Abstract
Background
Numerous factors influence late-life depressive symptoms in adults, many not thoroughly characterized. We addressed whether genetic and environmental influences on depressive symptoms differed by age, sex, and physical illness.
Methods
The analysis sample included 24,436 twins aged 40 through 90 drawn from the Interplay of Genes and Environment across Multiple Studies (IGEMS) consortium. Biometric analyses tested age, sex, and physical illness moderation of genetic and environmental variance in depressive symptoms.
Results
Women reported greater depressive symptoms than men. After age 60, there was an accelerating increase in depressive symptom scores with age, but this did not appreciably affect genetic and environmental variances. Overlap in genetic influences between physical illness and depressive symptoms was greater in men than in women. Additionally, in men extent of overlap was greater with worse physical illness (the genetic correlation ranged from near .00 for the least physical illness to nearly .60 with physical illness two SD above the mean). For men and women, the same environmental factors that influenced depressive symptoms also influenced physical illness.
Conclusions
Findings suggested that genetic factors play a larger part in the association between depressive symptoms and physical illness for men than for women. For both sexes, across all ages, physical illness may similarly trigger social and health limitations that contribute to depressive symptoms.
Keywords: depressive symptoms, twin studies, heritability, aged
Introduction
Depressive symptoms are common throughout adulthood. These symptoms often impair daily function, especially in older adults, even when their total number does not meet criteria for clinical diagnosis (Judd et al., 2002). Both cross-sectional and longitudinal reports have observed U-shaped relations between depressive symptom scores and age (Kessler et al., 1992; Sutin et al. 2013). Additionally, at most ages, women report more depressive symptoms than men, although the difference may diminish in very old age (e.g., Berkman et al., 1986; Forlani et al., 2014).
In older twins, genetic influences generally account for up to 30% of the variance in symptom scores (Johnson et al., 2002; Schnittker, 2014). Possible age and sex differences in heritability of depressive symptoms have been considered using twin designs. Most find similar heritability estimates of depressive symptoms for men and women (Gillespie et al., 2004). Some studies have observed greater heritability of depressive symptoms with higher age (e.g., Carmelli et al., 2000), although others did not find significant age differences in heritability (McGue & Christensen, 1997; Johnson et al., 2002). Because heritability estimates represent the relative proportions of genetic and environmental variance components, greater heritability may be a function of reduced environmental influences, increased genetic influences, or both.
Physical illness is a well-established risk factor for depressive symptoms, particularly in later life (e.g., Meeks et al., 2000; Djernes, 2006), with these observations not simply due to overlap with somatic symptoms used in diagnosing depression (Sutin et al., 2013). Depressive symptoms and depressive disorders are elevated among medical inpatients and primary care outpatients (Blazer, 2003). The association between depression and physical illness may lie in vascular, neuroendocrine, or inflammatory manifestations of physical illness that also contribute to risk for explicit depressive symptoms (Fiske et al., 2009; Steptoe, 2007). There may be common factors such as low education or high neuroticism that predispose to both physical illness and depression (Steptoe, 2007). Physical illness may occasion emotional distress and existential struggles that lead to elevated depressive symptoms (Steptoe, 2007). As well, physical illness may impose functional limitations and disruptions to daily living that potentiate depression, representing a behavioral pathway (Fiske et al., 2009).
Twin studies can be valuable in illuminating the extents to which genetic and environmental sources of influence explain observed covariation between depressive symptoms and physical illness. Vulnerability factors in common to physical illness and depressive symptoms, as well as proposed physiological pathways linking the two, suggest the possibility that genetic factors account for some of the association. Behavioral explanations support roles for environmental factors. Physical illness may also moderate genetic and environmental vulnerabilities to depressive symptoms, either uniquely or via shared pathways (Johnson, 2007).
In this paper, we explored the cross-sectional interplay among depressive symptoms, physical illness, sex, and age. Prior twin studies of depressive symptoms have not considered the role of physical illness, and research pointing to co-occurring physical illness and depressive symptoms in older adults has not addressed how genetic and environmental influences interplay. We thus examined whether genetic and environmental variance in depressive symptoms varies with sex, age, and different levels of physical illness.
Method
Participants
Summarized in Table 1, the sample was drawn from ten independent studies from the Interplay of Genes and Environments across Multiple Studies (IGEMS) consortium (Pedersen et al., 2013). A total of 24,436 individual twins (53.1% women) had relevant data. Depressive symptom data were taken from the first occasion at which each participant answered self-report depressive symptom questionnaires. Incomplete twin pairs were included, as they are informative about age differences in means and variances. Demographic characteristics in Table 1 include numbers of monozygotic (MZ), same-sex dizygotic (SSDZ), and opposite-sex dizygotic (OSDZ) pairs. Demographics by study are in Table S1.
Table 1.
Mean scores on depressive symptoms and physical illness (with standard deviations), and phenotypic correlations, intra-pair correlations, and cross-twin cross-trait correlations (with 95% confidence intervals)
| N pairs | Depressive symptoms mean(SD) | I-CIRS Mean (SD) | Phenotypic Correlation (95% CI) | Depressive symptoms intra-pair correlation (95% CI) | I-CIRS Intra-pair correlation (95% CI) | Cross-twin cross-trait correlation (95% CI) | |
|---|---|---|---|---|---|---|---|
| Men | 3605 | 19.43 (4.17) | 1.59 (1.66) | .20 (.18–.21) | .25 (.22–.28) | .27 (.24–.30) | .09 (.06–.12) |
| Women | 5331 | 20.42 (4.80) | 1.97 (1.80) | .17 (.15–.18) | .24 (.21–.26) | .29 (.26–.31) | .08 (.05–.11) |
| MZM | 1621 | 19.45 (4.34) | 1.55 (1.61) | .19 (.15–.22) | .33 (.28–.37) | .35 (.31–.39) | .08 (.03–.13) |
| MZW | 1762 | 20.30 (4.77) | 1.91 (1.80) | .16 (.13–.19) | .36 (.32–.40) | .38 (.34–.42) | .11 (.06–.15) |
| DZM | 1984 | 19.60 (4.15) | 1.63 (1.71) | .19 (.17–.22) | .18 (.14–.22) | .20 (.16–.24) | .10 (.05–.14) |
| DZW | 2416 | 20.69 (4.90) | 2.05 (1.84) | .15 (.13–.18) | .16 (.12–.20) | .24 (.20–.27) | .04 (.01–.08) |
| DZOS | 1153 | 19.11 (3.98) | 1.66 (1.58) | .25 (.22–.29) | .15 (.09–.21) | .24 (.19–.29) | .12 (.06–.17) |
| Total | 8936 | 19.95 (4.54) | 1.79 (1.75) | .19 (.18–.20) | .25 (.23–.27) | .29 (.27–.31) | .09 (.07–.11) |
Notes: I-CIRS = IGEMS revision of Cumulative Illness Rating Scale; MZM = monozygotic male pairs; MZW=monozygotic female pairs; DZM=same-sex dizygotic male pairs; DZW= same-sex dizygotic female pairs; DZOS=opposite-sex dizygotic pairs.
Twins in Swedish Adoption Twin Study of Aging (SATSA; Finkel & Pedersen, 2004), Origins of Variance in the Oldest-Old (OCTO-Twin; McClearn et al., 1997), Ageing in Women and Men: A Longitudinal Study of Gender Differences in Health Behaviour and Health among Elderly (GENDER; Gold et al., 2002), and Twin and Offspring Study in Sweden (TOSS; Neiderhiser & Lichtenstein, 2008) were ascertained from the population-based Swedish Twin Registry. SATSA includes all same-sex twins from the Swedish Twin Registry who were reared apart and a matched sample who were reared together, with baseline depressive symptoms assessed at the first questionnaire follow-up in 1987. OCTO-Twin includes same-sexed pairs who both survived until age 80, with baseline in 1991. GENDER consists of OSDZ twin pairs born 1906 – 1925, with baseline in 1994. Baseline for TOSS was 1997 or 2004.
Longitudinal Study of Aging Danish Twins (LSADT; Christensen et al., 1999) and Middle-Age Danish Twins (MADT; Skytthe et al., 2013) were ascertained from the Danish Twin Registry. LSADT includes same-sex twin pairs age 70 years or older, with baseline in 1995. MADT includes both same- and opposite-sex twin pairs born 1931 – 1952, with baseline in 1998.
The Minnesota Twin Study of Adult Development and Aging (MTSADA; Finkel & McGue, 1993) includes same-sex twin pairs aged 60 and older ascertained through Minnesota state birth records, with baseline data collected 1984 – 1994. The Vietnam Era Twin Study of Aging (VETSA; Kremen, et al., 2006) includes male twins aged 51 – 60 at baseline in 2003 – 2007. The twin subsample of Midlife in the United States (MIDUS; Kendler et al., 2000) includes same- and opposite-sex twin pairs, with depressive symptoms collected at follow-up in 2007.
The older Finnish Twin Cohort (FTC; Kaprio & Koskenvuo, 2002) is a registry of same-sex Finnish twin pairs with age range comparable to the Swedish and Danish twin registries. Depressive symptom data reported here come from wave 4 in 2011 (Kaprio, 2013).
All procedures contributing to this work complied with ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Previous publications from SATSA (Gatz et al., 1992), LSADT (McGue & Christensen, 1997), MADT (Johnson et al., 2002), VETSA (Franz et al., 2011), and FTC (Takkinen et al., 2004; Korhonen et al., 2007) have presented results from behavior genetic analyses of depressive symptoms. The benefits of the IGEMS consortium are a large sample of twins offering increased power to detect potentially small moderation effects of physical illness, age, and sex and inclusion of a large number of opposite-sex twin pairs to explore potential sex differences in genetic and environmental influences on depressive symptoms.
Zygosity
Specific methods for zygosity determination varied across studies, but in most cases involved use of standard questionnaires about physical similarity between twins and DNA analysis to resolve uncertain cases.
Measures
Depressive symptoms
Two measures of depressive symptoms were used in IGEMS studies: the Center for Epidemiologic Studies-Depression (CES-D) scale (Radloff, 1977) and a modified version of the Cambridge Mental Disorders of the Elderly Examination (CAMDEX; Roth et al., 1986). The CES-D score is the sum of 20 items, each answered from 0–3 to indicate frequency of experiencing the symptom during the past week. Four positively worded items were reverse-scored so that higher scores indicated more severe depressive symptoms. Previous research supports a 4-factor structure representing depressed mood, psychomotor retardation/somatic symptoms, lack of well-being (the 4 reverse-scored items), and interpersonal difficulties (Radloff, 1977; Gatz et al., 1992).
The CAMDEX was administered only to Danish twins and consists of 16 items assessing frequency of depressive symptoms. Items were answered from 1–3 to indicate frequency of experiencing the symptom, except for 2 items rated on a “yes/no” scale. Items were scored so that higher scores indicate more severe depressive symptoms. The CAMDEX consists of two factors: affective and somatic symptoms (McGue & Christensen, 1997).
To create a common depressive symptom metric, CESD and CAMDEX scales were administered to a separate data-harmonization sample. Item-response theory methods were applied to compare items from the two measures and create a conversion table between the scales (Gatz et al., 2015b). For substantive comparability, we retained only the 14 CESD items that loaded onto the depressed mood and psychomotor retardation/somatic symptoms subscales. The co-calibrated score was expressed in CAMDEX units, such that the total score could range from 16 for someone who endorsed no symptoms of depression to a maximum of 46 for someone who endorsed the most severe frequency of depressive symptoms. Because the harmonized measure did not include the same items for those who completed different scales, we calculated separate reliabilities for each scale’s harmonization-relevant items. For those who completed the CESD, alpha =.90 (N=16,177), while for those who completed the CAMDEX, alpha=.84 (N=8,718).
Physical illness
To create a harmonized index of physical illness (I-CIRS; Gatz et al., 2015a), we created a revised version of the Modified Cumulative Illness Rating Scale (CIRS: Salvi et al., 2008). I-CIRS is a list of 13 body systems, with a score that represents the number of systems with moderate disability or morbidity. All studies had self-reported lists of physical illnesses that we recoded to be consistent across IGEMS studies. I-CIRS categories included cardiac; hypertension; vascular (circulatory); respiratory; eye, ear, nose and throat; upper gastro-intestinal; hepatic; renal; musculoskeletal; neurological (excluding stroke which was rated in a separate category); endocrine/metabolic; cancer; and stroke. Each participant received a score from 0–13, with higher scores indicating more systems affected by illness.
Statistical Analysis
Depressive symptoms and I-CIRS were positively skewed, so scores were log-transformed to approximate assumptions of multivariate normality. Depressive symptoms and I-CIRS further were standardized (mean= 0, SD=10) to ease computational burden. Twin pairs younger than 40 (240 complete pairs) or older than 90 (10 complete pairs) were removed from analyses due to sparse coverage.
We examined the phenotypic means of depressive symptoms by sex and linear, quadratic, and cubic continuous age using SAS Proc Mixed (SAS Inc., Cary, NC 2013) to account for dependencies between members of twin pairs. Intra-pair correlations for depressive symptoms and cross-twin cross-trait correlations for depressive symptoms and physical illness were calculated by sex, age (by decade), and zygosity (see table S2). To evaluate cohort effects, we formed a sample of 50- to 59-year-olds tested between 1987 and 1991 (comprised of SATSA and MTSADA) and a sample of 50- to 59-year-olds tested between 2003 and 2007 (comprised of TOSS twins from 2004, MIDUS twins, and a random sample from VETSA to make the two samples similar in size).
Twin studies allow variance in a measure to be decomposed into additive genetic variance (A), shared environmental variance (C), dominant genetic variance (D), and nonshared environmental variance including error (E). Shared environment refers to environmental influences that contribute to twin pair similarity, such as rearing environment or contact as adults; nonshared environment encompasses influences contributing to differences within a pair. Conventional twin methods possess too few degrees of freedom to estimate all ACDE components, but preliminary models suggested no evidence of dominant genetic influences. Variance decomposition relies on assumptions that SSDZ and OSDZ twins share 50% of their segregating genes, on average, while MZ twins share 100% of their genes; and that genetic and environmental biometric components are independent. Based on these assumptions, twin (“ACE”) models specify 3 sets of equations based on degree of genetic relationship: MZ = covMZ = Va + Vc; SSDZ = covSSDZ = 0.5*Va + Vc; and OSDZ = covOSDZ=0.5*Va + Vc. All multivariate twin models were estimated in OpenMx 2.2.6 (Neale et al., 2015).
Qualitative sex differences (i.e., different sources of influences) were tested in univariate biometric models by freely estimating the genetic correlation in OSDZ pairs instead of constraining it to be .50 as well as freely estimating the shared environmental correlation instead of constraining it to be 1.00. An estimated genetic correlation between OSDZ twins significantly less than .50 suggests qualitative sex differences. Due to documented problems estimating quantitative sex-limitation in bivariate models (Neale et al., 2006), bivariate moderation models were estimated separately for males and females using only SSDZ twins. To test significance of sex differences we constrained parameter estimates for women to equal those for men. We compared this constrained model to the model allowing women’s parameter estimates to be freely estimated.
We fit age-moderated univariate biometric models (van der Sluis et al., 2012) to address whether and how continuous age moderated genetic and environmental variance components underlying depressive symptoms. Preliminary results suggested nonlinear age differences in depressive symptom scores. These were best characterized as two slopes with a turning point at age 75 (see Fig. S3). Therefore, we estimated two linear age effects centered at age 75 – with age recoded into two variables: age 40 to 75 (i.e., AgeA=age – 75; if age ≥ 75 then ageA=0) and aged 75 to 90 (AgeB= age – 75; if age < 75 then ageB=0).
Under the model, additive genetic, shared environmental, and nonshared environmental variances are expressed as:
The “a” parameter is the estimated additive genetic variance at the age-75 turning point, βa40 is the estimate of the linear slope on additive genetic variance for participants aged 40 – 75, and the βa75 is the second linear slope for participants aged 75 – 90. The C and E variance components are computed in the same way. Likelihood-ratio-tests (LRT) were used to compare nested models.
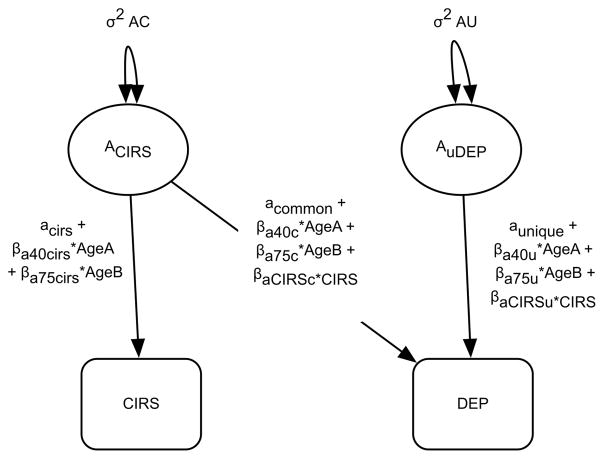
We fit continuous age and I-CIRS-moderated bivariate biometric models to test whether I-CIRS and age moderated genetic and environmental variance between I-CIRS and depressive symptoms (Johnson, 2007). Figure 1 depicts the additive genetic variance components of this model, which were expressed analogously for the shared and nonshared environmental components (not presented). The model estimated the genetic variance of I-CIRS (aCIRS), the genetic covariance between I-CIRS and depressive symptoms (acommon), and a genetic variance component unique to depressive symptoms (aunique). Age-moderating terms for 40–75 and 75–90 year olds on depressive symptoms and I-CIRS were included in the I-CIRS, common, and unique depressive symptoms paths. Genetic (rG) and environmental (rC and rE) correlations between I-CIRS and depressive symptoms were calculated to aid in interpreting the covariance. The equation for genetic correlation, for example, is:
Figure 1.
Diagram of the additive genetic components from the age and I-CIRS moderated bivariate biometric model examining physical health (CIRS) and depressive symptoms (DEP). Note that slope a40 pertains to individuals age 40–75 while slope a75 pertains to individuals 75 and older. The equation for calculating the genetic covariance between I-CIRS and depressive symptoms is the product of the pathway shared between CIRS and depressive symptoms and the genetic pathway on CIRS. The equation can be written as: (aCommon + βa40c * AgeA + βas75c * AgeB + βaCIRSc * CIRS) * (aCIRS + βa40CIRS * AgeA + βa40CIRS * AgeB)
A possible alternative explanation to the variance moderation model is the uniform nonlinear main effects model. To address this possible explanation we ran a nonlinear main effects model of I-CIRS on depressive symptoms (Van Hulle et al., 2013) and compared this model to the variance moderation model.
Results
Descriptive Statistics
Harmonized depressive symptom scores and I-CIRS scores by age group and sex, pooled across studies are shown in Table 1, and by age group in Table S2. Depressive symptom scores were positively correlated with physical illness. Results from mixed-effects regression models indicated significant age and sex associations with depressive symptoms. Women scored significantly higher than men, and both sexes showed accelerating (cubic) score increases with age (Fig. S3). The variability of depressive symptoms was greater in women than in men and was larger at older ages than at younger ages for both men and women (Table S2). There was no significant interaction between age and sex. I-CIRS scores increased quadratically with age in both sexes. The phenotypic association between depressive symptoms and physical illness was linear, and correlations showed no systematic differences with age.
There were differences in mean depressive symptom scores across participating studies (Table S4). However, differences were not systematic by country or by original measure of depressive symptoms, and adding study to the biometric models did not affect findings.
Next, we evaluated whether there were cohort differences in depressive symptoms (Table S5). We compared 50- to 59-year-olds tested between 1987 and 1991 with 50- to 59-year-olds tested between 2003 and 2007 and found no significant differences between the earlier cohort (M = 20.09, SD = 4.76, N = 402) and the later cohort (M = 20.05, SD = 4.56, N = 400). There was no evidence of interaction between sex and cohort.
Twin correlations (shown in Table 1) suggested genetic and nonshared environmental influences on both depressive symptoms and I-CIRS in male and female twins. For most ages there was little evidence of shared environmental influences; however, there was some evidence for them (i.e., DZ correlation greater than half the MZ correlation) on depressive symptoms at ages 80–90 (Table S2). The small but significant MZ and DZ cross-twin cross-trait correlations were similar for men but not for women. Similar correlations indicate overlapping shared environmental influences, while larger correlations for MZ than for DZ twins suggest that additive genetic effects likely mediate the association between the two traits.
Biometric Model-Fitting Results
Age-moderated univariate biometric models with qualitative sex-limitation suggested no qualitative sex differences in genetic influences or shared environmental influences on depressive symptoms (rG=.50, 95% CI = .39–.50; rC =1.00, 95% CI = 0.91–1.00). This result was expected given the similar correlations for OSDZ and SSDZ pairs. Consistent with greater variance in women, magnitudes of genetic and environmental influences could not be constrained equal without significant deterioration of model fit (see Table S6, with parameter estimates for the full univariate model in Table S7). Therefore, separate age-moderated bivariate biometric models were calculated for men and for women. Bivariate model-fitting results are shown in Table 2 (with additional details in S8 and S9 for males and females, respectively). For both sexes, shared environmental influences were very small, not significantly different from 0.00, and could be dropped without significant reduction in model fit (see Table 2, model 2). Table 3 presents the unstandardized parameter estimates from the best-fitting model, which was model 5 (parameter estimates for the full bivariate model are in Table S10).
Table 2.
Model fitting results for I-CIRS and age-moderated bivariate biometric models of depressive symptoms
| Model | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| −2LL | df | Comp | Δ−2LL (Δdf) | −2LL | df | Comp | Δ−2LL (Δdf) | ||
| 1. | Full ACE | 108024 | 14850 | - | - | 126727 | 17240 | - | - |
| 2. | AE | 108032 | 14861 | 1 | 8.14 (11) | 126742 | 17251 | 1 | 15.24 (11) |
| 3. | Drop all CIRS moderation | 108317 | 14865 | 2 | 285.03 (4)** | 126904 | 17255 | 2 | 161.91 (4)** |
| 4. | Drop age 40–75 covariance moderation | 108034 | 14863 | 2 | 2.29 (2) | 126744 | 17253 | 2 | 2.23 (2) |
| 5. | Drop age 75–90 covariance moderation | 108036 | 14865 | 4 | 1.19 (2) | 126744 | 17255 | 4 | 0.02 (2) |
| 6. | Drop age 40–75 unique depression moderation | 108072 | 14867 | 5 | 35.86 (2)** | 126759 | 17257 | 5 | 14.11 (2)** |
| 7. | Drop age 75–90 unique depression moderation | 108056 | 14867 | 5 | 20.17 (2)** | 126754 | 17257 | 5 | 9.02 (2)** |
Notes: More complete model-fitting results in Tables S8 and S9. −2LL = negative log likelihood multiplied by 2; df= degrees of freedom; Comp= model to which compared, Δ−2LL (Δdf) = the difference in log likelihood by difference in degrees of freedom between the model being fit and its comparison model; A = additive genetic variance; C = shared environmental variance; E = non-shared environmental variance; ACE = full model estimating additive genetic, shared environmental, and nonshared environmental variance components; AE= constrained model estimating only additive genetic and nonshared environmental variance components;
= significant deterioration in fit compared to comparison model. Best-fitting model is shown in bold.
Table 3.
Unstandardized parameter estimates (95% confidence intervals) from the best fitting bivariate biometric model from table 2 (model number 5) of depressive symptoms
| Parameter | Men | Women | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |||
| Lower | Upper | Lower | Upper | |||
| Additive genetic | ||||||
| aCIRS | 6.00 | 5.14 | 6.84 | 5.18 | 4.53 | 5.81 |
| acommon | 0.28 | 0.22 | 0.32 | 0.26 | 0.18 | 0.32 |
| aunique | 3.96 | 3.12 | 4.74 | 5.41 | 4.63 | 6.16 |
| βaCIRSc | 0.16 | 0.01 | 0.33 | 0.01 | −0.04 | 0.07 |
| βa40u | −0.06 | −0.10 | 0.01 | −0.01 | −0.06 | 0.03 |
| βa75u | 0.20 | −0.08 | 0.45 | 0.16 | −0.03 | 0.34 |
| βaCIRSu | 0.03 | −0.02 | 0.08 | 0.02 | −0.02 | 0.07 |
| Non-shared Environmental | ||||||
| eCIRS | 7.90 | 7.35 | 8.46 | 7.12 | 6.69 | 7.55 |
| ecommon | 0.14 | 0.10 | 0.17 | 0.15 | 0.12 | 0.19 |
| eunique | 6.80 | 6.30 | 7.32 | 7.59 | 7.10 | 8.10 |
| βeCIRSc | 0.09 | 0.05 | 0.13 | 0.11 | 0.07 | 0.15 |
| βe40u | −0.04 | −0.01 | −0.07 | −0.03 | −0.06 | −0.01 |
| βe75u | 0.16 | 0.01 | 0.33 | 0.03 | −0.09 | 0.16 |
| βeCIRSu | 0.10 | 0.07 | 0.13 | 0.06 | 0.03 | 0.09 |
Notes: aCIRS represents the additive genetic genetic path estimate to the additive genetic I-CIRS factor at age 75; acommon = the common pathway estimate of the additive genetic contributions of I-CIRS on depressive symptoms; aunique = the estimated variance unique to depressive symptoms at age 75; βaCIRSc = the estimate of the moderating effect of I-CIRS on the additive genetic covariance. βa40u = the estimate of the linear slope on the additive genetic variance unique to depressive symptoms for participants aged 40 to 75; βa75u = the estimate of the linear slope on the additive genetic variance unique to depressive symptoms for participants aged 75 to 90. βaCIRSu = the estimate of the moderating effect of I-CIRS on the additive genetic variance unique to depressive symptoms. The parameters denoted with an e represent corresponding parameter estimates for the non-shared environmental contributions.
Genetic and environmental influences on depressive symptoms
Results for sex and age moderation of depressive symptoms were similar in the univariate analyses of depressive symptoms and for influences unique to depressive symptoms in the bivariate model. In the full univariate model (Tables S7), additive genetic influences were greater for women than for men; in the best-fitting bivariate model (Table 3), this pattern held but with the confidence intervals somewhat overlapping.
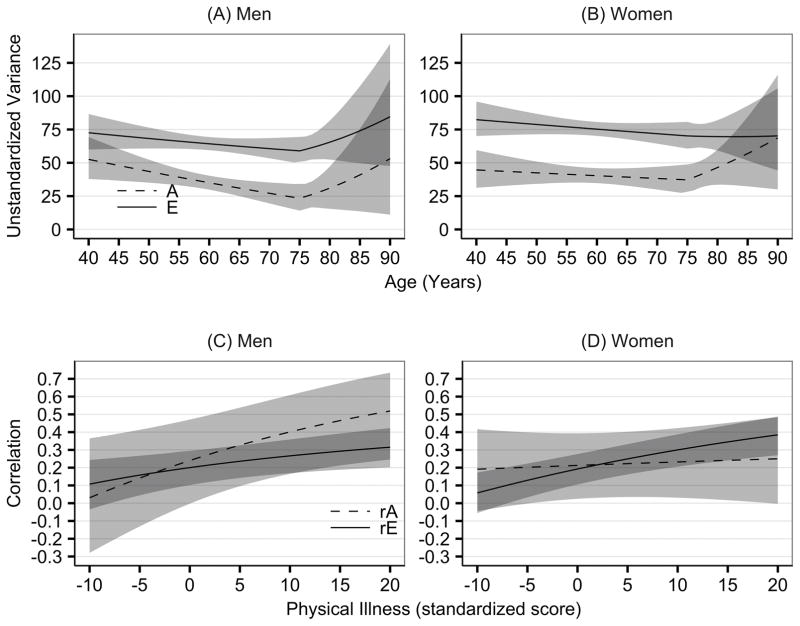
Figure 2a–b presents total (common plus unique) unstandardized A and E variance of depressive symptoms as a function of age. Although age moderation of influences unique to depression could not be dropped, the effects were quite small. Specifically, for both men and women, nonshared environmental variances were smaller with greater age until age 75. After age 75, for men only, nonshared environmental contributions accounted for larger amounts of variance at older than at younger ages. Heritability estimates, which represent relative proportions of genetic and environmental variance, were not significantly different for men and women: At age 75, h2 for men was 28.6% [95% CI = 21.7% – 33.7%] and for women 34.7% [95% CI = 30.6% – 37.8%].
Figure 2.
Graphs of the estimated unstandardized genetic and environmental variance components for depressive symptoms for men (panel A) and women (panel B) by age from combining the common and unique raw variance estimates from the AE age and I-CIRS moderated bivariate biometric model. Graphs of the estimated genetic, and non-shared environmental correlations between I-CIRS and depressive symptoms by I-CIRS score for men (panel C) and women (panel D) shown at age 75 for purposes of illustration.
Notes: A = additive genetic variance; E = non-shared environmental variance; rG = genetic correlation between depressive symptoms and I-CIRS; rE = non-shared environmental correlation between depressive symptoms and I-CIRS. Shaded area represents 95% confidence interval of the estimate. The I-CIRS score was standardized so the mean CIRS score equals zero and with standard deviation equal plus or minus 10.
GE interplay in association between depressive symptoms and physical illness
Physical illness significantly moderated genetic and environmental contributions to depressive symptoms. There was significant overlap in both genetic and nonshared environmental influences between I-CIRS and depressive symptoms. From the information in Table 3, we calculated the proportion of variance in depressive symptoms that was shared with physical illness. For men, at age 75, with a mean I-CIRS score, 15% (95% CI = 12% – 18%) of the genetic variance and 2% (95% CI = 1% – 4%) of the nonshared environmental variance in depressive symptoms was shared with physical illness. The analogous figures for women were 6% of the genetic variance (95% CI = 3% – 8%) and 2% (95% CI = 1% – 3%) of the nonshared environmental variance. These figures reflect both the greater phenotypic correlation in men and greater I-CIRS variance moderation.
Consistent with variance moderation, genetic correlations varied with level of physical illness for men but not women (Figures 2c and 2d). That is, for men there was greater overlap in additive genetic influences underlying I-CIRS and depressive symptoms at higher I-CIRS scores than at lower I-CIRS scores, regardless of age. The genetic correlation ranged from near .00 for the least physical illness to nearly .60 with physical illness two SD above the mean. Also referring to Figures 2c and 2d, environmental factors contributing to twin differences in I-CIRS scores accounted for more of the environmental variation in depressive symptoms at higher I-CIRS scores than at lower I-CIRS scores in both sexes. As well, referring to βeCIRSu in Table 3, for both men and women, greater physical illness was associated with greater nonshared environmental contributions unique to depressive symptoms.
Nonlinear and linear means moderation did not provide better model fit in either sex, suggesting that nonlinear uniform main effects of I-CIRS on depressive symptoms were not explaining the moderation detected in these models.
Discussion
We explored the cross-sectional interplay among depressive symptoms, physical illness, sex, and age in a combined sample of 24,436 twins aged 40–90. We highlight five findings. First, although additive genetic variance in depressive symptoms was greater in women than men, heritability of depressive symptoms was not significantly different for men and women. Finding no sex differences in heritability is consistent with past work with depressive symptoms (e.g., Johnson et al., 2002; Gillespie et al., 2004) although some research has reported greater heritability of depressive disorders in women than men (e.g., Bierut et al., 1999; Kendler et al., 2001; Kendler et al., 2006). The sex-limitation model indicated that the same genes influenced depression in women and men, although others have reported that different genes influenced depressive illness in men and women (Kendler & Gardner, 2014).
Second, for men but not for women, nonshared environmental variance in depressive symptoms (i.e., influences on depressive symptoms unique to one individual in a twin pair) was greater at higher ages. Some have suggested that men, in particular, may be increasingly sensitive to stressful life events—including health issues—with age (Sonnenberg et al., 2000; Kendler & Gardner, 2014; Forliani et al., 2014). This account might explain the patterns of nonshared environmental variances after age 75.
Third, physical illness significantly moderated nonshared environmental influences unique to depressive symptoms. Regardless of sex, greater self-reported physical illness was associated with greater nonshared environmental influences on the unique variance in depressive symptoms.
Fourth, overlap in genetic influences between physical illness and depressive symptoms was greater for men than for women. In men, as well, the genetic correlation between depressive symptoms and physical illness was higher with greater physical illness. These effects did not differ with age. Analyses of genetic correlations derived from summary statistics of common disorders from genome-wide association studies indicate shared genetic effects of major depressive disorder with multiple psychiatric, neurological and cardiovascular traits and risk factors (Anttila et al., 2016).
Fifth, nonshared environmental influences mediated the association between physical illness and depressive symptoms, suggesting small but potentially directly causal influences (Heath et al., 1993; Turkheimer & Harden, 2013). These environmental influences did not vary with age; however, they were stronger with greater physical illness. With poorer health, environmental influences that make people ill (e.g. poor diet, lack of physical exercise) may also contribute to experiencing more depressive symptoms.
We note several limitations. First, the data were cross-sectional so intra-individual changes in depressive symptoms over time could not be observed. Cohort differences, however, were not statistically significant. Additionally, previous research does not suggest secular diminution of CES-D scores in younger cohorts (Radloff, 1977; Berkman et al., 1986; Erlich & Isaakowitz, 2002; Twenge, 2015).
Second, reverse causality remains an alternative possibility in this study, including reciprocal processes between depressive symptoms and physical illness (Steptoe, 2007; Scott et al., 2016). Here, longitudinal data are preferable to cross-sectional data to disentangle direction of causation (Heath et al., 1993) and dual change mechanisms (McArdle & Hamagami, 2003). Third, twins’ histories of depression were unknown. Onset of depressive symptoms in late life might have different etiologies than chronic or recurring depressive symptoms (Fiske et al., 2009). Fourth, other potential risk factors for depression such as personality characteristics, smoking, use of alcohol, and stressful life events unrelated to illness were not included in the models. Fifth, results may have been influenced by differences in measurement or recruiting procedures among the IGEMS studies, although we were not able to identify any. Sixth, while the sample was large and diverse in many respects, population-based, and not selected for depressive symptoms or physical illness, ethnic and racial compositions were fairly homogenous.
Overall, results showed that co-occurring physical illness and depressive symptoms in older adults reflects both genetic and environmental mechanisms. Results suggesting shared genes may indicate common pathogenic pathways. Those for nonshared environmental influences suggest that physically ill twins may face experiences (e.g., functional limitations, pain, disruptions to daily living) that lead to decreased physical and social engagement, elevating depressive symptoms compared to their physically healthy co-twins. One implication is that alternations in physically ill older adults’ environments, such as better chronic-pain treatment, sleep therapy, assistive devices, and better overall disease management may offset these effects. Interventions directed at the physical symptoms might be paired with behavioral therapies for depression that emphasize learning new skills to adapt to physical limitations and challenges.
Supplementary Material
Acknowledgments
Members of the consortium on Interplay of Genes and Environment across Multiple Studies (IGEMS) include: Nancy L. Pedersen (Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, and Department of Psychology, University of Southern California, Los Angeles, CA), Kaare Christensen (Department of Epidemiology, University of Southern Denmark, Odense, Denmark), Anna Dahl Aslan (Institute of Gerontology, School of Health Sciences, Jönköping University, Jönköping, Sweden), Deborah Finkel (Department of Psychology, Indiana University Southeast, New Albany, IN), Carol E. Franz (Department of Psychiatry, University of California, San Diego, La Jolla, CA), Margaret Gatz (Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, and Department of Psychology, University of Southern California, Los Angeles, CA), Briana N. Horwitz (Department of Psychology, California State University, Fullerton, CA), Boo Johansson (Department of Psychology, University of Gothenburg, Gothenburg, Sweden), Wendy Johnson (Department of Psychology and Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, UK), Jaakko Kaprio, Department of Public Health, University of Helsinki, Helsinki, Finland), William S. Kremen (Department of Psychiatry, University of California, San Diego, and VA Center of Excellence for Stress and Mental Health, La Jolla, CA), Robert Krueger (Department of Psychology, University of Minnesota, Minneapolis, MN), Michael J. Lyons (Department of Psychological and Brain Sciences, Boston University, Boston, MA), Matt McGue (Department of Psychology, University of Minnesota, Minneapolis, MN), Miriam A. Mosing (Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden), (Jenae M. Neiderhiser (Department of Psychology, The Pennsylvania State University, University Park, PA), Matthew S. Panizzon (Department of Psychiatry, University of California, San Diego, La Jolla, CA), Inge Petersen (Department of Epidemiology, University of Southern Denmark, Odense, Denmark), and Chandra A. Reynolds (Department of Psychology, University of California-Riverside, Riverside, CA).
Financial Support
IGEMS is supported by the National Institutes of Health (grant number R01 AG037985). SATSA was supported by the National Institutes of Health (grants number R01 AG04563, R01 AG10175), the MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS; 97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141). OCTO-Twin was supported by the National Institutes of Health (grant number R01 AG08861). Gender was supported by the MacArthur Foundation Research Network on Successful Aging, The Axel and Margaret Ax:son Johnson’s Foundation, The Swedish Council for Social Research, and the Swedish Foundation for Health Care Sciences and Allergy Research. TOSS was supported by the National Institutes of Health (grant number R01 MH54610). The Danish Twin Registry is supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science and Innovation, the Velux Foundation and the National Institutes of Health (grant number P01 AG08761). The Minnesota Twin Study of Adult Development and Aging was supported by the National Institutes of Health (grant number R01 AG 06886). VETSA was supported by National Institutes of Health (grant numbers R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982) and resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. MIDUS was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by the National Institutes of Health (grant number P01 AG20166).
Footnotes
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of Interest
None
Contributor Information
Andrew J. Petkus, Department of Psychology, University of Southern California, Los Angeles, CA 90089
Christopher R. Beam, Department of Psychology & Davis School of Gerontology, University of Southern California, Los Angeles, CA 90089
Wendy Johnson, Centre for Cognitive Ageing and Cognitive Epidemiology and Department of Psychology, University of Edinburgh, EH-89JZ Edinburgh, UK.
Jaakko Kaprio, Department of Public Health, University of Helsinki, 00014 Helsinki, Finland. Institute for Molecular Medicine (FIMM), University of Helsinki, 00014 Helsinki, Finland.
Tellervo Korhonen, Department of Public Health, University of Helsinki, 00014 Helsinki, Finland. Institute for Molecular Medicine (FIMM), University of Helsinki, 00014 Helsinki, Finland. Institute of Public Health and Clinical Nutrition, University of Eastern Finland, 70211 Kuopio, Finland.
Matt McGue, Department of Psychology, University of Minnesota, Minneapolis, MN, 55455. The Danish Twin Registry, University of Southern Denmark, Institute of Public Health, Epidemiology, DK-5000 Odense C, Denmark.
Jenae M. Neiderhiser, Department of Psychology, Penn State University, University Park, PA 16802
Nancy L. Pedersen, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, SE 171 77 Stockholm, Sweden. Department of Psychology, University of Southern California, Los Angeles, CA 90089
Chandra A. Reynolds, Department of Psychology, University of California Riverside, Riverside, CA 92521
Margaret Gatz, Department of Psychology, University of Southern California, Los Angeles, CA 90089. Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, SE 171 77 Stockholm, Sweden.
References
- Anttila V, et al. Analysis of shared heritability in common disorders of the brain. bioRxiv. 2016 doi: 10.1101/048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Berkman CS, Kasl S, Freeman DH, Jr, Leo L, Ostfeld AD, Cornoni-Huntley J, Brody J. Depressive symptoms in relation to physical health and functioning in the elderly. American Journal of Epidemiology. 1986;124:372–388. doi: 10.1093/oxfordjournals.aje.a114408. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PA, Statham DJ, Dunne MP, Martin NG. Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Archives of General Psychiatry. 1999;56:557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: Review and commentary. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58A:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Kelly-Hayes M, Wolf PA, Reed T, Miller B. Longitudinal changes in the contribution of genetic and environmental influences to symptoms of depression in older male twins. Psychology and Aging. 2000;15:505–510. doi: 10.1037//0882-7974.15.3.505. [DOI] [PubMed] [Google Scholar]
- Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. Journal of Aging and Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatrica Scandinavia. 2006;113:372–387. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich BS, Isaacowitz DM. Does subjective well-being increase with age? Perspectives in Psychology. 2002;5:20–26. [Google Scholar]
- Finkel D, McGue M. The origins of individual differences in memory among the elderly: a behavior genetic analysis. Psychology and Aging. 1993;8:527–537. doi: 10.1037//0882-7974.8.4.527. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition Special Issue: Longitudinal studies of cognitive aging. 2004;11:325–345. [Google Scholar]
- Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annual Review of Clinical Psychology. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlani C, Morri M, Ferrari B, Dalmonte E, Menchetti M, De Ronchi D, Atti AR. Prevalence and gender differences in late-life depression: a population-based study. American Journal of Geriatric Psychiatry. 2014;22:370–380. doi: 10.1016/j.jagp.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Franz CE, Lyons MJ, O’Brien R, Panizzon MS, Kim K, Bhat R, Grant MD, Toomey R, Eisen S, Xian H, Kremen WS. A 35-Year Longitudinal Assessment of Cognition and Midlife Depression Symptoms: The Vietnam Era Twin Study of Aging. The American Journal of Geriatric Psychiatry. 2011;19:559–570. doi: 10.1097/JGP.0b013e3181ef79f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. The importance of shared genes and shared environments for symptoms of depression in older adults. Journal of Abnormal Psychology. 1992;101:701–708. doi: 10.1037//0021-843x.101.4.701. [DOI] [PubMed] [Google Scholar]
- Gatz M, Petkus A, Reynolds CA, Franz C, Kaprio J, Christensen K for the IGEMS Consortium. Age moderation of individual differences in chronic medical illness burden [abstract] Behavior Genetics. 2015a;45:657. [Google Scholar]
- Gatz M, Reynolds CR, Finkel D, Hahn CJ, Zhou Y, Zavala C. Data harmonization in aging research: not so fast. Journal of Experimental Aging Research. 2015b;41:475–495. doi: 10.1080/0361073X.2015.1085748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Kirk KM, Evans DM, Heath AC, Hickie IB, Martin NG. Do the genetic or environmental determinants of anxiety and depression change with age? A longitudinal study of Australian twins. Twin Research. 2004;7:39–53. doi: 10.1375/13690520460741435. [DOI] [PubMed] [Google Scholar]
- Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and health: a study of older unlike-sex twins. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2002;57B:S168–S176. doi: 10.1093/geronb/57.3.s168. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross sectional family data. Behavior Genetics. 1993;23:29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, Gaist D, Vaupel JW, Christensen K. Frequency and heritability of depression symptomatology in the second half of life: evidence from Danish twins over 45. Psychological Medicine. 2002;32:1175–1185. doi: 10.1017/s0033291702006207. [DOI] [PubMed] [Google Scholar]
- Johnson W. Genetic and environmental influences on behavior: capturing all the interplay. Psychological Review. 2007;114:423–440. doi: 10.1037/0033-295X.114.2.423. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Akiskal HS. The prevalence, clinical relevance, and public health significance of subthreshold depressions. Psychiatric Clinics of North America. 2002;25:685–698. doi: 10.1016/s0193-953x(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Kaprio J. The Finnish Twin Cohort Study: an update. Twin Research and Human Genetics. 2013;16:157–162. doi: 10.1017/thg.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Research and Human Genetics. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Sex differences in the pathways to Major Depression: a study of opposite-sex twin pairs. The American Journal of Psychiatry. 2014;171:426–435. doi: 10.1176/appi.ajp.2013.13101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychological Medicine. 2001;4:605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. American Journal of Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gilman SE, Kessler RC. Sexual orientation in a U.S. national sample of twin and nontwin sibling pairs. American Journal of Psychiatry. 2000;157:1843–1846. doi: 10.1176/appi.ajp.157.11.1843. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Foster C, Webster PS, House JS. The relationship between age and depressive symptoms in two national surveys. Psychology and Aging. 1992;7:119–126. doi: 10.1037//0882-7974.7.1.119. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Broms U, Varjonen J, Romanov K, Koskenvuo M, Kinnunen T, Kaprio J. Smoking behaviour as a predictor for depression among Finnish men and women – a prospective study of adult twins. Psychological Medicine. 2007;37:705–715. doi: 10.1017/S0033291706009639. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Structural equation models for evaluating dynamic concepts within longitudinal twin analyses. Behavioral Genetics. 2003;33:137–159. doi: 10.1023/a:1022553901851. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. Genetic and environmental contributions to depression symptomatology: evidence from Danish twins 75 years of age and older. Journal of Abnormal Psychology. 1997;106:439–448. doi: 10.1037//0021-843x.106.3.439. [DOI] [PubMed] [Google Scholar]
- Meeks S, Murrell SA, Mehl RC. Longitudinal relationships between depressive symptoms and health in normal older and middle-aged adults. Psychology and Aging. 2000;15:100–109. doi: 10.1037//0882-7974.15.1.100. [DOI] [PubMed] [Google Scholar]
- Neale M, Hunter M, Pritikin JN, Zahery M, Kickpatrick RM, Estabrook R, Bates TC, Maes H, Boker SM. Open Mx 2.0: extended structural equation and statistical modeling. Psychometrika. 2015 doi: 10.1007/s11336-014-9435-8. Available from: The Psychometric Society. [27 January 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and GxE interaction. Twin Research and Human Genetics. 2006;9:481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Lichtenstein P. The Twin and Offspring Study in Sweden: advancing our understanding of genotype-environment interplay by studying twins and their families. Acta Psychologica Sinica. 2008;40:1116–1123. [Google Scholar]
- Pedersen NL, Christensen K, Dahl A, Finkel D, Franz C, Gatz M, Horwitz BN, Johansson B, Johnson W, Kremen WS, Lyons MJ, Malmberg B, McGue M, Neiderhiser JM, Peterson I, Reynolds CA. IGEMS: The Consortium on Interplay of Genes and Environment across Multiple Studies. Twin Research and Human Genetics. 2013;16:481–489. doi: 10.1017/thg.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES---D scale: a self---report depressive mood scale for research in the general population. Applied Psychological Measurement. 1977;3:385–401. [Google Scholar]
- Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goodard R. CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. British Journal of Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- Salvi F, Miller MD, Grilli A, Giorgi R, Towers AL, Morichi V, Spazzafumo L, Mancinelli L, Espinosa E, Rappelli A, Dessì-Fulgheri P. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. Journal of the American Geriatrics Society. 2008;56:1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS 9.4. SAS Institute Inc; Cary, NC: 2013. [Google Scholar]
- Schnittker J. Natural Symptoms? The Intersection of Social, Biological, and Genetic Determinants of Depression in Later Life. 2014. Working paper: University of Pennsylvania Population, Aging Research Center. PARC Working Paper Series, WPS 14–01. [Google Scholar]
- Scott KM, Lim C, Al-Hamzawi A, Alonso J, Bruffaerts R, Caldas-de-Almeida JM, Florescu S, de Girolamo G, Hu C, de Jonge P, Kawakami N, Medina-Mora ME, Moskalewicz J, Navarro-Mateu F, O’Neill S, Piazza M, Pasada-Villa J, Torres Y, Kessler RC. Association of mental disorders with subsequent chronic physical conditions: world mental health surveys from 17 countries. JAMA Psychiatry. 2016;73:150–158. doi: 10.1001/jamapsychiatry.2015.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skytthe A, Christiansen L, Kyvik KO, Bødker FL, Hvidberg L, Petersen I, Nielsen MMF, Bingley P, Hjelmborg J, Tan Q, Holm NV, Vaupel JW, McGue M, Christensen K. The Danish Twin Registry: Linking surveys, national registers, and biological information. Twin Research and Human Genetics. 2013;16:104–111. doi: 10.1017/thg.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg CM, Beekman ATF, Deeg DJH, van Tilburg W. Sex differences in late life depression. Acta Psychiatrica Scandinavica. 2000;101:286–292. [PubMed] [Google Scholar]
- Steptoe A. Depression and physical illness. Cambridge University Press; New York: 2007. [Google Scholar]
- Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB. The trajectory of depressive symptoms across the adult lifespan. JAMA Psychiatry. 2013;70:803–811. doi: 10.1001/jamapsychiatry.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen S, Tolvanen A, Kaprio J, Berg S, Koskenvuo M, Rantanen T. The genetic and environmental effects on depressive symptoms among older female twins. Twin Research. 2004;7:626–636. doi: 10.1375/1369052042663904. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Harden KP. Behavior genetic research methods: testing quasi-causal hypotheses using multivariate twin data. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Personality and Social Psychology. John Wiley; New York: 2014. pp. 159–187. [Google Scholar]
- Twenge JM. Time period and birth cohort differences in depressive symptoms in the U.S., 1982–2013. Social Indicators Research. 2015;121:437–454. [Google Scholar]
- van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in GxE modelling of twin data. Behavior Genetics. 2012;42:170–186. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle C, Lahey B, Rathouz P. Operating characteristics of alternative statistical methods for detecting gene-by-measured environment interaction in the presence of gene-environment correlation in twin and sibling studies. Behavior Genetics. 2013;43:71–84. doi: 10.1007/s10519-012-9568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.