Abstract
Objectives
Atrioventricular conduction prolongation (AVCP) in cardiac pacing is measurable and results primarily from delayed atrial conduction. Noninvasive methods for measuring atrial conduction are lacking. Accordingly, AVCP was used to estimate atrial conduction and investigate its role on the paced atrioventricular delay (pAVD) during biventricular pacing (BiVP) optimization.
Design
Retrospective analysis of data collected as part of a randomized controlled study of temporary BiVP after cardiopulmonary bypass.
Setting
Single-center study at university-affiliated tertiary care hospital.
Participants
Cardiac surgical patients at risk of left ventricular failure after cardiopulmonary bypass.
Interventions
Temporary BiVP was optimized immediately after cardiopulmonary bypass. Vasoactive medication and fluid infusion rates were held constant during optimization.
Measurements and Main Results
For each patient the AVCP and the pAVD producing the optimum (highest) cardiac output (OptCO) and mean arterial pressure (OptMAP) were determined. Patients were stratified into long- and short-AVCP groups. Overall AVCP (mean ± standard deviation) was 64 ± 28 ms. For the short-AVCP group (<64 ms, n = 3), AVCP, OptCO, and OptMAP were 40 ± 11, 120 ± 0, and 150 ± 30 ms, respectively, and for the long-AVCP group (>64 ms, n = 4), these same parameters were 89 ± 10, 218 ± 44, and 218 ± 29 ms. OptCO and OptMAP were significantly less in the short-AVCP group (p = 0.015 and p = 0.029, respectively).
Conclusions
AVCP varies widely after cardiopulmonary bypass, affecting optimum pAVD. Failure to correct for this can result in the selection of inappropriately short and potentially deleterious pAVDs, especially when nominal pAVD is used, causing BiVP to appear ineffective.
Keywords: cardiac resynchronization therapy, electrophysiology, biventricular pacing, hemodynamics, perioperative management, atrioventricular delay
BASED ON POSITIVE DATA from numerous clinical trials,1–5 biventricular pacing (BiVP) currently is indicated as an adjunctive treatment for a subset of patients with medically refractory advanced heart failure.6,7 The observation that increases in cardiac output (CO), arterial pressure, stroke volume, and systolic function are seen as soon as the 1st beat augmented by BiVP8 has sparked interest in the use of BiVP for the treatment of acute heart failure as occurs in patients undergoing cardiac surgery.9–20 The effects of optimized temporary BiVP in patients undergoing cardiac surgery are currently being investigated by the authors’ lab as part of a randomized clinical trial (Biventricular Pacing After Cardiac Surgery [BiPACS] trial). The present report is a substudy analysis of the BiPACS trial.
Optimization of the interval between atrial and ventricular stimulation (paced atrioventricular delay [pAVD]) increases the hemodynamic benefits of chronic BiVP.21–24 Preliminary data from the BiPACS trial have demonstrated a similar benefit for temporary postoperative BiVP.25 Individualized optimization is crucial, because the optimal pAVD differs from patient to patient.26 Atrioventricular conduction prolongation (AVCP) is an often overlooked component of pAVD. AVCP results mostly from slower intra- and interatrial conduction and thus delayed atrial depolarization, with an artificially paced impulse compared with a naturally occurring impulse originating in the sinoatrial node27–29 (Fig 1). This primarily reflects tissue properties but also is affected by atrial lead location and decremental atrioventricular nodal conduction, with increased heart rate during atrial overdrive pacing. Because it is difficult to measure, AVCP frequently is ignored or assigned a nominal value, usually about 30 ms, in commercially available BiVP devices.30
Fig 1.

Schematic representation of electrocardiographic tracings in the dual-sensed, dual-paced (DDD; top) and ventricular-paced, dual-sensed mode (VDI; bottom). Atrioventricular conduction prolongation (AVCP), the difference between optimal paced atrioventricular delay (pAVD) and the optimal sensed atrioventricular delay (sAVD), occurs because of a longer delay to atrial depolarization (Ad) after an artificially generated atrial pulse (Ap) compared with atrial depolarization originating in the sinoatrial node. Vp, ventricular pace.
To investigate the appropriateness of a nominal AVCP, the authors developed a rapid assessment method using pacemaker timing intervals. They hypothesized that AVCP varies widely in BiPACS patients because of differences in preexisting atrial conduction properties and the extensive surgical manipulation of atrial tissue inherent to cardiac surgery. Because of these factors, the authors hypothesized that AVCP frequently is longer than nominal values. Furthermore, they postulated that variation in AVCP would account for some of the variability of optimal pAVD. They examined the hypothesis that short pAVDs in the presence of long AVCP can affect hemodynamics negatively.
METHODS
This study is a substudy analysis of the BiPACS trial, a randomized clinical trial investigating the effect of optimized temporary BiVP on CO in patients undergoing cardiac surgery. This study complied with the Declaration of Helsinki and was approved by the institutional review board. An investigational device exemption for temporary postoperative use of an InSync III 8042 permanent biventricular pacemaker (Medtronic, Inc, Minneapolis, MN) was obtained from the Food and Drug Administration. With approval from the attending surgeon, patients scheduled to undergo surgery on cardiopulmonary bypass (CPB) with preoperative ejection fraction ≤40% and QRS duration ≥100 ms or undergoing concomitant aortic and mitral valve procedures (repair or replacement), irrespective of their ejection fraction and QRS duration, were approached for enrollment. All patients gave informed consent for participation in the study. Patients were excluded for intracardiac shunts, reoperation, congenital heart disease, 2nd- or 3rd-degree heart block, atrial fibrillation, or postcardiopulmonary bypass heart rate >120 beats/min.
The anesthetic technique used for all patients was a general endotracheal anesthetic. After induction with midazolam, fentanyl, propofol or etomidate, and paralysis with vecuronium or rocuronium, anesthesia was maintained with isoflurane at a minimal alveolar concentration of 0.4 to 0.6. Fentanyl and midazolam were used as adjunct agents to attenuate autonomic responses. Inotropes, vasopressors, or vasodilators were used based on real-time transesophageal echocardiographic and hemodynamic monitoring.
Before separation from CPB, temporary bipolar epicardial pacing leads (Medtronic, Inc) were sewn to the right ventricle and the posterior-basal or lateral basal left ventricle. These sites were chosen because they are most likely to be efficacious based on prior clinical data. Paired temporary unipolar epicardial leads were sewn on, or near, the right atrial appendage and used for bipolar atrial pacing. All leads were attached by sterile extension cables to appropriate terminals on a custom-housed InSync III pacemaker under programmer control (CareLink 2090, Medtronic, Inc). Proper lead functions were confirmed, and outputs and sensitivities were adjusted. A carefully sized, scissor-type electromagnetic flow probe (Cliniflow II, Carolina Medical Electronics, East Bend, NC) was placed around the ascending aorta and connected to a flowmeter (Model 701 D, Carolina Medical Electronics). Aortic flow from the flowmeter, lead II from the surface electrocardiogram, and arterial pressure from a radial arterial catheter were sampled by an analog-to-digital converter (PowerLab, ADInstruments, Milford, MA) and recorded on a digital computer (iMac, Apple Computer, Cupertino, CA). A sample tracing from a representative patient is shown in Fig 2.
Fig 2.

Tracing depicting lead II of the surface electrocardiogram (top), arterial pressure (middle), and aortic flow (bottom) from a representative patient in the study. The dashed line indicates a change from optimized biventricular pacing mode to no pacing.
After separation from CPB, protamine administration, and decannulation, rates of administration of anesthetic agents, blood products, fluids, and continuous infusion medications were stabilized. No changes to administration rates for any of these were permitted during the optimization protocol.
Before optimization, the interval (as displayed on the programmer screen) between atrial and ventricular sensing with the pacemaker set to dual-sensed (ODO) mode was measured. Similarly, the interval between delivery of the atrial pacing impulse and subsequent ventricular sensing with the pacemaker set to atrial-paced dual-sensed (ADI) mode was noted. In post hoc analyses, AVCP was calculated as the difference between these 2 measurements (Fig 3). Before this substudy, these measurements were not performed routinely as part of the BiPACS study and thus were not available for many previously studied patients. Only patients for whom the 2 measurements were available were included in this substudy analysis.
Fig 3.
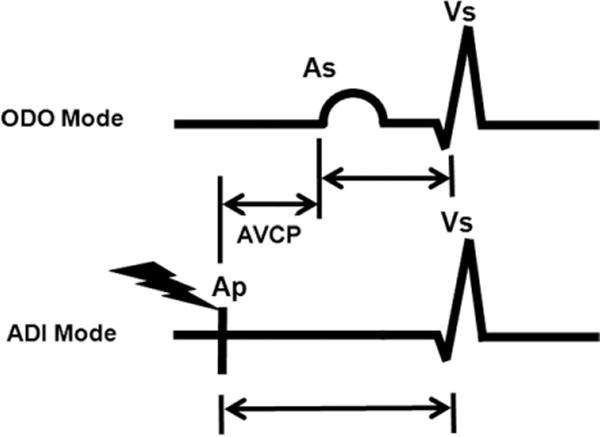
Schematic representation of electrocardiographic tracings in dual-sensed (ODO; top) and atrial paced, dual-sensed (ADI; bottom) modes as displayed on the pacemaker programmer screen. Atrial paced (Ap), atrial sensed (As), and ventricular sensed (Vs) markers are shown. Atrioventricular conduction prolongation (AVCP) is calculated as the difference between the Ap-to-Vs interval and the As-to-Vs interval.
Optimization of the pAVD was carried out in dual-paced dual-sensed (DDDOV) mode, with simultaneous biventricular pacing, at a heart rate of 90 beats/min or 10 beats/min above the patient’s intrinsic heart rate (≤120 beats/min). The pAVD was changed randomly across 7 settings (90, 120, 150, 180, 210, 240, and 270 ms), with each setting tested for 10 seconds. All settings then were retested in a new random order. Settings with pAVD exceeding the intrinsic delay between atrial pacing and the naturally conducted ventricular depolarization were excluded from analysis, because these would have resulted in a lack of ventricular pacing. The total time for pAVD optimization without exclusion of any settings was 2 minutes 20 seconds. This time decreased by 20 seconds for each excluded setting because each setting was tested in duplicate for 10 seconds each time.
In post hoc analysis, the CO and mean arterial pressure (MAP) for each beat were calculated using custom Matlab (MathWorks, Inc, Natick, MA) routines. Briefly, respiratory cycles were defined by minimal MAP and the CO and MAP for each setting were obtained by averaging all beats in 1 full respiratory cycle within each setting tested. To allow for stabilization of hemodynamics after programming changes, beats toward the end of each testing interval were used. Because each setting was tested 2 times, 2 CO and 2 MAP values were obtained for each pAVD. These were averaged together to obtain 1 CO and 1 MAP value per tested pAVD. The averaged values then were normalized by expressing them as a percentage of the mean CO, or MAP, for all the tested pAVDs in that patient. Normalization allowed for comparison of CO and MAP among patients with different baseline CO and MAP values. Curves of normalized CO (Fig 4) and MAP (Fig 5) as a function of pAVD were created for each patient. The pAVDs resulting in the optimum (highest) CO (OptCO) and MAP (OptMAP) then were determined.
Fig 4.

Plots of cardiac output (CO) as a function of paced atrioventricular delay (pAVD) for the short (left) and long (right) atrioventricular conduction prolongation (AVCP) groups. In each patient, the CO has been normalized to the mean CO of all pAVDs for that patient. The pAVDs exceeding the intrinsic atrioventricular conduction were not tested (ie, 240 and 270 ms were not tested in patient number 2 because the time from delivery of an atrial pace impulse to the resultant naturally occurring ventricular depolarization was 210 to 240 ms). A decrease in CO is observed at shorter pAVDs for patients in the long-latency group, whereas short pAVDs result in optimal CO for patients in the short-latency group.
Fig 5.
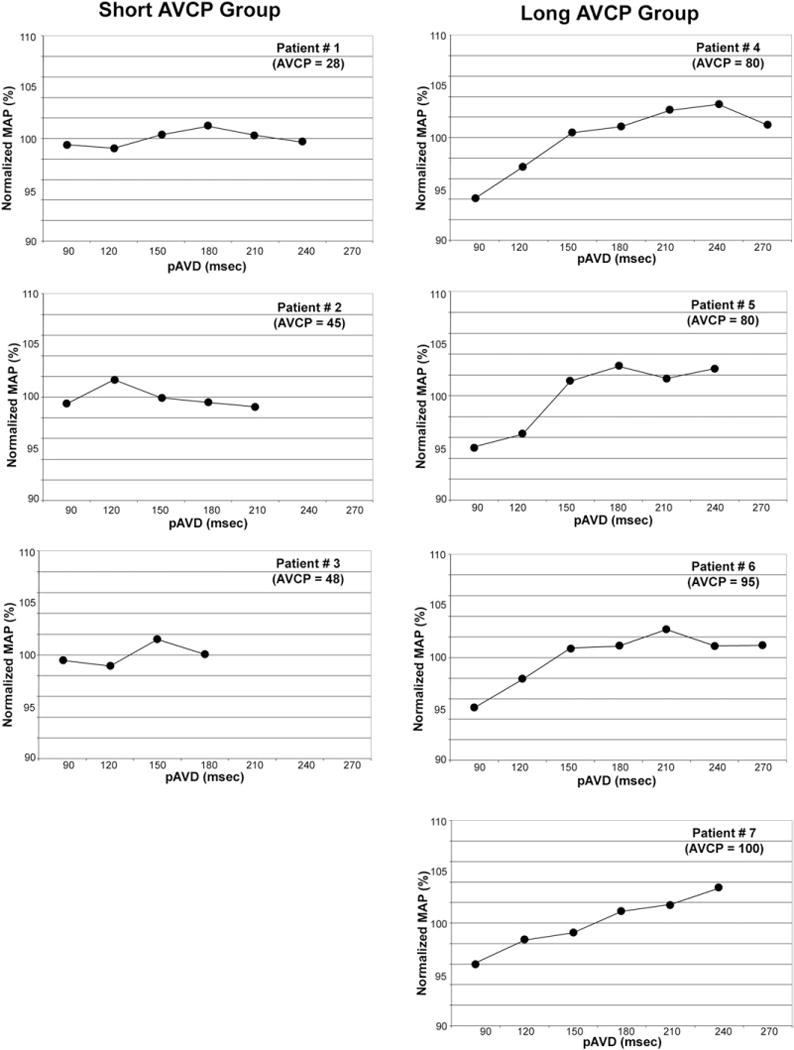
Plots of mean arterial pressure (MAP) as a function of paced atrioventricular delay (pAVD) for the short (left) and long (right) atrioventricular conduction prolongation (AVCP) groups. In each patient, the MAP has been normalized to the mean MAP of all pAVDs for that patient. The pAVDs exceeding the intrinsic atrioventricular conduction were not tested (ie, 240 and 270 ms were not tested in patient number 2 because the time from delivery of an atrial pace impulse to the resultant naturally occurring ventricular depolarization was 210 to 240 ms). A decrease in MAP is observed at shorter pAVDs for patients in the long-latency group, whereas short pAVDs result in optimal MAP for patients in the short-latency group.
The associations between AVCP and the OptCO and OptMAP were investigated through linear regression models. The OptCO and Opt-MAP were compared between short-AVCP (<50th percentile of study group) and long-AVCP groups using 1-way analysis of variance models. The analysis was performed in SAS 9.2 (SAS Institute, Cary, NC). A p value <0.05 was considered statistically significant. All continuous variables were summarized as mean ± standard deviation.
RESULTS
Of the 42 studied patients, data required for this analysis (measured AV intervals in ODO and ADI modes) were available in 7 patients. Patients’ average age was 62 ± 13 years (range 49–83 years). Four were men. Left ventricular ejection fraction averaged 31 ± 13% (range 20–57%). The average QRS duration was 115 ± 22 ms (range 94–150 ms). Two patients underwent combined aortic and mitral valve replacement or repair, 3 underwent coronary artery bypass grafting, 1 underwent combined coronary artery bypass grafting and aortic valve replacement, and 1 underwent coronary artery bypass grafting and mitral valve repair.
The mean AVCP was 64 ± 28 ms (range 28–100 ms). There were positive correlations for OptCO versus AVCP (R2 = 0.77) and OptMAP versus AVCP (R2 = 0.53; Fig 6).
Fig 6.

Scatterplots relating atrioventricular conduction prolongation (AVCP) to optimum paced atrioventricular delay determined using cardiac output (OptCO; left) and mean arterial pressure (OptMAP; right). Linear trends have been superimposed.
Patients were segregated into short- and long-AVCP groups, which were differentiated by the mean AVCP (64 ms). For the short-AVCP group (n = 3), the mean AVCP was 40 ± 11 ms; for the long-AVCP group (n = 4), it was 89 ± 10 ms. The mean OptCO and OptMAP values were 120 ± 0 and 150 ± 30 ms, respectively, for the short-AVCP group compared with 218 ± 44 and 218 ± 29 ms, respectively, for the long-AVCP group. The OptCO (p = 0.015) and OptMAP (p = 0.029) were significantly lower for the short-AVCP group compared with the long-AVCP group (Fig 7).
Fig 7.
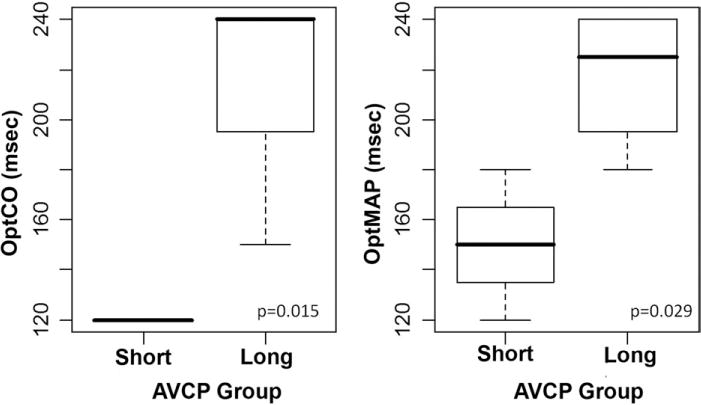
Boxplots depicting the distribution of optimal paced atrioventricular delays obtained by cardiac output measurement (OptCO; left) and mean arterial pressure measurement (OptMAP; right) for the short and long atrioventricular conduction prolongation (AVCP) groups.
DISCUSSION
The utility of BiVP for the management of acute left ventricular failure in patients undergoing cardiac surgery is an active area of investigation. Preliminary results from clinical trials have indicated that BiVP improves hemodynamics in this patient population.9–11,13,14,17 Interim analyses of data from the BiPACS trial have highlighted the importance of perioperative optimization of pacing parameters. Of the pacing parameters studied, optimization of the pAVD was found to yield the greatest hemodynamic benefits.25
Experience with AV pacing optimization during permanent endocardial pacemaker insertion has shown that, in a given patient, the optimum programmed AV delay in atrial-sensed mode is shorter than the optimum delay in atrial-paced mode (pAVD; Fig 3).27–29 Artificially generated impulses from a pacing lead first must propagate by cell-to-cell conduction before reaching the interatrial (ie, Bachmann bundle) and intra-atrial conduction tracts, whereas naturally occurring impulses from the sinoatrial node are conducted directly by these tracts. Factors that affect atrial conduction, such as conduction velocity, electrolyte balances, atrial scarring and size, type of surgery performed, and lead location, will affect AVCP. Furthermore, because AV-nodal conduction time lengthens at higher heart rates, this also will increase the AVCP during atrial pacing. Described methods for estimating AVCP have relied on invasive monitoring of left atrial electrical activity using a sensing electrode placed in the coronary sinus31,32 or in the esophagus.33,34 Intervals to left atrial sensing after right atrial pacing and after an intrinsic right atrial depolarization are measured, and the difference between the 2 intervals is used to define interatrial conduction delay. These techniques fail to account for the AV-nodal prolongation component of AVCP seen at faster heart rates. AVCP was measured using intervals to right ventricular sensing as opposed to left atrial sensing. Because right ventricular electrodes are a necessary component of BiVP systems, this method for AVCP measurement requires no extra instrumentation, expertise, equipment, or time.
Not only is AVCP rarely measured, but pAVD optimization often is neglected because of concerns about added time or lack of appropriate equipment or expertise. Commercially recommended nominal pAVDs are 120 to 150 ms. In contrast, the optimal pAVD in the BiPACS trial has averaged 171 ms.25 Nominal settings assume AVCP is the same in all patients. The nominal pace-sense offset correction for AVCP is 30 ms for most implantable BiVP devices.31 Assumed values can markedly underestimate the true AVCP.26,27,30,33 In the present analysis, AVCP ranged from 28 to 100 ms, with an average of 64. All but 1 patient had an AVCP >45 ms. The assumption of a short AVCP and use of a short default pAVD can be detrimental, because continued pacing at a short pAVD in patients with a long AVCP worsens hemodynamics owing to an inappropriately short delay between atrial and ventricular contractions.35 Selection of a suboptimal pAVD for continued pacing, therefore, also may be a factor in the failure to improve hemodynamics in some patients. Some of the reported “nonresponders” to BiVP might be converted to responders if their AVCP values were measured and the pAVD adjusted accordingly.36
Protocols for pAVD optimization rely on the measurement of hemodynamic indices during pAVD variation. Optimization across a narrow range of pAVD risks missing the optimum value, whereas optimization across a wide range is time-consuming. The speed and efficiency of optimization protocols were especially important in the BiPACS trial, in which concerns of operative and anesthesia time were present. The present data demonstrate a positive correlation between the optimal pAVD and AVCP, with which patients with a long AVCP tend to benefit from a longer pAVD. The present data also demonstrate the negative impact on CO and MAP of a short pAVD in patients with a long AVCP. Determination of AVCP before optimization thus aids in the selection of an appropriate optimization range. It is prudent to shift this range toward a longer pAVD in patients with long AVCP. Because the upper limit of the testable range is limited by the patient’s intrinsic AV conduction, shifting the range shortens the testing protocol by decreasing the number of pAVDs tested. This increases the speed and efficiency of pAVD optimization and avoids settings that are detrimental to hemodynamics.
As AVCP increases, the range of usable pAVD decreases because of the upper limit imposed by the intrinsic AV conduction time. Atrio-biventricular pacing is not possible when the pAVD is greater than or equal to the time from atrial pacing to the resultant naturally conducted ventricular depolarization. Only atrial sensed modes can be used in such cases. Although some factors affecting AVCP (atrial scarring, size, and conduction velocity) are unalterable, the effects of atrial lead location on AVCP require further study. AVCP then might prove useful in optimizing the atrial pacing site.37,38
The present study was limited by a small number of patients (measurements required to calculate AVCP were implemented only recently) and its retrospective design. Prospective, preoptimization determination of AVCP would be preferable. The small number of patients also made it difficult to analyze patients undergoing valvular procedures separately from those undergoing coronary artery bypass procedures; as more patients are recruited, it would be interesting to look for differences in AVCP between these 2 groups. BiPACS data for pAVD optimization at 3 time points over the 1st postoperative day suggest that the optimal pacing protocol changes over time. AVCP may change as the atria and conduction system recover from the effects of ischemia and reperfusion. Further studies are needed to measure temporal changes in AVCP and pAVD optimization.
The present results demonstrate that AVCP varies widely after CPB, affecting optimum pAVD. Failure to correct for this can result in the selection of an inappropriately short and potentially deleterious pAVD. Future progress will determine whether the present study of temporary epicardial pacing in a selected population after cardiopulmonary bypass translates directly to optimization of permanent BiVP with endocardial leads. However, the present data demonstrate that AVCP can be estimated using commercial programmers and that this AVCP can define the optimal range for pAVD optimization and decrease pAVD optimization time. AVCP measurement also may prove helpful with optimization of an atrial pacing site. These general principles are applicable regardless of the setting and details of BiVP implementation.
Acknowledgments
The authors thank the patients for their participation in the BiPACS trial and the members of the Division of Cardiothoracic Surgery at New York Presbyterian Hospital–Columbia University Medical Center for their continued support.
This work was supported by the National Institutes of Health (grant 5T32GM008464-17 to A.R., T32 HL007854 to D.Y.W., and RO1 HL080152 to H.M.S.).
References
- 1.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 2.St John Sutton MG, Plappert T, Abraham WT, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 7.Strickberger SA, Conti J, Daoud EG, et al. Patient selection for cardiac resynchronization therapy: From the Council on Clinical Cardiology Subcommittee on Electrocardiography and Arrhythmias and the Quality of Care and Outcomes Research Interdisciplinary Working Group, in collaboration with the Heart Rhythm Society. Circulation. 2005;111:2146–2150. doi: 10.1161/01.CIR.0000161276.09685.4A. [DOI] [PubMed] [Google Scholar]
- 8.Nelson GS, Berger RD, Fetics BJ, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–3059. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 9.Weisse U, Isgro F, Werling Ch, et al. Impact of atrio-biventricular pacing to poor left ventricular function after CABG. J Thorac Cardiovasc Surg. 2002;50:131–135. doi: 10.1055/s-2002-32403. [DOI] [PubMed] [Google Scholar]
- 10.Berberian G, Quinn TA, Kanter JP, et al. Optimized biventricular pacing in atrioventricular block after cardiac surgery. Ann Thorac Surg. 2005;80:870–875. doi: 10.1016/j.athoracsur.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 11.Dzemali O, Bakhtiary F, Dogan S, et al. Perioperative biventricular pacing leads to improvement of hemodynamics in patients with reduced left-ventricular function—Interim results. Pacing Clin Electrophysiol. 2006;29:1341–1345. doi: 10.1111/j.1540-8159.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt C, Frielingsdorf J, Debrunner M, et al. Acute biventricular pacing after cardiac surgery has no influence on regional and global left ventricular systolic function. Europace. 2007;9:432–436. doi: 10.1093/europace/eum042. [DOI] [PubMed] [Google Scholar]
- 13.Pichlmaier M, Bagaev E, Lichtenberg A, et al. Four-chamber pacing in patients with poor ejection fraction but normal QRS durations undergoing open heart surgery. Pacing Clin Electrophysiol. 2008;31:184–189. doi: 10.1111/j.1540-8159.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 14.Muehlschlegel JD, Peng YG, Lobato EB, et al. Temporary biventricular pacing post-cardiopulmonary bypass in patients with reduced ejection fraction. J Cardiovasc Surg. 2008;23:324–330. doi: 10.1111/j.1540-8191.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 15.Eberhardt F, Heringlake M, Massalme MS, et al. The effect of biventricular pacing after coronary artery bypass grafting: A prospective randomized trial of different pacing modes in patients with reduced left ventricular function. J Thorac Cardiovasc Surg. 2009;137:1461–1467. doi: 10.1016/j.jtcvs.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Foster AH, Gold MR, McLaughlin JS. Acute hemodynamic effects of atrio-biventricular pacing in humans. Ann Thorac Surg. 1995;59:294–300. doi: 10.1016/0003-4975(94)00878-b. [DOI] [PubMed] [Google Scholar]
- 17.Flynn MJ, McComb JM, Dark JH. Temporary left ventricular pacing improves haemodynamic performance in patients requiring epicardial pacing post-cardiac surgery. Eur J Cardiothorac Surg. 2005;28:250–253. doi: 10.1016/j.ejcts.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Hajiseyedjavadi O, Pasque M, Moon M, et al. Coronary artery bypass grafting and biventricular pacing efficacy: Do past trials dictate a change in future practice? J Thorac Cardiovasc Surg. 2006;132:974–975. doi: 10.1016/j.jtcvs.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 19.Evonich RF, Stephens JC, Merhi W, et al. The role of temporary biventricular pacing in the cardiac surgical patient with severely reduced left ventricular systolic function. J Thorac Cardiovasc Surg. 2008;136:915–921. doi: 10.1016/j.jtcvs.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Flynn M, Dark JH, McComb JM. Biventricular pacing after cardiac surgery. J Thorac Cardiovasc Surg. 2009;138:259–260. doi: 10.1016/j.jtcvs.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 22.Stellbrink C, Breithardt OA, Franke A, et al. Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol. 2001;38:1957–1965. doi: 10.1016/s0735-1097(01)01637-0. [DOI] [PubMed] [Google Scholar]
- 23.Auricchio A, Stellbrink C, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026–2033. doi: 10.1016/s0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 24.Sawhney NS, Waggoner AD, Garhwal S, et al. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004;1:562–567. doi: 10.1016/j.hrthm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Wang DY, Richmond ME, Quinn TA, et al. Optimized temporary biventricular pacing acutely improves intraoperative cardiac output after weaning from cardiopulmonary bypass: A substudy of a randomized clinical trial. J Thorac Cardiovasc Surg. 2011;141:1002–1008. doi: 10.1016/j.jtcvs.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold MR, Niazi I, Giudici M, et al. A prospective comparison of AV delay programming methods for hemodynamic optimization during cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2007;18:490–496. doi: 10.1111/j.1540-8167.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 27.Cha YM, Nishimura RA, Hayes DL. Difference in mechanical atrioventricular delay between atrial sensing and atrial pacing modes in patients with hypertrophic and dilated cardiomyopathy: An electrical hemodynamic catheterization study. J Interv Card Electrophysiol. 2002;6:133–140. doi: 10.1023/a:1015311416232. [DOI] [PubMed] [Google Scholar]
- 28.Ausubel K, Klementowicz P, Furman S. Interatrial conduction during cardiac pacing. Pacing Clin Electrophysiol. 1986;9:1026–1031. doi: 10.1111/j.1540-8159.1986.tb06665.x. [DOI] [PubMed] [Google Scholar]
- 29.Wish M, Fletcher RD, Gottdiener JS, et al. Importance of left atrial timing in the programming of dual-chamber pacemakers. Am J Cardiol. 1987;60:566–571. doi: 10.1016/0002-9149(87)90306-7. [DOI] [PubMed] [Google Scholar]
- 30.Gold M, Leman R, Niazi I, et al. Is nominal AV delay offset optimal for heart failure patients receiving cardiac resynchronization therapy? Europace. 2005;7:168A. [Google Scholar]
- 31.Levin V, Nemeth M, Colombowala I, et al. Interatrial conduction measured during biventricular pacemaker implantation accurately predicts optimal paced atrioventricular intervals. J Cardiovasc Electrophysiol. 2007;18:290–295. doi: 10.1111/j.1540-8167.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 32.Bogaard MD, Dijkman B, Loh P, et al. Individualized paced/sensed offset for the atrioventricular delay in cardiac resynchronization therapy is recommended. Heart Rhythm. 2010;7:S88–S130A. [Google Scholar]
- 33.Binkley PF, Bush CA, Kolibash AJ, et al. The anatomic relationship of the esophageal lead to the left atrium. Pacing Clin Electrophysiol. 1982;5:853–859. doi: 10.1111/j.1540-8159.1982.tb06567.x. [DOI] [PubMed] [Google Scholar]
- 34.Binkley PF, Bush CA, Fleishman BL, et al. In vivo validation of the origin of the esophageal electrocardiogram. J Am Coll Cardiol. 1986;7:813–818. doi: 10.1016/s0735-1097(86)80341-2. [DOI] [PubMed] [Google Scholar]
- 35.Grant SC, Bennett DH. Atrial latency in a dual chambered pacing system causing inappropriate sequence of cardiac chamber activation. Pacing Clin Electrophysiol. 1992;15:116–118. doi: 10.1111/j.1540-8159.1992.tb02907.x. [DOI] [PubMed] [Google Scholar]
- 36.Flaker G, Weachter R. In cardiac resynchronization therapy: Should we opt for AV optimization? J Cardiovasc Eletrophysiol. 2007;18:296–297. doi: 10.1111/j.1540-8167.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 37.Papageorgiou P, Monahan K, Boyle NG, et al. Site-dependent intra-atrial conduction delay. Relationship to initiation of atrial fibrillation. Circulation. 1996;94:384–389. doi: 10.1161/01.cir.94.3.384. [DOI] [PubMed] [Google Scholar]
- 38.Hettrick DA, Euler DE, Pagel PS, et al. Atrial pacing lead location alters the effects of atrioventricular delay on atrial and ventricular hemodynamics. Pacing Clin Electrophysiol. 2002;25:888–896. doi: 10.1046/j.1460-9592.2002.00888.x. [DOI] [PubMed] [Google Scholar]


