Abstract
Introduction
Pancreatic cancer (PC) is characterized by mucin overexpression. MUC4 is the most differentially overexpressed membrane-bound mucin that plays a functional role in disease progression and therapy resistance.
Area covered
We describe the clinicopathological significance of MUC4, summarize mechanisms contributing to its deregulated expression, review preclinical studies aimed at inhibiting MUC4, and discuss how MUC4 overexpression provides opportunities for developing targeted therapies. Finally, we discuss the challenges for developing MUC4-based therapeutics, and identify areas where efforts should be directed to effectively exploit MUC4 as a therapeutic target for PC.
Expert opinion
Studies demonstrating that abrogation of MUC4 expression reduces proliferation and metastasis of PC cells and enhances sensitivity to therapeutic agents affirm its utility as a therapeutic target. Emerging evidence also supports the suitability of MUC4 as a potential immunotherapy target. However, these studies have been limited to in vitro, ex vivo or in vivo approaches using xenograft tumors in immunodeficient murine models. For translational relevance,MUC4-targeted therapies should be evaluated in murine models with intact immune system and accurate tumor microenvironment. Additionally, future studies evaluating MUC4 as a target for immunotherapy must entail characterization of immune response in PC patients and investigate its association with immunosuppression and survival.
Keywords: MUC4, miRNA, natural products, pancreatic cancer (PC), therapeutic target, vaccine
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related mortality in the United States, projected to cause an estimated 43,090 deaths in 2017 [1]. The high mortality rate of PDAC results from asymptomatic early disease resulting in clinical presentation at advanced stages, high incidence of postoperative recurrence, poor sensitivity to chemotherapy and radiotherapy, and lack of specific therapeutic targets. Thus, the quest for novel targets and approaches for effective screening and curative therapeutic interventions to improve the survival of PDAC patients is an ongoing endeavor.
Mucins, a family of 21 high-molecular-weight glycoproteins which are either secreted or remain tethered to the plasma membrane, are predominantly produced by the epithelial surfaces of the respiratory, gastrointestinal, and genitourinary systems [2,3]. Under physiologic conditions, mucins keep the epithelial surfaces hydrated and lubricated, and serve as a barrier protecting the underlying epithelia from pathogens, acids, and digestive enzymes. Furthermore, by sequestering growth factors and cytokines, mucins serve as molecular sieves to regulate the diffusion of biomolecules to and from the epithelia, thereby playing a critical role in epithelial homeostasis [2,4]. Aberrant overexpression of various mucins is associated with, and contributes to, several inflammatory and malignant pathologies.
MUC4 is the most differentially overexpressed transmembrane mucin in PDAC [5-7] and has been demonstrated to functionally contribute to the pathobiology and aggressiveness of the disease [8,9]. In contrast to undetectable expression in normal pancreatic ducts, MUC4 expression is observed in the earliest pancreatic intraepithelial neoplasms (PanIN lesions), and its expression progressively increases with the progression of disease to pancreatic ductal adenocarcinoma (PDAC) [5,10]. Functionally, MUC4 expression induces neoplastic transformation [11], promotes tumorigenesis and metastasis [2,8,12], modulates the interaction of tumor cells with the components of tumor microenvironment [9,12,13], and contributes to the resistance of tumor cells to chemotherapeutic agents [14–17]. These diverse functions of MUC4 have been attributed to its ability to interact with multiple proteins including epidermal growth factor receptor (EGFR) family members [18], extracellular matrix components like fibulin-2 [19], and circulating, metastasis-promoting factors like soluble galectin-3 [13]. Furthermore, the expression of MUC4 on PDAC cells has been demonstrated to compromise the immune effector response by facilitating apoptosis of antigen-specific cytotoxic T cells [20]. Due to its differential overexpression and functional involvement in various aspects of PDAC pathobiology, MUC4 has emerged as a potential therapeutic target for directly abrogating MUC4-mediated progression and for enhancing the efficacy of chemotherapeutic and immunotherapeutic regimens. In addition, high MUC4 expression in tumors was found to be an independent determinant of poor prognosis in PDAC patients [21]. Although the clinical utility of MUC4-targeted therapeutic modalities remains to be demonstrated, early preclinical studies have provided a glimpse of various approaches that can be employed to exploit MUC4 as a therapeutic target. Herein, we provide a brief overview of the MUC4 structure, summarize the functional relevance of its deregulated overexpression, and discuss the approaches that have been tested to abrogate or modulate MUC4 in PDAC. We also describe the existing challenges that need to be met to fully realize the therapeutic potential of targeting MUC4 in PDAC.
2. MUC4 structure and physiological expression
Human MUC4 is a multi-domain transmembrane glycoprotein with a high degree of allelic polymorphism in the characteristic variable number tandem repeat (VNTR) domain due to which the molecular weight of the apoprotein backbone can range between ~550 and ~930 kDa [22]. MUC4 is synthesized as a single polypeptide, and is putatively cleaved at Gly-Asp-Pro-His (GDPH) site in an autocatalytic manner into two subunits: a large N-terminal MUC4α and a smaller membranetethered MUC4β (Figure1) [3,22,23]. MUC4α consists of a central polypeptide core composed of a VNTR region rich in serine, threonine, and proline residues and is extensively decorated with characteristic mucin-type O-linked oligosaccharide side chains of variable size, composition, and structure. Downstream of VNTR region are the nidogen-like domain (NIDO) and adhesion-associated domain in MUC4 and other proteins (AMOP) [2,4]. MUC4β contains a von Willebrand factor type D domain (vWD), three EGF-like domains, and a transmembrane domain followed by a short cytoplasmic tail (Figure 1). The genomic organization, domain architecture, and evolution of MUC4 have been reviewed previously [2], while the functions of its various domains in the context of pathobiology of PDAC are discussed in subsequent sections.
Figure 1.
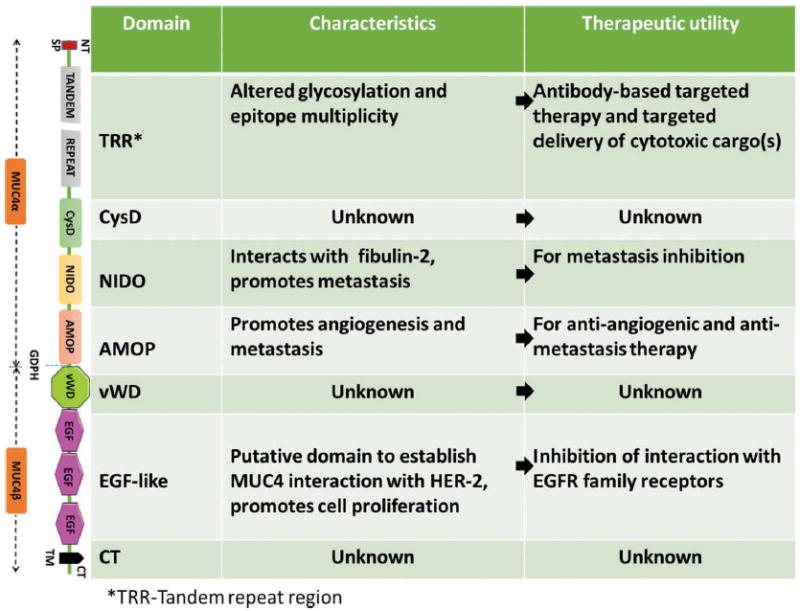
Schematic representation of structural organization of various MUC4 domains, their functional role, and putative therapeutic utility. Presented here are the different domains of high molecular weight glycosylated MUC4 from N-terminal (NT) to C-terminal (CT) (top to bottom). Understanding their structure and function may facilitate the development of targeted [domain-specific] therapeutics.
MUC4 is expressed in several tissues, both during development and into adulthood. While MUC4 is the first mucin expressed in the developing lung [24], it is also expressed in the developing esophagus, jejunum, ileum, and colon, but not in the hepato-pancreatico-biliary tract [25,26]. MUC4 expression has been reported in the salivary glands, trachea and bronchioles, reproductive tract, colon, and mammary epithelium [2,27]. Although MUC4 is a transmembrane mucin, it can be detected in secretory products including saliva, milk, and tears, possibly due to its putative cleavage at GDPH site, or as a part of membranous structures (like milk fat globules or salivary exosomes) [28-30]. Physiologically, the functions of MUC4 largely overlap with those of other mucins that include epithelial renewal, cell–cell adhesion, cell signaling, and protection from physical and biochemical insults [2].
3. MUC4 expression in PDAC
In contrast to undetectable expression in normal pancreas and inflammatory diseases of the pancreas, MUC4 is expressed de novo in pancreatic neoplasms as early as PanIN-1 lesions, and its expression increases progressively with disease progression from invasive to metastatic PDAC [5,31]. Immunohistochemical analyses of human pancreatic tissue samples indicated a progressive increase in MUC4 expression from 17% in PanIN-I to 89% in PDAC [10]. In addition, a twofold increase in MUC4 expression was reported in PDACs compared to intraductal papillary mucinous neoplasms (IPMNs) [31]. In efforts to distinguish PDAC from chronic pancreatitis, MUC4 expression was analyzed along with mesothelin, clustein-β, and survivin in fine needle aspirates (FNAs). MUC4 was expressed in 91% of the FNAs from PDAC patients and exhibited 100% specificity [32]. Moreover, there is a high concordance in MUC4 expression in primary tumors and paired lymph node metastases, suggesting that MUC4 expression is retained during metastasis [33]. In addition to its aberrant overexpression, deregulation in the glycosylation [34] and splicing machineries [35-37] during carcinogenesis may result in the expression of aberrant glycoforms and splice variants of MUC4 which may exhibit unique tumor-specific expression patterns and functions.
4. Role of MUC4 in the pathobiology of PDAC
Due to the loss of cellular polarity during carcinogenesis, and its overexpression, MUC4, which is typically expressed on apical surface, gets uniformly distributed on the plasma membrane and interacts with the growth factor receptors of EFGR family, particularly HER2 and HER3, which are normally expressed on the basolateral side on the normal epithelium. These interactions are mediated by the EGF-like domains present in MUC4. Carraway et al. demonstrated that of the two EGF-like domains present in rat Muc4/Asgp2/Sialomucin complex, interaction with ErbB2 was mediated by EGF1 domain [38]. Human MUC4 contains three EGF domains, and a glutathione S-transferase (GST) fusion construct containing EGF3–EGF1–EGF2 domains of MUC4 forms a stable heterodimer with HER2, while in the HER2 knockdown cells MUC4 can interact with HER3 [39,40]. These interactions subsequently activate various signaling cascades including MAPK, JNK, and STAT-1 that promote cell proliferation and migration in vitro and enhance tumorigenicity and metastasis in vivo (Figure 2) [8,9,12,18,39,40]. Additionally, MUC4 can modulate E-cadherin and N-cadherin signaling to promote contact-independent proliferation and drive epithelial-to-mesenchymal transition [9,41]. In addition, the large oligosaccharide side chains create steric hindrance and disrupt normal cell–cell and cell–matrix interactions by preventing ligand–receptor interaction, further suggesting the role of oligosaccharide side chains in contactindependent proliferation [42]. With these attributes, MUC4 regulates a multitude of cellular processes to promote the proliferation and metastasis of cancer cells in PDAC [43].
Figure 2.
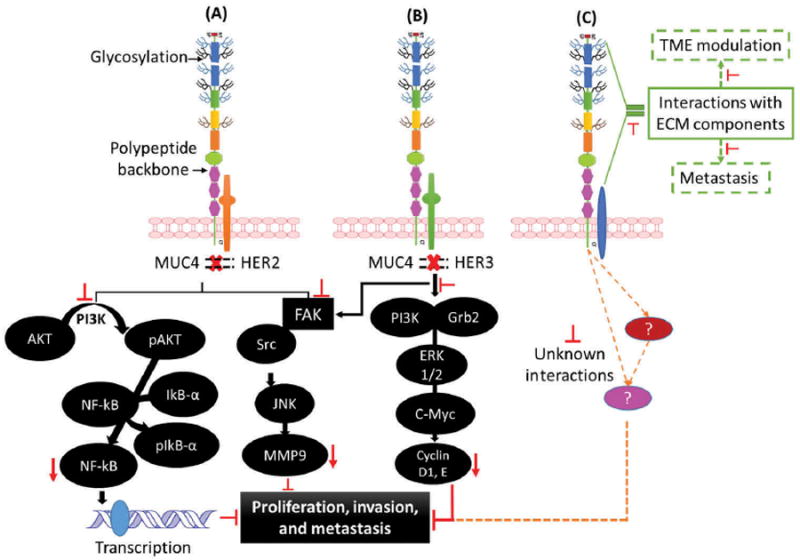
MUC4 signaling and interactions in PDAC: (A) Inhibition of MUC4-HER2 interaction or abrogation of MUC4 expression may result in the downregulation of AKT phosphorylation and lead to transcription down-regulation through NF-kB inhibition. (B) In HER2 low cells, inhibition of MUC4-HER3 interaction leads to downregulation of ERK and C-myc via PI3K inhibition, resulting in lower proliferation, invasion, and metastasis. Moreover, inhibition of MUC4-HER3 interaction leads to downregulation of FAK signaling ultimately inhibiting cell proliferation, invasion, and metastasis. (C) Extracellular MUC4 interaction with ECM also promotes metastasis; inhibition of these known and unknown interactions can abrogate metastasis. Red arrows indicate the inhibition of downstream targets which impact various cellular processes. Full color available online.
4.1. MUC4 modulates tumor microenvironment
Due to its large molecular size, MUC4 extends up to 2.0 μm from the cell surface and is thus uniquely endowed to interact with acellular and cellular components of the tumor microenvironment (TME) including extracellular matrix (ECM) proteins, immune cells, and vascular endothelium [4]. Ectopic expression of MUC4 in MiaPaCa2, a PDAC cell line with no endogenous MUC4 expression, resulted in increased invasiveness of the cells in vitro and metastasis in vivo, by enhancing their ability to disrupt the basement membrane (BM) barrier. This was due to the ability of the NIDO domain of MUC4 to interact with BM protein fibulin-2 and competitively abrogate its interaction with ECM proteins like entactin which is essential for BM integrity [19]. Similarly, silencing MUC4 in CD18/HPAF cells increased integrin-mediated interaction of cells with various ECM proteins including, LI-cadherin, CEACAM6, RAC1, thrombomodulin, epiregulin, and neuregulin or basement membrane components like laminin, fibronectin, and collagens I and IV [9]. Together, these studies suggest that MUC4 can facilitate and regulate the interactions of PDAC cells with the components of TME during tumor growth and metastasis.
MUC4 also modulates the interaction of PDAC cells with other cell types in the TME. Multiple studies have investigated the interaction of MUC4 with immune cells. Komatsu et al. demonstrated decreased killing of mammary adenocarcinoma cells overexpressing sialomucin complex (Rat MUC4 homologue) by lymphokine-activated killer (LAK) cells. This was attributed to the large molecular size of Muc4 that prevented the interaction of cancer cells with LAK cells by steric hindrance and resulted in masking of cancer-associated epitopes, thereby facilitating immune evasion [44]. In pancreatic cancer cells, MUC4 has been implicated in promoting the apoptosis of MUC4-specific cytotoxic T-lymphocytes (CTL), in a cell contact dependent and Fas ligand independent manner [20]. This is an interesting observation that may explain the suboptimal cytotoxic response of effector T cells, but in-depth investigation is required to further validate the role of MUC4 in mediating tumor cell-cytotoxic T-lymphocyte (CTL) interactions. In addition to infiltrating immunosuppressive and inflammatory immune cells [45], pancreatic tumors are characterized by low abundance of effector CD8+ T cells which in part, can be attributed to MUC4-mediated apoptosis. Therefore, MUC4 in PDAC might play a crucial role in establishing an immunosuppressive TME [45,46].
Mucins have been implicated in promoting the adhesion of cancer cells to endotheliumand facilitating extravasation via interactions ofmucin glycans with endothelial cell-surface proteins like E-selectin, P-selectin, and intracellular adhesionmolecule-1 (ICAM-1) [47]. MUC4 was shown to bind to circulating galectin-3: this interaction promoted MUC4 clustering and increased accessibility of surface adhesion molecules for endothelial cell interaction to facilitate metastasis [13]. Clinically, patients with metastatic disease have higher levels of serum galectin-3, which further supports the involvement of MUC4-galectin 3 interaction in promoting tumor cell dissemination in PDAC patients [13]. Another study found that the AMOP domain of MUC4/Y impacts the expression of VEGF A and ang-2, and its deletion significantly reduced the angiogenic and metastatic properties of PC cells in mice [48]. Thus, the AMOP domain functionally contributes to PDAC metastasis and is a potential target site for developing MUC4-based therapies to reduce metastasis in PDAC. Recently, Zhu et al. created a series of MUC4/Y constructs where NIDO, AMOP, and vWD domains were deleted either individually or simultaneously. While the deletion of each of these domains reduced the oncogenic activity of MUC4/Y in Panc1 cells, simultaneous deletion suggested that the three domains promote proliferation and metastasis in a synergistic manner, possibly by modulating MUC4/EGF–ERB2–ERB3 signaling and downstream activation of MAPK and other singling networks [49].
4.2. MUC4 mediates drug resistance
MUC4 has been shown to mediate resistance to gemcitabine treatment, which is a first-line therapy for the advanced PDAC [17]. MUC4 reduced cytochrome c release in a HER2-dependent manner in response to gemcitabine treatment, leading to inhibition of apoptosis. Furthermore, investigations by Skrypek et al. revealed that knockdown of MUC4 in PDAC cells lines enhanced their sensitivity to gemcitabine with elevation in pro-apoptotic marker Bax and decreased levels of anti-apoptotic marker BclXL. This was attributed to an increased expression of concentrative nucleoside transporter-1 (hCNT-1), which is one of the transporters involved in the uptake of gemcitabine in cancer cells. In PDAC patient tissue samples, MUC4 expression negatively correlate with hCNT-1 [15]. Chemoresistance in PDAC has also been attributed to high expression of drug efflux proteins like members of multidrug resistance proteins (MRPs) family members like MRP 5 and transporters involved in drug uptake in cancer cells like members of hCNT family [50]. Knockdown of MUC4 expression resulted in a decrease in cancer stem cells, which have inherently high levels of MRP proteins and exhibit poor sensitivity to gemcitabine [14]. Similarly, apicidin treatment epigenetically abrogated the expression of MUC4 and sensitized PDAC cells to gemcitabine [51]. Wissniowski et al. also demonstrated that silencing of MUC4 expression by siRNA enhanced the sensitivity of PDAC cells to gemcitabine; however, parental cells were found to be more sensitive to bortezomib, a proteasome inhibitor [16]. The clinical significance of MUC4 expression in response to therapy was recently demonstrated by Urey et al. [52], who found that in patients with resectable tumors and receiving gemcitabine, low MUC4 expression was associated with improved survival. Collectively, these studies have established the involvement of MUC4 in chemotherapy resistance suggesting its potential utility as a biomarker for treatment stratification and/or prognostication and make a case for targeting MUC4 in combination with standard chemotherapeutic regimens.
5. Molecular determinants of MUC4 expression in PDAC
Multiple pathways regulate MUC4 expression through the binding of various transcription factors at the MUC4 promoter [42,53]. There are four transcriptional initiation sites in the MUC4 promoter. The distal region contains three transcriptional start sites (-2603, -2604, and -2605) and putative binding sites for Sp1, GATA, GR, cAMP-responsive element binding protein, AP-1/-2/-4, and STAT transcription factors [53]. The presence of these binding sites on MUC4 promoter suggests cytokine-mediated regulation of MUC4 expression (Figure 3) [54]. Indeed, interferon-ɣ (IFN-ɣ) and retinoic acid (RA) synergistically upregulate the expression of MUC4 in PC cell lines through activation of STAT-1 and transforming growth factor beta-2 (TGFβ2) pathways, respectively [55,56]. Classically, TGFβ receptor activates signaling cascades involving Smad2 and Smad4, which cooperatively upregulate MUC4 expression; however, Smad3, Smad7, and c-Ski inhibit Smad4-mediated MUC4 upregulation [57]. TGFβ-mediated MUC4 upregulation is not strictly Smad4 dependent because in Smad4-deficient cells, TGFβ receptor activation resulted in activation of p42MAPK, PI3K, and PKA signaling pathways leading to MUC4 upregulation [57]. Similarly, attenuation of MUC4 upregulation by TGFβ-neutralizing antibody in response to RA treatment suggested the role of TGFβ2 as an interim mediator of MUC4 regulation [58]. In addition, interleukin (IL)-4/IL-9 and IL-6 also upregulate MUC4 expression through JAK/STAT pathway in a dose- and time-dependent manner [59,60]. MUC4 expression is induced by IL-6 and IL-24 treatment in gastric and colon cancer cells in a STAT3-dependent manner [61,62]; however, their effect on MUC4 expression has not been investigated in PDAC. Additionally, combined treatment with tumor necrosis factor-α (TNFα) and IFN-ɣ was shown to upregulate MUC4 expression through NF-κB and JAK/STAT pathways (Figure 3). Cumulatively, these data demonstrate that in addition to cytokines, RA and its receptors RA receptor alpha and RXRs are important regulators of MUC4 expression in PDAC cells [58].
Figure 3.
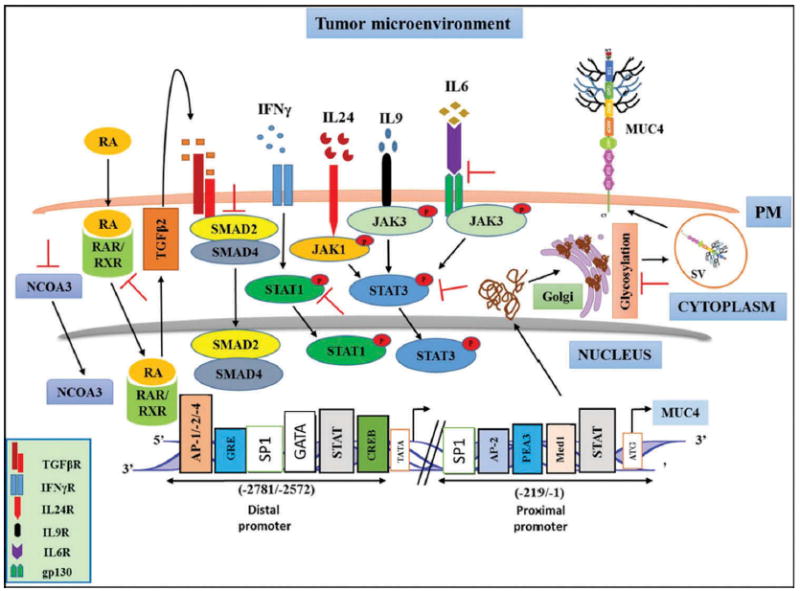
Targeting MUC4 regulatory elements in PDAC cells. In the tumor microenvironment, MUC4 regulation is under the control of different cytokines, ligands and receptors. Depending upon the cytokine milieu, various downstream signaling pathways are activated leading to MUC4 overexpression. Inhibiting (┬) these regulatory switches (receptor ligand interactions, intracellular signaling pathways, transcription factors, post-translational modifications) with natural products or pharmacological agents may abrogate MUC4-mediated processes and can be of therapeutic significance against PDAC.
Recent studies have provided new insights into the regulation of MUC4 expression in PDAC and suggest that MUC4 expression can be regulated by oncogenes (mutant Kras), epigenetic modifications, factors in the tumor microenvironment (bile acids and hypoxia), and signaling pathways (Wnt/β-catenin), at both transcriptional and posttranscriptional levels. As mentioned earlier, de novo MUC4 expression is associated with the earliest neoplastic lesions of the pancreas, and oncogenic mutation in K-ras is a well recognized initiating event in PDAC. Recently, Vasseur et al. demonstrated that oncogenic K-rasG12D mutation regulates MUC4 at both transcriptional and posttranscriptional levels. While transcriptional regulation of MUC4 was mediated via the activation of MAPK, JNK, and NF-κB signaling followed by downstream binding of AP-1 and NF-κB on MUC4 promoter, posttranscriptional regulation was mediated by RalB GTPase [63]. Mucin genes are also subjected to epigenetic regulation by DNA methylation and histone acetylation [64]. Epigenetic regulation of MUC4 in PDAC cell lines by histone acetylation was suggested by Jonckheere and coworkers [57], while the association between MUC4 expression and CpG methylation in the 5′-region of MUC4 gene was indicated by Yamada et al. [65]. Subsequently, using MUC4-expressing (Capan 1 and Capan 2) and nonexpressing (Panc1) PDAC cell lines, Vincent et al. demonstrated the involvement of DNA methyltransferases (DNMT3A and DNMT3B) and histone deacetylases (HDAC1 and HDAC3) in the epigenetic modification of the MUC4 promoter [66]. Recently, Yokoyama et al. demonstrated that MUC4 promoter is hypomethylated in PDAC patient samples and promoter hypomethylation correlated with MUC4 overexpression and poor prognosis [67]. We recently identified nuclear receptor co-activator 3 (NCOA3) as an important regulator of MUC4 expression in PDAC cells, which acts by modulating the accessibility of MUC4 promoter to transcription factors [68]. Furthermore, due to its ability to regulate enzymes involved in mucin glycosylation, NCOA3 also potentially regulates MUC4 and other mucins at the posttranslational level. Transcriptional regulation of MUC4 by canonical Wnt signaling in PDAC cells was also demonstrated recently. Three T-cell factor/lymphoid enhancer factor (TCF/LEF)-binding sites were identified on the MUC4 promoter and the binding of β-catenin/ TCF4 was demonstrated on MUC4 promoter [69]. Pancreatic ductal cells are also exposed to high levels of bile acids, particularly in cases of biliary obstruction. Bile acids were found to upregulate MUC4 in PDAC cell lines via activation of FAK-c-Jun signaling with possible upstream regulation by FXR. In patient tumors, MUC4 and FXR expression was positively correlated [70]. Hypoxia is another hallmark of pancreatic TME, and we recently observed that hypoxia negatively regulates MUC4 expression by promoting MUC4 degradation by autophagy pathway [71]. While other regulators like cytokines can compensate to upregulate MUC4 expression, these findings could also explain the intratumoral heterogeneity of MUC4 expression that is observed in pancreatic tumors. Together, these studies suggest that there are several layers of regulation of MUC4 in PDAC cells. Both cell-intrinsic factors like oncogenes and epigenetic modifications, and cell-extrinsic factors like hypoxia and bile acids, orchestrate MUC4 expression at transcript and protein levels. These factors on one hand provide multiple opportunities to target MUC4 expression for therapeutic intervention and on the other hand make it equally challenging due to the existence of possible compensatory mechanisms that can restore MUC4 expression.
6. MUC4-based therapeutic approaches in PDAC
Due to greater understanding of the molecular mechanisms contributing to deregulated overexpression of MUC4 in PDAC, and its functional involvement in the pathobiology, its promise as a therapeutic target is beginning to be realized. There are several approaches by which MUC4 can be exploited for the treatment of PDAC. Since MUC4 is aberrantly expressed in PDAC, the most straightforward approach to abrogate MUC4 function is to downregulate its expression. This can be achieved directly by gene silencing, i.e. targeting MUC4 transcripts with siRNA, shRNA, and miRNAs or indirectly by pharmacological inhibitors or natural products that can inhibit the signaling pathways involved in MUC4 upregulation. Therapeutic strategies can also be designed to elicit MUC4-specific antitumor immune response using vaccines or exploiting overexpression of MUC4 for targeting cytotoxic or chemotherapeutic agents with anti-MUC4 antibodies.
6.1. Gene-silencing approaches
6.1.1. RNA interference (RNAi)
One of the first insights into the role of MUC4 in the pathogenesis of PDAC came from our studies involving stable silencing of MUC4 expression using plasmid-encoding antisense-MUC4 RNA in PDAC cell lines [8]. A combination of in vitro and in vivo assays demonstrated that silencing of MUC4 impacted cell growth and motility and significantly reduced tumorigenicity and metastasis. These antitumor effects were, in part, attributed to reduced HER2 levels upon MUC4 silencing. MUC4 expression was also silenced transiently, using MUC4-targeted siRNA oligonucleotides [72], or stably, using a retroviral vector encoding MUC4-targeted shRNA [40], to validate the interaction of MUC4 with HER2 and determine its impact on HER2 stability. Retroviral vector-mediated knockdown of MUC4 expression was also used to demonstrate the involvement of MUC4 in gemcitabine resistance [15] and elucidate the metastasis-promoting molecular mechanisms mediated by MUC4 in PDAC cell lines [41]. Recently, antitumorigenic and antimetastatic effects of MUC4 silencing were also demonstrated using lentiviral vector encoding MUC4-targeted shRNA [73]. While these RNAi-based approaches have helped in establishing MUC4 as a valid target for therapeutic intervention, to date none of these approaches have been tested to downregulate MUC4 in vivo in established tumors. The development and acceptance of retroviral and lentiviral vectors for human use remains a challenge, while the clinical translation of synthetic oligonucleotide-based RNAi approaches is compromised by inefficient delivery [74,75]. We recently demonstrated that MUC4 expression can be indirectly downregulated by silencing NCOA3, one of the master regulators of MUC4 and other mucins [68] using retroviral shRNA vector. More importantly, our subsequent studies demonstrated the feasibility of downregulating MUC4 expression in established tumors by delivering NCOA3-directed siRNA oligonucleotides polyplexed with tumor-targeted polymeric CXCR4 antagonist [76]. A combination of CXCR4 inhibition and NCOA3 silencing in orthotopic tumors resulted in reduced tumor growth, decreased metastasis, and enhanced perfusion along with a marked decrease in the expression of MUC4 [76].
6.1.2. Micro RNAs (miRs)
Micro-RNAs (mi-RNAs or miRs) are naturally occurring, noncoding, small RNAs that function in gene regulation. The importance of mi-RNAs is underscored by high interspecies conservation, and the myriad of roles they play in the pathophysiology of multiple cancers [77]. By base pairing with partially complementary mRNAs, these mi-RNAs either inhibit protein translation or promote the degradation of the mRNA. Due to alterations in their expression patterns during various stages of carcinogenesis, and the ability of a single miRNA to modulate the expression of multiple targets, miRs have emerged as a novel class of biomarkers and therapeutic targets for various malignancies including PDAC.
Several studies have demonstrated that the expression of various mucins under physiological and pathological conditions can be subjected to regulation by miRs. Several miRs capable of regulating MUC4 expression have been identified in PDAC (Table 1). We recently characterized alterations in miRs during the development and progression of PDAC in genetically engineered mouse model and validated these findings using human PDAC cell lines and tissue samples [78]. In silico analysis indicated that miR-Let-7b, one of the most consistently downregulated miRs in murine model, human cell lines, and tissue samples, potentially targets KRas oncogene, NCOA3, and MUC4. In fact, restoration of Let-7b expression using lentiviral vector resulted in the downregulation of MUC4 and Kras in human PDAC cell lines [78]. miR-150 is another MUC4-regulating miR that was one of the top differentially downregulated miRs, as observed in different PDAC studies [78,79]. Srivastava et al. first described the presence of a highly conserved miR-150-binding site in the 3′-UTR of MUC4 [80] (Table 1). Transfection of PDAC cell line with miR-150 mimic resulted in the downregulation of MUC4 expression along with decrease in HER2 and its downstream signaling. In vitro assays indicated that restoration of miR-150 results in the suppression of malignant behavior of the cells. Using lentiviral vectors for stable restoration of miR-150 by overexpression or abrogation of its expression with siRNA, Yang et al. demonstrated that in addition to MUC4, miR-150 regulates c-Myb expression [79]. Importantly, restoration of miR-150 in PDAC cell lines resulted in decreased tumor growth, while knockdown of miR-150 promoted tumorigenicity in vivo [79] along with reciprocal alterations in MUC4 levels in the tumors. Similarly, miR-200c was demonstrated to target MUC4 and MUC16 exon sequences which consequently decreased the mRNA and protein expression of both of these mucins in PDAC cell lines [81] (Table 1). Although the inhibitory role of miR-200c in PDAC growth and metastasis is more relevant therapeutically due to its ability to simultaneously target two mucins implicated in PDAC pathobiology, the functional consequences of MUC4 and MUC16 with miR-200c remain to be determined. Using bioinformatics approaches, Lahdaoui et al. identified two binding sites for a unique miR-219-1-3p in the MUC4 3′-UTR [82]. miR-219-1-3p is downregulated in human PDAC tissues and cell lines, and restoration of its expression in PDAC cell lines resulted in the downregulation of MUC4 protein and decreased cell proliferation, survival, and migration in vitro (Table 1). Importantly, intratumoral injection of miR-219-1-3p expressing vectors in the subcutaneous xenograft tumors resulted in reduction in tumor volume and decreased MUC4 expression in the tumors [82].
Table 1.
Effect of different natural products, miRs, and pharmacological agents on MUC4 expression, pancreatic cancer progression, and metastasis
| Agent | Effect on MUC4 | Pathway | Observed effects on PDAC cells | Reference |
|---|---|---|---|---|
| Guggulsterone (4,17(20)-pregnadiene-3,16-dione | Downregulation | JAK/STAT and FAK/Src/MM9 pathway inhibition | Reduced proliferation and invasion | Macha et al. 2013 |
| Thymoquinone (2-isopropyl-5-methylbenzo-1,4-quinone) | Downregulation | Synergistic to gemcitabine and oxaliplatin | Enhanced apoptosis, reduced proliferation and metastasis | Torres et al. 2010; Banerjee et al. 2009; Wu et al. 2011 |
| Graviola extract (Annona muricata) | Downregulation | Not known | Antiproliferative and antimetastatic | Torres et al. 2012 |
| miR-150 | Direct-targeting MUC4 leading to inhibition | Reduces pEGFR and HER-2 downstream signaling | Antiproliferative and antimetastatic | Yang et al. 2014; Srivastava et al. 2011; Arora et al. 2014 |
| miR-200c | Downregulate MUC4 along with MUC16 | Not known | Reduced cell proliferation | Radhakrishnan et al. 2013 |
| miR-219-1-3p | Downregulation | Cyclin D1/ERK/AKT downregulation | Decrease of cyclin D1 expression and decreased Erk and Akt ativation | Lahdaoui et al. 2015 |
| Canertinib | Downregulation | Through EGFR and HER2 | Antiproliferative and antimetastatic | Seshacharyulu et al. 2015 |
| Afatinib | Downregulation |
Although several miRs have been demonstrated to target MUC4 and abrogate MUC4-mediated cellular processes, limited studies have tested their ability to target MUC4 in vivo in established tumors. Delivery of the miRs to the tumor represents a significant challenge to the implementation of miRbased therapies. The small size of miR-mimetics results in their rapid clearance from the body and thus mandates the development of efficient delivery or vector systems to target DNA sequences encoding miRs to tumor site. Recently, Arora et al. developed a poly(lactic-co-glycolic acid) (PLGA)-based nanoparticle formulation to encapsulate miR-150 and demonstrated its utility to downregulate MUC4 and HER2 in vitro [83]. Although the miR-, and RNAi-based approaches to silence MUC4 expression have shown promise, for clinical translation, the significant first step would be to test these approaches in immunocompetent animal models that express human MUC4 in normal physiological context.
6.2. Pharmacological agents
Corticosteroids appear to modulate MUC4 expression in a context-dependent manner. Dexamethasone was one of the first pharmacological agents shown to modulate MUC4 expression. In epithelial Ishikawa cells, dexamethasone treatment resulted in upregulation of MUC4 transcripts [84]. In contrast, in the corneal epithelial cells [85], cultured nasal polyps, and nasal polyp-derived epithelial cells [86], dexamethasone treatment was found to inhibit MUC4 expression. However, treatment of patients with nasal polyposis with oral (prednisone) or intranasal (budesonide) corticosteroids resulted in the upregulation of MUC1 and MUC4, and downregulation of MUC5AC in polyps [87]. In yet another study, dexamethasone was found to have no effect on MUC4 expression in nasal mucosa [88]. Dexamethasone is often given as an antiemetic agent during surgery in PDAC patients, and its ability to modulate MUC4 expression can be of potential utility. However, given the variable effects of corticosteroids on MUC4 in other systems, in the context of PDAC, it will be of interest to systematically evaluate the impact of corticosteroids on MUC4 expression. In addition to corticosteroids, we also evaluated the impact of two pan-EGFR family inhibitors canertinib and afatinib on MUC4 expression in PDAC cells [18]. Although both inhibitors decreased MUC4 levels, only the irreversible inhibitor canertinib targeted EGFR–STAT1 signaling to downregulate MUC4. Consequently, canertinib treatment resulted in decreased tumor growth and reduced MUC4 expression in the tumors.
6.3. Natural products
Due to their ability to target signaling pathways involved in inflammation, several natural products have been demonstrated to modulate mucin expression [89]. In PDAC models, several natural compounds have been shown to exhibit therapeutic effects that are putatively mediated by downregulation of MUC4 [89]. Guggulsterone (GS) [(4,17(20)-pregnadiene-3,16-dione], a plant polyphenol derived from Commiphora mukul, has been traditionally used as a remedy for obesity, hypertension, and atherosclerosis. GS was shown to decrease the proliferation and metastatic behavior of PDAC cells [90] (Table 1). Importantly, these effects were shown to be associated with decreased activation of the JAK/STAT pathways and subsequent downregulation of MUC4. These results suggest that GS may be an effective means of targeting MUC4 in PDAC. Additionally, concomitant use of GS with gemcitabine increased the sensitivity of PDAC cells to chemotherapy [91]. Thymoquinone (TQ), a compound derived from Nigella sativa, has also been demonstrated to exhibit antineoplastic properties against PDAC cell lines. In CD18/HPAF and FG/ COLO357 cells, treatment with TQ resulted in decreased expression of MUC4 via increased proteasomal degradation [92,93] (Table 1). This downregulation of MUC4 resulted in increased cancer cell apoptosis and decreased migration. These effects were mediated by the activation of p38 and c-Jun-(NH2)-terminal kinase. Transient knockdown of MUC4 expression was sufficient to activate these pathways, thereby directly implicating MUC4 as a critical target of TQ [93]. Consistent with its mechanism of increased MUC4 degradation, subsequent studies demonstrated that TQ reduces metastasis and sensitizes PDAC cells to conventional chemotherapeutics in vitro and in vivo, suggesting a role for TQ in multidrug regimens for the treatment of PDAC [92,94,95]. Annona muricata, commonly known as graviola, has been used for the treatment of various inflammatory conditions like, rheumatism, neuralgia, diabetes, hypertension, and insomnia. We recently evaluated the effect of graviola extract on PDAC cell lines in vitro and in vivo. Graviola extract inhibited cellular metabolism to induce necrosis by downregulating genes associated with hypoxia and glycolysis [96]. More importantly, graviola extract exhibited antitumor effects in vitro and in vivo accompanied by MUC4 downregulation [96] (Table 1).
6.4. MUC4-based vaccine design and immunotherapy
There has been an interest in developing immunotherapybased approaches for the treatment of a wide range of malignancies. In PDAC, the development of immunotherapies is in its infancy due to poorly defined antigenic targets. Only recently, the neoantigen landscape of PDAC was defined [97]. The criteria for the selection of antigens for immunotherapy include: (1) tumor-specific expression as neoantigen; (2) expression in sufficient amount; and (3) high immunogenicity to elicit a strong immune response. Given these criteria, MUC4 appears to be a promising antigen for immunotherapy of PDAC. While MUC4 is expressed in various normal tissues and overexpressed in PDAC, its expression is undetectable in normal pancreas, both, in humans and in murine models [4-6,98]. Further, due to aberrant glycosylation and splicing, MUC4 expressed on cancer cells is likely to be distinct from that expressed on normal tissues [34,37]. Thus, MUC4 can be exploited as a tumor-associated antigen (TAA) for immunotherapeutic approaches. Overexpression and aberrant glycosylation of MUC4 appears to elicit MUC4-specific immune response as suggested by the presence of MUC4-specific autoantibodies in patients with colorectal cancer, and elevated levels of both Th1 and Th2 cytokines in MUC4 expressing pancreatic tumors [56,99]. Presentation of TAA-derived peptides to T-lymphocytes by antigen presenting cells (APCs) is the first and the most critical step in eliciting an effective antitumor immune response. Human Leukocyte Antigen (HLA)-restricted MUC4 peptide epitopes identified in silico were evaluated for their ability to elicit CTL response. Adenoviral vectors encoding HLA-A1- and HLA-A2 restricted MUC4 epitopes in conjunction with a universal T-helper epitope were used to transduce dendritic cells (DC). The transduced DC exhibited efficient antigen presentation and elicited potent CTL response [100], which indicates that MUC4-based epitopes can be used to develop DC-based vaccines. Furthermore, Chen et al. demonstrated that a tumor vaccine based on DCs transfected with TAA mRNA elicited strong CTL response in vitro in PDAC. When both MUC4 and survivin mRNA were transfected into DCs, the elicited CTL response was greater than that elicited by either mRNA alone [101]. These results suggest that MUC4 alone or in combination with other immuno-dominant antigens can be used to design vaccines for PDAC. In another study, sub-nanometer sized particles displaying MUC4 glycopeptides along with Thomsen Friedenreich (TF) antigen and C3d, a complement proteinderived peptide adjuvant system was used which elicited a potent immune response, suggesting that nanoparticle-based delivery might be a viable method for vaccine delivery [102]. Overall, MUC4 is an effective antigen to develop vaccines against PDAC and should be optimized with respect to better adjuvant systems and antitumor CTL responses. Evaluation of MUC4 for the immunotherapy of PDAC and other cancers is hindered due to the lack of appropriate murine models. Efforts are underway to develop murine models that recapitulate the normal and tumor-specific MUC4 expression, which can be used to investigate MUC4-specific antitumor response at the primary and metastatic site and evaluate the ability of the MUC4 vaccines to overcome the barrier of self-tolerance.
In addition to vaccines, antibodies are promising agents both for the diagnosis and therapy of several cancers including PDAC [103,104]. Considering the selective expression, tumor-specific alterations, and antigenicity of MUC4 in PDAC, antibodies against MUC4 can be exploited as potential therapeutic agents. In addition to stimulating anti-PDAC immune response, MUC4-specific antibodies can be utilized to image the tumor and selectively deliver therapeutic radionuclides and cytotoxic agents to the tumor. We have generated a panel of MUC4-specific antibodies targeting different regions of the protein including tandem repeat domain (mAb 8G7), non-tandem repeat N-terminal region (mAb 2214) [105,106], and beta domain (mAb 6E8)(unpublished). MAb 8G7 has shown high specificity for human MUC4 in immunohistochemical analysis, immunoblotting, and fluorescence assays and is widely used for deciphering various aspects of MUC4 biology and examining its potential as a biomarker [105-107]. We are presently evaluating the utility of multiple MUC4-specific antibodies for imaging, drug delivery, and as MUC4 function-neutralizing or antibody-mediated cellular cytotoxicity (ADCC)-inducing therapeutic agents against PDAC.
7. Expert opinion
Due to its differential expression and functional involvement in pathogenesis, MUC4 has emerged as a promising diagnostic and therapeutic target. From early PanINs to PDAC, MUC4 expression gradually increases and contributes to progression and metastasis of PDAC by modulating critical signaling pathways, cell–cell interactions, and cell–matrix interactions. Overall, MUC4-mediated alterations augment the proliferative and metastatic capacity of PDAC cells and imply that MUC4 is a critical molecule in PDAC pathobiology. This central role of MUC4 in PDAC development and progression has led to considerable interest in MUC4 as a therapeutic target.
A series of in vitro and in vivo studies have validated the suitability of MUC4 as a therapeutic target for PDAC. Among several approaches tested, pharmacological inhibitors, natural products, and several miRs have emerged as viable options for MUC4-targeted PDAC therapies. In vitro and/or in vivo studies have clearly demonstrated the potential of these agents for targeting MUC4 at preclinical level with significant antitumor effects. Unfortunately, translating MUC4 as a therapeutic target in clinical settings is still a challenge. MUC4-regulating signaling pathways that have been targeted by pharmacological agents like afatinib also regulate other cellular processes, and caution should be exercised while attributing the antitumor effects of such agents solely to MUC4 downregulation. Furthermore, with such agents, stringent analysis of off-target effects and nontarget toxicity should be carefully considered in the study design. Lack of efficient delivery systems capable of delivering MUC4-targeting miRs to primary and disseminated PDAC is a significant barrier to the translation of miR-based therapeutic approaches. Another challenge in miR-based therapeutics is the existence of several splice variants of MUC4 [37]. Although the existence of most splice forms of MUC4 has not been established at protein levels, presence of a spectrum of transcripts from these splice variants can reduce the efficacy of miRbased therapies. Alternatively, pathologically significant splice variants like MUC4/Y can escape miRs designed to target fulllength MUC4. Clinical development will further entail profiling of splice variants and polymorphisms individually in patients to design personalized MUC4-targeting miRs.
The structural and functional diversity of various MUC4 domains, and the presence of various glycoforms due to aberrant glycosylation in cancer, add further complexity in designing therapeutic strategies seeking to inhibit MUC4 function or exploit it as a target for immunotherapy. While the roles of some MUC4 domains have been determined, these studies have been conducted using truncated MUC4 constructs. Even the cleavage of MUC4 into two subunits has not been conclusively demonstrated. Recent availability of novel domain-specific antibodies developed by others and us should help address these issues and aid in determining what proportion of MUC4 remains associated with cell surface. The multiplicity of epitopes in the tandem repeats region of MUC4 offers a promising avenue for targeted therapies. Antibodies or peptides generated against these epitopes can be tagged with cytotoxic agents or radionuclides for treatment or imaging purposes, respectively. Availability of high affinity antibodies capable of detecting cell-surface-associated MUC4 can provide opportunities for delivering cytotoxic cargoes to PDAC cells in a MUC4-specific manner, while neutralizing antibodies can be used to inhibit MUC4 mediated mechanisms that promote proliferation and metastasis. Given the involvement of MUC4 in chemoresistance, it will be of utmost interest to evaluate MUC4-targeting antibodies and other agents in conjunction with chemotherapeutic agents.
Although MUC4 is one of the most differentially overexpressed membrane bound mucins in PDAC, studies exploring MUC4 as a target for immunotherapy are still in very early stages. Thus far, a few ‘cherry-picked’ immunodominant epitopes of MUC4 have been demonstrated to elicit specific CTL response using in vitro and ex vivo approaches. However, MUC4-based immunotherapy requires optimization of adjuvant systems, identification of helper epitopes, and development of delivery systems. Antigenicity profiling of MUC4 using bioinformatics approaches needs to be performed to identify immunodominant T- and B-cell epitopes, and MUC4-associated neoantigens in the context of altered glycosylation and splicing. It also remains to be determined if MUC4 contributes to immunosuppressive tumor microenvironment. It will be of interest to examine the correlation of MUC4 expression with the immune-phenotype of resected tumors. Another significant step towards the development of MUC4-based immunotherapy will be to characterize the quality and magnitude of anti-MUC4 immune response in PDAC patients to gauge the impact of anti-MUC4 immune response on prognosis. A major hurdle in the development of MUC4-based targeted and immunotherapeutic approaches is the unavailability of human-MUC4 transgenic mice (hMUC4-tg mice), syngeneic MUC4 expressing cell lines, and therapeutic grade anti-MUC4 antibodies. Development of these models and reagents is mandatory to evaluate MUC4-targeted approaches in a clinically translatable manner. MUC4-based immunotherapy can potentially be more effective in combination with other immunotherapeutic approaches like checkpoint blockade agents, cytokine administration, and depletion of suppressor immune cells from the TME.
Despite these challenges, MUC4 is a promising candidate for the development of targeted PDAC therapy and efforts need to be directed towards the development of effective MUC4-targeting agents and suitable animalmodels to evaluateMUC4-based therapies. These efforts should be guided by emerging technologies like CRISPR-based gene editing and silencing; high throughput screening of libraries containing natural products and synthetic compounds; development of screening assays and models for evaluating MUC4 functions; antibody engineering for humanization and development of CAR-T cells; bioinformatics approaches for predicting MUC4 immunogenicity and identifying interacting partners; and, development of genetically engineered murine models expressing human MUC4 in autochthonous pancreatic tumors. More importantly, MUC4-based therapeutics should be evaluated in combination with existing regimens involving chemotherapy and radiation therapy. Successful development of MUC4-based therapeutics can have positive impact on the the management of patients with PDAC and other malignancies where MUC4 functionally contributes to pathogenesis.
Highlights.
MUC4 is one of the most differentially overexpressed membranebound mucins in pancreatic cancer and functionally contributes to tumor growth, metastasis, chemoresistance, and poor survival.
Well-characterized mechanisms of MUC4 deregulation, its altered glycosylation, existence of multiple splice variants, and multiplicity of TR epitopes make it an attractive target for therapy of pancreatic cancer.
Inhibition of MUC4 expression with miRNAs, siRNAs, pharmacological agents and natural products in pancreatic cancer cells reduces their proliferative and invasive properties in vitro and in vivo and restores sensitivity to chemotherapeutic agents.
Several challenges need to be overcome to develop MUC4-based targeted therapies, and overcoming those impediments will require development of novel reagents, models and delivery platforms.
Understanding the interplay of MUC4 with the immune system and its role in establishing an immunosuppressive tumor microenvironment, characterization of MUC4-specific immune responses in pancreatic cancer patients, and development of human MUC4-expressing transgenic models are critical for realizing the true potential of MUC4 as a target for immunotherapy.
Acknowledgments
Funding
S. Batra receives funding from grants R01 CA183459, RO1 GM113166, SPORE P50 CA127297 and EDRN U01 CA200466 from the National Institutes of Health. M. Jain receives funding from grant R01 CA195586 from the National Institutes of Health.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;(67):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moniaux N, Escande F, Porchet N, et al. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- 4.Kaur S, Kumar S, Momi N, et al. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 6.Balagué C, Audié J-P, Porchet N, et al. In situ hybridization shows distinct patterns of mucin gene expression in normal, benign, and malignant pancreas tissues. Gastroenterology. 1995;109:953–964. doi: 10.1016/0016-5085(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 7.Hollingsworth MA, Strawhecker JM, Caffrey TC, et al. Expression of MUC1, MUC2, MUC3 and MUC4 mucin mrnas in human pancreatic and intestinal tumor cell lines. Int J Cancer. 1994;57:198–203. doi: 10.1002/ijc.2910570212. [DOI] [PubMed] [Google Scholar]
- 8.Singh AP, Moniaux N, Chauhan SC, et al. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. •• First report that demonstrated the functional contribution of human MUC4 in tumor cell growth and metastasis in vitro and in vivo. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 10.Swartz MJ, Batra SK, Varshney GC, et al. MUC4 Expression Increases Progressively in Pancreatic Intraepithelial Neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 11.Bafna S, Singh AP, Moniaux N, et al. MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 2008;68:9231–9238. doi: 10.1158/0008-5472.CAN-08-3135. • First experimental evidence suggesting that MUC4 can act as an oncogene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moniaux N, Chaturvedi P, Varshney GC, et al. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97:345–357. doi: 10.1038/sj.bjc.6603868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senapati S, Chaturvedi P, Chaney WG, et al. Novel interaction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin Cancer Res. 2011;17:267–274. doi: 10.1158/1078-0432.CCR-10-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mimeault M, Johansson SL, Senapati S, et al. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skrypek N, Duchene B, Hebbar M, et al. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32:1714–1723. doi: 10.1038/onc.2012.179. •• This report unraveled one of the mechanisms involved in MUC4-mediated chemoresistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wissniowski TT, Meister S, Hahn EG, et al. Mucin production determines sensitivity to bortezomib and gemcitabine in pancreatic cancer cells. Int J Oncol. 2012;40:1581–1589. doi: 10.3892/ijo.2012.1337. [DOI] [PubMed] [Google Scholar]
- 17.Bafna S, Kaur S, Momi N, et al. Pancreatic cancer cells resistance to gemcitabine: the role ofMUC4mucin. Br J Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. • This study provided the experimental evidence supporting the involvement of MUC4 in gemcitabine resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshacharyulu P, Ponnusamy MP, Rachagani S, et al. Targeting EGFreceptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6:5164–5181. doi: 10.18632/oncotarget.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senapati S, Gnanapragassam VS, Moniaux N, et al. Role of MUC4-NIDO domain in the MUC4-mediated metastasis of pancreatic cancer cells. Oncogene. 2012;31:3346–3356. doi: 10.1038/onc.2011.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Zhang JJ, Liang WB, et al. Pancreatic cancer counterattack: MUC4 mediates Fas-independent apoptosis of antigen-specific cytotoxic T lymphocyte. Oncol Rep. 2014;31:1768–1776. doi: 10.3892/or.2014.3016. [DOI] [PubMed] [Google Scholar]
- 21.Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. Journal of Clinical Pathology. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moniaux N, Nollet S, Porchet N, et al. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338(Pt 2):325–333. [PMC free article] [PubMed] [Google Scholar]
- 23.Moniaux N, Escande F, Batra SK, et al. Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur J Biochem. 2000;267:4536–4544. doi: 10.1046/j.1432-1327.2000.01504.x. [DOI] [PubMed] [Google Scholar]
- 24.Buisine MP, Devisme L, Copin MC, et al. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol. 1999;20:209–218. doi: 10.1165/ajrcmb.20.2.3259. [DOI] [PubMed] [Google Scholar]
- 25.Buisine MP, Devisme L, Degand P, et al. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000;48:1667–1676. doi: 10.1177/002215540004801210. [DOI] [PubMed] [Google Scholar]
- 26.Reid CJ, Harris A. Developmental expression of mucin genes in the human gastrointestinal system. Gut. 1998;42:220–226. doi: 10.1136/gut.42.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillem P, Billeret V, Buisine MP, et al. Mucin gene expression and cell differentiation in human normal, premalignant and malignant esophagus. Int J Cancer. 2000;88:856–861. doi: 10.1002/1097-0215(20001215)88:6<856::aid-ijc3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Offner GD, Nunes DP, et al. MUC4 is a major component of salivary mucin MG1 secreted by the human submandibular gland. Biochem Biophys Res Commun. 1998;250:757–761. doi: 10.1006/bbrc.1998.9390. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Perez A, Yasin M, et al. Presence of MUC4 in human milk and at the luminal surfaces of blood vessels. J Cell Physiol. 2005;204:166–177. doi: 10.1002/jcp.20277. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Begne M, Lu B, Han X, et al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonezawa S, Higashi M, Yamada N, et al. Precursor Lesions of Pancreatic Cancer. Gut and Liver. 2008;2:137–154. doi: 10.5009/gnl.2008.2.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jhala N, Jhala D, Vickers SM, et al. Biomarkers in Diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 33.Ansari D, Urey C, Gundewar C, et al. Comparison of MUC4 expression in primary pancreatic cancer and paired lymph node metastases. Scand J Gastroenterol. 2013;48:1183–1187. doi: 10.3109/00365521.2013.832368. [DOI] [PubMed] [Google Scholar]
- 34.Chugh S, Gnanapragassam VS, Jain M, et al. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim Biophys Acta. 2015;1856:211–225. doi: 10.1016/j.bbcan.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudhury A, Moniaux N, Winpenny JP, et al. Human MUC4 mucin cDNA and its variants in pancreatic carcinoma. J Biochem. 2000;128:233–243. doi: 10.1093/oxfordjournals.jbchem.a022746. [DOI] [PubMed] [Google Scholar]
- 36.Choudhury A, Moniaux N, Ringel J, et al. Alternate splicing at the 3′-end of the human pancreatic tumor-associated mucin MUC4 cDNA. Teratog Carcinog Mutagen. 2001;21:83–96. doi: 10.1002/1520-6866(2001)21:1<83::aid-tcm8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Cruz E, Joshi S, et al. Genetic Variants of Mucins: unexplored Conundrum. Carcinogenesis. 2016 doi: 10.1093/carcin/bgw120. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carraway KL, 3rd, Rossi EA, Komatsu M, et al. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 39.Lakshmanan I, Seshacharyulu P, Haridas D, et al. Novel HER3/MUC4 oncogenic signaling aggravates the tumorigenic phenotypes of pancreatic cancer cells. Oncotarget. 2015;6:21085–21099. doi: 10.18632/oncotarget.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonckheere N, Skrypek N, Merlin J, et al. The mucin MUC4 and its membrane partner ErbB2 regulate biological properties of human CAPAN-2 pancreatic cancer cells via different signalling pathways. Plos ONE. 2012;7:e32232. doi: 10.1371/journal.pone.0032232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rachagani S, Macha MA, Ponnusamy MP, et al. MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis. 2012;33:1953–1964. doi: 10.1093/carcin/bgs225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh AP, Chauhan SC, Andrianifahanana M, et al. MUC4 expression is regulated by cystic fibrosis transmembrane conductance regulator in pancreatic adenocarcinoma cells via transcriptional and posttranslational mechanisms. Oncogene. 2007;26:30–41. doi: 10.1038/sj.onc.1209764. [DOI] [PubMed] [Google Scholar]
- 43.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- 45.Basso D, Gnatta E, Plenani M. Pancreatic cancer fostered immunosuppression privileges tumor growth and progression. J Clin Cell Immunol. 2014;5:278–293. [Google Scholar]
- 46.Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World J Gastroenterol. 2014;20:11160–11181. doi: 10.3748/wjg.v20.i32.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geng Y, Marshall JR, King MR. Glycomechanics of the metastatic cascade: tumor cell-endothelial cell interactions in the circulation. Ann Biomed Eng. 2012;40:790–805. doi: 10.1007/s10439-011-0463-6. [DOI] [PubMed] [Google Scholar]
- 48.Tang J, Zhu Y, Xie K, et al. The role of the AMOP domain in MUC4/Y-promoted tumour angiogenesis and metastasis in pancreatic cancer. J Exp Clin Cancer Res. 2016;35:91. doi: 10.1186/s13046-016-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Zhang JJ, Peng YP, et al. NIDO, AMOP and vWD domains of MUC4 play synergic role in MUC4 mediated signaling. Oncotarget. 2017;8:10385–10399. doi: 10.18632/oncotarget.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagmann W, Faissner R, Schnolzer M, et al. Membrane Drug Transporters and Chemoresistance in Human Pancreatic Carcinoma. Cancers. 2011;3:106–125. doi: 10.3390/cancers3010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ansari D, Urey C, Hilmersson KS, et al. Apicidin sensitizes pancreatic cancer cells to gemcitabine by epigenetically regulating MUC4 expression. Anticancer Res. 2014;34:5269–5276. [PubMed] [Google Scholar]
- 52.Urey C, Andersson B, Ansari D, et al. Low MUC4 expression is associated with survival benefit in patients with resectable pancreatic cancer receiving adjuvant gemcitabine. Scand J Gastroenterol. 2017;52:595–600. doi: 10.1080/00365521.2017.1290134. •• This report provided clear evidence using clinical samples that low MUC4 expression correlates with better response in PDAC patients receiving gemcitabine. [DOI] [PubMed] [Google Scholar]
- 53.Perrais M, Pigny P, Ducourouble MP, et al. Characterization of human mucin gene MUC4 promoter: importance of growth factors and proinflammatory cytokines for its regulation in pancreatic cancer cells. J Biol Chem. 2001;276:30923–30933. doi: 10.1074/jbc.M104204200. •• This study characterized MUC4 promoter and demonstrated the presence of binding sites for various transcription factors involved in growth factor and cytokine signaling. [DOI] [PubMed] [Google Scholar]
- 54.Chapela PJ, Broaddus RR, Hawkins SM, et al. Cytokine stimulation of MUC4 expression in human female reproductive tissue carcinoma cell lines and endometrial cancer. J Cell Biochem. 2015;116:2649–2657. doi: 10.1002/jcb.25213. [DOI] [PubMed] [Google Scholar]
- 55.Andrianifahanana M, Agrawal A, Singh AP, et al. Synergistic induction of the MUC4 mucin gene by interferon-gamma and retinoic acid in human pancreatic tumour cells involves a reprogramming of signalling pathways. Oncogene. 2005;24:6143–6154. doi: 10.1038/sj.onc.1208756. [DOI] [PubMed] [Google Scholar]
- 56.Andrianifahanana M, Chauhan SC, Choudhury A, et al. MUC4-expressing pancreatic adenocarcinomas show elevated levels of both T1 and T2 cytokines: potential pathobiologic implications. Am J Gastroenterol. 2006;101:2319–2329. doi: 10.1111/j.1572-0241.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 57.Jonckheere N, Perrais M, Mariette C, et al. A role for human MUC4 mucin gene, the ErbB2 ligand, as a target of TGF-beta in pancreatic carcinogenesis. Oncogene. 2004;23:5729–5738. doi: 10.1038/sj.onc.1207769. [DOI] [PubMed] [Google Scholar]
- 58.Choudhury A, Singh RK, Moniaux N, et al. Retinoic acid-dependent transforming growth factor-beta 2-mediated induction of MUC4 mucin expression in human pancreatic tumor cells follows retinoic acid receptor-alpha signaling pathway. J Biol Chem. 2000;275:33929–33936. doi: 10.1074/jbc.M005115200. [DOI] [PubMed] [Google Scholar]
- 59.Damera G, Xia B, Ancha HR, et al. IL-9 modulated MUC4 gene and glycoprotein expression in airway epithelial cells. Biosci Rep. 2006;26:55–67. doi: 10.1007/s10540-006-9000-5. [DOI] [PubMed] [Google Scholar]
- 60.Damera G, Xia B, Sachdev GP. IL-4 induced MUC4 enhancement in respiratory epithelial cells in vitro is mediated through JAK-3 selective signaling. Respir Res. 2006;7:39. doi: 10.1186/1465-9921-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mejias-Luque R, Peiro S, Vincent A, et al. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochim Biophys Acta. 2008;1783:1728–1736. doi: 10.1016/j.bbamcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Andoh A, Shioya M, Nishida A, et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol. 2009;183:687–695. doi: 10.4049/jimmunol.0804169. [DOI] [PubMed] [Google Scholar]
- 63.Vasseur R, Skrypek N, Duchêne B, et al. The mucin MUC4 is a transcriptional and post-transcriptional target of K-ras oncogene in pancreatic cancer. Implication of MAPK/AP-1, NF-κB and RalB signaling pathways. Biochimica Et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2015;1849:1375–1384. doi: 10.1016/j.bbagrm.2015.10.014. •• This study comprehensively described the regulation of MUC4 by oncogenic kRas in pancreatic cancer cells. [DOI] [PubMed] [Google Scholar]
- 64.Yamada N, Kitamoto S, Yokoyama S, et al. Epigenetic regulation of mucin genes in human cancers. Clinical Epigenetics. 2011;2:85–96. doi: 10.1007/s13148-011-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamada N, Nishida Y, Tsutsumida H, et al. Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. British Journal of Cancer. 2009;100:344–351. doi: 10.1038/sj.bjc.6604845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent A, Ducourouble M-P, Van Seuningen I. Epigenetic regulation of the human mucin gene MUC4 in epithelial cancer cell lines involves both DNA methylation and histone modifications mediated by DNA methyltransferases and histone deacetylases. The FASEB Journal. 2008;22:3035–3045. doi: 10.1096/fj.07-103390. •• This study demonstrated that MUC4 expression in pancreatic cancer cells is deregulated as a consequence of epigenetic modifications of MUC4 promoter by DNA methylation and histone acetylation. [DOI] [PubMed] [Google Scholar]
- 67.Yokoyama S, Higashi M, Kitamoto S, et al. Aberrant methylation of MUC1 and MUC4 promoters are potential prognostic biomarkers for pancreatic ductal adenocarcinomas. Oncotarget. 2016;7:42553–42565. doi: 10.18632/oncotarget.9924. •• This study provided clinical validation of findings of Ref no. 66 and demonstrated that promoter hypomethylation correlates with MUC4 expression and poor prognosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar S, Das S, Rachagani S, et al. NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer. Oncogene. 2015;34:4879–4889. doi: 10.1038/onc.2014.409. •• This report explains why despite the presence of cytokines in both pancreatitis and pancreatic cancer, MUC4 is only upregulated specifically in pancreatic cancer. The MUC4 promoter accessibility is regulated by NCOA3 and it is upregulated only in pancreatic cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pai P, Rachagani S, Lakshmanan I, et al. The canonical Wnt pathway regulates the metastasis-promoting mucin MUC4 in pancreatic ductal adenocarcinoma. Molecular Oncology. 2016;10:224–239. doi: 10.1016/j.molonc.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joshi S, Cruz E, Rachagani S, et al. Bile acids-mediated overexpression of MUC4 via FAK-dependent c-Jun activation in pancreatic cancer. Molecular Oncology. 2016;10:1063–1077. doi: 10.1016/j.molonc.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joshi S, Kumar S, Ponnusamy MP, et al. Hypoxia-induced oxidative stress promotes MUC4 degradation via autophagy to enhance pancreatic cancer cells survival. Oncogene. 2016;35:5882–5892. doi: 10.1038/onc.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaturvedi P, Singh AP, Chakraborty S, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Wu C, Chen T, et al. Effects of RNAi-mediated MUC4 gene silencing on the proliferation and migration of human pancreatic carcinoma BxPC-3 cells. Oncol Rep. 2016;36:3449–3455. doi: 10.3892/or.2016.5152. [DOI] [PubMed] [Google Scholar]
- 74.Sepp-Lorenzino L, Ruddy MK. Challenges and opportunities for local and systemic delivery of siRNA and antisense oligonucleotides. Clin Pharmacol Ther. 2008;84:628–632. doi: 10.1038/clpt.2008.174. [DOI] [PubMed] [Google Scholar]
- 75.Watts JK, Corey DR. Gene silencing by siRNAs and antisense oligonucleotides in the laboratory and the clinic. The Journal of Pathology. 2012;226:365–379. doi: 10.1002/path.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Kumar S, Rachagani S, et al. Polyplex-mediated inhibition of chemokine receptor CXCR4 and chromatin-remodeling enzyme NCOA3 impedes pancreatic cancer progression and metastasis. Biomaterials. 2016;101:108–120. doi: 10.1016/j.biomaterials.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacFarlane L-A, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Current Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rachagani S, Macha MA, Menning MS, et al. Changes in microRNA (miRNA) expression during pancreatic cancer development and progression in a genetically engineered KrasG12D;Pdx1-Cre mouse (KC) model. Oncotarget. 2015;6:40295–40309. doi: 10.18632/oncotarget.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang K, He M, Cai Z, et al. A decrease in miR-150 regulates the malignancy of pancreatic cancer by targeting c-Myb and MUC4. Pancreas. 2015;44:370–379. doi: 10.1097/MPA.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 80.Srivastava SK, Bhardwaj A, Singh S, et al. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. • This well-designed study provided a clear evidence of a miRNA directly targeting MUC4 in pancreatic cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radhakrishnan P, Mohr AM, Grandgenett PM, et al. MicroRNA-200c modulates the expression of MUC4 and MUC16 by directly targeting their coding sequences in human pancreatic cancer. Plos One. 2013;8:e73356. doi: 10.1371/journal.pone.0073356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lahdaoui F, Delpu Y, Vincent A, et al. miR-219-1-3p is a negative regulator of the mucin MUC4 expression and is a tumor suppressor in pancreatic cancer. Oncogene. 2015;34:780–788. doi: 10.1038/onc.2014.11. [DOI] [PubMed] [Google Scholar]
- 83.Arora S, Swaminathan SK, Kirtane A, et al. Synthesis, characterization, and evaluation of poly (D,L-lactide-co-glycolide)-based nanoformulation of miRNA-150: potential implications for pancreatic cancer therapy. Int J Nanomedicine. 2014;9:2933–2942. doi: 10.2147/IJN.S61949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gollub EG, Waksman H, Goswami S, et al. Mucin genes are regulated by estrogen and dexamethasone. Biochem Biophys Res Commun. 1995;217:1006–1014. doi: 10.1006/bbrc.1995.2870. [DOI] [PubMed] [Google Scholar]
- 85.Seo KY, Chung SH, Lee JH, et al. Regulation of membrane-associated mucins in the human corneal epithelial cells by dexamethasone. Cornea. 2007;26:709–714. doi: 10.1097/ICO.0b013e31804f5a09. [DOI] [PubMed] [Google Scholar]
- 86.Bai CH, Song SY, Kim YD. Effect of glucocorticoid on the MUC4 gene in nasal polyps. Laryngoscope. 2007;117:2169–2173. doi: 10.1097/MLG.0b013e31813e5fef. [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Anton A, De Bolos C, Alobid I, et al. Corticosteroid therapy increases membrane-tethered while decreases secreted mucin expression in nasal polyps. Allergy. 2008;63:1368–1376. doi: 10.1111/j.1398-9995.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- 88.Woo HJ, Bae CH, Song SY, et al. Expression of membrane-bound mucins in human nasal mucosa: different patterns for MUC4 and MUC16. Arch Otolaryngol Head Neck Surg. 2010;136:603–609. doi: 10.1001/archoto.2010.71. [DOI] [PubMed] [Google Scholar]
- 89.Macha MA, Krishn SR, Jahan R, et al. Emerging potential of natural products for targeting mucins for therapy against inflammation and cancer. Cancer Treat Rev. 2015;41:277–288. doi: 10.1016/j.ctrv.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Macha MA, Rachagani S, Gupta S, et al. Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett. 2013;341:166–177. doi: 10.1016/j.canlet.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahn DW, Seo JK, Lee SH, et al. Enhanced antitumor effect of combination therapy with gemcitabine and guggulsterone in pancreatic cancer. Pancreas. 2012;41:1048–1057. doi: 10.1097/MPA.0b013e318249d62e. [DOI] [PubMed] [Google Scholar]
- 92.Woo CC, Kumar AP, Sethi G, et al. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 93.Torres MP, Ponnusamy MP, Chakraborty S, et al. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9:1419–1431. doi: 10.1158/1535-7163.MCT-10-0075. • A study demonstrating the utility of a natural product targeting MUC4 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu ZH, Chen Z, Shen Y, et al. Anti-metastasis effect of thymoquinone on human pancreatic cancer. Yao Xue Xue Bao. 2011;46:910–914. [PubMed] [Google Scholar]
- 95.Banerjee S, Azmi AS, Padhye S, et al. Structure-activity studies on therapeutic potential of Thymoquinone analogs in pancreatic cancer. Pharm Res. 2010;27:1146–1158. doi: 10.1007/s11095-010-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torres MP, Rachagani S, Purohit V, et al. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29–40. doi: 10.1016/j.canlet.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bailey P, Chang DK, Forget MA, et al. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma. Sci Rep. 2016;6:35848. doi: 10.1038/srep35848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rachagani S, Torres MP, Kumar S, et al. Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: potential implications for diagnosis and therapy. J Hematol Oncol. 2012;5:68. doi: 10.1186/1756-8722-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pedersen JW, Gentry-Maharaj A, Nøstdal A, et al. Cancer-associated autoantibodies to MUC1 and MUC4-a blinded case–control study of colorectal cancer in UK collaborative trial of ovarian cancer screening. Int J Cancer. 2014;134:2180–2188. doi: 10.1002/ijc.28538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei J, Gao W, Wu J, et al. Dendritic cells expressing a combined PADRE/MUC4-derived polyepitope DNA vaccine induce multiple cytotoxic T-cell responses. Cancer Biother Radiopharm. 2008;23:121–128. doi: 10.1089/cbr.2007.0427. • This study along with Ref. 101, provided evidence that MUC4-based vaccines can generate CTL response in vitro. [DOI] [PubMed] [Google Scholar]
- 101.Chen J, Guo XZ, Li HY, et al. Generation of CTL responses against pancreatic cancer in vitro using dendritic cells co-transfected with MUC4 and survivin RNA. Vaccine. 2013;31:4585–4590. doi: 10.1016/j.vaccine.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 102.Brinas RP, Sundgren A, Sahoo P, et al. Design and synthesis of multifunctional gold nanoparticles bearing tumor-associated glycopeptide antigens as potential cancer vaccines. Bioconjug Chem. 2012;23:1513–1523. doi: 10.1021/bc200606s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chames P, Kerfelec B, Baty D. Therapeutic antibodies for the treatment of pancreatic cancer. Scientific World J. 2010;10:1107–1120. doi: 10.1100/tsw.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang ZQ, Buchsbaum DJ. Monoclonal antibodies in the treatment of pancreatic cancer. Immunotherapy. 2009;1:223–229. doi: 10.2217/1750743X.1.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moniaux N, Varshney GC, Chauhan SC, et al. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 106.Jain M, Venkatraman G, Moniaux N, et al. Monoclonal antibodies recognizing the non-tandem repeat regions of the human mucin MUC4 in pancreatic cancer. Plos One. 2011;6:e23344. doi: 10.1371/journal.pone.0023344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang G, Lipert RJ, Jain M, et al. Detection of the potential pancreatic cancer marker MUC4 in serum using surface-enhanced Raman scattering. Anal Chem. 2011;83:2554–2561. doi: 10.1021/ac102829b. [DOI] [PMC free article] [PubMed] [Google Scholar]


