Abstract
Immunodeficient mice transplanted with human hematopoietic stem cells (HSCs) have been referred to as “Human Immune System” (HIS) mice and are a translational platform for studying human immune responses in vivo. Human HSC sources used in generating HIS mice include fetal liver (FL), umbilical cord blood (CB), and adult bone marrow (BM). Since HSCs from FL, CB, and BM are produced at various stages of human development, we tested whether mice transplanted with these three HSCs differ in their immune responses. We found that compared with CB HSCs or FL HSCs, adult BM HSCs reconstitute the immune system poorly. The resulting HIS mice do not mount an antibody response to Borrelia hermsii infection and as a consequence suffer persistently high levels of bacteremia. While both CB and FL HSCs yield comparable levels of immune reconstitution of HIS mice resulting in robust anti-B. hermsii immune responses, FL HSC-transplanted mice exhibited a discernable difference in their human B cell maturity as identified by an increased frequency of CD10+ immature B cells and relatively smaller lymphoid follicles compared with CB HSC-transplanted mice. Although CB HSC-transplanted mice generated robust antibody responses to B. hermsii and specific protein antigens of B. hermsii, they failed to respond to Salmonella typhi Vi polysaccharide, a classical T cell-independent antigen. This situation resembles that seen in human infants and young children. Therefore, CB HSC-transplanted mice may serve as a translation platform to explore approaches to overcome the impaired antipolysaccharide responses characteristic of human infants.
Keywords: : hematopoietic stem cell, Borrelia hermsii, Salmonella, B cells, bacterial polysaccharides, typhoid
Introduction
B cells early and late in life originate from fetal liver (FL) and bone marrow (BM) lymphopoiesis, respectively. Analysis of knockout and transgenic mice has revealed that Fms-like tyrosine kinase 3L (Flt3L, also known as Flk2L)-mediated B lymphopoiesis occurs early, whereas interleukin-7 (IL-7)-driven lymphopoiesis contributes to B development in the BM later in life [1]. In fact, mice deficient in both Flt3L and IL-7 completely lack mature B cells [1]. In mice, mature B cells are phenotypically, developmentally, and functionally distinct and are composed of B2 (follicular), marginal zone, and B1a and B1b subsets [2,3]. B1a cells but not B2 cells are generated efficiently from FL hematopoietic stem cells (HSCs), while B2 cells but not B1a cells are generated efficiently from BM HSCs [4,5]. Interestingly, B1b cells are generated efficiently from both FL as well as adult BM lymphopoiesis [6]. B1a and B1b cell subsets generated early in life can persist throughout life by self-renewal [6,7], and therefore, the B cell population in adult mice is developmentally heterogeneous. The B1a subset contributes to natural antibody production [8], while the B1b subset contributes to T cell independent antibody responses to a variety of bacterial protein and polysaccharide antigens [3]. B2 cells play a major role in T cell-dependent responses [2]. Thus, each B cell subset in mice occupies a distinct functional niche. Although rodent models have been exceptionally useful for elucidating the developmental aspects of each mature B cell subset and the protective responses they mount to bacterial infections, caution must be applied when interpreting these findings since the responses observed in mice may not reflect those that take place in humans.
Immune responses are complex biological processes and require in vivo analysis. Studies of human immunobiology in vivo are severely limited by both technical and ethical constraints. Immunodeficient mice such as NOD.Cg-Prkdcscid/IL2rγtm1Wjl/SzJ (NSG) mice xenografted with CD34+ human HSCs reconstitute many compartments of the human immune system [9–11]. These mice have been referred to as “Human Immune System” (HIS) mice and have become an attractive tool for studying infectious diseases specific to humans. For example, Salmonella enterica serovar typhi (S. typhi), the causative agent of typhoid fever in humans, is entirely host-adapted to humans and does not cause typhoid disease in mice [12,13]. However, HIS mice are permissive to lethal S. typhi infection, suggesting that HIS mice can serve as a model to identify the mechanism of protection against typhoid in humans [14–17]. We have shown that the characteristics of B cell responses to Borrelia hermsii in HIS mice mirror those of humans [18]. B. hermsii is a causative agent of relapsing fever in humans, is uniquely adapted to grow in the blood and causes recurrent episodes of bacteremia. We found that HIS mice are capable of controlling both primary and secondary bacteremic episodes and produce a specific IgM response to B. hermsii [18]. In mice, B1b cells mount a specific antibody response to Factor H-binding protein A, (FhBA), an outer membrane protein of B. hermsii in a T cell-independent manner [19]. Interestingly, IgM from B. hermsii-infected individuals or from B. hermsii-infected HIS mice display an identical reactivity to FhBA [19,20]. We have also found that the presumed equivalent of human B1 cells (CD20+CD27+CD43+CD70−) develop in HIS mice. Therefore, B. hermsii infection in HIS mice can serve as a translational platform for analyzing human B cell responses to T cell-independent antigens [18].
Given the above considerations, we reasoned that HSCs isolated from different human tissues and stages of development might reconstitute the B cell compartments of HIS mice in distinct manners, impacting the ability of these compartments to respond efficiently to particular antigens and infectious pathogens. In this study, we show that G-CSF mobilized HSCs from adult BM reconstitute the B cell compartment of HIS mice poorly. In contrast, HSCs isolated from human umbilical cord blood (CB) and FL both effectively reconstituted the B cell compartment of HIS mice. However, CB HSC HIS mice failed to mount an antibody response to S. typhi Vi polysaccharide, a characteristic of both neonatal and young children and young wild-type mice. We provide possible explanations for this and discuss how the development of more mature B cell compartments in HIS mice might be promoted.
Materials and Methods
Mice
All animals were housed in microisolator cages in a pathogen-free facility at Thomas Jefferson University. C57BL/6J (stock no. 00664), NOD.Cg-PrkdcscidIL2rγtm1Wjl/SzJ (NSG; stock no.005557), and NOD.Cg-Rag1tm1Mom IL2rγtm1Wjl/SzJ (NRG; stock no.007799) mice were purchased from the Jackson Laboratories, Bar Harbor, ME and bred in-house. The studies were reviewed and approved by the Institutional Animal Care and Use Committee.
Isolation of CD34+ HSCs
Human umbilical CB was obtained from healthy deliveries (Department of Obstetrics and Gynecology, Thomas Jefferson University) as approved by the Institutional Review Board. Human FL samples (18–19 week gestation) were purchased from Advanced Biomedical Resources (Alameda, CA). Livers were processed into single-cell suspensions using collagenase/dispase (Roche). CD34+ cells were isolated using a CD34+ isolation kit (Miltenyi Biotec). Cells were frozen in 95% FBS 5% DMSO at −80°C and then transferred to liquid nitrogen for storage until use. Human granulocyte-colony stimulating factor (G-CSF) mobilized CD34+ cells in the adult human peripheral blood, referred to as BM-derived HSCs were obtained from the Clinical Laboratory for Cellular Therapy, Thomas Jefferson University as approved by the Institutional Review Board. Cells were washed and frozen in Synth-a-Freeze cryopreservation medium (Life Technologies) at −80°C and then transferred to liquid nitrogen for storage until use. The purity of HSCs (CD34+) from G-CSF mobilized peripheral blood, and umbilical CB was comparable (∼90%), whereas the purity of HSCs from FL is 70%–90%. The contamination of B (CD19+) cells in all the three HSC preparations is ≤1%. The contaminating T (CD3+) cells in umbilical CB HSC preparation is 1%–2%, and this is minimal in G-CSF mobilized peripheral blood (0.04%) and FL HSC preparations (0.01%).
Transplantation of HSCs
Twenty-four to 48 h after birth, NSG pups were given whole body irradiation at a dose of 1.5 Gy (150 Rads), while NRG mice received 4 Gy (400 Rads). These doses were chosen because previously we found that they yielded similar engraftment levels between the strains, and the mice remained healthy. Five hours after irradiation, 105 HSCs derived from BM, CB, or FL were injected intrahepatically in 25 μL of PBS using a 30-gauge needle. Mice were weaned at 3 weeks of age and randomly distributed among different experimental groups.
Bacterial infections and immunizations
Eight- to 12-week-old mice were infected i.p. with 5 × 104 B. hermsii bacteria, strain DAH-p19 and bacteremia was determined by dark-field microscopy as described [18]. For whole bacterial immunization, mice were injected i.p. with 3 × 108 heat-killed S. typhi strain Ty2, which expresses Vi polysaccharide (ViPS) antigen. Blood samples were obtained 0, 7, 14, 21, or 28 days following immunization.
Enzyme-linked immunosorbent assay
B. hermsii-specific human IgM was measured by coating 96 well EIA/RIA plates (Costar 9017; Corning Incorporated, Corning, NY) with B. hermsii DAH (105 wet bacteria/well). FhbA-specific human IgM was measured by coating 96-well plates with 1 μg/mL recombinant FhbA (rFhbA) [19]. ViPS-specific human IgM was measured by incubating 96-well microtiter plates (Nunc MultiSorp 467340; Thermo Fisher Scientific Nunc A/S, Roskilde, Denmark) with 2 μg/mL of ViPS in DPBS overnight at room temperature. All plates were washed and blocked with 2% BSA in PBS pH 7.2 for 2 h at room temperature. Blood samples of immunized mice were diluted 1:50 and were centrifuged (16,000 g for 10 min), and the supernatant was used. Bound human IgM was measured using HRP-conjugates of goat anti-human IgM as described [18]. Bound mouse IgM or IgG was measured using HRP-conjugated goat anti-mouse IgM or IgG (Bethyl Laboratories, Montgomery, TX). Specific antibody levels were interpreted as ng/μL equivalents using human or mouse IgM and IgG standards.
Flow cytometry
Evaluation of human lymphocyte reconstitution in the HSC recipient mice was performed using flow cytometry. Ten microliters of peripheral blood was collected from each mouse at 8 weeks of age and put into tubes containing 40 U heparin in 20 μL PBS. Red blood cells were lysed using ammonium chloride-potassium lysis buffer and cells were stained with anti-human CD45-FITC (2D1), CD3-Percp-Cy-5.5 (OKT3), and CD20-APC (2H7) (eBioscience). AccuCount (1 × 106 beads/mL, Spherotech, Inc.) beads were added to each sample before flow cytometry to determine total cell counts. Two hundred thousand events were recorded per sample and data were analyzed using the FlowJo software (Tree Star).
To determine the frequency of various B cell populations, spleen cells of NRG mice engrafted with CB or FL HSCs were sacrificed and their spleens were removed and cut in half for phenotyping and histology. BM HSC-engrafted mice did not have enough human cells to accurately phenotype all of their B cell subsets. Cells were harvested from individual mouse spleens in staining medium [Minimum Essential Medium Eagle with Earle's salts and without l-glutamine and phenol red (Corning Cellgro, Manassas, VA) with 3% newborn calf serum (HyClone Laboratories, Inc., Logan, UT), 2 mM EDTA]. After blocking murine Fc receptors with 2.4G2 antibody and human Fc receptors with FC block® (BD Biosciences), an aliquot of 50 μL (106 cells) of cells was incubated in a microtiter plate with appropriately diluted antibody. The following additional fluorescent-conjugated monoclonal antibodies specific for the indicated human antigens were used: CD19-PE-Cy7 (Hib19), CD20-PerCP-Cy5.5 (2H7), CD43-APC (CD43-10G7), CD10-FITC (HI10a), and CD69-BV421 (FN50) conjugates were purchased from BioLegend; CD70-FITC (Ki-24), CD27-APC-H7 (M-T271), IgM-Percp-Cy5.5 (G20-127), and IgD-APC (IA6-2) conjugates were purchased from BD Pharmingen. CD34-FITC (4H11) was purchased from eBioscience. After washing, the stained cells were analyzed by flow cytometry (LSR II; BD Biosciences, San Jose, CA). At least two hundred thousand events were acquired per sample and analyzed using the FlowJo software (Tree Star, Ashland, OR).
Histology
Spleens were flash frozen in O.C.T. compound (Fisher HealthCare) using liquid nitrogen, and then placed at −20°C for storage. Eight micron sections were cut from each spleen using a Leica CM1900 cryostat and were placed on clear microscope slides for staining. Slides were stained with fluorescent-labeled anti-human IgM-FITC (Invitrogen), CD3-PE (OKT3; BioLegend), and CD11c-APC (3.9; BioLegend) antibodies. Images of lymphoid microenvironments were taken on a Leica DM5000b fluorescent microscope.
Statistical analysis
Data presented throughout depict pooled data from at least two independent experiments. Statistics were performed using the Prism 5 software program (GraphPad Software, Inc., La Jolla, CA).
Results
Human adult BM-derived HSCs do not reconstitute the lymphoid compartment or humoral immune responses in HIS mice as efficiently as human CB- or FL-derived HSCs
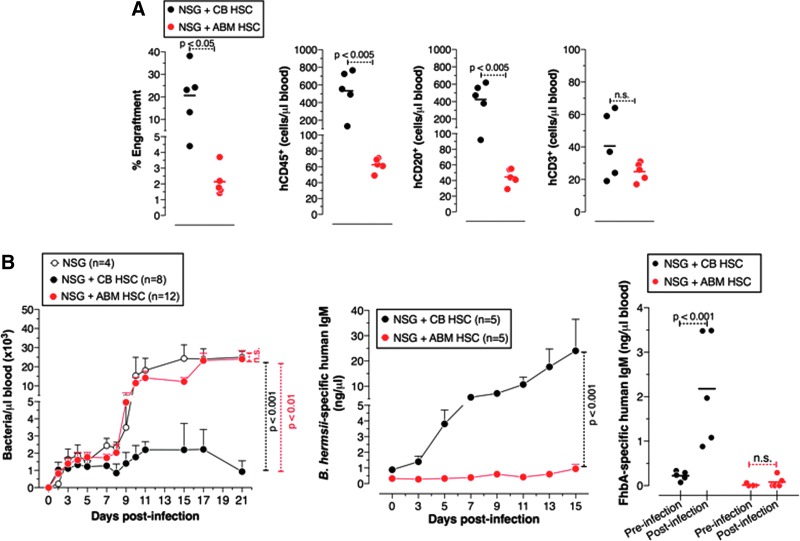
Control of B. hermsii infection is mediated by T cell-independent B cell responses [21]. We have previously shown that HIS mice generated using CB HSCs possess several B cell subsets and control B. hermsii bacteremia, but they do so less efficiently than adult wild-type (C57BL/6) mice [18]. This may be due to a suboptimal reconstitution of the B cells that mediate the anti-B. hermsii response by CB HSCs. To test whether HIS mice generated from human adult BM-derived HSCs (ie, G-CSF mobilized adult CD34+ cells) or FL HSCs posses a more robust humoral arm, we have compared the degree of B cell reconstitution and the function of the B cells in these HIS mice with that of HIS mice generated using CB HSCs. We found that the development of the B cell compartment in BM-HSC HIS mice was very poor compared with CB-HSC HIS mice (Fig. 1A). This poor reconstitution was not improved even with a two-fold increase in the number of BM-HSCs injected. Consistent with this, BM-HSC HIS mice failed to generate an antibody response to B. hermsii or FhbA, an outer membrane protein of B. hermsii, and as a consequence suffered persistent bacteremia that was indistinguishable from that of unreconstituted NSG mice (Fig. 1B).
FIG. 1.
Human adult bone marrow HSCs poorly reconstitute functional immunity in mice compared to human umbilical cord blood HSCs. (A) The relative reconstitution of human lymphocytes in the peripheral blood is referred to as percent engraftment. (B) Mice were infected with 5 × 104 Borrelia hermsii bacteria i.p., and bacteremia, and anti-B. hermsii responses at the indicated days postinfection were determined by dark-field microscopy and ELISA, respectively. Specific IgM responses to B. hermsii outer membrane protein (FhbA) were measured on 14 days postinfection. The difference in lymphocyte reconstitution was analyzed using Student's t-test (two-tailed). Statistics were done using two-way ANOVA with Bonferroni posttest. Statistically significant differences in bacteremia were seen from day 10 to 21 postinfection and for anti-B. hermsii IgM responses from day 11 to 15. For bacteremia and the antibody response the mean ± SEM is shown. Pooled data from two independent experiments are shown. HSC, hematopoietic stem cell. n.s., not significant.
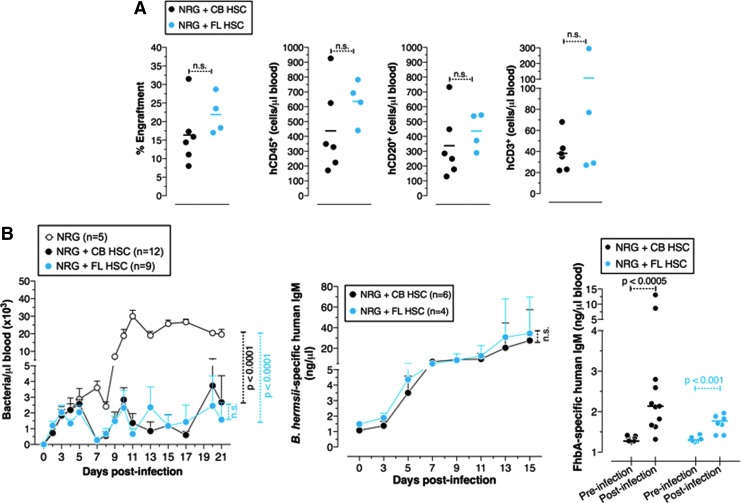
Unlike BM HSCs, HSCs derived from FL proliferate extensively and exhibit a robust metabolism [22]. Since BM HSCs do not reconstitute the B cell compartment of HIS mice well, we tested whether FL HSCs reconstitute the B cell compartment of these mice better than CB HSCs. Another variable in the generation of HIS mice is the dose of radiation used to “condition” the NSG recipients before human HSC injection. An increase in radiation dose might enhance the efficiency of HSC engraftment and human B cell reconstitution. To test this, we utilized NRG mice as recipients, which unlike NSG mice can tolerate high doses of radiation. We found that an increase in dose from 1.5 Gy (used for NSG recipients) to 4 Gy (used for NRG recipients) did not result in an enhancement of B cell reconstitution (Fig. 1A vs. Fig. 2A). We found that quantitative reconstitution of the B cell compartment in CB HSC HIS mice and FL HSC HIS mice was not significantly different (Fig. 2A). Consistent with this, both mice controlled B. hermsii bacteremia and generated a comparable antibody response to B. hermsii as well as to FhBA (Fig. 2B).
FIG. 2.
Comparable reconstitution of protective immune responses in mice transplanted with human umbilical cord blood and fetal liver HSCs. (A) The relative reconstitution of human lymphocytes in the peripheral blood is referred to as percent engraftment. (B) Mice were infected with 5 × 104 B. hermsii bacteria i.p., and bacteremia and anti-B. hermsii responses at the indicated days postinfection were determined by dark-field microscopy and ELISA, respectively. Specific IgM responses to B. hermsii outer membrane protein (FhbA) were measured on 14 days postinfection. The difference in lymphocyte reconstitution was analyzed using Student's t-test (two-tailed). Statistics were done using two-way ANOVA with Bonferroni posttest. Statistically significant differences in bacteremia were seen from day 10 to 21 postinfection. For bacteremia and the antibody responses the mean ± SEM is shown. Pooled data from two independent experiments are shown. n.s., not significant.
The B cell compartment reconstituted by FL HSCs appears less mature than that reconstituted by UCB HSCs
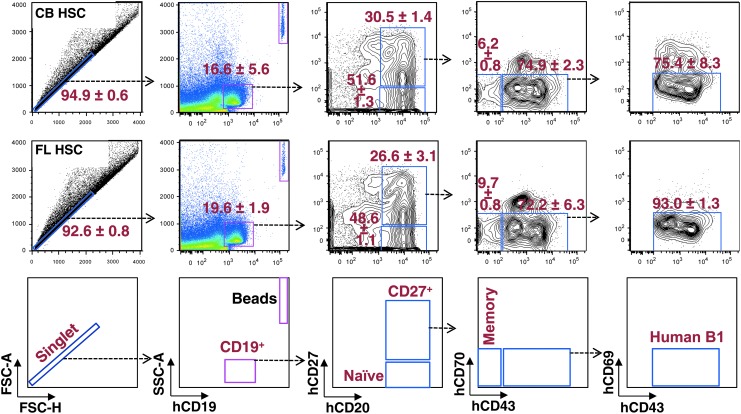
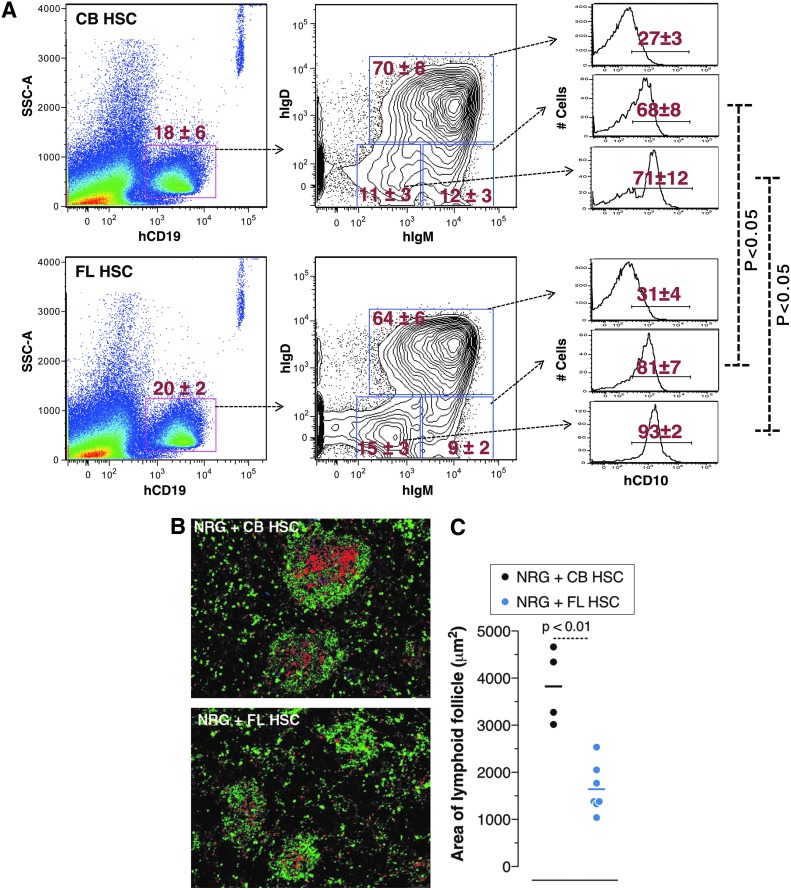
In mice, B1b cells produce antibody responses to FhBA [19]. In humans, a subset of B cells was identified (CD19+CD20+CD27+CD43+CD70−CD69−) in umbilical CB as well as adult peripheral blood that exhibit many functional characteristics of murine B1 cells [23]. Although we have not found direct evidence that human B1 cells control B. hermsii, we have previously shown that the phenotypic equivalents of human B1 cells develop in UCB HSC HIS mice [18]. To test whether FL HSC HIS mice also develop human “B1-like” cells, we analyzed the composition of B cell subsets in the spleens of CB HSC and FL HSC HIS mice. We found that the development of a number of B cell subsets, including human “B1-like” cells in these two mice, is comparable (Fig. 3). In mice, B1 cells are known to arise during fetal B cell lymphopoiesis [4,24]. Therefore, the presence of B1-like cells in FL HSC HIS mice suggests that B1 cells in humans also develop from fetal B cell lymphopoiesis. Interestingly, we found that compared to CB-HSC HIS mice, the FL-HSC HIS mice have a relatively higher frequency of CD19+IgMlo/hiIgDlo cells, and these cells are mainly of immature phenotype as indicated by their CD10 expression profile (Fig. 4A). Although this difference was consistent, it was somewhat subtle. We therefore analyzed the splenic lymphoid microenvironments of these two mice by immunohistochemistry. We found that lymphoid follicular architecture was less well defined in FL HSC HIS mice compared with CB HSC HIS mice. Moreover, the size of B lymphoid follicles in FL-HSC HIS mice was significantly smaller compared with CB-HSC HIS mice (Fig. 4B, C).
FIG. 3.
Development of various human B cell subsets in mice reconstituted with HSCs from human umbilical cord blood and fetal liver. Figures were derived from a single sample representative of each population observed (n = 4 for each). Cells were isolated from the spleen and labeled with anti-human CD19, CD20, CD27, CD43, CD69, and CD70 fluorescent antibodies. The stained cells were then analyzed by flow cytometry. A total of 200,000 events were recorded. Differences in B cell subset sizes between the two types of HIS mice are not statistically significant. HIS, human immune system.
FIG. 4.
Mice engrafted with human fetal liver HSCs show an increased frequency of immature B cells and smaller lymphoid follicles compared with mice engrafted with cord blood HSCs. (A) Figures were derived from a single sample representative of each population observed (n = 4 for each). Cells were isolated from the spleen and labeled using anti-human CD10, CD19, IgM, and IgD fluorescent antibodies. Analysis of stained cells was performed by flow cytometry. A total of 200,000 events were recorded. The difference in CD10 expression on IgMhi IgDlo or IgMlo IgDlo B cells in the two types of HIS mice is statistically significant by Student's t-test. (B) Spleen histology images are representative of each type of HIS mouse and are stained with anti-human IgM-FITC (green; B cells), CD3-PE (red; T cells) and CD11c-APC (blue; dendritic cells) antibodies. (C) Area of lymphoid microenvironments within the spleen. The sizes of the lymphoid follicles in the histology sections were scored blinded and follicle areas were estimated using width and height measurements of individual follicles in histological images. Each dot represents an individual mouse, and the statistical significance was determined by Student's t-test.
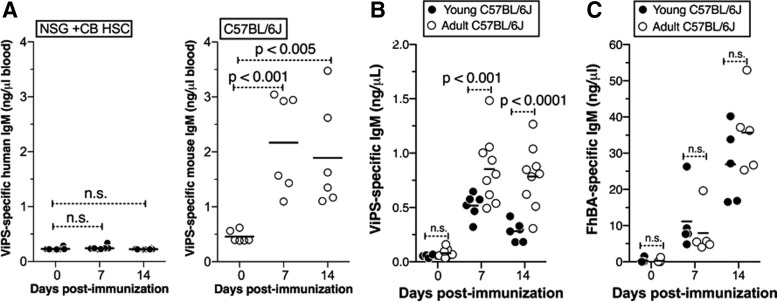
In mice, Flt3L and IL-7 promote B lymphopoeisis early and late in life, respectively [1,25]. We have previously shown that IL-7-dependent B cells are required for antibody responses to polysaccharide antigens [26], but not for controlling B. hermsii bacteremia [27]. In BM, IL-7 is mainly derived from stromal cells rather than the cells of the hematopoietic lineages. Since FL HSCs and CB HSCs reconstitute B lymphopoiesis in the absence of human IL-7 in HIS mice, we predicted that these mice would not respond to bacterial polysaccharides as efficiently as they respond to B. hermsii. Indeed, we showed that CB HSC HIS mice respond poorly to S. typhi Vi polysaccharide compared to adult wild-type mice (Fig. 5A). Unlike bacterial polysaccharides, which are regarded as classical TI antigens, B. hermsii induces an atypical TI antibody response that is IL-7 independent [3]. It is known that human infants or young children do not respond to purified polysaccharide antigens. Therefore, we predicted that young mice and adult mice would respond to B. hermsii comparably, whereas adult mice but not young mice would generate an efficient response to polysaccharide antigens. Indeed, we found that the response to FhBA of B. hermsii in young and adult wild-type mice is indistinguishable. In contrast, young wild-type mice generate a significantly lower response to Vi polysaccharide than adult mice (Fig. 5B). Thus, a lack of response to polysaccharide antigens in HIS mice suggests that their humoral immune system recapitulates that of human infants.
FIG. 5.
Mice reconstituted with human umbilical cord blood HSCs do not respond to bacterial polysaccharide antigens. (A) Mice transplanted with human umbilical cord blood HSCs or adult C57BL6 mice were immunized with 3 × 108 heat-killed S. typhi strain Ty2 and ViPS-specific human and mouse IgM responses were measured by ELISA. Three-week-old (young) or 14-week-old (adult) mice were (B) immunized with ViPS or (C) infected with B. hermsii and anti-ViPS IgM and anti-FhBA responses were measured by ELISA. ViPS, Vi polysaccharide. n.s., not significant.
Discussion
In the present study, we show that HSCs derived from CB reconstitute a far more complete and responsive B cell compartment in HIS mice compared with adult BM HSCs. However, CB-HSC HIS mice completely fail to respond to Vi polysaccharide of S. typhi, indicating that CB HSC HIS mice in their current form are not suitable for studying classical TI B cell responses. Several aspects of B cell development could account for this defect. One possibility is the relatively high percentage of CD10+ immature B cells in CB HSC HIS mice (Fig. 4A), perhaps due to lack of appropriate cross stimulation of human receptors necessary for human peripheral B cell maturation and survival by mouse cytokines and chemokines. A likely candidate in this regard would be B cell activating factor (BAFF, also known as BLyS), which plays an important role in the function and maintenance of mature B cells [28,29]. It was previously shown that human peripheral blood B cells transplanted into immunodeficient mice require exogenous human BAFF for their survival as well as their ability to mount an antipneumococcal polysaccharide response [30]. Murine BAFF does not efficiently support the survival and function of human B cells [30]. Since BAFF is not required for the survival of pre-B cells and immature B cells [28,29,31], the further development of immature B cells might be promoted in HIS mice by supplementation with human BAFF. In fact, the human cytokine knock-in approach has been shown to increase the efficiency of human CD34+ cell engraftment in mice [32].
The lack of a response to polysaccharides in HIS mice could also be due to a limitation in IL-7-driven B lymphopoiesis. This possibility is supported by the fact that neither young wild-type mice nor IL-7-deficient adult mice respond to polysaccharide antigens [26]. On the contrary, we showed that transgenic expression of IL-7 in young mice permits an antibody response to pneumococcal polysaccharide [26] and Vi polysaccharide of S. typhi (article submitted). Similar to the mouse and human BAFF axes, mouse IL-7 is ∼100-fold less efficient than human IL-7 in stimulating the human IL-7 receptor [33]. In addition, IL-7 is largely derived from nonhematopoietic cells, namely stromal cells in the BM. Thus, human B lymphopoiesis in HIS mice is presumably occurring in a human IL-7-limiting environment, similar to human perinatal B lymphopoiesis. Perinatal B lymphopoiesis in mice is known to be driven by the FLT3L-FLT3 axis [1]. Thus, the B cells generated in HIS mice are likely developmentally and functionally similar to those in human newborns and infants.
Although both CB HSCs and FL HSCs reconstituted the B lymphoid compartments of HIS mice fairly well, BM HSCs did not. This might also be explained by functional deficiencies of IL-7 and BAFF in BM-HSC HIS mice. It is known that mice deficient in IL-7 have an arrest in B cell development in the BM [34–36] but not in fetal or perinatal B cell development that predominantly produces B1 cells [26,36]. B cells that develop in the presence of IL-7 are mainly B2 cells, which are more dependent on BAFF than B1 cells [28,37]. Therefore, we hypothesize that supplementation of HIS mice with human IL-7 and human BAFF will increase B cell production and maintenance, respectively, thereby enhancing the overall reconstitution of a functionally mature B cell compartment in HIS mice generated using adult BM HSCs. It has been shown that efficient human B cell maturation in HIS mice also requires T cells [38]. Since IL-7 is a crucial cytokine for T cells, supplementation of human IL-7 could promote a more efficient T and B cell reconstitution and functionality in HIS mice.
Collectively, the data we present strongly suggest that HIS mice generated with CB and FL HSCs are excellent models for examining the functionality of the B cell compartment of human neonates and young children. Moreover, such mice will allow in vivo studies of how current vaccination strategies for childhood illnesses might be modified to achieve greater vaccine efficacy. We suggest that the generation of HIS mice with B cell compartments more similar to adult humans will require substantial alterations of the current technology. Modifications likely to promote the development of the B cell compartment from “neonatal-like” to “adult-like” include the expression of the human versions of IL-7 and BAFF by recipient mice. Reconstitution of such mice with HSCs from adults might also be required to ensure that the appropriate precursors of adult B lymphopoiesis become durably engrafted in the chimeric mice.
Acknowledgments
We thank all residents and staff of Labor and Delivery, Jefferson Dept. of Obstetrics & Gynecology for their continued efforts in collecting and providing cord blood samples for this study. We also thank the staff of the Jefferson Clinical Laboratory for Cellular Therapy for providing purified adult bone marrow hematopoietic stem cells. This work was supported by an NIH R21AI097677 grant to T.L.M. and an NIH RO3 AI112624 grant to K.R.A.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Sitnicka E, Brakebusch C, Martensson IL, Svensson M, Agace WW, Sigvardsson M, Buza-Vidas N, Bryder D, Cilio CM, et al. (2003). Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. J Exp Med 198:1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin F. and Kearney JF. (2001). B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol 13:195–201 [DOI] [PubMed] [Google Scholar]
- 3.Alugupalli KR. (2008). A distinct role for B1b lymphocytes in T cell-independent immunity. Curr Top Microbiol Immunol 319:105–130 [DOI] [PubMed] [Google Scholar]
- 4.Kantor AB, Stall AM, Adams S, Herzenberg LA. and Herzenberg LA. (1992). Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A 89:3320–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung JW, Mrazek MD, Yang Y, Herzenberg LA. and Herzenberg LA. (2006). Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci U S A 103:6293–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stall AM, Adams S, Herzenberg LA. and Kantor AB. (1992). Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann N Y Acad Sci 651:33–43 [DOI] [PubMed] [Google Scholar]
- 7.Kantor AB, Stall AM, Adams S, Watanabe K. and Herzenberg LA. (1995). De novo development and self-replenishment of B cells. Int Immunol 7:55–68 [DOI] [PubMed] [Google Scholar]
- 8.Martin F. and Kearney JF. (2000). B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev 175:70–79 [PubMed] [Google Scholar]
- 9.Brehm MA, Shultz LD. and Greiner DL. (2010). Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes 17:120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shultz LD, Ishikawa F. and Greiner DL. (2007). Humanized mice in translational biomedical research. Nat Rev Immunol 7:118–130 [DOI] [PubMed] [Google Scholar]
- 11.Pearson T, Greiner DL. and Shultz LD. (2008). Humanized SCID mouse models for biomedical research. Curr Top Microbiol Immunol 324:25–51 [DOI] [PubMed] [Google Scholar]
- 12.Jones C, Darton TC. and Pollard AJ. (2014). Why the development of effective typhoid control measures requires the use of human challenge studies. Front Microbiol 5:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waddington CS, Darton TC, Woodward WE, Angus B, Levine MM. and Pollard AJ. (2014). Advancing the management and control of typhoid fever: a review of the historical role of human challenge studies. J Infect 68:405–418 [DOI] [PubMed] [Google Scholar]
- 14.Libby SJ, Brehm MA, Greiner DL, Shultz LD, McClelland M, Smith KD, Cookson BT, Karlinsey JE, Kinkel TL, et al. (2010). Humanized nonobese diabetic-scid IL2rgammanull mice are susceptible to lethal Salmonella Typhi infection. Proc Natl Acad Sci U S A 107:15589–15594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA. and Galan JE. (2010). A mouse model for the human pathogen Salmonella Typhi. Cell Host Microbe 8:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firoz Mian M, Pek EA, Chenoweth MJ. and Ashkar AA. (2011). Humanized mice are susceptible to Salmonella Typhi infection. Cell Mol Immunol 8:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mian MF, Pek EA, Chenoweth MJ, Coombes BK. and Ashkar AA. (2011). Humanized mice for Salmonella Typhi infection: new tools for an old problem. Virulence 2:248–252 [DOI] [PubMed] [Google Scholar]
- 18.Vuyyuru R, Liu H, Manser T. and Alugupalli KR. (2011). Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A 108:20707–20712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombo MJ. and Alugupalli KR. (2008). Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol 180:4858–4864 [DOI] [PubMed] [Google Scholar]
- 20.Hovis KM, Schriefer ME, Bahlani S. and Marconi RT. (2006). Immunological and molecular analyses of the Borrelia hermsii factor H and factor H-like protein 1 binding protein, FhbA: demonstration of its utility as a diagnostic marker and epidemiological tool for tick-borne relapsing fever. Infect Immun 74:4519–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T. and Gerstein RM. (2004). B1b Lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379–390 [DOI] [PubMed] [Google Scholar]
- 22.Manesia JK, Xu Z, Broekaert D, Boon R, van Vliet A, Eelen G, Vanwelden T, Stegen S, Van Gastel N, et al. (2015). Highly proliferative primitive fetal liver hematopoietic stem cells are fueled by oxidative metabolic pathways. Stem Cell Res 15:715–721 [DOI] [PubMed] [Google Scholar]
- 23.Griffin DO, Holodick NE. and Rothstein TL. (2011). Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med 208:67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayakawa K, Hardy RR, Herzenberg LA. and Herzenberg LA. (1985). Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 161:1554–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen CT, Kharazi S, Boiers C, Cheng M, Lubking A, Sitnicka E. and Jacobsen SE. (2008). FLT3 ligand and not TSLP is the key regulator of IL-7-independent B-1 and B-2 B lymphopoiesis. Blood 112:2297–2304 [DOI] [PubMed] [Google Scholar]
- 26.Shriner AK, Liu H, Sun G, Guimond M. and Alugupalli KR. (2010). IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol 185:525–531 [DOI] [PubMed] [Google Scholar]
- 27.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT. and Leong JM. (2003). The resolution of relapsing fever Borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol 170:3819–3827 [DOI] [PubMed] [Google Scholar]
- 28.Mackay F. and Schneider P. (2009). Cracking the BAFF code. Nat Rev Immunol 9:491–502 [DOI] [PubMed] [Google Scholar]
- 29.Treml JF, Hao Y, Stadanlick JE. and Cancro MP. (2009). The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys 53:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD. and Woodland RT. (2008). Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One 3:e3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay F. and Schneider P. (2008). TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev 19:263–276 [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Ellegast JM, Rafiei A, Song Y, Kull D, Heikenwalder M, Rongvaux A, Halene S, Flavell RA. and Manz MG. (2016). Peripheral blood CD34+ cells efficiently engraft human cytokine knock-in mice. Blood 128:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombetti S, Levy F. and Chapatte L. (2009). IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood 113:6629–6637 [DOI] [PubMed] [Google Scholar]
- 34.Hao Z. and Rajewsky K. (2001). Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med 194:1151–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE. and Murray R. (1995). Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med 181:1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J. and Vieira P. (2001). Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7-/- mice. J Exp Med 194:1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O'Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, et al. (2008). BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A 105:15517–15522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang J, Kelly M, Freed BM, McCarter MD, Kedl RM, Torres RM. and Pelanda R. (2013). Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J Immunol 190:2090–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]