Abstract
Sudden unexpected death in epilepsy (SUDEP) is a significant public health burden. The mechanisms of SUDEP are elusive, although cardiorespiratory dysfunction is a likely contributor. Clinical and animal studies indicate that seizure-induced respiratory arrest (S-IRA) is the primary event leading to death in many SUDEP cases. Our prior studies demonstrated that intraperitoneal (IP) injection of atomoxetine, a norepinephrine reuptake inhibitor (NRI) widely used to treat attention deficit hyperactivity disorder, suppresses S-IRA in DBA/1 mice. In the current study, we injected atomoxetine intracerebroventricularly (ICV) and measured its effect on S-IRA in DBA/1 mice to determine its central effects. Additionally, to test our hypothesis that atomoxetine reduces S-IRA via altering cardiorespiratory function, we examined the effect of atomoxetine on respiratory and cardiac function using non-invasive plethysmography and ECG in anesthetized DBA/1 mice, and on blood pressure and heart rate using a tail-cuff system in conscious DBA/1 mice. ICV administration of atomoxetine at 200–250 nmol significantly reduced S-IRA evoked by acoustic stimulation in DBA/1 mice, consistent with a central atomoxetine effect on S-IRA. Peripheral atomoxetine administration at a dosage that reduces S-IRA (15 mg/kg, IP) slightly increased basal ventilation and the ventilatory response to 7% CO2, but exerted no effect on heart rate in anesthetized DBA/1 mice. IP injection of atomoxetine produced no effect on the heart rate and blood pressures in conscious mice. These data suggest that atomoxetine suppresses S-IRA through direct effects on the CNS and potentially through enhanced lung ventilation in DBA/1 mice.
Keywords: Generalized seizures, NRI, intracerebroventricular, ventilation, heart rate, blood pressures
1. Introduction
Sudden unexpected death in epilepsy (SUDEP) is a major burden on public health (Thurman et al., 2014), as the risk of sudden death in younger patients with epilepsy is increased more than 20-fold (Ficker et al., 1998; Tomson et al., 2016). Clinical and animal studies demonstrate that seizure-induced respiratory arrest (S-IRA) is the primary event leading to death after generalized tonic-clonic seizures in many cases (Bateman et al., 2008; Blum, 2009; Bravo et al., 2015; Buchanan et al., 2014; Faingold et al., 2010; Langan et al., 2000; Pezzella et al., 2009; Ryvlin et al., 2013; So et al., 2000; Zhang et al., 2016), although cardiac dysfunction may also contribute to seizure-induced sudden death (Frasier et al., 2016; Kalume et al., 2013). Previous studies implicate serotonergic and adenosinergic neurotransmission in the pathogenesis of S-IRA in animal models of SUDEP, including the DBA/1 mouse (Feng and Faingold, 2015; Richerson et al., 2016). Our recent data demonstrate that enhanced norepinephrine (NE) availability in the synapses by intraperitoneal (IP) administration of the NE reuptake inhibitor (NRI) atomoxetine reduces S-IRA evoked by either acoustic stimulation or pentylenetetrazole in DBA/1 mice (Zhang et al., 2017). However, it is unknown how atomoxetine suppresses S-IRA. We hypothesized that atomoxetine reduces S-IRA by altering cardiorespiratory function in the DBA/1 mouse model of SUDEP.
2. Materials and methods
2.1. Animals
Experimental procedures were approved by Massachusetts General Hospital Institutional Animal Care and Use Committee, and all studies were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. DBA/1 mice were originally purchased from Envigo (Indianapolis, IN) and were housed and bred in the animal facility at Massachusetts General Hospital under a temperature- and humidity-controlled environment (12-h light/dark cycle), provided with rodent food and water ad libitum. DBA/1 mice were “primed” by daily exposure to acoustic stimulation using an electric bell (96 dB SPL, UC4-150, Zhejiang People’s Electronics, China) for 3–4 days, starting from postnatal day 26–28, to establish consistent susceptibility to audiogenic seizures (AGSz) and S-IRA (Zhang et al., 2016). The same acoustic stimulus parameters were used in priming and induction of AGSz. DBA/1 mice of both sexes at approximately 2 months of age were used in the intracerebroventricular (ICV) experiments, and those at approximately 1–2 months of age were used in non-invasive plethysmography and tail-cuff experiments. Animals were randomly assigned to a treatment group. Some mice were reused in the ICV experiments (9 out of 21 mice used in the experiments), and none were reused in the other experiments. When a DBA/1 mouse was reused in the ICV experiments, the animal rested in the animal facility for at least one week to ensure the clearance of administered drugs, and the susceptibility of this mouse to S-IRA was always confirmed 24 hr prior to the next test.
2.2. The effect of ICV injection of atomoxetine on S-IRA
The guide cannula was implanted as previously described (Faingold et al., 2016; Feng et al., 2007). Briefly, a DBA/1 mouse was anesthetized using ketamine/xylazine (100/10 mg/kg, IP), and a guide cannula (26G, Plastics One, Roanoke, VA) was stereotaxically implanted into the left lateral ventricle (AP – 0.4 mm; ML – 1.0 mm; V – 2.0 mm) (Paxinos and Franklin, 2013).
One week after the surgery, the mouse was subjected to acoustic stimulation to confirm that it was still susceptible to S-IRA and was resuscitated using a rodent ventilator (Harvard Apparatus 680, Holliston, MA). Twenty-four hr after the confirmation of S-IRA, microinjection was performed using a minipump (11 Elite Nanomite, Harvard Apparatus) and a Hamilton syringe (Harvard Apparatus) connected to the infusion cannula (33G, Plastics One) by a polyethylene tubing (PE10, Harvard Apparatus). Atomoxetine (Y0001586, Sigma-Aldrich, St. Louis, MO) (150, 200 or 250 nmol) or vehicle, 50% dimethyl sulfoxide (DMSO), at 2 μl volume was administered ICV at a rate of 0.5 μl/min in different groups of DBA/1 mice, respectively. After completion of the microinjection, the infusion cannula remained inside the guide cannula for an additional one minute and was then slowly withdrawn to avoid back flow.
AGSz and S-IRA were examined 2 hr after atomoxetine or vehicle injection and videotaped for offline analysis. For those DBA/1 mice in which S-IRA was reduced by atomoxetine, susceptibility to S-IRA was tested 24 hr after microinjection or at 24 hr intervals thereafter until susceptibility to S-IRA returned to confirm the reversibility of the drug effect on S-IRA.
2.3. Histology
At the end of the microinjection experiment, ICV injection of fast green was performed to mark the ventricular space; guide cannula placement was verified using histology. For brain harvest, each mouse was deeply anesthetized with an overdose of ketamine/xylazine and transcardially perfused with 10 ml PBS (pH 7.4), followed by 10 ml 4% paraformaldehyde. After removal, the brain was stored in 4% paraformaldehyde at 4°C. Each brain was sectioned into 50-μm thickness of coronal slices with a freezing microtome (CM 1850 UV, Leica, Buffalo Grove, IL), and the location of the guide cannula track was observed using a Nikon Eclipse TS100 light microscope (Nikon Instruments, Melville, NY).
2.4. The effect of atomoxetine on respiratory function and heart rate in anesthetized DBA/1 mice
Modulation of atomoxetine on respiratory function was examined using nose-only plethysmography in anesthetized DBA/1 mice, as previously described (Zeng et al., 2015). In brief, the nose of a primed DBA/1 mouse under 1.5% isoflurane (Baxter Healthcare, Deerfield, IL) anesthesia was inserted into a custom-built breathing chamber through a 0.25-inch hole in a latex diaphragm (McMaster Carr, Robbinsville, NJ). The chamber was flushed with stable flow of fresh room air (1 l/min), and the composition of gas in the chamber was continuously monitored using a Capnomac Ultima medical gas analyzer (GE Healthcare, Buckinghamshire, UK). Changes in gas pressure induced by mouse breathing inside the breathing chamber were detected by a Model 8420 pneumotachometer (Hans Rudolph, Shawnee, KS), which were converted to an analog signal by a CD15 differential pressure transducer and an MP45-14-871 demodulator (Validyne Engineering, Northridge, CA). The system was calibrated using a rodent ventilator (Harvard Apparatus 683). The body temperature of the mouse was monitored using a rectal thermistor and maintained at 37°C by a heat lamp.
ECG recordings were performed in anesthetized DBA/1 mice as previously described (Zhang et al., 2016). Briefly, three subdermal needle electrodes (Model F-E2, Grass Instruments, Warwick, RI) were inserted into the skin across the thorax, and cardiac electrical activity was recorded using a P511 AC amplifier (Grass Technologies, Warwick, RI) with the following parameters: gain 20,000; bandpass filter 0.3–3,000 Hz.
Minute ventilation (VE), tidal volume (VT) and respiratory frequency (fR) (VE = VT × fR) as well as heart rate were recorded from the anesthetized DBA/1 mouse for 30 min in the absence of any treatment, and the data for the last 10 min were used for baseline normalization. Ventilatory response to CO2 was recorded for 10 min by switching from room air to a 7% CO2 gas mixture. The normalized response of each parameter was compared between the drug and vehicle groups. Drugs (i.e., atomoxetine, doxapram or atropine) or vehicle (saline) was administered IP through a 24-gauge angiocatheter inserted into the abdomen prior to data collection. Doxapram and atropine served as positive control for ventilation and heart rate in these experiments, respectively. Custom software written using LabView 2013 (National Instruments, Austin, TX) was used for data acquisition, analysis and gas flow control.
2.5. The effect of atomoxetine on blood pressures and heart rate in awake DBA/1 mice
Non-invasive measurements of heart rate and blood pressures at 37°C were performed in conscious DBA/1 mice treated with either atomoxetine or vehicle using tail-cuff system (BP-2000 Blood Pressure Analysis System, Visitech Systems, Apex, NC) (Buys et al., 2008). DBA/1 mice were habituated to the tail-cuff system for 7 days with daily subjection to three cycles of 20 measurements of blood pressures. Atomoxetine or vehicle (saline) was administered IP, and the effect of atomoxetine or vehicle on heart rate and blood pressures was determined.
2.6. Statistical analysis
Data are reported as mean ± SEM. Statistical analyses were performed using Prism 6 software (GraphPad Software, La Jolla, CA). The incidence of S-IRA between atomoxetine and vehicle control was compared using Mann-Whitney test. One-way ANOVA and post-hoc Tukey’s test were used to compare the latency to AGSz among drug and control groups. The effect of atomoxetine on VE, VT, fR, heart rate and blood pressures at 2 hr after atomoxetine administration was compared with vehicle control using unpaired Student t test. Statistical significance was inferred if p < 0.05.
3. Results
3.1. ICV injection of atomoxetine suppresses S-IRA in DBA/1 mice
Our recent study demonstrated that S-IRA evoked by either acoustic stimulation or pentylenetetrazole in DBA/1 mice was reduced 2 hr after IP administration of atomoxetine (Zhang et al., 2017). In the current study, we examined if atomoxetine suppresses S-IRA mainly via its action in the brain by ICV administration of the chemical. S-IRA evoked by acoustic stimulation in primed DBA/1 mice was significantly suppressed 2 hr after ICV injection of atomoxetine at 200 nmol (40%, n = 10; p < 0.01) and 250 nmol (0%, n = 6; p < 0.001) as compared with vehicle control (100%, n = 12). However, S-IRA was unaffected by atomoxetine at 150 nmol (100%, n = 6) (Fig 1A). For those DBA/1 mice in which S-IRA was reduced by atomoxetine, susceptibility to S-IRA returned 24–48 hr after microinjection.
Fig. 1. ICV injection of atomoxetine reduces S-IRA in DBA/1 mice.
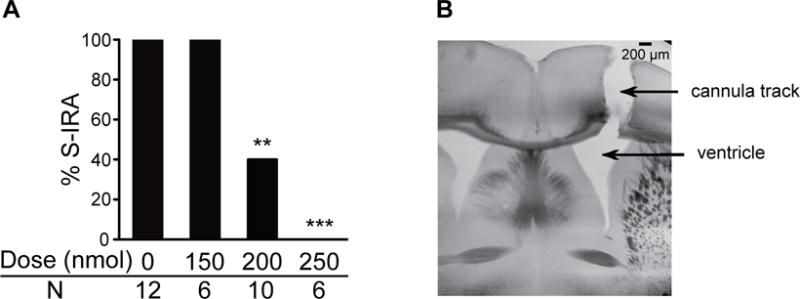
A, ICV administration of atomoxetine at 200–250 nmol reduced S-IRA in primed DBA/1 mice 2h after microinjection. B, a representative example of histology showing that the vehicle or the chemical was successfully delivered into the ventricle.
** p < 0.01; *** p < 0.001: Significantly different from vehicle control.
ICV injection of atomoxetine at 200 nmol and 250 nmol specifically blocked S-IRA without altering AGSz characteristics in 66.7% and 66.7% of DBA/1 mice tested, respectively. However, the tonic AGSz component was blocked in the other 33.3% of DBA/1 mice at both dosages. All of these mice did still exhibit wild running and/or clonic seizures, suggesting that atomoxetine does not block AGSz susceptibility of DBA/1 mice. The latency to AGSz after ICV injection of atomoxetine at 150 nmol (4.4 ± 0.8 sec), 200 nmol (4.7 ± 0.7 sec) or 250 nmol (5.7 ± 0.8 sec) was not significantly altered as compared with that in vehicle control (4.0 ± 0.7 sec) in DBA/1 mice.
Only data from the DBA/1 mice with correct guide cannula placement were included in ICV data analysis. A representative example of histology showing that the guide cannula hit the lateral ventricle is presented in Fig 1B. The cannula hit the ventricle correctly in 21 out of 27 implanted mice. Microinjection of atomoxetine did not produce any effect on S-IRA in the 6 mice in which the cannula missed the target ventricle as determined by histology.
3.2. Atomoxetine enhances breathing in anesthetized DBA/1 mice
We studied the effect of atomoxetine on breathing in primed DBA/1 mice in the absence of seizures using non-invasive nose-only plethysmography (Zeng et al., 2015). After IP injection of atomoxetine at a dose (15 mg/kg) known to reduce S-IRA (Zhang et al., 2017), the VE gradually increased within 5 min, with the effects lasting more than 2 hours (Fig 2B). As compared with vehicle control (n = 7), VE was significantly increased by 16.4% 2 hr after IP administration of atomoxetine (n = 7; p < 0.01). However, atomoxetine did not significantly alter the individual components of ventilation, VT and fR, as compared with vehicle control (Fig 2C).
Fig. 2. Atomoxetine enhances lung ventilation in anesthetized DBA/1 mice.
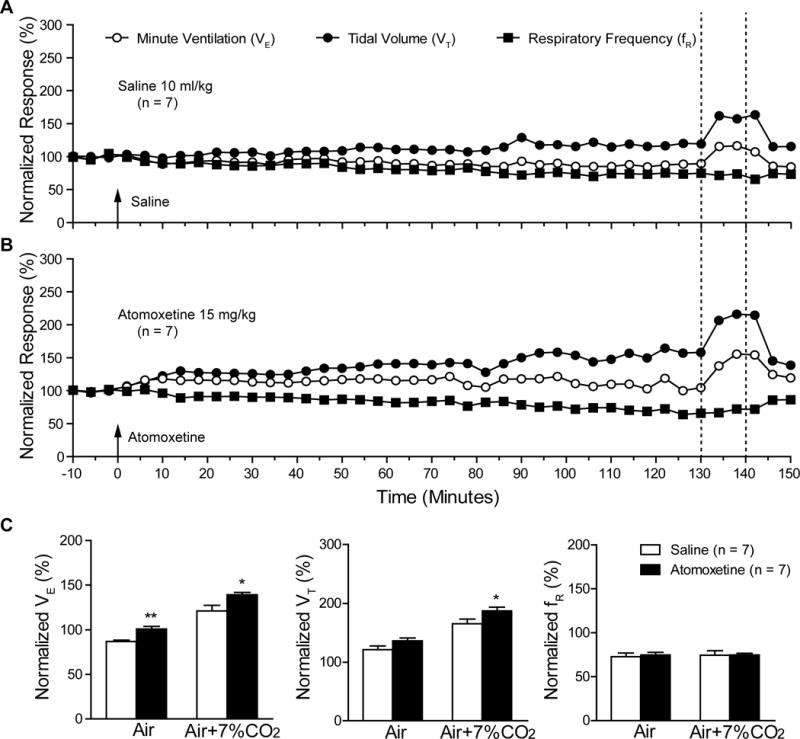
A, B, representative traces of minute ventilation (VE), tidal volume (VT) and respiratory frequency (fR) from anesthetized DBA/1 mice treated with vehicle (saline, 10 ml/kg, IP) or atomoxetine (15 mg/kg, IP), respectively, in room air or room air plus 7% CO2. Data were normalized to the averaged baseline value of VE, VT or fR. Averaged baseline values of VE, VT and fR were 13.3 ± 1.4 ml/20 g/min, 0.15 ± 0.02 ml/20 g/min and 96.3 ± 11.2 breaths/min, respectively. Traces between the two dotted lines denote the effect in response to room air plus 7% CO2. C, comparison of the vehicle and atomoxetine effects on the normalized VE, VT or fR in room air and in room air plus 7% CO2 in anesthetized DBA/1 mice at the period of 120–130 min (mean value of 10-min recordings) after IP injection, at which atomoxetine at this dosage is known to reduce S-IRA (Zhang et al., 2017).
* p < 0.05; ** p < 0.01: Significantly different from the vehicle control.
The effect of atomoxetine on the ventilatory response to 7% CO2 was also examined (Fig 2B). In the presence of atomoxetine, 7% CO2 resulted in significant increases in VE (p < 0.05) and VT (p < 0.05) but not in fR as compared with vehicle control (Fig 2C).
As reference, we also examined the effect of doxapram, a classic breathing stimulant (Cotten, 2013), on respiratory function; and consistent with a previous report (Zeng et al., 2015), VE gradually increased within 5 min and reached a peak value approximately 15 min after doxapram administration (50 mg/kg, IP). Doxapram significantly increased VE by 82.1%, VT by 47.2% and fR by 22.8% (n = 3; p < 0.01) as compared with vehicle control in room air.
3.3. Atomoxetine exerts no effect on heart rate in anesthetized DBA/1 mice
It is well known that NE produces a chronotropic response. We therefore examined atomoxetine effects on heart rate by ECG analysis in anesthetized DBA/1 mice following IP administration of atomoxetine at 15 mg/kg. As compared with vehicle control (n = 6), heart rate in anesthetized DBA/1 mice was not significantly altered following atomoxetine administration (n = 5), including the time point of 2 hr used in our earlier behavioral S-IRA studies (Fig 3A, B).
Fig. 3. Atomoxetine produces no effect on the heart rate in anesthetized DBA/1 mice.
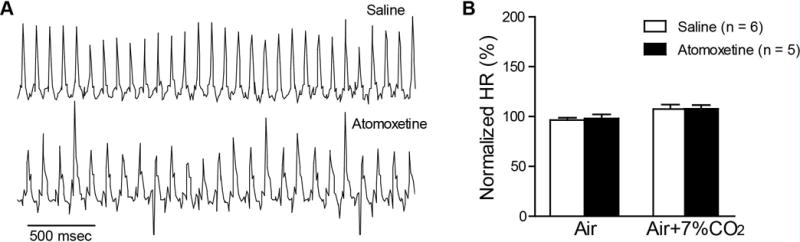
A, representative ECG traces in anesthetized DBA/1 mice treated with vehicle (saline 10 ml/kg, IP) or atomoxetine (15 mg/kg, IP) in room air. B, comparison of the normalized mean heart rate between vehicle and atomoxetine in room air and in room air plus 7% CO2 at the period of 120–130 min (mean value of 10-min recordings) after injection of vehicle/atomoxetine, at which atomoxetine at this dosage is known to reduce S-IRA (Zhang et al., 2017).
As a positive control, we examined the effect of atropine on heart rate in anesthetized DBA/1 mice. Similar to what others have reported (Mesirca et al., 2013), heart rate was significantly increased by 10.2% 30 min after IP injection of atropine (1 mg/kg) (n = 4) as compared with vehicle control (n = 6) (p < 0.01).
3.4. Atomoxetine exerts no effects on heart rate and blood pressures in conscious DBA/1 mice
The effect of atomoxetine on the heart rate and blood pressures was evaluated in conscious primed DBA/1 mice using a non-invasive tail-cuff system. Following 7 days of acclimation (Buys et al., 2008), four cardiac parameters, i.e., systolic blood pressure, diastolic blood pressure, mean arterial pressure and heart rate were quantified in mice following treatment with vehicle or atomoxetine (15 mg/kg, IP). None of the parameters differed between the vehicle control group (n = 8) and the atomoxetine group (n = 7) at 30 min or 2 hr time points after atomoxetine treatment (Fig 4A, B).
Fig. 4. Atomoxetine produces no effect on the heart rate and blood pressures in conscious DBA/1 mice.
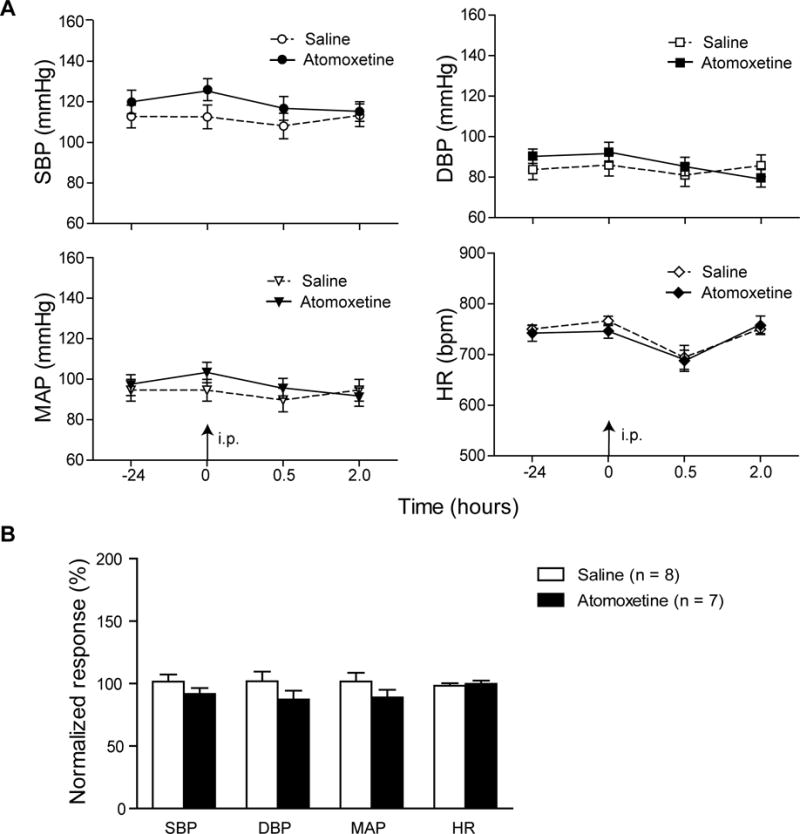
A, changes in mean systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and the heart rate prior to and after IP injection of vehicle or atomoxetine in conscious DBA/1 mice. B, comparison of the normalized mean SBP, DBP, MAP and heart rate between vehicle and atomoxetine in behaving DBA/1 mice at the period of 120–130 min (mean value of 10-min recordings) after injection of vehicle/atomoxetine, at which atomoxetine at this dosage is known to reduce S-IRA (Zhang et al., 2017).
4. Discussion
In the current studies, we demonstrate that ICV administration of atomoxetine significantly reduces S-IRA evoked by acoustic stimulation in DBA/1 mice, suggesting that atomoxetine exerts its effects on S-IRA via direct effects on the CNS. Systemic administration of atomoxetine, at a dose known to inhibit S-IRA (Zhang et al., 2017), slightly enhances lung ventilation but exerts no effect on heart rate in anesthetized DBA/1 mice and no effects on heart rate and blood pressures in conscious DBA/1 mice in the absence of seizures.
ICV administration of atomoxetine at certain dosages blocked the tonic component of AGSz in about 1/3 of DBA/1 mice in the present study. The reasons why tonic AGSz were specifically blocked by atomoxetine are unknown. Studies indicate that periaqueductal gray is involved in the tonic component of AGSz (Faingold, 2012). We speculate that atomoxetine is more likely to accumulate at periaqueductal gray to reach a higher concentration than that in other brain structures that are involved in wild running and/or clonic components of AGSz. This could lead to more specific blockade of tonic AGSz, as atomoxetine at very high concentrations is reported to exert protection against seizures (Torres et al., 2008).
Our preliminary data observed that S-IRA was not consistently modulated 1 hr after IP or ICV injection of atomoxetine but was considerably reduced 2 hr after IP or ICV injection. This delay in response is not observed in studies using selective serotonin reuptake inhibitors (SSRIs), in which S-IRA is reduced 30 min after IP administration of SSRIs (Faingold et al., 2011; Tupal and Faingold, 2006; Zeng et al., 2015). Interestingly, a mouse microdialysis study determined that maximal elevation of NE brain levels occurs approximately 2 hr after IP injection of atomoxetine (Koda et al., 2010), suggesting that atomoxetine may reduce S-IRA via a CNS effect. Consistent with this, we observed that ICV administration of atomoxetine inhibits S-IRA without affecting seizure susceptibility. Although atomoxetine readily passes the blood-brain barrier, the reduction of S-IRA evoked by ICV administration of atomoxetine is unlikely achieved by its peripheral actions due to “leak” of the chemical into the systemic circulation, as the amount of atomoxetine applied ICV is substantially smaller than that administered IP to block S-IRA; the ICV dosage was less than 1/5 of that used IP. Therefore, atomoxetine likely exerts its effect on S-IRA by blocking NE transporters in the brain. Of note, ICV and IP administration of atomoxetine suppresses S-IRA in a similar time frame after drug injection, indicating that the delayed inhibitory effect of atomoxetine on S-IRA is unlikely due to pharmacokinetics, but possibly due to delayed pharmacodynamic effects of atomoxetine on NE transporter inhibition. In addition to selectively binding to NE transporters, atomoxetine also binds with low affinity to serotonin and dopamine transporters (Bymaster et al., 2002; Koda et al., 2010). However, microdialysis studies demonstrated that atomoxetine did not elevate serotonin or dopamine levels in the brain except for the prefrontal cortex where dopamine increased (Bymaster et al., 2002). Further studies are needed to investigate if enhanced dopamine in the prefrontal cortex is involved in the pathogenesis of S-IRA.
Death following S-IRA evoked by acute AGSz in DBA/1 mice is prevented by mechanical ventilation of mice with a rodent ventilator (Faingold et al., 2011; Zeng et al., 2015), and oxygenation prevents S-IRA in DBA/2 mice (Venit et al., 2004), suggesting that occurrence of S-IRA may be related to respiratory dysfunction. Other studies have also demonstrated that S-IRA is the primary event causing sudden death following evoked generalized seizures in most cases (Buchanan et al., 2014; Faingold et al., 2010; Zhang et al., 2016). Therefore, S-IRA is a likely contributor to some SUDEP cases. In the current study, we demonstrated that atomoxetine, at a dosage known to suppress S-IRA, slightly but significantly enhanced basal ventilation (enhanced basal ventilation by ~16%), suggesting that atomoxetine may reduce S-IRA, at least in part, by augmenting ventilation. However, it is unlikely that atomoxetine suppresses S-IRA by directly acting on respiratory effectors for several reasons. Compared with the effective breathing stimulant doxapram, which enhanced basal ventilation by ~82%, the enhancement of ventilation by atomoxetine is small. Interestingly, fluoxetine, an SSRI known to reduce S-IRA (Feng and Faingold, 2015), does not enhance basal ventilation in DBA/1 mice (Zeng et al., 2015). Moreover, in our previous study, doxapram and PK-THPP, another potent breathing stimulant, exerted no effect on S-IRA in DBA/1 mice (Zeng et al., 2015). However, our data do suggest that atomoxetine may activate specific neuronal pathways to block S-IRA. Noradrenergic neurotransmission plays an important role in modulating respiration and arousal (Berridge et al., 2012; Doi and Ramirez, 2008). Consistent with this, stimulation of noradrenergic neurons in the medulla produces arousal and enhanced respiratory activity (Burke et al., 2014). Thus, it is possible that atomoxetine may reduce S-IRA by promoting arousal to regulate breathing. In addition, it was reported that brainstem depression due to seizure-induced spreading depolarization can compromise cardiorespiratory function, leading to SUDEP (Aiba and Noebels, 2015). Therefore, it is also possible that atomoxetine may reduce S-IRA by preventing brainstem depression. Clearly, further studies are needed to dissect the neuronal circuitry involved in protection from S-IRA by atomoxetine.
Atomoxetine is an NRI prescribed for attention deficit hyperactivity disorder that was approved in the USA in 2002 and that is widely used in 97 countries (Reed et al., 2016). It is well known that the sympathetic neurotransmitter NE evokes chronotropic and hypertensive effects, and seizure-induced enhancement of sympathetic drive is a risk factor for cardiac SUDEP (Schomer et al., 2014; Verrier et al., 2016). Thus, we evaluated the effects of atomoxetine on heart rate and systemic blood pressures, surrogates for cardiovascular function. However, atomoxetine at a dosage that suppresses S-IRA does not affect heart rate or blood pressures in anesthetized or conscious DBA/1 mice in the absence of seizures. Our findings are in general agreement with a prior rat study (Li et al., 2010). We speculate that the dosages of atomoxetine used in our study may not reach the threshold to trigger a cardiac response in DBA/1 mice. Notably, atomoxetine was reported to decrease baseline sympathetic tone and/or elevate cardiac parasympathetic tone (Li et al., 2010). Although small increases in mean systolic blood pressure and heart rate were reported in patients treated with atomoxetine, the benefits of this medication in treatment of attention deficit hyperactivity disorder are widely accepted (Martinez-Raga et al., 2013).
In conclusion, this is the first study to explore the mechanisms underlying suppression of S-IRA by atomoxetine. Our data indicate that atomoxetine reduces S-IRA through direct effects on the CNS and potentially through modest effect on lung ventilation. Atomoxetine, at a dosage that inhibit S-IRA, produced no detectable effects on the cardiovascular system.
Acknowledgments
We thank James Boghosian for technical assistance on respiratory functional studies and Dr. Emmanuel Buys for guidance on measuring cardiovascular function. This work was supported by R03NS078591, R21NS101311 and CURE (Citizens United for Research in Epilepsy) foundation to HJF and R01HL117871 to JFC. We thank the support from the Massachusetts General Hospital Department of Anesthesia, Critical Care and Pain Medicine. Haiting Zhao is a recipient of fellowship from China Scholarship Council (CSC 201506370075).
Abbreviations
- AGSz
Audiogenic seizures
- DMSO
Dimethyl sulfoxide
- ICV
Intracerebroventricular(ly)
- IP
Intraperitoneal(ly)
- NE
Norepinephrine
- S-IRA
Seizure-induced respiratory arrest
- NRI
Norepinephrine reuptake inhibitor
- SSRI
Selective serotonin reuptake inhibitor
- SUDEP
Sudden unexpected death in epilepsy
Footnotes
Conflict of interest
None of the authors has any conflict of interest to disclose.
References
- Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Science translational medicine. 2015;7:282ra246. doi: 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain: a journal of neurology. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep medicine reviews. 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AS. Respiratory physiology of seizures. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2009;26:309–315. doi: 10.1097/WNP.0b013e3181b7f14d. [DOI] [PubMed] [Google Scholar]
- Bravo E, Kim Y, Richerson GB. Ventilatory arrest is the primary initiating event that leads to sudden death after heat-induced seizures in a Dravet mouse model. AES Annual Meeting; 2015. p. 3.364. Abstract. [Google Scholar]
- Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592:4395–4410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PG, Abbott SB, Coates MB, Viar KE, Stornetta RL, Guyenet PG. Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats. American journal of respiratory and critical care medicine. 2014;190:1301–1310. doi: 10.1164/rccm.201407-1262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovascular research. 2008;79:179–186. doi: 10.1093/cvr/cvn068. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Cotten JF. TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth Analg. 2013;116:810–816. doi: 10.1213/ANE.0b013e318284469d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respiratory physiology & neurobiology. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL. Brainstem Networks: Reticulo-Cortical Synchronization in Generalized Convulsive Seizures. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy & behavior : E&B. 2010;17:436–440. doi: 10.1016/j.yebeh.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Zeng C, Peng S, Long X, Feng HJ. Serotonergic agents act on 5-HT3 receptors in the brain to block seizure-induced respiratory arrest in the DBA/1 mouse model of SUDEP. Epilepsy & behavior : E&B. 2016;64:166–170. doi: 10.1016/j.yebeh.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, Tupal S, Randall M. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy & behavior : E&B. 2011;22:186–190. doi: 10.1016/j.yebeh.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Faingold CL. Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy & behavior : E&B Aug 10. 2015 doi: 10.1016/j.yebeh.2015.06.008. pii:S1525-5050(15)00339-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Yang L, Faingold CL. Role of the amygdala in ethanol withdrawal seizures. Brain Res. 2007;1141:65–73. doi: 10.1016/j.brainres.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Ficker DM, So EL, Shen WK, Annegers JF, O’Brien PC, Cascino GD, Belau PG. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology. 1998;51:1270–1274. doi: 10.1212/wnl.51.5.1270. [DOI] [PubMed] [Google Scholar]
- Frasier CR, Wagnon JL, Bao YO, McVeigh LG, Lopez-Santiago LF, Meisler MH, Isom LL. Cardiac arrhythmia in a mouse model of sodium channel SCN8A epileptic encephalopathy. Proc Natl Acad Sci U S A Oct 16. 2016 doi: 10.1073/pnas.1612746113. pii: 201612746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of Dravet syndrome. The Journal of clinical investigation. 2013;123:1798–1808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–270. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: a series of witnessed deaths. Journal of neurology, neurosurgery, and psychiatry. 2000;68:211–213. doi: 10.1136/jnnp.68.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WY, Strang SE, Brown DR, Smith R, Silcox DL, Li SG, Baldridge BR, Nesselroade KP, Jr, Randall DC. Atomoxetine changes rat’s HR response to stress from tachycardia to bradycardia via alterations in autonomic function. Autonomic neuroscience : basic & clinical. 2010;154:48–53. doi: 10.1016/j.autneu.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS drugs. 2013;27:15–30. doi: 10.1007/s40263-012-0019-9. [DOI] [PubMed] [Google Scholar]
- Mesirca P, Marger L, Toyoda F, Rizzetto R, Audoubert M, Dubel S, Torrente AG, Difrancesco ML, Muller JC, Leoni AL, Couette B, Nargeot J, Clapham DE, Wickman K, Mangoni ME. The G-protein-gated K+ channel, IKACh, is required for regulation of pacemaker activity and recovery of resting heart rate after sympathetic stimulation. J Gen Physiol. 2013;142:113–126. doi: 10.1085/jgp.201310996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. 4th. Academic Press; New York: 2013. [Google Scholar]
- Pezzella M, Striano P, Ciampa C, Errichiello L, Penza P, Striano S. Severe pulmonary congestion in a near miss at the first seizure: further evidence for respiratory dysfunction in sudden unexpected death in epilepsy. Epilepsy & behavior : E&B. 2009;14:701–702. doi: 10.1016/j.yebeh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Reed VA, Buitelaar JK, Anand E, Day KA, Treuer T, Upadhyaya HP, Coghill DR, Kryzhanovskaya LA, Savill NC. The Safety of Atomoxetine for the Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Comprehensive Review of Over a Decade of Research. CNS drugs. 2016;30:603–628. doi: 10.1007/s40263-016-0349-0. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Boison D, Faingold CL, Ryvlin P. From unwitnessed fatality to witnessed rescue: Pharmacologic intervention in sudden unexpected death in epilepsy. Epilepsia. 2016;57(Suppl 1):35–45. doi: 10.1111/epi.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet neurology. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- Schomer AC, Nearing BD, Schachter SC, Verrier RL. Vagus nerve stimulation reduces cardiac electrical instability assessed by quantitative T-wave alternans analysis in patients with drug-resistant focal epilepsy. Epilepsia. 2014;55:1996–2002. doi: 10.1111/epi.12855. [DOI] [PubMed] [Google Scholar]
- So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia. 2000;41:1494–1497. doi: 10.1111/j.1528-1157.2000.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–1485. doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- Tomson T, Surges R, Delamont R, Haywood S, Hesdorffer DC. Who to target in sudden unexpected death in epilepsy prevention and how? Risk factors, biomarkers, and intervention study designs. Epilepsia. 2016;57(Suppl 1):4–16. doi: 10.1111/epi.13234. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–26. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- Venit EL, Shepard BD, Seyfried TN. Oxygenation prevents sudden death in seizure-prone mice. Epilepsia. 2004;45:993–996. doi: 10.1111/j.0013-9580.2004.02304.x. [DOI] [PubMed] [Google Scholar]
- Verrier RL, Nearing BD, Olin B, Boon P, Schachter SC. Baseline elevation and reduction in cardiac electrical instability assessed by quantitative T-wave alternans in patients with drug-resistant epilepsy treated with vagus nerve stimulation in the AspireSR E-36 trial. Epilepsy & behavior : E&B. 2016;62:85–89. doi: 10.1016/j.yebeh.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Zeng C, Long X, Cotten JF, Forman SA, Solt K, Faingold CL, Feng HJ. Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy & behavior : E&B. 2015;45:1–7. doi: 10.1016/j.yebeh.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Feng HJ. Atomoxetine, a norepinephrine reuptake inhibitor, reduces seizure-induced respiratory arrest. Epilepsy & behavior : E&B. 2017;73:6–9. doi: 10.1016/j.yebeh.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Yang X, Xue Q, Cotten JF, Feng HJ. 5-Hydroxytryptophan, a precursor for serotonin synthesis, reduces seizure-induced respiratory arrest. Epilepsia. 2016;57:1228–1235. doi: 10.1111/epi.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]


