Abstract
The blood–brain barrier (BBB) restricts the delivery of therapeutics into the central nervous system (CNS). This limitation is due largely to localization of drug transport mechanisms to the brain endothelial cells (BECs). Although mammalian and in vitro models are currently used to study drug transport, zebrafish (Danio rerio) are advantageous for dissecting complex biological problems such as the BBB. This report focuses on zebrafish as a suitable model system for studying the BBB and drug transport.
PHARMACOLOGICAL BARRIERS TO THE CNS
The central nervous system (CNS) maintains a homeostatic environment by using three CNS barriers: the blood–brain barrier (BBB), the choroid plexus, and the arachnoid barrier.1 Although these barriers are vital for proper CNS function, they pose a challenging obstacle for the treatment of CNS diseases. Physically, barriers contain tight junctions, prohibiting paracellular diffusion of macromolecules.2 Chemically, barriers express drug transporters, regulating the access of compounds into the brain.3 In fact, it has been estimated that >98% of small molecules cannot penetrate the BBB.4 By gaining a comprehensive understanding of the development and regulation of the brain barriers, more effective methods for drug delivery may be accomplished.
Several classes of transporters are expressed at the blood– CNS barriers and regulate the distribution of drugs, xenobiotics, and nutrients.5 Although different transporters are distributed among the blood–CNS barriers, transporters at the BBB are the best characterized. With more than 40 individual members of the adenosine triphosphate-binding cassette (ABC) family, ABC transporters are the most widely studied drug transporters at the BBB. Of the ABC transporters, ABCB1, also known as multidrug-resistance protein 1 (MDR1), and P-glycoprotein, play a prominent role in excluding compounds from the CNS. Knockout mice of the MDR1 mouse ortholog MDR1a revealed higher sensitivities to both neurotoxic ivermectin and chemotherapeutic vinblastine as compared with wild-type littermates, indicating a protective role of MDR1a at the mouse BBB6 (Supplementary References 6–38 are provided as Supplementary References online.). Since its discovery, many groups have attempted to modulate MDR1 function as a means to alter therapeutic bioavailability and to overcome multidrug resistance. Other ABC family members, such as BCRP (ABCG2) and MRP4 (ABCC4), are also highly expressed at the BBB and play important roles in drug transport.7
In addition to drug transporters, Glucose Transporter 1 (GLUT1) is also vital for proper brain function. Because glucose is impermeant to the BBB and the brain uses ~20% of whole-body glucose,8 GLUT1 is highly expressed in brain endothelial cells (BECs) and is often used as a marker for the BBB. In fact, GLUT1 is one of the earliest BBB markers9 and has been used by several recent studies as a functional indicator of BBB formation.10–13
MODELS FOR STUDYING THE BBB
Current and emerging models
Although the concept of the BBB has been around for more than a century, an ideal model has yet to be established. In their pioneering experiments, Erhlich and Goldmann used animal models to characterize the access of water-soluble dyes between the bloodstream and CNS14,15, an experimental paradigm still in use today. Currently, mice are the most used animal models; they possess transporters, cell types, and size-selective properties similar to those of the human BBB.16–18 In vitro models are also used to examine drug efflux and have recently become more sophisticated at accurately modeling in vivo BBB properties.19,20 In addition, the fruit fly,21 grasshopper,22 zebrafish,23–25 and other animal models have emerged as valuable tools for dissecting the BBB. These organisms are more suitable for high-throughput screening assays and may contribute to CNS drug discovery and development.26 Although each model system has its own unique advantages, the limitations of each model should also be considered.
Advantages of the zebrafish model
Zebrafish possess many characteristics suitable for the study of drug transporters and the BBB. Zebrafish are transparent and develop rapidly outside of the mother, making them ideal for observing complex in vivo processes. Zebrafish also produce large numbers of offspring, providing the opportunity for large-scale mutagenesis and small-molecule screens. These high-throughput assays offer an unbiased approach for identifying novel genes and molecular targets for drug discovery. For example, genetic screens have uncovered zebrafish mutants involved in diverse biological processes such as early development,27 organogenesis,28 behavior,29 and cancer.30 Furthermore, small-molecule screens in zebrafish have identified compounds that inhibit developmental pathways,31 angiogenesis,32 and tumor growth,33 demonstrating their potential usefulness in discovering new drugs for human disease.
Zebrafish and the BBB
It has recently been established that zebrafish possess a BBB functionally homologous to humans. Jeong et al. demonstrated that the large hydrophilic compound sulfo-NHS-biotin and the protein horseradish peroxidase are excluded from the zebrafish brain following intravenous injection.23 As with mammals, zebrafish BECs express the tight junction protein Claudin 5,23,25,34 which is largely responsible for the size-selective properties of the BBB.18 In a recent seminal study, Xie et al. created a transgenic zebrafish to examine BBB permeability in vivo.25 In this model, green fluorescent protein is secreted from the liver into the circulation. By examining the penetration of green fluorescent protein into the brain and eye, they concluded that the BBB and blood–retinal barrier form simultaneously at 3 days postfertilization and confirmed that barrier function is dependent on the presence of tight junctions.
In addition to a physical barrier, zebrafish possess a chemical barrier in the form of solute and drug transporters. Using Glut1 as a marker, Tam et al. showed that death receptors DR6 and TROY are key regulators of CNS angiogenesis and barriergenesis in both zebrafish and mice.24 This study further demonstrated that DR6 and TROY are downstream targets of canonical Wnt/β-catenin signaling, an essential pathway for CNS angiogenesis.11,13,35 Taken together, these studies clearly show that zebrafish recapitulate many mammalian barrier properties and highlight the usefulness of zebrafish for studying the BBB.
ZEBRAFISH DRUG TRANSPORTERS
Identification of zebrafish MDR1
To date, drug transporters in zebrafish remain poorly characterized, and this field is in its infancy. Understanding the spatiotemporal expression of zebrafish transporters such as Mdr1 could be useful for performing high-throughput screens. For example, assays could be designed to identify compounds that diminish expression or inhibit function of Mdr1 in a live animal. These compounds could be coadministered with therapeutic agents, allowing more effective drug penetration into the CNS.
To determine whether zebrafish express Mdr1, we mined the zebrafish genome using the human MDR1 protein sequence. We identified a zebrafish homolog of Mdr1 with conserved synteny in the genome. Next, we examined whether Mdr1 is expressed at the BBB. We performed immunohistochemistry using the anti-MDR1 monoclonal antibody C219, which has been shown to localize MDR1 to BECs in humans and rodents.7 The epitopes for C219, VQAALD, and VQEALD are highly conserved in zebrafish Mdr1. For these experiments, we used the transgenic line Tg(fli1a:EGFP)y1, which expresses green fluorescent protein in all endothelial cells.36 Frozen sections from whole embryos at 2 days postfertilization, whole larvae at 6 days postfertilization, and adult brains were examined. As shown in Figure 1, Mdr1 is expressed in BECs as early as 2 days postfertilization and continues to be expressed throughout larval and adult stages. This is the first demonstration of drug transporter expression at the zebrafish BBB and further illustrates the utility of zebrafish for understanding the development, maintenance, and function of the BBB. Future experiments will be aimed at determining the substrate specificity of zebrafish Mdr1 and its functional similarities to human.
Figure 1.
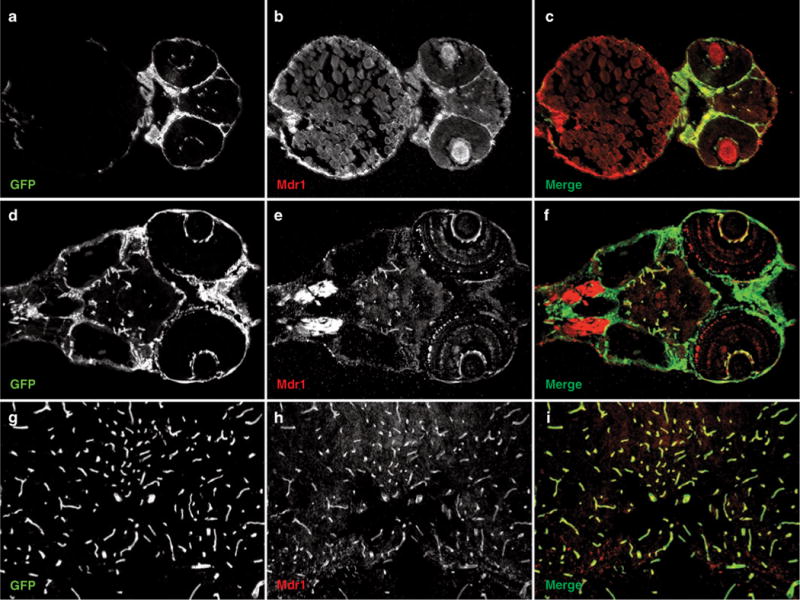
Zebrafish express Multidrug Resistance Protein 1 (Mdr1) at the blood–brain barrier. Frozen sections (12 μm) from Tg(fli1a:EGFP)y1 zebrafish, which express green fluorescent protein (GFP) in all endothelial cells, were stained with the anti-MDR1 antibody C219 at the (a–c) embryonic, (d–f) larval, and (g–i) adult stages. Following staining, images were captured on a fluorescence microscope, and the green and red channels were superimposed to create a merged image. Note the colocalization of Mdr1 (red) with GFP-labeled brain endothelial cells (green) at all stages of development.
Drug bioavailability in zebrafish
In an attempt to determine whether zebrafish Mdr1 is functional, we tested the bioavailability of the chemotherapeutic agent doxorubicin. Doxorubicin is a known substrate for MDR1 and provides an advantage for in vivo visualization due to its fluorescent properties.37 We added doxorubicin (10 μmol/l) directly to the water of zebrafish larvae. Following 1-h incubation, we visualized the distribution of doxorubicin by confocal microscopy. As shown in Figure 2a, doxorubicin was found only within the lumen of the gut. To determine whether this exclusion could be caused by Mdr1, we performed immunohistochemistry and found high levels of Mdr1 at the intestinal epithelium (Figure 2b). This experiment clearly demonstrates that zebrafish effectively prohibit the absorption of specific compounds and indirectly indicates that Mdr1 may play an important role in this process. Although this result does not demonstrate the functionality of Mdr1 at the BBB, the intestinal barrier and BBB share many similar mechanisms to maintain tissue homeostasis.38 By screening for genes or small molecules that allow doxorubicin to penetrate into the larvae and across the BBB, perhaps new inhibitors of MDR1 could be identified. However, we emphasize that our observations are purely speculative at this point, as we have not directly demonstrated Mdr1 function in zebrafish.
Figure 2.
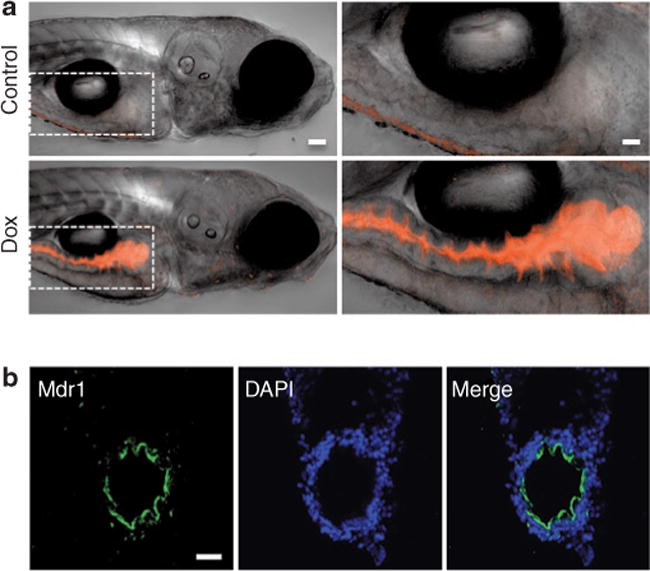
Doxorubicin is excluded from zebrafish larvae. (a) Doxorubicin (10 μmol/l), a fluorescent chemotherapeutic agent, was added directly to the water of zebrafish larvae at 6 days post-fertilization (dpf). Images were acquired using confocal microscopy, and single-view two-dimensional reconstructions of z-series stacks were created by an overlay of transmitted light and red fluorescence channels. Control larvae show no fluorescence (top panels). Treated larvae contain doxorubicin fluorescence only in the gut (bottom panels). The right panels are ×2.5 magnifications of the left panels (dashed box). Bar = 50 μm (left panels) and 125 μm (right panels). (b) Multidrug Resistance Protein 1 (Mdr1) localizes to the intestinal epithelium. Transverse frozen sections (12 μm) from zebrafish larvae (6 dpf) were stained with anti-MDR1 (green) and nuclei counterstained with 4′,6-diamidino-2-phenylindole (blue). Images were acquired on a confocal fluorescence microscope, and the green and blue channels were superimposed to create a merged image. Bar = 20 μm.
CONCLUSIONS
Although the BBB was first discovered more than 100 years ago, many complex problems and unresolved questions remain with respect to CNS drug delivery, BBB development, and BBB damage. As stated in a recent review by Saunders et al., “A particular reason why the field of brain barrier biology has been in decline is a lack of innovation and an adherence to methods that have been around for decades.”1 By offering a reductionist approach to this problem, zebrafish provide a novel in vivo tool for gaining a fundamental understanding of the BBB. We predict that zebrafish will be used to answer the following longstanding questions, and others as well. When and how does barriergenesis occur? Are immature barriers more permeable? What is the link between CNS angiogenesis and barriergenesis? What cell autonomous and nonautonomous signals regulate these processes?
Ultimately, we anticipate that zebrafish will be used to identify new genes and small molecules that modulate the blood–CNS barriers, leading to the discovery of drugs that permit the controlled access of therapeutics into the brain and drugs that repair damaged barriers.
Supplementary Material
Acknowledgments
We thank Jennifer Peters of the St Jude Children’s Research Hospital Cell and Tissue Imaging facility for help with confocal imaging. This research was supported by St Jude Children’s Research Hospital, American Lebanese Syrian Associated Charities, and The Hartwell Foundation.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
References
- 1.Saunders NR, Ek CJ, Habgood MD, Dziegielewska KM. Barriers in the brain: a renaissance? Trends Neurosci. 2008;31:279–286. doi: 10.1016/j.tins.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun H, Dai H, Shaik N, Elmquist WF. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55:83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


