibition/exhaustion of T cell immune responses which may in turn critically influence the clinical outcome of the treatment.
Figure 3.
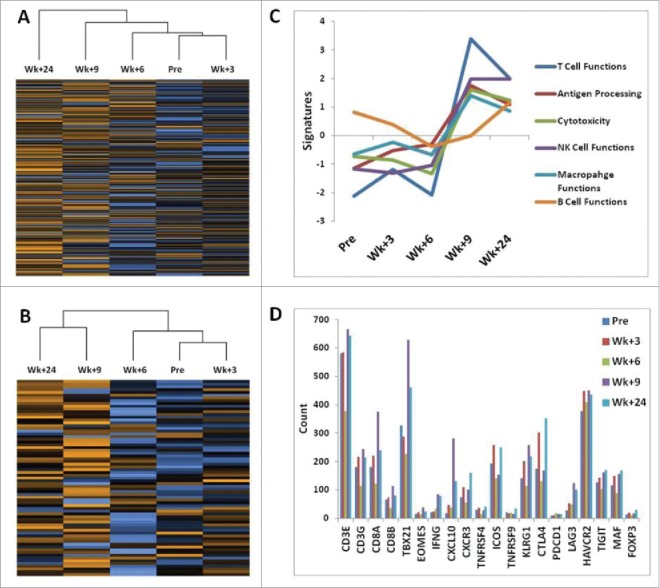
Multiplexed gene expression analysis of PBMC samples using nCounter PanCancer Immune Profiling Panel. (A) shows hierarchic clustering of 5 PBMC samples for 730 immune profiling genes and (B) shows clustering for 71 selected genes to assess T cell functions. The heat map in (B) shows complete segregation of the PBMC samples at weeks 9 and 24 after initiation of treatment from the remaining samples at weeks 6, 3, and Pre-treatment. Heat maps present normalized data scaled to give all genes equal variance (Fig. S2). Orange indicates high expression; blue indicates low expression. (C) depicts immune function pathway scores plotted to show how they vary across time during treatment. Lines show each pathway's average score of their transcriptomes. The T cell functions and associated immune response categories peak at week 9. The list of genes that define pathways is included in the supplementary Table S1. (D) shows the expression of genes encoding selected stimulatory and inhibitory molecules that play a major role in pathways determining the balance between activation/duration and inhibition/exhaustion of T cell immune responses.
Discussion
This report describes a rapid clinical response after targeting of overexpressed tumoral p53 in combination with PD-1 blockade. This response was sustained for 6 months and led to a dramatic improvement in quality of life for the patient. While it is difficult to distinguish the relative effects of the vaccine and pembrolizumab, a specific anti-p53 response was stimulated by combined therapy and demonstrated by increased frequencies of p53-responsive T cells detected in vitro (Fig. 2 A and 2B). The clinical response was associated with upregulation of multiple immune response genes in peripheral blood cells most prominently those associated with T cell functions (Fig. 3).
An interesting aspect of p53 as a target antigen for the immune system is its mutational status. The TP53 gene is mutated in over 80% of TNBC (as opposed to 25% of other breast cancers) patients15,16 providing an additional rationale for targeting the p53 protein in this novel approach for the treatment of TNBC. This high mutation rate and p53 protein overexpression have been associated with poor response to chemotherapy and reduced survival. The patient presented in this study responded successfully to the p53MVA/pembrolizumab administration with the generation of p53-specific T cell responses directed against wild type p53 epitopes (Fig. 2 A and 2B). However, there is a possibility that T cell responses were also directed against neoantigenic epitopes derived from mutated sequences of the p53, especially after activation of systemic and local immune responses with enhanced antigen presenting functions (Fig. 3 C). TP53 genomic alterations identified in this patient included D281E missense and R209fs* deletion/frameshift mutations. While deletion or nonsense mutations lead to low or no expression of p53, missense mutations typically lead to the production of full-length altered p53 protein with a prolonged half-life.17 We have attempted to identify potential neoepitopes in the mutated sequence of the p53 using prediction algorithms of the Immune Epitope Database and Analysis Resource (http://www.iedb.org). Single amino acid substitution D281E did not produce any predicted epitopes that would bind to patient's HLA class I molecules with high, medium, or even low affinity. The best binder identified was a 10-mer CPGRERRTEE that would potentially bind to HLA-B*0702 with very low affinity (IC50 = 2536 nM). In contrast, this analysis predicted 27 HLA class I epitopes in the wild type p53 sequence that would bind with high affinity at IC50 <50 nM (Table S2). The patient's class I HLA type is also shown in the box with the Table S2. Based on the results of this in silico analysis we decided not to pursue any functional studies directed at identification of T cell clones specific for putative neoepitopes. p53MVA vaccine with the TP53 transgene that has no patient-specific mutated sequences, has the characteristics of a “universal” vaccine that can function across HLA restriction barriers to activate patient-specific anti-p53 immune responses.
A considerable hurdle to successful immunotherapy is the intrinsic and acquired immuno-suppression seen in cancer patients. This dramatic, complete dermal response showed that a cancer antigen-specific vaccine given in combination with PD-1 immune checkpoint blockade can stimulate an immune response that is associated with durable clinical benefit in heavily pretreated patients. Progressive loss of the ability to respond to TCR stimuli and upregulation of inhibitory coreceptors that desensitize T cells to tumor antigens are well described phenomena. This may be particularly relevant in older cancer patients with senescent immune systems, subjected to multiple rounds of immune suppressive anti-cancer treatments. The TCR stimulation by tumor antigens during the immune checkpoint blockade therapy acquires even greater significance in light of recent reports showing that tumor antigen-specific CD8+ T cells targeted by anti-PD-1 therapies require CD28 costimulation to be rescued from exhaustion.18,19 In this respect, the p53MVA vaccine offers an efficient way to naturally stimulate TCR/CD28 signaling by professional antigen-presenting cells and to benefit from its combination with PD-1 blockade.
There are currently several clinical studies examining cancer vaccines combined with PD-1 blockade (listed by ClinicalTrials.gov). However the majority of these studies use cell and peptide based vaccines. Using a viral based vaccine to deliver a tumor antigen is a more physiologic and potentially efficient way to generate a complete immune response involving innate and adaptive components and durable immune memory. This dramatic response, in a patient who had no further treatment options, justifies further exploration of this combination therapy. Moreover, in our ongoing phase I clinical trial of p53MVA and pembrolizumab (NCT02432963) we have been monitoring elevated p53-specific T cell immunity in 2 patients showing stable metastatic disease for 7 months (TNBC patient) and 6 months (patient with head and neck squamous cell carcinoma).
Materials and methods
Study patient and treatment regimen
City of Hope Institutional Review Board (IRB #15002) approved the study registered with ClinicalTrials.gov (NCT02432963). All patients provided written informed consent for participation in this study, including treatment, collection of blood, and data analysis in accordance with the ethical institutional standards and with the 1975 Helsinki Declaration. The primary objective is to establish safety and tolerability of the p53MVA vaccine in combination with pembrolizumab. The secondary objectives are to provide evidence of enhanced cellular immunity to p53 and evaluate the response rate and progression free survival. The treatment schedule for this study is shown in Fig. 1 A. p53MVA and pembrolizumab are given concurrently for 3 doses, every 3 weeks. Pembrolizumab is administered first IV (200 mg), followed by IM injection of p53MVA (5.6 × 108 pfu) at least 30 minutes later. This was followed by 4 doses of pembrolizumab alone every 3 weeks. The patient was assessed for toxicity and clinical response. Immunological assessments were performed on peripheral blood mononuclear cells (PBMC) and skin biopsy tissue. Adverse events were classified using the NCI Common Toxicity Criteria for Adverse Events (CTCAE) v4.3. Imaging was performed pre-study, and every 2 months according to standard of care. Histopathology examination of skin punch biopsies pre- and post-therapy by standard H&E staining was also performed.
Trial agents
p53MVA was manufactured using GMP-grade materials at the Center for Biomedicine and Genetics at City of Hope. The final product was diluted in PBS with 7.5% lactose at a concentration of 5.6 × 108 pfu/ml. Pembrolizumab (Keytruda, Merck & Co., Inc.) was provided through the clinical trial and continued with the compassionate use program from Merck upon completion of the trial.
Monitoring of T cell immune responses
Peripheral blood samples were obtained at study entry and at weeks 3, 6, 9 and 24 after initiation of therapy. PBMC prepared by ficoll gradient separation were cryopreserved until analysis. In the initial analysis PBMC were thawed and plated at 2 × 105 cells/0.2 ml/well in media (RPMI, FBS 10%, glutamine 2 mM, sodium pyruvate 1 mM, non-essential amino acids) with one of the following stimuli: media alone, p53MVA, MVA, pool of 96 15-mer overlapping peptides spanning the entire length of p53 (p5396; 5 μg/ml; synthesized in-house), and a pool of 138 peptides derived from CMV pp65 protein epitopes (pp65138; 2 μg/ml; BEI Resources, NIH, Bethesda). After 24 h of culture, the cells were stained with the following antibodies: CD3, CD4, CD8, CD137 (BD Biosciences, San Diego, CA) and analyzed by flow cytometry (BD FACSCelesta, BD Biosciences, San Jose, CA). Data acquired in FACSDiva (BD Bioscience) were analyzed in FlowJo (Flowjo LLC, Ashland, OR).
Multiplexed immunohistochemistry analysis of skin punch biopsies
FFPE sample blocks were cut into 3-μm thick slides and labeled with combinations of the following antibodies: CD3, CD4, CD8, CD103, CD137, and PD-1 by using the multiplex IHC opal method.20 Approximately 10–20 FOV (field-of-view: 0.70 × 0.52 mm) containing either tumor cells and/or immune cells were selected for image acquisition and cell counting using PerkinElmer Vectra automated quantitative pathology imaging system and inForm software analysis (PerkinElmer, Waltham, MA).
Profiling of immune function gene expression
Total RNA was isolated from patient PBMC samples using miRNeasy mini kit (Qiagen, Valencia, CA). RNA concentration was assessed with the Nanodrop spectrophotometer ND-1000 and Qubit 3.0 Fluorometer (Thermo Scientific, Waltham, MA). RNA fragmentation and quality control was determined by 2100 Bioanalyzer (Agilent, Santa Clara, CA) (Fig. S1). All samples were normalized to 20 ng/μL. RNA expression was analyzed by NanoString nCounter platform (NanoString Technologies, Seattle, WA) by digitally detecting and counting in a single reaction without amplification. nCounter PanCancer Immune Profiling Panel (Cat XT-CSO-HIP1–12) from NanoString was used. This 770-plex gene expression panel covers innate and adaptive immune responses, inflammation, adhesion molecules, chemokines, cytokines and pattern recognition receptors. Each assay included 6 positive and 6 negative RNA assay controls, plus 40 mRNA housekeeping controls.100 ng of RNA was first hybridized with codeset from the gene panel at 65 °C for 16 hours. Post-hybridization probe-target mixture was quantified with nCounter Digital Analyzer and all data analyzed in nSolver software package (NanoString).
Supplementary Material
Disclosure of potential conflicts of interest
All authors declare no conflict of interest.
Acknowledgments
We thank the following City of Hope staff members and departments: The Investigational Drug Service, Center for Biomedicine and Genetics, The Office of IND Development and Regulatory Affairs, and Molecular Pathology Core. We are grateful to the staff of the Clinical Molecular Diagnostic Laboratory for allowing us to use the NanoString nCounter instrumentation. We thank Bernard Moss (National Institutes of Health) for allowing access to 1974-MVA and the NIAID for agreeing to its transfer by MTA for clinical use. The RAID program of the NCI is acknowledged for partial support of the original derivation of the p53MVA vaccine.
Funding
This work was supported by funds from the Hope Portfolio Fund, R21CA114889, NCI-SAIC 25XS061, FAMRI 042275, 2 K12 CA001727 and the Phase One Foundation. Research reported in this publication included work performed in the Molecular Pathology Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nature Rev Clin Oncol. 2016;13(11):674-90. doi: 10.1038/nrclinonc.2016.66. PMID:27184417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, et al.. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19(19):5533-40. doi: 10.1158/1078-0432.CCR-13-0799. PMID:23948975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arpino G, Generali D, Sapino A, Del Mastro L, Frassoldati A, de Laurentis M, Paolo P, Mustacchi G, Cazzaniga M, De Placido S, et al.. Gene expression profiling in breast cancer: a clinical perspective. Breast. 2013;22(2):109-20. doi: 10.1016/j.breast.2013.01.016. PMID:23462680 [DOI] [PubMed] [Google Scholar]
- 4.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2(1):a001008. doi: 10.1101/cshperspect.a001008. PMID:20182602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueter M, Gasser M, Lebedeva T, Benichou G, Waaga-Gasser AM. Influence of p53 on anti-tumor immunity (review). Int J Oncol. 2006;28(2):519-25. PMID:16391808 [PubMed] [Google Scholar]
- 6.Song GY, Gibson G, Haq W, Huang EC, Srivasta T, Hollstein M, Daftarian P, Wang Z, Diamond D, Ellenhorn JD. An MVA vaccine overcomes tolerance to human p53 in mice and humans. Cancer Immunol Immunother. 2007;56(8):1193-205. doi: 10.1007/s00262-006-0270-3. PMID:17219151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song GY, Srivastava T, Ishizaki H, Lacey SF, Diamond DJ, Ellenhorn JD. Recombinant modified vaccinia virus ankara (MVA) expressing wild-type human p53 induces specific antitumor CTL expansion. Cancer Invest. 2011;29(8):501-10. doi: 10.3109/07357907.2011.606248. PMID:21843052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Peralta EA, Ellenhorn JD, Diamond DJ. Targeting of human p53-overexpressing tumor cells by an HLA A*0201-restricted murine T-cell receptor expressed in Jurkat T cells. Cancer Res. 2000;60(3);693-701. PMID: 10676655 [PubMed] [Google Scholar]
- 9.Hardwick NR, Carroll M, Kaltcheva T, Qian D, Lim D, Leong L, Chu P, Kim J, Chao J, Fakih M, et al.. p53MVA therapy in patients with refractory gastrointestinal malignancies elevates p53-specific CD8+ T-cell responses. Clin Cancer Res. 2014;20(17):4459-70. doi: 10.1158/1078-0432.CCR-13-3361. PMID:24987057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, et al.. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361-70. doi: 10.1158/2326-6066.CIR-13-0127. PMID:24764583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraia K, Aktan G, Cheng JD, et al.. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460-7. doi: 10.1200/JCO.2015.64.8931. PMID:27138582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartkopf AD, Taran FA, Wallwiener M, Walter CB, Kramer B, Grischke EM, Brucker SY. PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care. 2016;11(6):385-90. doi: 10.1159/000453569. PMID:28228704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardwick N, Chung V, Cristea M, Ellenhorn JD, Diamond DJ. Overcoming immunosuppression to enhance a p53MVA vaccine. Oncoimmunology. 2014;3(10):e958949. doi: 10.4161/21624011.2014.958949. PMID:25941580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275-87. doi: 10.1038/nrc.2016.36. PMID:27079802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, et al.. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395-9. doi: 10.1038/nature10933. PMID:22495314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumors. Nature. 2012;490(7418):61-70. doi: 10.1038/nature11412. PMID:23000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26(12):1268-86. doi: 10.1101/gad.190678.112. PMID:22713868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, et al.. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355(6332):1423-7. doi: 10.1126/science.aaf0683. PMID:28280249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, et al.. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428-33. doi: 10.1126/science.aaf1292. PMID:28280247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: A review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2024;70(1):46-58. doi: 10.1016/j.ymeth.2014.08.016. PMID:25242720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


