ABSTRACT
Dendritic cell (DC)-based vaccines pulsed with high hydrostatic pressure (HHP)-inactivated tumor cells have recently been shown to be a promising tool for prostate cancer chemoimmunotherapy. In this study, DC-based vaccines, both pulsed and unpulsed, were as effective as docetaxel (DTX) in reducing prostate tumors in the orthotopic transgenic adenocarcinoma of the mouse prostate (TRAMP) model. However, we did not observe any additive or synergic effects of chemoimmunotherapy on the tumor growth, while only the combination of DTX and pulsed dendritic cells resulted in significantly lower proliferation detected by Ki67 staining in histological samples. The DC-based vaccine pulsed with HHP-treated tumor cells was also combined with another type of cytostatic, cyclophosphamide, with similar results. In another clinically relevant setting, minimal residual tumor disease after surgery, administration of DC-based vaccines after the surgery of poorly immunogenic transplanted TRAMP-C2, as well as in immunogenic TC-1 tumors, reduced the growth of tumor recurrences. To identify the effector cell populations after DC vaccine application, mice were twice immunized with both pulsed and unpulsed DC vaccine, and the cytotoxicity of the spleen cells populations was tested. The effector cell subpopulations were defined as CD4+ and NK1.1+, which suggests rather unspecific therapeutic effects of the DC-based vaccines in our settings. Taken together, our data demonstrate that DC-based vaccines represent a rational tool for the treatment of human prostate cancer.
KEYWORDS: dendritic cells, docetaxel, high hydrostatic pressure, immunotherapy, prostate cancer
Introduction
Prostate cancer remains the most common diagnosed non-skin malignancy in elderly men and the second leading cause of cancer-related death in Western countries.1 Up to 40% of men diagnosed with prostate cancer will eventually develop metastatic disease, and although most respond to initial medical or surgical castration, progression to castration resistance is universal.2 Docetaxel (DTX), a widely used chemotherapeutic drug, has represented the first-line chemotherapy for metastatic castration-resistant prostate cancer since 2004.3 DTX has also been suggested to have immune enhancing properties against tumors and was shown to antagonize myeloid-derived suppressor cells (MDSCs) by their polarization toward M1 macrophages.4 In the past few years, cancer immunotherapy has made significant strides due to improved understanding of the underlying principles of tumor biology and immunology.5-7 It is an attractive approach to cancer treatment, especially when combined with other therapeutic modalities such as chemotherapy. Synergic effects of combinations of immunotherapy and chemotherapy have been demonstrated in several pre-clinical and clinical studies.8,9 Dendritic cells (DCs) are key players in the immune response as they are able to capture antigens with their pattern-recognition receptors, to process and present them to naïve T-cells, inducing their activation,10 and thus building an essential bridge between innate and adaptive responses. The possibility of their generation in vitro enabled their use for immunotherapy of cancer,11 and several clinical trials have been performed in the last decade.12,13 The first cellular immunotherapy based on activated peripheral blood mononuclear cells, Sipuleucel T, has been FDA-approved.14 Typically, an autologous dendritic cell-based vaccine represents in vitro cultured dendritic cells loaded with tumor antigens that can be in the form of tumor cells with subsequent DC maturation. For DC pulsing, tumor cells can be inactivated by different ways, and selection of the optimal inactivation method can be crucial for DC vaccine optimization.15,16 High hydrostatic pressure (HHP) has been demonstrated as a method for tumor cell inactivation preserving their immunogenic capacity17 and HHP-treated cells were able to induce monocyte-derived DC maturation, and DC co-cultured with HHP-treated tumor cells were able to induce T cell activation in vitro. These results showed HHP as a convenient tool for tumor cell inactivation before their use for DC pulsing.17 In our previous work, we demonstrated, using murine tumor models, that HHP was able to induce immunogenic cell death of both TC-1 and TRAMP-C2 tumor cells, representing murine models for human papilloma virus-associated tumors and prostate cancer, respectively. HHP-treated cells were successfully used for preparation of a DC vaccine that is based on DC pulsed with HHP-treated tumor cells inducing high immune responses. In combination with docetaxel chemotherapy, these vaccines significantly inhibited not only growth of immunogenic TC-1, but also poorly immunogenic TRAMP-C2 tumors.18 Here, we investigated the therapeutic capacity of the HHP cell-pulsed DC vaccines using a clinically more relevant, orthotopic model for prostate cancer treatment, transgenic adenocarcinoma of the mouse prostate (TRAMP) mice, males that spontaneously develop prostate tumors following the onset of puberty.19 We have also demonstrated the DC vaccine efficacy in a previously established therapeutic setting, minimal residual tumor disease after surgery.20-23
Material and methods
Mice
Female heterozygous C57BL/6/TGN TRAMP mice, line PB Tag 8247NG were purchased from The Jackson Laboratory (Bar Harbor, ME). Transgenic males for the studies were routinely obtained as [TRAMP x C57BL/6/TGN]F1 or [TRAMP x C57BL/6/TGN]F2 offspring. The genotypes of TRAMP mice were confirmed by PCR-based screening using tail biopsies. We added 0.3 ml 50 mM NaOH to the tail biopsies and incubated them for 90 minutes at 95°C, neutralized by adding 0.4 ml Tris buffer (pH 7.2, 20 mM) and centrifuged (4000 g, 3 min). We prepared aliquots of the master mix (Aptamer hot start master mix, Top-Bio) for each PCR reaction with primers specific for the transgene (fw GCGCTGCTGACTTTCTAAACATAAG, rev GAGCTCACGTTAAGTTTTGATGTGT), for positive control (fw CTAGGCCACAGAATTGAAAGATCT, rev GTAGGTGGAAATTCTAGCATCATCC) and finally, we added 2.5 µl of 10x diluted DNA in water. C57BL/6/TGN (B6) male mice, 6–8 weeks old, were obtained from AnLab Co., Prague, Czech Republic. Experimental protocols were approved by the Institutional Animal Care Committee of the Institute of Molecular Genetics, Prague.
Tumor cell lines
The TC-1 tumor cell line (obtained from ATCC collection) was developed by co-transfection of murine C57BL/6/TGN lung cells with HPV16 E6/E7 genes and activated (G12V) Ha-ras plasmid DNA.24 TRAMP-C2 tumor cells (obtained from ATCC collection), MHC class I-deficient, were established from a heterogeneous 32-week tumor of the transgenic adenocarcinoma mouse prostate (TRAMP) model.25 TC-1 cells were maintained in RPMI 1640 medium (Sigma-Aldrich GmbH) supplemented with 10% FCS (PAN Biotech GmbH), 2 mM L-glutamine and antibiotics; TRAMP-C2 cells were maintained in D-MEM medium (Sigma-Aldrich GmbH) supplemented with 5% FCS, 5%Nu-Serum IV (Corning), 0.005 mg/ml human insulin (Sigma-Aldrich GmbH), 10 nM dehydroisoandrosterone (DHEA, Sigma-Aldrich GmbH) and antibiotics. Both cell lines were cultured at 37°C in a humidified atmosphere with 5% CO2.
High hydrostatic pressure treatment
Tumor cells were treated by 200 MPa in the custom-made device (Resato International BV, Netherlands) that is located in the GMP manufacturing facility, SOTIO, a.s., Prague. This device allows reliable treatment of tumor cells by the defined levels of HHP for specified periods of time (10 minutes in the case of 200 MPa).17
Dendritic cell preparation
Dendritic cells (DC) were prepared from bone marrow precursors as described by Lutz 26,27 with slight modifications.28 Briefly, the bone marrow cells were cultured for 7 d in the complete RPMI 1640 medium supplemented with 2 × 10−5 M mercaptoethanol (Calbiochem), 10 ng/ml GM-CSF and IL-4 (R&D Systems). On day 5, the DC were pulsed with HHP-treated tumor cells by 48 h incubation in a ratio of 2:1 (DC/tumor cells, 106 DC/ml). Some DC were also left unpulsed. DC pulsed or unpulsed with the tumor cells were treated for 24 h with unmethylated CpG containing phosphorothioate-modified oligodeoxynucleotide CpG ODN 1826 (5’-TCCATGACGTTCCTGACGTT-3’)29 at a final concentration of 5 μg/ml (Generi Biotech), that were sulfur-modified in their backbone (phosphorothioate) and synthesized under endotoxin-free conditions. On day 7, non-adherent cells were harvested. These cells, designated as DC, pulsed or unpulsed, contained approximately 60–70% CD11c+ cells. For mouse therapeutic experiments, DC were washed twice with PBS and injected subcutaneously (s.c.) in PBS, 300 μl/2 × 106 cells/mouse.
Therapeutic experiments with TRAMP mice
Approximately 8-week-old male TRAMP mice were used for the therapeutic experiments. In each experiment, 10–12 mice per experimental group were used. Docetaxel, 30 mg/kg (Actavis) was repeatedly administered on weeks 8, 10, 12 and 14 intraperitoneally (i.p.). Dendritic cells were s.c. administered on weeks 9, 11, 13 and 15. Tumor growth was followed by palpation. When the animals were 28 weeks old, the experiment was terminated. Mice underwent autopsy, their genitourinary tracts (GUT) were dissected and weighed, and fixed in 4% paraformaldehyde in PBS for 24 h, then embedded and mounted for histopathology analysis. In case of the combination experiment where CY treatment was used, CY, 200 mg/kg (Endoxan, Baxter Oncology GmbH) was administered i.p. only once, on week 8, and the vaccine on weeks 9, 11, 13 and 15. For evaluation of this part, the values of untreated group and the group treated with pulsed dendritic cells from DTX combination experiment were used, as allowed by the spontaneous tumor model.
Treatment of surgical minimal residual tumor disease
To obtain the minimal residual tumor disease after surgery, B6 mice were inoculated s.c. with TC-1 (5 × 104 cells) or TRAMP-C2 (106 cells). After approximately 28 days, when the transplanted tumors reached ∼5–10 mm in diameter, the tumors were excised under i.p. anesthesia, leaving no macroscopically visible tumor residuum.30 The hypothetical microscopic tumor residua after surgery were designated as surgical minimal residual tumor disease. Mice were randomly divided into 3 experimental groups. One experimental group was left without treatment as a control group (operated-only mice). Two groups of experimental mice were s.c. treated with DC-based vaccines, one group with pulsed and one group with unpulsed cells (2 × 106 cells/mouse). Vaccines were administered on days 7 and 21 in the site of previous tumor surgery. In one experiment, mice were also pretreated with pulsed DC vaccine on day -7 in case of TC-1 surgery and -21, -7 in case of TRAMP-C2 surgery. Mice were observed twice a week and the size of the tumors was recorded. Two perpendicular diameters of the tumors were measured with a caliper and the tumor size was expressed as the tumor area (cm2).
Histopathology analysis
Morphology of the therapeutic response
Semi-thin sections of 5 µm were obtained from 3 different regions of the dissected tumor and stained with hematoxylin and eosin in an autostainer (Ventana Symphony, entana Medical Systems). Analysis for response assessed either diminished amount tumor infiltrates in the section and/or presence of neoplastic cell degeneration, necrosis, or apoptotic bodies. Grading of the response to different therapeutic regimens was done evaluating 3 regions: the primary neoplasm in the dorsal prostate, the secondary infiltrate in the proximal part of the seminal vesicle, and the tertiary: presence of infiltrate in the distal part of the seminal vesicle. The scoring system used complete response (equals 2; includes complete neoplastic cell degeneration in the respective region), incomplete response (equals 1; partial degeneration of the tumor) and absence (equals 0; the neoplasm is not affected and represents typical morphology) of the neoplasm in any of the 3 regions evaluated, i.e. the tumor vitality score. A cumulative score of minimum 0 and maximum of 6 was calculated as a sum of individual scores in every single mouse.
Immunohistochemistry
Immunohistochemistry was performed on sections of the primary neoplasia to evaluate the Ki67 labeling index. In brief, 3 µm thick sections were submitted to automated rehydration, antigen damasking, and immune reaction in a Ventana Discovery ULTRA automated slide stainer. The variable points in the procedure are epitope retrieval for 32 min in CC1 buffer (Ventana Medical Systems Tucson AZ; pH 9.0), automatic application of anti Ki67 antibody (Clone SP6, 1:500; Thermo Scientific) at 37 ˚C for 32 min. The detection was done by a secondary anti-rabbit polymer system conjugated with peroxidase (Zytomed GmbH) after its manual application and incubation in the stainer at 37˚C for 32 min. The reaction was developed by the amino-ethyl-carbazole ready-to-use solution (DAKO) at 37˚C for 20 min. Counterstaining was performed by the autostainer using Gill's hematoxylin II. All slides were consecutively coverslipped in Ventana Symphony using its coverslipper function.
Immunohistochemistry evaluation and scoring
Scoring was done using quantitative and qualitative approach. In brief, 5 individual high power fields (40fold magnification) were analyzed for presence of Ki67-positive reaction by manual counting of 4 different intensity levels: negative (0), weakly positive (1), moderately positive (2) and strongly positive (3) as a normalizer the total count of the nuclei in every respective field was counted. At least 250 cells were counted per field. The score was calculated as a proportion of the maximum possible score per field, meaning as sum of the individual cell numbers multiplied by their group intensity (n1 x0, for negative cells; n2 x1 for weak positive cells; n3 x2 for moderate positive and n4 x3 for strong positive cells), divided by the maximum score per field:
Flow cytometry
Expression of cell surface molecules on the DC was determined by flow cytometry. The expression of CD11c, MHC class II, and CD86 was analyzed using the following antibodies: APC anti-CD11c (HL3), FITC anti-MHCII (AF6–120.1) and PE anti-CD86 (GL1) (RB6–8C5). The percentage of CD44+ CD62 L− of CD8+ and CD4+ lymphocytes in splenocytes from immunized mice was determined by flow cytometry using the following antibodies: PE anti-CD44 (IM7), APC-CY7 anti-CD62L (MEL-14), PE-CF594 anti-CD8 (53–6.7), and FITC anti-CD4 (RM4–5). Relevant unspecific isotype controls were used. All products were purchased from BD Biosciences. FACS analysis was performed using an LSR II flow cytometer (BD Biosciences) and analyzed by FlowJo 7.6.5 software.
Immunization/challenge experiments with dendritic cells
Mice, 3 animals per group, were twice immunized with 2 × 106 cells of pulsed and unpulsed DC-based vaccine in a 2-week interval. Control mice received vehiculum only. Ten day after the second immunization, mice were killed, single-cell suspensions from the spleens were prepared and the cells were used for further analysis by FACS, chromium release assay, and ELISPOT.
Chromium release microcytotoxicity assay
The cytolytic activity of effector cells was tested in 18 h 51Cr release assay, as described earlier.21,31 Briefly, spleen cells from control and immunized mice that served as effector cells were treated with ammonium chloride-potassium lysing buffer (1 min) to deplete erythrocytes. The mixtures of effector cells with relevant 51Cr-labeled tumor targets were incubated in selected target/effector cell ratios (1:25, 1:50, 1:100, 1:200) in triplicate in 96-well round bottom microtiter plates (Nunc). The percentage of specific 51Cr release was expressed according to the formula: [cpm experimental release – cpm control release / cpm maximum release /cpm control release] × 100.
For depletion of selected spleen cell subpopulations, 5 × 10 and 2.5 × 105 spleen cells/well were seeded in 96-well round bottom microtiter plates (Nunc) and incubated for 1 h at 37°C with 5 µg/ml of anti-CD8 (2.43), anti-CD4 (GK1.5) or anti-NK 1.1 (PK136) antibodies (EXBIO) with addition of the same volume of RPMI 1640 media and Baby Rabbit Complement (Cedarlane) diluted according to the manufacturer's instruction. After the incubation, plates were washed 2 times with RPMI 1640 and used for the microcytotoxicity assay.
For positive selection of effector cells, CD8-positive, CD4-positive and NK1.1-positive cells from the spleens of immunized animals were isolated using anti mouse CD8 (Ly-2) CD4 (L3T4), or CD49b (DX5) NK1.1 antibodies conjugated to magnetic MicroBeads (Miltenyi Biotec), in accordance with the manufacturer's instructions, as described previously.32,33 Cell separation was performed with the autoMACS system (Miltenyi Biotec). The purity of cells was verified by FACS analysis. The percentage of CD8+, CD4+ and NK1.1+ cells achieved 86–91%. 5 × 105 and 2.5 × 105 spleen cells/well were seeded in 96-well round bottom microtiter plates (Nunc) and used for microcytotoxicity assay.
ELISPOT
To determine the amount of IFNγ-secreting cells, an ELISPOT kit for detection of murine IFNγ (BD Biosciences) was used. Spleen cells were cultured for 48 h and then placed into the wells of ELISPOT plates (concentration 1 × 105, 5 × 104, 5 × 103 cells/well) for 24 h. The plates were then processed according to the manufacturer's instructions (BD Biosciences). Colored spots were counted with CTL Analyzer LLC (CTL) and analyzed using the ImmunoSpot Image Analyzer software.
Statistical analyses
For statistical analyses of in vitro experiments, Student′s t-test was used. For evaluation of in vivo experiments, Analysis of Variance (ANOVA) from the NCSS, Number Cruncher Statistical System (Kaysville, Utah, USA) statistical package was used. Standard deviations are indicated in the figures.
Results
Combined chemoimmunotherapy of TRAMP mice with docetaxel and DC-based vaccine inhibited tumor growth
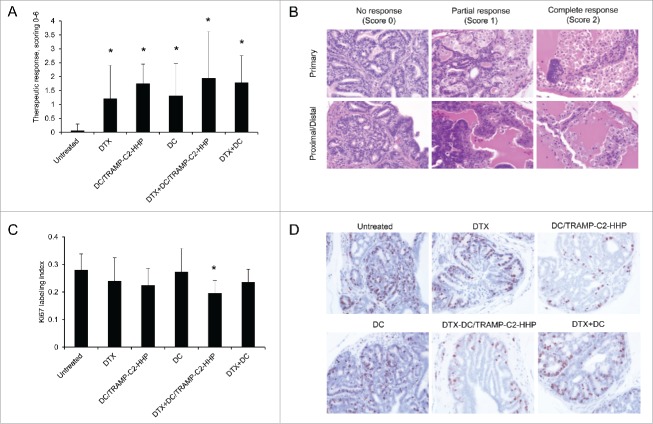
The therapeutic efficacy of HHP-treated tumor cell-pulsed or unpulsed matured DC used either as monotherapy or in combination with the DTX treatment was tested in TRAMP mice. Approximately 8-week-old male TRAMP mice were repeatedly treated with DTX alone, antigen-pulsed or unpulsed DC-based vaccine, or with their combination (Fig. 1A). Tumor growth was verified by palpation and 28 weeks old mice were autopsied. The tumor development was quantified by genitourinary tract weight evaluation, as well as by scoring of the therapeutic response using histopathology analysis. As can be seen in Fig. 1B, genitourinary tract weights expressed as weight GUT per mouse body weight were significantly decreased after the treatments with DTX or both DC pulsed with HHP-treated tumor cells and unpulsed (*P<0.05). Combined chemoimmunotherapy using DTX and DC vaccines also resulted in significantly decreased GUT weights. However, we did not observe any additive or synergic effects of the combined treatments. Therapeutic responses were confirmed by histopathology scoring of the neoplasms analyzed in 3 different positions of the primary site, proximal part of the seminal vesicle and distal part of the vesicular gland, as described in Material and Methods. Histopathology evaluation showed a significantly higher therapeutic response to DC-based vaccine, both pulsed and unpulsed, as well as to their combination with DTX (Fig. 2A). Figure 2B shows representative pictures of scoring. The trend toward less advanced cancer was also confirmed by immunochemistry focused on tumor cell proliferation. As shown in Fig. 2C, the Ki67 labeling index in the group treated with DTX in combination with the pulsed DC vaccine was the only significantly lower index when compared with untreated controls. Figure 2D shows representative pictures of Ki67 staining of the samples from all treated groups and untreated group.
Figure 1.
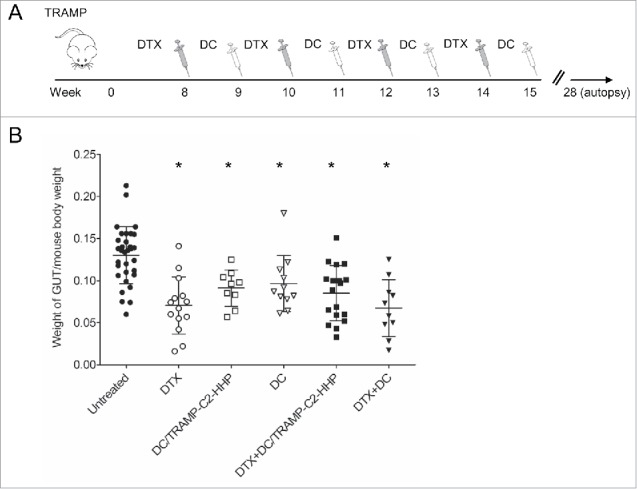
Combined chemoimmunotherapy of TRAMP mice with docetaxel and DC-based vaccines. (A) Approximately 8-week-old male TRAMP mice were treated with DTX alone (30 mg/kg), pulsed or unpulsed DC-based vaccines (2 × 106 cells/mouse), or with their combination. (B) Dotplots of GUT expressed as weight of genitourinary tract (GUT) per mouse body weight. *P<0.05 vs. control (t-test).
Figure 2.
Histopathology analysis of TRAMP mice treated with docetaxel and DC-based vaccines. (A) Quantitative analysis of therapeutic response scoring in TRAMP mice treated with docetaxel and DC-based vaccines. (B) Representative pictures of scoring. (C) Ki67 labeling index and representative pictures of all treated groups and untreated control (D). *P<0.05 vs. control (t-test).
Chemoimmunotherapy of TRAMP mice with cyclophosphamide and pulsed DC-based vaccine inhibited tumor growth
For comparison, the therapeutic efficacy of HHP-treated tumor cell-pulsed matured DC was thereafter tested in the TRAMP model using a combination with another cytostatic agent, cyclophosphamide. Approximately 8-week-old male TRAMP mice were treated either with CY alone using a previously optimized therapeutic scheme,20 or in combination with the pulsed DC-based vaccine (Fig. 3A). As can be seen in Fig. 3B, after the treatment with CY or pulsed DC-based vaccine, or after their combination as well, genitourinary tract weights expressed as weight of genitourinary tract (GUT) per mouse body weight significantly decreased (*P<0.05). Therapeutic responses were confirmed by histopathology scoring of the neoplasms. The histopathology evaluation surprisingly showed a significantly higher therapeutic response in groups treated with monotherapy (both CY and DC), while the therapeutic response score in the combination group did not reach the statistical significance (Fig. 3C).
Figure 3.
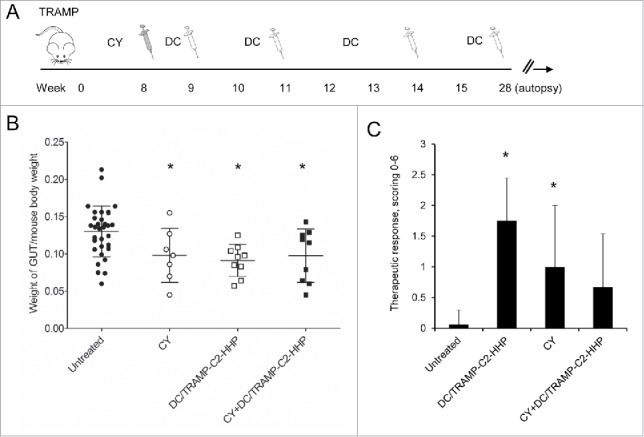
Combined chemoimmunotherapy of TRAMP mice with CY and DC-based vaccines. (A) Approximately 8-week-old male TRAMP mice were treated with CY alone (200 mg/kg), pulsed DC-based vaccine (2 × 106 cells/mouse), or with their combination. (B) Dotplots of GUT expressed as weight of genitourinary tract (GUT) per mouse body weight. (C) Quantitative analysis of therapeutic response scoring in TRAMP mice treated with CY and DC-based vaccines. *P<0.05 vs. control (t-test).
Immunotherapy of surgical minimal residual tumor disease of TC-1 and TRAMP-C2 tumors with DC-based vaccine inhibited growth of recurrent tumors
The therapeutic efficacy of HHP-treated tumor cell-pulsed or unpulsed matured DC was then tested in the therapeutic setting when immunotherapy with DC-based vaccine was used for the treatment of surgical minimal residual tumor disease of TC-1 and TRAMP-C2 tumors. Mice were s.c. transplanted with TC-1 or TRAMP-C2 and underwent tumor excision when the transplanted tumors reached ∼5–10 mm in diameter. The DC-based vaccine was administered one and three weeks after the surgery. In selected experiments, mice were pretreated with DC 7 d before surgery (or in case of TRAMP-C2 also 21) (Fig. 4A). Figure 4B indicates that the growth of tumor recurrences of poorly immunogenic TRAMP-C2 tumors was inhibited by both unpulsed and pulsed DC-based vaccines. Similar results were obtained for the immunogenic TC-1 tumor model (*P<0.05 vs. control) (Fig. 4C). Interestingly, the therapeutic effect of the vaccine was abolished by DC vaccine pretreatment in both TRAMP-C2 and TC-1 tumor models.
Figure 4.
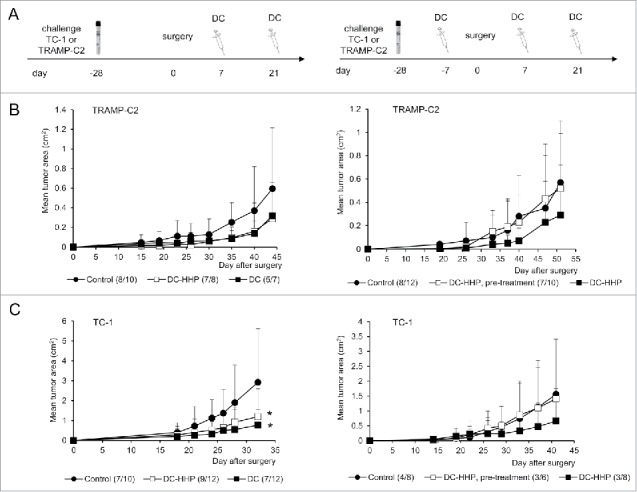
Immmunotherapy of surgical minimal residual tumor disease of TC-1 and TRAMP-C2 tumors with DC-based vaccine. (A) Mice were inoculated s.c. with TC-1 (5 × 104 cells) or TRAMP-C2 (106 cells). When the transplanted tumors reached ∼5–10 mm in diameter, the tumors were excised. DC-based vaccines (2 × 106 cells/mouse) were administered on days 7 and 21 after surgery (left panel). In the case of pretreatment, DC-based vaccines (2 × 106 cells/mouse) were additionally administered on days -7 (TC-1, TRAMP-C2) and -21 (TRAMP-C2) before surgery (right panel) (B) Growth of TRAMP-C2 tumor recurrences after treatment with unpulsed and pulsed DC-based vaccine (left panel) and the growth of TRAMP-C2 tumor recurrences after pre-treatment or treatment with pulsed DC-based vaccine (right panel). (C) Growth of TC-1 tumor recurrences after treatment with unpulsed and pulsed DC-based vaccine (left panel) and the growth of TC-1 tumor recurrences after pre-treatment or treatment with pulsed DC-based vaccine (right panel). *P<0.05 vs. control (Analysis of Variance).
Analysis of splenocytes from mice immunized with pulsed and unpulsed DC vaccines
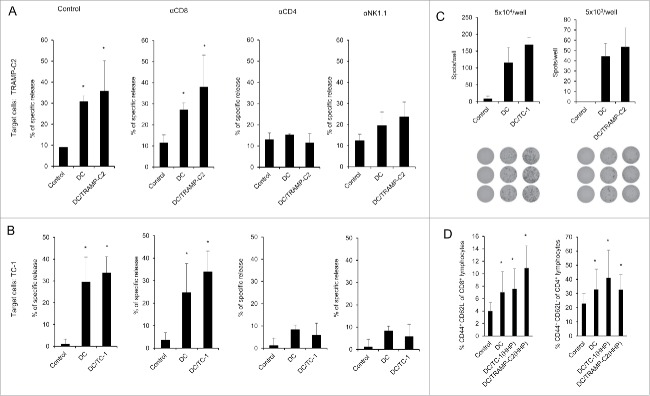
As both pulsed and unpulsed DC-based vaccines displayed similar anti-tumor effects, we characterized the immune effector cell subpopulations. Mice were twice immunized with 2 × 106 cells of DC-based vaccine, both pulsed and unpulsed with HHP-treated TRAMP-C2 or TC-1 tumor cells, in a 2-week interval. Ten days after the second immunization, spleen cells were used for further analysis by FACS, chromium release assay, and ELISPOT. In vitro analyses of the spleen effector cells depleted with antibodies against CD8, CD4 and NK1.1 showed no difference between the groups immunized by pulsed or unpulsed DC, suggesting induction of unspecific immune responses. The cytotoxic effect was mediated by CD4+ and NK1.1+ effector cell subpopulations (Fig. 5AB). Both mice immunized with DC unpulsed and pulsed with HHP-treated tumor cells (TRAMP-C2 or TC-1) displayed significantly increased numbers of IFNγ-producing cells detected by the ELISPOT assay (Fig. 5C). On the other hand, flow cytometry analysis of the spleen cells (Fig. 5D) revealed that the percentage of effector memory T cells (CD44+ CD62L− of CD8+ and CD4+ T lymphocytes) in the spleens from mice immunized with pulsed or unpulsed DC vaccines was significantly increased. The results obtained in immunization experiments using the TRAMP-C2 model were comparable with the results of experiments using the TC-1 tumor model. These results suggest rather unspecific therapeutic effects of the DC-based vaccines in our settings, mediated by NK1.1+ and CD4+ spleen cells as the effector cell population.
Figure 5.
Analysis of splenocytes from mice immunized with pulsed and unpulsed DC-based vaccine. Mice were immunized 2 times in a 2-week interval with 2 × 106 DC pulsed with HHP-treated TC-1 or TRAMP-C2 tumor cells. Ten days after the last immunization, pooled splenocytes of 3 mice were used for in vitro analysis. (A) 51Cr microcytotoxicity assay of depleted splenocytes (αCD8, αCD4, αNK1.1) from mice immunized with DC-based vaccines unpulsed or pulsed with HHP-treated TRAMP-C2 tumor cells or (B) TC-1 tumor cells. (C) The number of IFNγ-producing cells (ELISPOT assay). (D) Percentage of CD44+ CD62L− of CD8+ lymphocytes or CD4+ lymphocytes in splenocytes from mice immunized with pulsed or unpulsed DC-based vaccines. *P<0.05 vs. control. Statistical significances were determined by Student's t-test. Results are representative of 2 independent experiments.
Discussion
We have recently demonstrated that the DC-based vaccine loaded by co-culture with stressed, HHP-treated tumor cells inhibited growth of both transplanted syngeneic tumors TRAMP-C2 and the TC-1 tumors.18 Further, we have observed additive-synergic effects with chemotherapy in this setting. In this study, we assessed the therapeutic capacity of this cellular vaccine using a clinically more relevant TRAMP transgenic murine model, which spontaneously developed prostate tumors. Using this experimental model, the DC vaccine was able to slow down the tumor growth when used either as monotherapy or in combination with DTX chemotherapy to the same extent as DTX alone. Indeed, in all these treatment settings, immunotherapy and chemotherapy only, as well as combined chemoimmunotherapy, we observed significant tumor growth inhibition when compared with the untreated tumor-bearing animals. Similar results were obtained when the DC vaccine treatment was combined with another chemotherapeutic agent, CY. These results suggest that the DC therapeutic vaccination is effective when used as monotherapy during long-term tumor development, with an efficacy corresponding to the effects of chemotherapy. On the other hand, in this model we did not observe any additive of synergic effects when combined with immunotherapy, according to the GUT weight analysis, in contrast to the therapy of small transplanted tumors. We have to consider that the long-term development of spontaneously arisen tumors differs from the relatively fast growth of transplanted tumors. However, our cell proliferation analysis indicates that combined DTX chemotherapy with the Ag pulsed DC vaccine can be beneficial in the later stages of the tumor growth. So far, only a few studies focused on the DC-based immunotherapy have been performed in TRAMP mice. Ricupito et al. have shown that DC pulsed with the SV40 T Ag-derived immunodominant peptide Tag404–411 restored immune competence and induced tumor shrinkage in TRAMP mice that had been previously sublethally irradiated and subjected to HSCT from congenic females and received donor lymphocyte infusions from female donors presensitized against male antigens.34
Further, we compared the therapeutic effectiveness of the DC vaccines either pulsed with the HHP-treated TRAMP-C2 cells or unpulsed. We noticed no significant differences in the GUT size when these 2 different vaccines were used. As mentioned above, the only difference was seen in histological analysis focused on Ki67 positive replicating cells, in which tumors treated with the DTX and pulsed DC combination displayed significantly lower proliferation. These results suggest that the effective anti-tumor immune responses elicited by the DC-based vaccination are either unspecific, or that the specific immunity is induced by in vivo antigen processing and presentation after DC administration. Our analysis of the spleen cells from mice immunized with pulsed or unpulsed vaccines documents that the major effector cells belonged to the NK1.1+ and CD4+ populations, and cytotoxic tests revealed non-specificity of effector cells. Notably, tumor surveillance dependence on NK cells in TRAMP mice has been demonstrated,35 and our data support the idea that engagement of activated NK cells can be crucial for DC vaccine-induced antitumor immunity.36-38
On the other hand, our data showing that the proliferation capacity of tumor cells was lower in tumors from animals treated with the combination of pulsed DC and chemotherapy, compared with all other treatment combinations, indicate the potential beneficial effect and specific immunity induction in the long-term perspective.
However, the therapeutic potential of the Ag-unloaded DC remains a controversial topic in tumor immunology. In our laboratory, we have previously investigated both pulsed and unpulsed bone marrow-derived DC prepared by the protocols similar to that used in this study in several settings and we found that unpulsed DC were effective in some therapeutic settings,39 while in immunization-challenge protocols the protective effects were seen on mice treated with pulsed DC only.40
In a second experiment focused on DC-based immunotherapy combined with chemotherapy using TRAMP mice, we evaluated the efficacy of the DC treatments combined with CY. In these experiments, we used the setting in which CY that was administered once in a dose that displayed antitumor effects in previous experiments.20 It is noteworthy that this treatment leads to induction of myeloid-derived suppressive cells that are potent inhibitors of T cell and NK cell proliferation.32 This could explain the fact that the results with CY combination were not as convincing as those with DTX. Contrary to CY, DTX is a chemotherapeutic that is able to efficiently inhibit myeloid-derived suppressor cells.4 The character of the tumor microenvironment, meaning its immunosuppressive status, might have influenced the effect of the immunotherapy used.
The effectiveness of both pulsed and unpulsed DC vaccines observed in the TRAMP model was also demonstrated using the clinically relevant model for therapy of minimal residual tumor disease after surgery. The only situation in which the vaccine did not bring any effect was the scheme in which the vaccine was also used before surgery and this administration abolished the effects of subsequent post-surgery vaccinations. It corresponds with our previous results using a murine model that the treatment before surgery with irradiated IL-2 producing tumor cells used as a cellular vaccine was without effect.30 These facts show that proper timing of DC vaccine administration (or immunotherapy in general) and surgery can be crucial for the final therapeutic output. Notably, in humans, possible clinical benefit has been suggested for adjuvant immunotherapy using autologous DC loaded with autologous tumor lysate after revision in patients with relapsed glioblastoma multiforme. 41
Collectively, our data indicate that DC-based immunotherapy, either using DC loaded with HHP-treated tumor cells or unpulsed DC, was effective against spontaneously developing prostate tumors, as well as in the model for therapy of minimal residual tumor disease. The fact that the unpulsed DC were also effective can be advantageous and justify the therapeutic use of dendritic cells loaded with a spectrum of antigens that might not be overlapping with the antigens expressed in growing tumors. On the other hand, our data do not exclude the possible superiority of DC loaded with HHP-treated tumor cells, and their possible long-term effect has to be particularly considered.
Disclosure of potential conflict of interest
Jirina Bartunkova and Radek Spisek are minority shareholders of SOTIO, a.s., a biotech company developing DC based immunotherapy.
Acknowledgments
This work was supported by research grant provided by SOTIO, a.s., and in part by the Ministry of Education, Youth and Sports (MEYS, LM2015040, Czech Center for Phenogenomics), Academy of Sciences of the Czech Republic (RVO 68378050), the project “BIOCEV – Biotechnology and Biomedicine Center of the Academy of Sciences and Charles University” (CZ.1.05/1.1.00/02.0109) and “Higher quality and capacity for transgenic models” (CZ.1.05/2.1.00/19.0395) by the Ministry of Education, Youth and Sports and the European Regional Development Fund and research grant No. 15–24769S from the Czech Science Foundation. The work of the Department of Immunology of Charles University is supported by Ministry of Health, Czech Republic-Conceptual Development of Research Organization (University Hospital Motol, Prague, Czech Republic, 00064203) and grant AZV ČR (agency for medical research, Czech Republic) 16–28135A.
The authors are grateful to Mrs. Renáta Turečková and Markéta Pícková for skillful technical assistance and to Dr. Šárka Takáčová for editorial help.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30. doi: 10.3322/caac.21387. PMID:28055103 [DOI] [PubMed] [Google Scholar]
- 2.Beltran H, Beer TM, Carducci MA, de Bono J, Gleave M, Hussain M, Kelly WK, Saad F, Sternberg C, Tagawa ST, et al.. New therapies for castration-resistant prostate cancer: Efficacy and safety. Eur Urol. 2011;60:279-90. doi: 10.1016/j.eururo.2011.04.038. PMID:21592649 [DOI] [PubMed] [Google Scholar]
- 3.Maluf FC, Smaletz O, Herchenhorn D. Castration-resistant prostate cancer: Systemic therapy in 2012. Clinics (Sao Paulo, Brazil). 2012;67:389-94. doi: 10.6061/clinics/2012(04)13. PMID:22522765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583-94. doi: 10.1158/1078-0432.CCR-10-0733. PMID:20702612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Emens LA. Chemoimmunotherapy: Reengineering tumor immunity. Cancer Immunol Immunother. 2013;62:203-16. doi: 10.1007/s00262-012-1388-0. PMID:23389507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309-35. doi: 10.3322/caac.20132. PMID:22576456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emens LA. Chemoimmunotherapy. Cancer J (Sudbury, Mass). 2010;16:295-303. doi: 10.1097/PPO.0b013e3181eb5066. PMID:20693839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: A new perspective. Cancer Immunol Immunother. 2008;57:1523-9. doi: 10.1007/s00262-008-0531-4. PMID:18488219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: Improving vaccines? Adv Drug Deliv Rev. 2006;58:975-90. doi: 10.1016/j.addr.2006.04.002. PMID:17005292 [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-52. doi: 10.1038/32588. PMID:9521319 [DOI] [PubMed] [Google Scholar]
- 11.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-77. doi: 10.1038/nrc3258. PMID:22437871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1:1111-34. doi: 10.4161/onci.21494. PMID:23170259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Sochorova K, Hromadkova H, Kayserova J, Vavrova K, Lastovicka J, et al.. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015;6:18192-205. doi: 10.18632/oncotarget.4145. PMID:26078335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al.. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22. doi: 10.1056/NEJMoa1001294. PMID:20818862 [DOI] [PubMed] [Google Scholar]
- 15.Vandenberk L, Belmans J, Van Woensel M, Riva M, Van Gool SW. Exploiting the immunogenic potential of cancer cells for improved dendritic cell vaccines. Front Immunol. 2015;6:663. PMID:26834740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adkins I, Fucikova J, Garg AD, Agostinis P, Spisek R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology. 2014;3:e968434. doi: 10.4161/21624011.2014.968434. PMID:25964865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, Fialova A, Sojka L, Cartron PF, Houska M, et al.. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer. 2014;135:1165-77. doi: 10.1002/ijc.28766. PMID:24500981 [DOI] [PubMed] [Google Scholar]
- 18.Mikyskova R, Stepanek I, Indrova M, Bieblova J, Simova J, Truxova I, Moserová I, Fučíková J, Bartůňková J, Špíšek R, et al.. Dendritic cells pulsed with tumor cells killed by high hydrostatic pressure induce strong immune responses and display therapeutic effects both in murine TC-1 and TRAMP-C2 tumors when combined with docetaxel chemotherapy. Int J Oncol. 2016;48:953-64. PMID:26718011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70-5. doi: 10.1038/sj.pcan.4500296. PMID:12496841 [DOI] [PubMed] [Google Scholar]
- 20.Mikyskova R, Indrova M, Simova J, Jandlova T, Bieblova J, Jinoch P, Bubeník J, Vonka V. Treatment of minimal residual disease after surgery or chemotherapy in mice carrying HPV16-associated tumours: Cytokine and gene therapy with IL-2 and GM-CSF. Int J Oncol. 2004;24:161-7. PMID:14654953 [PubMed] [Google Scholar]
- 21.Indrova M, Mikyskova R, Jandlova T, Vonka V, Bubenik J, Bieblova J. Adjuvant cytokine treatment of minimal residual disease after surgical therapy in mice carrying HPV16-associated tumours: Cytolytic activity of spleen cells from tumour regressors. Folia Biol (Praha). 2003;49:217-22. PMID:14748435 [PubMed] [Google Scholar]
- 22.Bubenik J, Mikyskova R, Vonka V, Mendoza L, Simova J, Smahel M, Indrová M. Interleukin-2 and dendritic cells as adjuvants for surgical therapy of tumours associated with human papillomavirus type 16. Vaccine. 2003;21:891-6. doi: 10.1016/S0264-410X(02)00537-6. PMID:12547599 [DOI] [PubMed] [Google Scholar]
- 23.Reinis M, Indrova M, Mendoza L, Mikyskova R, Bieblova J, Bubenik J, Símová J. HPV16-associated tumours: Therapy of surgical minimal residual disease with dendritic cell-based vaccines. Int J Oncol. 2004;25:1165-70. PMID:15375569 [PubMed] [Google Scholar]
- 24.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21-6. PMID:8548765 [PubMed] [Google Scholar]
- 25.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325-30. PMID:9269988 [PubMed] [Google Scholar]
- 26.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77-92. doi: 10.1016/S0022-1759(98)00204-X. PMID:10037236 [DOI] [PubMed] [Google Scholar]
- 27.Indrova M, Reinis M, Bubenik J, Jandlova T, Bieblova J, Vonka V, Velek J. Immunogenicity of dendritic cell-based HPV16 E6/E7 peptide vaccines: CTL activation and protective effects. Folia Biol (Praha). 2004;50:184-93. PMID:15709713 [PubMed] [Google Scholar]
- 28.Stepanek I, Indrova M, Bieblova J, Fucikova J, Spisek R, Bubenik J, Reinis M. Effects of 5-azacytidine and trichostatin A on dendritic cell maturation. J Biol Regul Homeost Agents. 2011;25:517-29. PMID:22217985 [PubMed] [Google Scholar]
- 29.Yi AK, Krieg AM. CpG DNA rescue from anti-IgM-induced WEHI-231 B lymphoma apoptosis via modulation of I kappa B alpha and I kappa B beta and sustained activation of nuclear factor-kappa B/c-Rel. J Immunol. 1998;160:1240-5. PMID:9570540 [PubMed] [Google Scholar]
- 30.Vlk V, Rossner P, Indrova M, Bubenik J, Sobota V. Interleukin-2 gene therapy of surgical minimal residual tumour disease. Int J Cancer. 1998;76:115-9. doi:. PMID:9533770 [DOI] [PubMed] [Google Scholar]
- 31.Bubenik J, Zeuthen J, Indrova M, Bubenikova D, Simova J. Kinetics and function of peritoneal-exudate cells during local IL-2 gene-therapy of cancer. Int J Oncol. 1994;4:13-6. PMID:21566882 [DOI] [PubMed] [Google Scholar]
- 32.Mikyskova R, Indrova M, Pollakova V, Bieblova J, Simova J, Reinis M. Cyclophosphamide-induced myeloid-derived suppressor cell population is immunosuppressive but not identical to myeloid-derived suppressor cells induced by growing TC-1 tumors. J Immunother. 2012;35:374-84. doi: 10.1097/CJI.0b013e318255585a. PMID:22576342 [DOI] [PubMed] [Google Scholar]
- 33.Mikyskova R, Indrova M, Vlkova V, Bieblova J, Simova J, Parackova Z, Pajtasz-Piasecka E, Rossowska J, Reiniš M. DNA demethylating agent 5-azacytidine inhibits myeloid-derived suppressor cells induced by tumor growth and cyclophosphamide treatment. J Leukoc Biol. 2014;95:743-53. doi: 10.1189/jlb.0813435. PMID:24389335 [DOI] [PubMed] [Google Scholar]
- 34.Ricupito A, Grioni M, Calcinotto A, Hess Michelini R, Longhi R, Mondino A, Bellone M. Booster vaccinations against cancer are critical in prophylactic but detrimental in therapeutic settings. Cancer Res. 2013;73:3545-54. doi: 10.1158/0008-5472.CAN-12-2449. PMID:23539449 [DOI] [PubMed] [Google Scholar]
- 35.Chin AI, Miyahira AK, Covarrubias A, Teague J, Guo BC, Dempsey PW, Cheng G. Toll-like Receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010;70:2595-603. doi: 10.1158/0008-5472.CAN-09-1162. PMID:20233880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lion E, Smits ELJM, Berneman ZN, Van Tendeloo VFI. NK Cells: Key to success of DC-based cancer vaccines? Oncologist. 2012;17:1256-70. doi: 10.1634/theoncologist.2011-0122. PMID:22907975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu K, Fujii SI. DC therapy induces long-term NK reactivity to tumors via host DC. Eur J Immunol. 2009;39:457-68. doi: 10.1002/eji.200838794. PMID:19180466 [DOI] [PubMed] [Google Scholar]
- 38.Bonaccorsi I, Pezzino G, Morandi B, Ferlazzo G. Novel perspectives on dendritic cell-based immunotherapy of cancer. Immunol Lett. 2013;155:6-10. doi: 10.1016/j.imlet.2013.09.021. PMID:24076312 [DOI] [PubMed] [Google Scholar]
- 39.Mendoza L, Indrova M, Hajkova R, Reinis M, Smahel M, Vonka V, Bubeník J, Jandlová T. Peritumoral administration of antigen-unstimulated bone marrow-derived dendritic cells inhibits tumour growth. Folia Biol-Prague. 2000;46:91-7. [PubMed] [Google Scholar]
- 40.Reinis M, Stepanek I, Simova J, Bieblova J, Pribylova H, Indrova M, Bubenik J. Induction of protective immunity against MHC class I-deficient, HPV16-associated tumours with peptide and dendritic cell-based vaccines. Int J Oncol. 2010;36:545-51. doi: 10.3892/ijo_00000528. PMID:20126973 [DOI] [PubMed] [Google Scholar]
- 41.De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M, et al.. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098-104. doi: 10.1158/1078-0432.CCR-07-4875. PMID:18483377 [DOI] [PubMed] [Google Scholar]