Abstract
Geographic variation in sensory traits is usually influenced by adaptive processes because these traits are involved in crucial life-history aspects including orientation, communication, lineage recognition and mate choice. Studying this variation can therefore provide insights into lineage diversification. According to the Sensory Drive Hypothesis, lineage diversification may be driven by adaptation of sensory systems to local environments. It predicts that acoustic signals vary in association with local climatic conditions so that atmospheric attenuation is minimized and transmission of the signals maximized. To test this prediction, we investigated the influence of climatic factors (specifically relative humidity and temperature) on geographic variation in the resting frequencies of the echolocation pulses of Geoffroy’s horseshoe bat, Rhinolophus clivosus. If the evolution of phenotypic variation in this lineage tracks climate variation, human induced climate change may lead to decreases in detection volumes and a reduction in foraging efficiency. A complex non-linear interaction between relative humidity and temperature affects atmospheric attenuation of sound and principal components composed of these correlated variables were, therefore, used in a linear mixed effects model to assess their contribution to observed variation in resting frequencies. A principal component composed predominantly of mean annual temperature (factor loading of -0.8455) significantly explained a proportion of the variation in resting frequency across sites (P < 0.05). Specifically, at higher relative humidity (around 60%) prevalent across the distribution of R. clivosus, increasing temperature had a strong negative effect on resting frequency. Climatic factors thus strongly influence acoustic signal divergence in this lineage, supporting the prediction of the Sensory Drive Hypothesis. The predicted future increase in temperature due to climate change is likely to decrease the detection volume in echolocating bats and adversely impact their foraging efficiency.
Introduction
Evolutionary biologists strive to understand the historical processes driving lineage divergence. However, the mechanisms by which lineages diverge are still among the least understood biological phenomena [1]. Nevertheless, there is a broad consensus among evolutionary biologists that, in sexually reproducing clades, new lineages arise largely by allopatric divergence [2]. When two populations become geographically isolated for long, they follow different evolutionary pathways. Typically, different geographic regions are likely to impose different selective pressures on such isolated populations due to differences in environmental conditions including climate, resources, predators and competitors. Such differences may result in the selection of divergent traits that are locally adaptive in each population. Indeed, there is evidence that geographic isolation caused by adaptation to different habitats has played a major role in lineage divergence [2].
Geographic variation manifests itself in traits such as morphology and physiology but also in behavioural ones such as acoustic signals (here used in the sense of animal sounds which carry information) [3]. Geographic variation in acoustic signals has been reported in birds [4–6], anurans [7] and bats [8–12]. Variation in sensory traits is of particular interest because these traits, whether visual, acoustic or olfactory, are involved in orientation [13, 14], communication [15], mate choice [16] and conspecific recognition [17, 18]. Divergence in these traits is therefore usually mediated by adaptive processes, rather than random processes like genetic drift [19]. This has led to the formulation of the Sensory Drive Hypothesis, which proposes that lineage diversification may be driven by environmentally-mediated differences in sensory signals [20, 21]. This hypothesis predicts a close association between geographic variation of sensory signals and environmental variables.
The function of sensory traits is dependent on the signals on which they are based (e.g. light, sound, chemicals) and transmission of these signals is in turn influenced by the local environment through which they are transmitted [19]. Geographic variation in acoustic signals, specifically, has been observed in a number of studies, many of which attributed signal divergence to current ecological conditions, providing support for the Sensory Drive Hypothesis (reviewed in Thorpe [22]). For instance, Tobias et al. [23] demonstrated that song divergence of bamboo-specialist birds was correlated with the sound transmission properties of their habitats, and diverged predictably across ecological gradients, suggesting that songs have become adapted to local ecological gradients.
Like bird song and frog calls, echolocation is an acoustics-based system but, unlike bird song and frog calls, it is primarily used for orientation and foraging [24, 25] rather than advertisement. However, mounting evidence suggests that social information is encoded in echolocation pulses as well [17, 18, 26–28, 29] and that echolocation may be implicated in mate choice and therefore lineage divergence [16]. In addition, echolocation is known to vary geographically over the distributional ranges of many lineages ([8, 21, 30–32]; see Jiang et al. 2015 [3] for a review) and this is likely due to environmental conditions. Echolocation thus provides opportunities to test the Sensory Drive Hypothesis.
Bat echolocation is influenced by several acoustic properties of the atmosphere through which it is propagated, affecting the operating range of echolocation [33]. This is due to atmospheric attenuation, which refers to the decrease in the energy of sound caused by scattering and absorption as it travels through the atmosphere [34]. Such atmospheric sound attenuation is a nonlinear function of the sound frequency, temperature, humidity and air pressure [33–35]. In general, atmospheric attenuation increases with an increase in signal frequency and relative humidity [33, 36]. Signal frequency is therefore likely to be negatively correlated with relative humidity to reduce atmospheric attenuation and optimize the operational range of echolocation. Guillén et al. [9], for example, demonstrated that echolocation frequencies among populations of Noack's roundleaf bat, Hipposideros ruber [37], in the Gulf of Guinea were inversely related with environmental humidity. However, the effect of relative humidity is mediated by a complex non-linear interaction with temperature and the interactive effects of these climatic variables [33, 35] must be considered. Such an approach was used by Mutumi et al. [21]. They found that, besides humidity, temperature and other climatic variables associated with latitude and altitude may play key roles in the geographic variation in bat echolocation call frequencies.
Geoffroy's horseshoe bat (Rhinolophus clivosus Cretzschmar, 1828) is ideal for testing the Sensory Drive Hypothesis because it has a wide geographic distribution [38] and echolocates at relatively high frequencies. This lineage, thus, presents an opportunity to test whether geographic variation in its calls exist and, if so, whether this variation can be explained by differences in climatic variables across its range. R. clivosus echolocates at relatively high frequencies (a mean of 91.7 ± 1 kHz in southern Africa; Jacobs et al. [39]), meaning that the impact of climatic variables on the attenuation of its echolocation calls should be relatively higher than on the calls of bats that echolocate at lower frequencies [33, 35].
Echolocation frequency of many insectivorous bats is generally known to decline as body size increases [39]. We therefore also considered the influence of body size on geographic variation in echolocation frequency. Furthermore, the call frequency of R. clivosus is much higher than expected from its body size (66 kHz) and lies outside of the 95% confidence limits of the allometric relationship between body size and call frequency for the Rhinolophidae [39]. It is possible, therefore, that climatic factors may have driven the evolution of these anomalous pulse frequencies especially because pulse frequencies of this lineage are reported to be lower in areas outside southern Africa [40].
We used a modelling approach based on Akaike’s Information Criterion (AICc) [41] to test the validity of sensory drive as an explanation for acoustic signal divergence and the anomalously high call frequencies of R. clivosus. We tested the prediction that environmental and, in particular, climatic variables are good predictors of call frequency across the distributional range of R. clivosus in eastern and southern Africa. Our objectives were to: 1) document geographic divergence, if any, in call frequency within R. clivosus and 2) assess the relative contributions of body size and environmental variables to call frequency divergence in this lineage. Our study provides insights into the climatic influence on the divergence of sensory traits and reveals potential impacts of future climatic change on faunal sensory signals.
Materials and methods
Ethical statement
Capture and handling of animals complied with the guidelines recommended by the American Society of Mammalogists [42], and sampling guidelines compiled by Aegerter et al. [43] and Kunz and Parsons [44], and were approved by the Science Faculty Animal Ethics Committee at the University of Cape Town (Clearance Number 2013/2011/V6/DJ). All workers handling bats were vaccinated against rabies and were required to use protective gloves when handling bats. Rhinolophus clivosus is a non-protected lineage in all countries of capture and was captured on both privately owned and protected areas under the relevant authorities as follows: Botswana (Ministry of Environment, Wildlife and Tourism, EWT 8/36/4 XVI– 78); Kenya (Kenya Wildlife Service, KWS/4001); Malawi (Department of Forestry Licence NO: 1/06/2013/1); South Africa (Northern Cape Province, Fauna 64/2010; Mupumalanga Tourism & Park Agency, MPB 5253; Cape Nature, 0035-AAA007-00081); Zimbabwe (Parks and WildlifeManagement Authority, Permit [23 (1) (C) (II) 25/2011; 19/2012 and 16/2013].
Study animals
Geoffroy’s horseshoe bat (R. clivosus) is morphologically variable and geographically widespread with a continuous range in eastern and southern Africa, more patchy occurrences further north, and an extension into the Arabian Peninsula [38, 45–47]. We focused on the lineage distributed in eastern and southern Africa which has been shown to form a single clade distinct from populations to the north [48] The lineage exists in different habitat types across its range including deserts, savannah woodlands and fringes of forests with climates that range from arid and tropical biomes with summer rainfall areas to Mediterranean biomes with winter rainfall [38].
R. clivosus is a medium-sized bat with a mass of around 18 g [39]. It is insectivorous and forages in and around dense foliage at low heights above ground [39, 49]. R. clivosus uses high duty cycle (HDC) echolocation. The duty cycle of a periodic pulse is defined as the ratio between the duration of the pulse and its period, which is the time between the onset of successive pulses [50]. The echolocation pulses of R. clivosus pulses are typically dominated by a constant frequency (CF) component and begin and/or end with a short-frequency modulated (FM) sweep (S1 Fig; Fenton [50]; Fenton et al. [51]). A CF component of an echolocation pulse or tone is a narrowband signal where the sound stays constant at one frequency throughout its duration while an FM component or sweep is a broadband signal, which contains a sweep through a range of frequencies (that is frequency increases or decreases evenly within a few milliseconds).
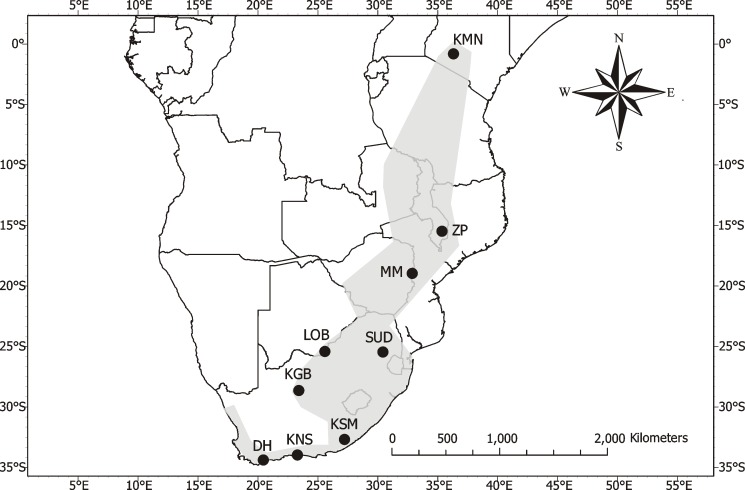
Bats were sampled from 11 different locations across the distribution of R. clivosus in Africa (Fig 1). Bats were captured at their roosts using either hand nets during the day, or mist nets at night. Sex was determined visually. We determined female reproductive condition by palpating the abdomen and inspecting the mammae [52]. Age-class was determined by examining the degree of epiphyseal–diaphyseal fusion [53]. Only adult bats were used in subsequent analyses. For ethical reasons, juveniles, pregnant or lactating bats were immediately released at the site of capture.
Fig 1. Sites across southern and eastern Africa from which R. clivosus bats were sampled.
Site codes: DH = De Hoop Nature Reserve (34.42°S, 20.35°E), KMN = Kariandusi Mine (0.45°S, 36.28°E), KNS = Knysna (34.06°S, 23.22°E), KGB = Koegelbeen (28.65°S, 23.35°E), KSM = Kokstad (32.68°S, 27.19°E), LOB = Lobatse (25.24°S, 25.51°E), MM = Monaci Mine (18.88°S, 32.72°E), SUD = Sudwala (25.38°S, 30.69°E), ZP = Zomba Plateau (15.33°S, 35.28°E).
We chose forearm length (FA) as a proxy for body size because body mass varies seasonally and diurnally in bats (e.g. Rughetti and Toffoli [54]). FA was measured to the nearest 0.1 mm using dial callipers. Echolocation pulses were recorded from bats held 30 cm away from the microphone of an ultrasound D1000X detector (Pettersson Elektronik AB, Uppsala, Sweden - www.batsound.se) at sampling frequencies of 384 kHz and 500 kHz. We used resting frequency (RF), recorded from handheld bats, as opposed to frequency measurements from flying individuals to avoid the variation in pulse frequency caused by horseshoe bats compensating for Doppler shifts in frequency during flight [49]. Pulses of hand-held horseshoe bats, in contrast, have stable CF components and the inter-pulse frequency variation is low [55].
Ten to twelve pulses with the best signal to noise ratios were measured for each of 112 R. clivosus individuals (Table 1). These pulses were not chosen from the first 10 pulses of a recording because horseshoe bats are known to tune into their resting frequencies from lower frequencies after periods of silence [56, 57]. The chosen pulses were analysed using Avisoft SASLab Pro automatic measurement function (Avisoft Bioacoustics, Version 4.2, Glienicke, Germany). A Hanning window was used to eliminate the effects of background noise. The frequency at the centre of the CF component of each pulse (S1 Fig) was measured at a threshold of 18 dB below maximum. The measurements were averaged over each individual’s pulses for use in further analyses.
Table 1. Resting frequency, forearm length, environmental variables, and detection distances for sites sampled in this study.
Means (±S.D.) and the ranges (in parentheses below the means) are given for resting frequency.
| Study site | n RF Range (F) |
n RF range (M) |
RF RF-Range (kHz) All |
Forearm length (mm) | Relative humidity (%) | Mean annual temperature (°C) | Altitude (m a.s.l) |
Detection range point reflector (m) | Detection range tree (m) |
|---|---|---|---|---|---|---|---|---|---|
| KMN | 13 99.5–100.5 |
1 | 100.06 ± 0.42 (99.50–100.60) | 53.34 ± 1.21 | 58.41 | 15.3 | 1856.18 | 7.1 | 11.9 |
| LOB | 5 91.3–92.7 |
5 92.1–92.7 |
92.28 ± 0.42 (91.30–92.70) |
53.88 ± 1.71 | 46.49 | 18.45 | 1250.68 | 7.6 | 12.9 |
| ZP | 1 | 2 79.4–81 |
80.80 ± 1.31 (79.40–82.00) |
53.10 ± 1.18 | 60.39 | 19.95 | 972.62 | 7.3 | 12.3 |
| MM | 0 | 2 87.8–88.8 |
88.30 ± 0.71 (87.80–88.80) |
54.40 ± 1.13 | 60.84 | 17.7 | 1221.39 | 7.4 | 12.4 |
| SUD | 22 89.8–92.8 |
15 89.6–92.5 |
91.46 ± 0.80 (89.60–92.80) |
54.32 ± 1.40 | 59.12 | 16.68 | 1195.31 | 7.4 | 12.5 |
| DH | 15 90.4–92.2 |
15 91.3–92.2 |
91.70 ± 0.41 (91.30–92.20) |
54.83 ± 1.33 | 63.46 | 16.82 | 137.91 | 7.0 | 11.6 |
| KNS | 2 90.8–92.7 |
5 91.7–92.7 |
91.86 ± 0.66 (90.80–92.70) |
52.68 ± 1.08 | 61.29 | 15.98 | 280.06 | 7.3 | 12.2 |
| KGB | 4 89.8–90.1 |
1 | 89.88 ± 0.13 (89.80–90.10) |
57.22 ± 1.01 | 43.1 | 16.2 | 1463.94 | 8.4 | 14.7 |
| KSM | 2 90.8–91.7 |
2 91.7–91.7 |
91.48 ± 0.45 (90.80–91.70) |
53.85 ± 1.01 | 58.9 | 14.54 | 957.86 | 7.8 | 13.4 |
Detection ranges
The distances at which bats detected a point reflector (e.g. small insect) and tree (e.g. background vegetation) were calculated for each site using Stilz and Schnitzler’s [34] online echolocation range calculator. Source levels (emitted intensity referenced to a standard distance) were originally calculated at 10 cm in front of the bats’ mouths following methods from Holderied and Helversen [58] but were adjusted to 1 meter in front of the bats’ mouths for use in the online calculator. A source level of 115 dB (n = 2 passes; unpublished data) was used across sites. This was the maximum source level measured for R. clivosus at one of our study sites (De Hoop Nature Reserve). Source levels for this lineage at the other sites are not currently available. Detection range calculations incorporated the following information: (1) climatic conditions (e.g., AMT, RH, and atmospheric pressure) at each sampled site, (2) resting frequency (Hz) of each individual bat; (3) the dynamic range, which is the difference between peak intensity (dB SPL) measured at 1 m and the auditory threshold of the bat (assumed to be 0 dB SPL for horseshoe bats [58]; (4) C1, the reflection loss which accounts for the fraction of the energy reflected, and (5) C2, the geometric spreading loss, which quantifies the loss of energy due to spreading multiplied by 2 for both outgoing emitted pulses and the returning echo. The values of the latter two factors are dependent on the geometry of the reflected wave and the web calculator therefore generates C1 and C2 depending on the target selected. Atmospheric pressure at each site was kept at that for normal atmospheric conditions, taken as 101.325 pascal.
Environmental variables
The environmental variables used in the analyses included mean annual temperature (AnnTemp), relative humidity (RH), longitude (Long) and altitude (Alt). Altitude was identified as an important environmental variable because of the decrease in temperature (and subsequent effects on relative humidity) with increasing altitude [59]. Altitude is also a proxy for atmospheric pressure, which has a significant relationship with RF [21, 60, 61]. In Gillam et al. [61], bats used lower frequency pulses at higher altitudes and lower atmospheric pressure.
Climatic variables and altitude were derived using the geographic information system (GIS) software for analyzing geographic information, ArcGIS, following Mutumi et al. [21]. Average values corresponding to a 20-km radius around each sampling site were extracted. Geographic variation in any phenotypic character would take many generations to evolve and it is the conditions that many generations experience in their local habitats that influence how populations diverge. Hence, we used climatic data averaged over several years/generations (see Mutumi et al. [21]). Many radio-tracking studies demonstrate that medium-sized bats, like R. clivosus, forage within a radius of 10 km from their roosts (e.g., Bontadina et al. [62]; Goiti et al. [63]), and therefore we considered a 20-km zone around each roost reasonable to account for the environmental conditions to which the bats were exposed.
Sudwala (Fig 1) consisted of Sudwala and two other sites, Echo Cave (25.37°S, 30.70°E) and Elandshoek (25.38°S, 30.69°E), that were situated so close together that a 20-km radius around each of them almost entirely overlapped, meaning that the extracted averages for relative humidity and annual temperature were almost identical for these three sites. Additionally, the sites exist in the same biome [64]. Consequently, the three sites were regarded as one site centered at Sudwala for subsequent analyses.
Statistical analyses
One-way ANOVA was used to test for differences in resting frequencies among sites, with resting frequency (RF) as the dependent variable and relative humidity (RH), mean annual temperature (AnnTemp), altitude (Alt), Latitude (Lat) and Longitude (Long) as independent variables. Data were log-transformed [ln (x + 1)] to adjust for a non-normal distribution. To account for potential multicollinearity among climatic variables, unequal variances across sites and spatial autocorrelation, we followed the statistical methods in Mutumi et al. [21]. Since relative humidity, annual temperature and altitude are correlated [35], we accounted for potential multicollinearity amongst these variables by extracting uncorrelated variables in the form of principal component scores using principal components analysis (PCA), as in Dormann et al. [65]. Furthermore, the component scores also account for the interactive effects of temperature and relative humidity [35] by combining their effects in single components. The PCA was performed on RH, AnnTemp and Alt in R [66] using the stats package MASS and the extracted principal component scores used in subsequent models. We tested both linear mixed effects (LME) and generalized least squares (GLS) models using the nlme package in R [67, 68]. These model structures were each fitted with different spatial autocorrelation functions to derive the best combination of model structure and spatial function to reduce autocorrelation. We used the exponential, spherical and gausian functions with and without sampling locality as a random effect (with distances defined by Latitude and Longitude). These models (including all the variables PC1, PC2, FA, Lat, Long and Sex) were weighted using the Akaike Information Criterion (AICc) values. The best model structure (with the lowest AICc value) was a linear mixed effects with site specified as a random effect and without any spatial autocorrelation function. This model structure sufficiently accounted for the possibility of non-normality, unequal variances (S2 Fig) and spatial auto-correlation (S3 Fig).
To test whether body size (forearm length), sex, or environmental factors (relative humidity, annual temperature and altitude) best explained the variation in RF across sites, a stepwise model selection by AICc was performed using the package MASS in R (http://www.R-project.org/.) and the stepAIC function. The best model was tested statistically using ANOVA to determine which predictor variables significantly contributed to the variation in RF.
To further explore the interactive relationship between climatic variables and RF, variables deemed significant after ANOVA on the best model were used to predict the effect of each variable on RF when the other variables were held constant [21]. To do so, we generated a new data set of PC scores as follows (see Mutumi et al. [21]). We held all except one variable constant at their mean values (calculated across all localities) and for the remaining variable we used the raw data from each locality. We then performed a PCA on these data to derive a new set of standardised PCs for each of the significant variables identified in our final best model. We ran the PC scores from this PCA in another linear mixed effects model.
Results
Geographic variation in resting frequencies
The RF of R. clivosus varied significantly across the study sites, ranging from means of 80 to 100 kHz (F8,103 = 424.235; P < 0.001; Table 1). However, latitude appeared to be a weak predictor of RF. Although latitudinal differences between Zomba Plateau and Kariandusi Mine were smaller than between Zomba Plateau and De Hoop Nature Reserve, the difference in RF was greater between Zomba Plateau and Kariandusi Mine than between Zomba Plateau and De Hoop Nature Reserve (Fig 2, Table 1). Additionally, although latitude varied among South African sites, there was little variability in the resting frequencies across these sites (Fig 1; Table 1). Although our sample sizes are small for some populations, the range of echolocation frequencies within populations is low even where we had many individuals (Table 1). We therefore think that we have covered a sufficient portion of the range in RF even where we have low sample sizes.
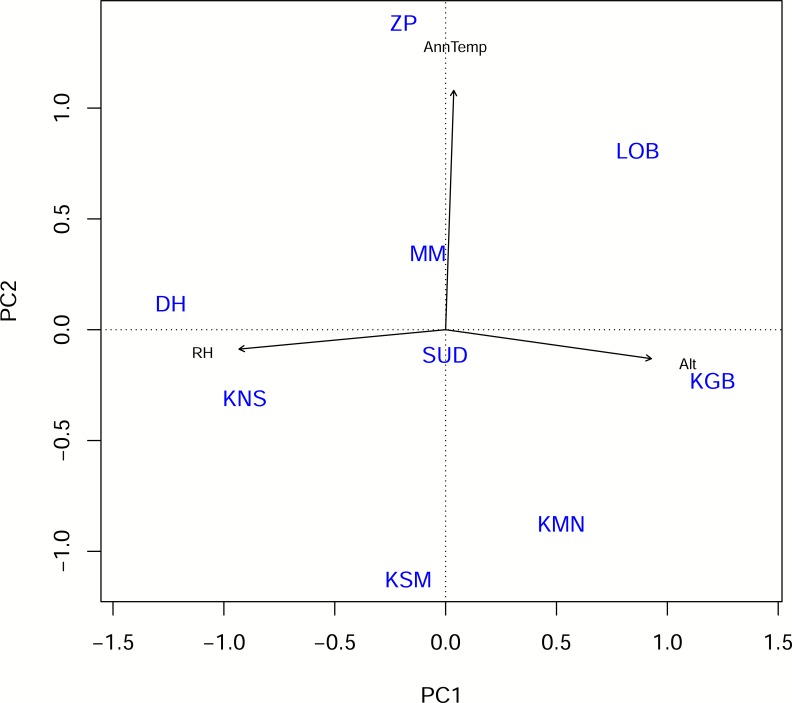
Fig 2. Principal components 1 and 2 extracted from PCA on relative humidity (RH) mean annual temperature (AnnTemp) and altitude (Alt).
Site abbreviations are the same as in Fig 1.
Principal components analysis (PCA)
The PCA yielded three principal component loadings (PC1, PC2 and PC3). PC1, on which Alt and RH loaded highest, accounted for 49% of the variation between sites while PC2, on which AnnTemp loaded the highest, explained 34% of the variation. In total, these two PCs therefore accounted for 83% of the variation (Table 2; Fig 2). PC3 accounted for only a small proportion of variance (17%) and was therefore excluded from further analyses.
Table 2. Factor loadings of each variable on each principle component derived from a PCA on climatic variables.
| PC1 (49%) | PC2 (34%) | PC3 (17%) | |
|---|---|---|---|
| Cumulative proportion | 0.489 | 0.826 | 1.000 |
| Variable factor loading (PC1) | Variable factor loading (PC2) | Variable factor loading (PC3) | |
| Relative Humidity | 0.60 | 0.07 | 0.59 |
| Annual Temperature | 0.02 | 0.64 | 0.09 |
| Altitude | 0.49 | 0.08 | 0.48 |
Stepwise regression
The best model (with the lowest AICc value; Table 3) only included PC1 + PC2 + Lat + Long + PC1:PC2 as predictor variables. Applying ANOVA on this model showed that only PC2 was a significant predictor of RF across sites (Table 3). Forearm length, sex, longitude, RH and Alt (Table 2) were not therefore good predictors of RF across sites.
Table 3. The ‘best’ model from forward-backward stepwise model selection on the global model of environmental variables, body size and sex against resting frequency for R. clivosus.
Statistics are only presented for variables maintained in the best model.
| numDF | denDF | ANOVA F-value | P-value | |
|---|---|---|---|---|
| PC1 (RH & Alt) | 1 | 103 | 0.93 | 0.407 |
| PC2 (AnnTemp) | 1 | 103 | 19.22 | < 0.05 |
| Lat | 1 | 103 | 1.91 | 0.261 |
| Long | 1 | 103 | 7.53 | 0.071 |
| PC1: PC2 | 1 | 103 | 4.49 | 0.124 |
| Total N = 112 | ||||
| Number of Groups: 9 |
Abbreviations: Alt = Altitude; RH = relative humidity; AnnTemp = annual temperature; Lat = Latitude; Long = Longitude; numDf = numerator degrees of freedom; denDF = denominator degrees of freedom. Please note that the best model did not retain forearm length and sex.
Predictive analysis
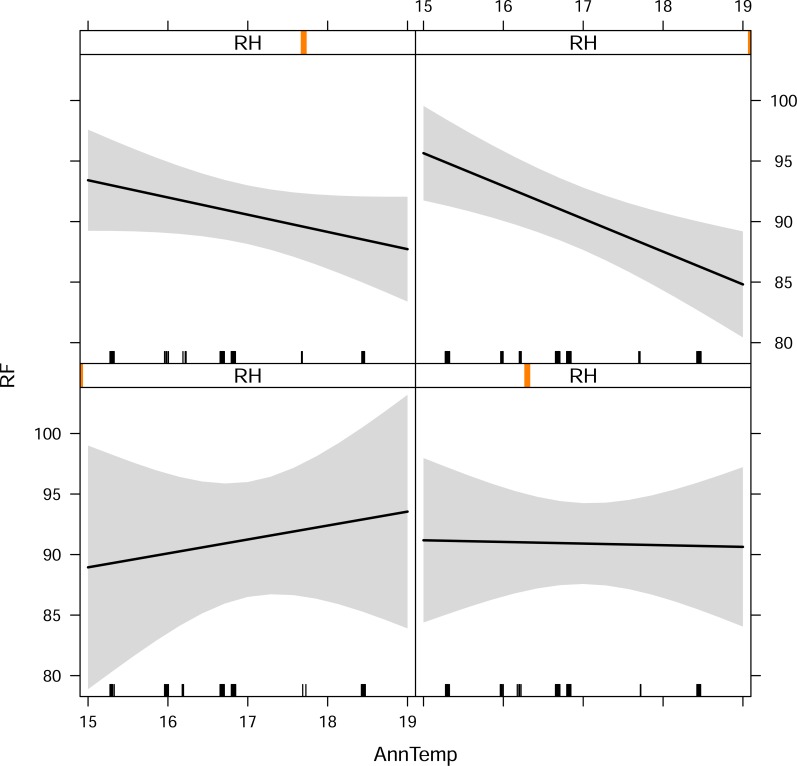
There was a complex nonlinear relationship between RF and two climatic variables—temperature and relative humidity (Fig 3). As the effect plots in Fig 3 demonstrate, the effect of temperature on RF was mediated by RH. At moderate to high relative humidity, increasing temperature led to a decrease in the RF used by R. clivosus (Fig 3, top two panels). At moderately low RH, there was little or no change in the RF (Fig 3, bottom right panel). In contrast, at extremely low relative humidity (Fig 3, bottom left panel), increasing temperature led to an increase in the RFs used by R. clivosus.
Fig 3. Interactive effects of relative humidity (RH; %) and mean annual temperature (AnnTemp; °C) on resting frequency (RF; kHz).
Relative humidity is represented by the vertical red bars at four levels of intensity ranging from high (top left and right panels), moderate (bottom right panel) to low (bottom left plot). Details are given in the main text.
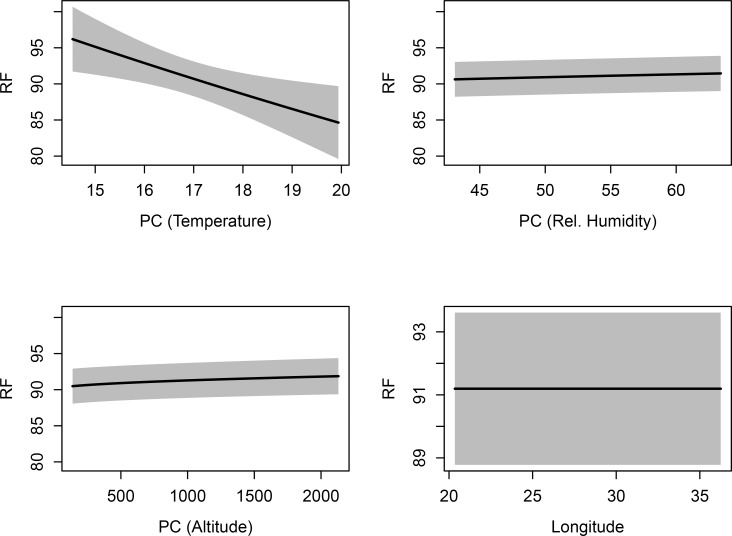
These trends differed slightly when the effects of the climatic variables were considered together in the form of principal components (note: in this case the PCs incorporate altitude; Fig 4). The effect plots in Fig 4 revealed an inverse relationship between RF and temperature when Alt and RH were held constant at their mean values (Fig 4, top left panel). This trend was similar to the relationship between temperature and RF at high RH (Fig 3, two top panels). The reverse was true for the relationships between RF and RH (when temperature and altitude are held constant, Fig 4, top right panel) as well as RF and altitude (when temperature and RH are held constant; Fig 4, bottom left panel). A positive relationship was found when RH, temperature, or altitude was varied while temperature and altitude or temperature and RH were held constant at their mean values (Fig 4, top right and bottom left panels, respectively). There was little effect of longitude on RF when all climatic variables were held constant (Fig 4, bottom right).
Fig 4. Predictive analysis plots showing how RF varies as each of AnnTemp (PC-Temperature), RH (PC-Rel. Humidity), Alt (PC-Altitude) and Longitude are varied whilst the remaining two climatic variables are kept at their mean values (calculated across study sites).
Explanatory details are given in the main text.
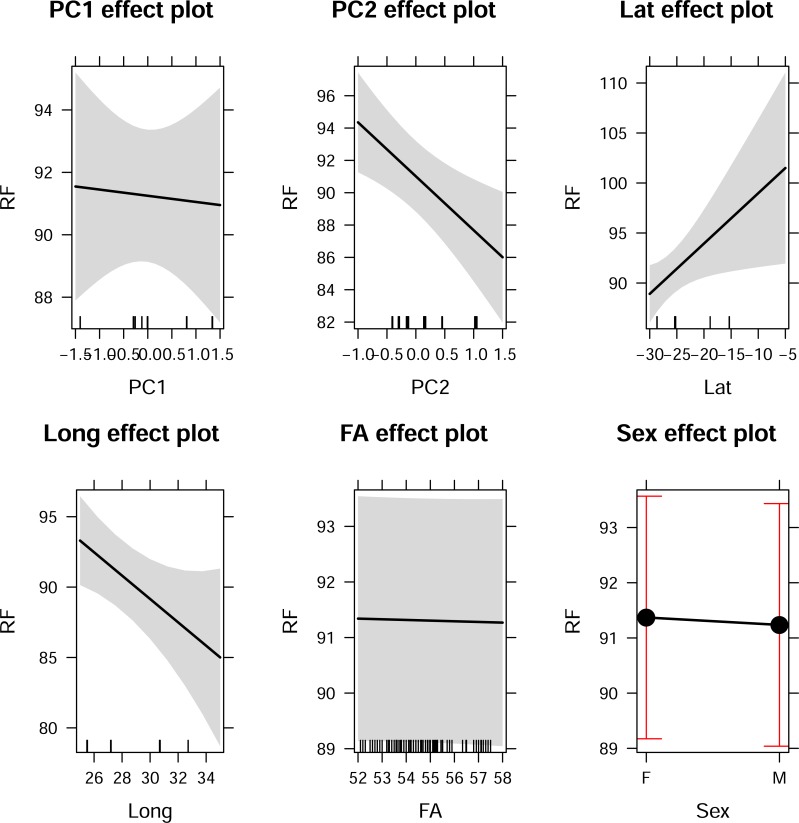
Analyses of the individual effects of PCs showed that PC1 (RH and altitude), despite accounting for 49% of the variation in the climatic data (Table 3) had little effect on RF (Fig 5, top left panel). In contrast, PC2 (AnnTemp) which accounted for slightly less of the variation in the climatic data (34%, Table 3), strongly influenced RF divergence–as temperature increased, RF decreased (Fig 5, top center panel). There were spatial effects in both longitude (Fig 5, bottom left panel) and latitude (Fig 5, top right panel) on the divergence of RF pulses incorporated in the climatic effect (Fig 5). However, this spatial effect became more variable towards the equator (confidence bands became broader at lower latitudes; Fig 5, top right panel) and towards the east (confidence bands become broader; Fig 5, bottom left panel). Both of these trends may however be a sampling artefact because we had relatively few populations in the east and closer to the equator (Fig 1). Neither body size (Fig 5, bottom center panel) nor sex (Fig 5, bottom left panel) had an effect on RF.
Fig 5. The relationship between RF and PC1 and PC2 in R. clivosus.
Note that the range of frequencies covered in each diagram are not the same. Explanatory details are given in the main text.
Detection distances
Detection distances varied little across sites and the difference between the maximum and minimum detection distances for a point reflector and vegetation was 1.4 m and 3.1 m, respectively (Table 1). These differences between maximum and minimum detection distances are largely due to the anomalously long detection distances in the Koegelbeen (KGB) population which is probably necessitated by the anomalously large body size of R. clivosus at this site (Table 1). Excluding this population reduces the differences to 0.7 m and 1.8 m for a point reflector and background vegetation, respectively (Table 1).
Discussion
Our study tested the Sensory Drive Hypothesis by investigating acoustic divergence in resting frequency (RF) of populations of the Geoffroy's horseshoe bat (R. clivosus) across its range in eastern and southern Africa. In agreement with previous studies of other bat lineages and other taxa (e.g. Jiang et al. [69, 70]; Snell-Rood [36]; Sun et al. [71], Mutumi et al. [21]), we found that the variation in the resting frequencies of R. clivosus across its geographic range were predominantly climate driven, thus confirming the prediction of the Sensory Drive Hypothesis. Temperature was the best predictor of RF (Table 3), but its effects were mediated by RH (Fig 4). In contrast, latitude and longitude were poor predictors of RF (Table 3) but there were spatial effects across sites (Fig 5) suggesting that the effects of other environmental variables not considered here may be uncovered through improved sampling. Although the RF of R. clivosus at some localities dropped to 79 kHz, this was still higher than that expected from its body size [39]). This suggests that sensory drive cannot explain the anomalously high frequencies used by R. clivosus (see Jacobs et al. [39]).
The implication of our results is that the resting frequency of R. clivosus may be adapted to various temperature and relative humidity gradients across its geographic range. Although annual temperature showed the strongest effect (PC2; Fig 5), this was conditional on the degree of relative humidity at which the population occurred (Fig 3). This supports the idea that variation in RFs used by bats is the result of a complex interaction between pulse frequency, temperature and humidity [21, 35]. Environmental factors that impact on these three factors may thus exert an influence on the RFs of bats. For instance, despite being situated close to the equator, where conditions are usually warm and humid and selection should favour low pulse frequencies (high frequencies are expected to attenuate more rapidly under such conditions; [35]), bats from Kariandusi Mine in Kenya had the highest mean RF (100.06 kHz). However, this is probably because this site had relatively low mean annual temperature (15.30°C) and low relative humidity of 58.41% because of it having the highest altitude (1856.41 m above sea level). In support of this, Zomba Plateau with the highest mean annual temperature (19.95°C) and one of the highest values for relative humidity (60.39%), recorded the lowest frequency (80.80 kHz). This reinforces the fact that the synergistic effects of temperature, humidity and altitude on RF of R. clivosus had more influence than any one of the three climatic variables alone. Our study affirms earlier evidence by Mutumi et al. [21] in support of climate exerting a major influence on resting frequency divergence among geographically-isolated populations of two other horseshoe bats, Rhinolophus simulator and R. swinnyi.
Similar to the findings of Mutumi et al. [21], the variation in RF in R. clivosus also had a spatial component in that RF appeared to follow both a longitudinal and latitudinal cline (Fig 5). This suggests that spatial structuring of populations along the distributional range of a lineage may expose populations to additional environmental factors, besides climate (e.g. stochastic factors such as drift and/or deterministic factors such as selection under competition from conspecifics), which may also exert an influence on RFs.
An interesting possibility is that bats may be flexible enough to respond to temperature and RH changes over a single night and adjust their echolocation pulses accordingly. Low duty cycle bats (LDC) that do not have an acoustic fovea and do not use Doppler shift compensation [49, 55] apparently vary their echolocation frequencies by a few kHz in response to changes in temperature and RH [72]. However, apart from not being within the scope of the present study, which tries to explain geographic variation across the range of a lineage, the acoustic fovea of HDC echolocating bats and the Doppler shift compensation that their echolocation system relies upon may limit the extent to which HDC echolocating bats can adjust their pulses to deal with changes in RH and temperature. Jacobs et al. [39] found little variation in RF within individuals. Nevertheless this would be an interesting field for future research.
The geographic variation reported in RF in this study is not the result of the existence of cryptic lineages. Populations of R. clivosus from northern Africa and Arabia were not analysed because recent work has demonstrated that southern African populations are as genetically distinct from those further north as they are from its sister lineage, R. ferrumequinum [47]. This distinctiveness led researchers to propose a taxonomic revision across the range of R. clivosus to identify any cryptic lineages [47, 73]. Indeed, genetic analyses (as well as phenotypic characters such as forearm length) suggest that North African populations of R. clivosus may represent cryptic lineages [47] or may be divergent populations of R. ferrumequinum [47, 74]. Compared to the patchy distribution of R. clivosus in northern Africa, the eastern and southern African populations of R. clivosus form a continuous range in distribution [47], and are genetically grouped in a single clade without separation by other lineages [74]. It is therefore valid to consider the divergence in resting frequencies within the eastern and southern African populations of R. clivosus as geographic variation within the same lineage.
Contrary to the prediction of James' Rule [74] and in agreement with Mutumi et al. [21], RF did not vary with body size across the distributional range of R. clivosus, indicating that variation in body size due to climatic factors did not have an effect on RF. According to James’ rule, animals in cool, dry areas generally have larger body sizes than those in cool, humid areas, and that those in hot, humid areas have the smallest body sizes. If so, then it is expected that bats in hot, humid areas should have high echolocation frequencies because of the inverse relationship between body size and echolocation frequency in bats [39, 75]. However, this outcome contradicts what would be expected, considering that sound is attenuated to a greater degree in humid environments. We found no support for James’ rule, but instead our results emphasized the influence of climatic variables on echolocation frequency.
Sex was also not included in the best model (Table 3) and is therefore not a good predictor of RF variation. This result contrary to what has been reported for three other southern African rhinolophid lineages where sex was a major predictor of RF variation [21, 76]. This is probably because RF does not differ appreciably between males and females in R. clivosus (Table 1).
These results could have strong implications for insectivorous bats because of their reliance on echolocation for foraging. Frequency, in addition to source level intensity, plays a key role in the distance at which bats can detect obstacles and prey in their environment [58]. Detection ranges were surprisingly similar across sites but this is based solely on differences in pulse frequency because we used the same source level intensities across sites. It is likely that the incorporation of source level intensities for each site might yield greater variation in detection ranges. If so, then it suggests that climatic factors could affect the foraging efficiency (high ratio of prey capture to prey detection) of bats through their detection distances. The distances at which bats detect prey, especially if they use the high frequencies such as those used by R. clivosus, would be sensitive to increasing temperatures due to atmospheric attenuation of sound [35]. Luo et al. [35] proposed a “crossover frequency”, which is dependent on local climatic conditions, at which bats experience no change in prey detection volume in the event of increasing temperatures due to climate change. Bats that echolocate below the crossover frequency for their environment will experience an increase in their detection volume whereas bats that echolocate above the crossover frequency will experience a decrease in their detection volume as temperatures rise. For R. clivosus, this crossover frequency is at around 40 kHz [35]. This is well below the frequencies at which this lineage emits its pulses, suggesting that the detection range of R. clivosus may be sensitive to increases in temperature and relative humidity. It seems likely, therefore, that the foraging efficiency of this lineage may be negatively impacted by predicted increases in temperature as a result of global climate change. For example, if mean temperatures were to increase by only 2°C within the geographic range of R. clivosus, its prey detection volume is predicted to decrease by around 15% [35]. This could severely and negatively impact bat populations living in the tropical and sub-tropical areas where temperature is predicted to increase in the following decades. The expected warming all over Africa is very likely to be larger than the global annual mean warming throughout the continent and in all seasons, with drier subtropical regions warming more than the moister tropics [77]. These areas are part of the distributional range of R. clivosus and other Old World bats that use high duty cycle (HDC) echolocation, and therefore rising temperatures are likely to have deleterious effects on the foraging efficiency of such lineages. Unfortunately, given their reliance on an acoustic fovea and Doppler shift compensation these bats are unlikely to have sufficient flexibility to respond to rapid climatic change.
Non-adaptive explanations for acoustic signal divergence across habitats have also been proposed. If acoustic signals have a learned component, then random geographical variation is increased leading to the formation of local dialects–a form of cultural drift. This type of signal divergence has been observed in oscine passerines [23]. Although there is lack of conclusive evidence for this process occurring in rhinolophid bats, if resting frequencies are determined to some extent through a learned component and the acoustic signals of various populations are different enough to influence mate preference, gene flow between populations may be restricted, enhancing non-adaptive acoustic signal divergence [78]. However, given the strong association between climatic variables and RF that we report here and supported by other studies [9, 36, 69–71], it is likely that geographic variation in RF is the result of adaptation to local conditions rather than drift [79]. Furthermore, the anomalously high frequencies used by R. clivosus may be the result of socially mediated selection on pulse frequency for discrete frequency bands in the context of intraspecific communication [18]. If so, the evolution of high pulse frequencies in this lineage may be mediated by competition from other sympatric rhinolophid lineages for discrete frequency bands. This is a potentially fruitful area for future research.
In summary, this study provides support for the Sensory Drive Hypothesis in that RF varied with climatic factors. Because bats rely on acoustic signals for foraging, communication and mate acquisition, the climate-mediated divergence in these traits is likely adaptive, meaning that it could lead to lineage diversification over time, a topic in much need of further research. Further research is also needed to determine what effects rising temperatures due to climate change may have on bats, considering how their echolocation is influenced by climatic variables. Such impacts could include range contraction due to effects of increasing temperature on prey detection volume and thus bat foraging efficiency. Future studies should strive to include larger sample sizes, and possibly incorporate other environmental variables such as mean annual precipitation as well as habitat types to further tease apart the relative influence of climate on echolocation frequencies.
Supporting information
(TIF)
The top panels show the linear-mixed-effects model before correction for spatial autocorrelation. The bottom panels show the best model after all spatial autocorrelation structures with and without study sites as a random effect have been tested.
(TIF)
(TIF)
Acknowledgments
We would like to thank Tinyiko Maluleke, Beryl Makori, Dedan Ngatia and Simon Masika for assistance in data collection. This research was supported by a grant to DSJ from the South African Research Chair Initiative (SARChI) of the Department of Science and Technology (http://www.dst.gov.za), administered by the National Research Foundation (http://www.nrf.ac.za), grant number–GUN 64798. SC received a student assistantship from the National Research Foundation. PWW was supported by an overseas grant of the British Ecological Society (http://www.britishecologicalsociety.org) and the International Foundation of Science (http://www.ifs.se/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are available from figshare at: https://doi.org/10.25375/uct.5549944.v1.
Funding Statement
This research was supported by a grant to DSJ from the South African Research Chair Initiative (SARChI) of the Department of Science and Technology (http://www.dst.gov.za), administered by the National Research Foundation (http://www.nrf.ac.za), grant number – GUN 64798. SC received a student assistantship from the National Research Foundation. PWW was supported by an overseas grant of the British Ecological Society (http://www.britishecologicalsociety.org) and the International Foundation of Science (http://www.ifs.se/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Coyne JA, Orr HA. Speciation Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- 2.Sobel JM, Chen GF, Watt LR, Schemske DW. The biology of speciation. Evolution. 2010;64(2):295–315. doi: 10.1111/j.1558-5646.2009.00877.x [DOI] [PubMed] [Google Scholar]
- 3.Jiang T, Wu H, Feng J. Patterns and causes of geographic variation in bat echolocation pulses. Integrative Zoology. 2015;10(3):241–56. doi: 10.1111/1749-4877.12129 [DOI] [PubMed] [Google Scholar]
- 4.Zann R. Variation in song structure within and among populations of Australian zebra finches. The Auk. 1993;110:716–26. [Google Scholar]
- 5.Warren PS. Geographic variation and dialects in songs of the bronzed cowbird (Molothrus aeneus). The Auk. 2002;119(2):349–61. [Google Scholar]
- 6.Shieh B-S. Song Structure and Microgeographic Variation in a Population of the Greycheeked Fulvetta (Alcippe morrisonia) at Shoushan Nature Park, Southern Taiwan. Zoological Studies-Taipei-. 2004;43(1):132–41. [Google Scholar]
- 7.Wilczynski W, Ryan MJ. Geographic variation in animal communication systems In: Foster S, Endler JA, editors. Geographic variation in behavior: perspectives on evolutionary mechanisms. Oxford: Oxford University Press; 1999. p. 234–61 [Google Scholar]
- 8.Barclay RMR, Fullard JH, Jacobs DS. Variation in the echolocation calls of the hoary bat (Lasiurus cinereus): influence of body size, habitat structure, and geographic location. Canadian Journal of Zoology. 1999;77(4):530–4. [Google Scholar]
- 9.Guillen J. Variation in the frequency of the echolocation calls of Hipposideros ruber in the Gulf of Guinea: an exploration of the adaptive meaning of the constant frequency value in rhinolophoid CF bats. The Journal of Evolutionary Biology. 2000;13:70–80. [Google Scholar]
- 10.Law BS, Reinhold L, Pennay M. Geographic variation in the echolocation calls of Vespadelus spp.(Vespertilionidae) from New South Wales and Queensland, Australia. Acta Chiropterologica. 2002;4(2):201–15. [Google Scholar]
- 11.Armstrong KN, Coles RB. Echolocation call frequency differences between geographic isolates of Rhinonicteris aurantia (Chiroptera: Hipposideridae): implications of nasal chamber size. Journal of Mammalogy. 2007;88(1):94–104. [Google Scholar]
- 12.Gillam EH, McCracken GF. Variability in the echolocation of Tadarida brasiliensis: effects of geography and local acoustic environment. Animal Behaviour. 2007;74(2):277–86. [Google Scholar]
- 13.Griffin DR. Listening in the dark: the acoustic orientation of bats and men. Connecticut: Yale University press; 1958. [Google Scholar]
- 14.Fenton MB. Eavesdropping on the echolocation and social calls of bats. Mammal Review. 2003;33(3–4):193–204. [Google Scholar]
- 15.Jones G, Siemers BM. The communicative potential of bat echolocation pulses. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2010;197(5):447–57. doi: 10.1007/s00359-010-0565-x [DOI] [PubMed] [Google Scholar]
- 16.Puechmaille SJ, Borissov IM, Zsebok S, Allegrini B, Hizem M, Kuenzel S, et al. Female mate choice can drive the evolution of high frequency echolocation in bats: a case study with Rhinolophus mehelyi. PLoS One. 2014;9(7):e103452 doi: 10.1371/journal.pone.0103452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuchmann M, Siemers BM. Behavioral evidence for community-wide species discrimination from echolocation calls in bats. The American Naturalist. 2010;176(1):72–82. doi: 10.1086/652993 [DOI] [PubMed] [Google Scholar]
- 18.Bastian A, Jacobs DS. Listening carefully: increased perceptual acuity for species discrimination in multispecies signalling assemblages. Animal Behaviour. 2015;101(2015):141–54. [Google Scholar]
- 19.Boughman JW. How sensory drive can promote speciation. Trends in Ecology & Evolution. 2002;17(12):571–7. [Google Scholar]
- 20.Endler JA. Signals, signal conditions, and the direction of evolution. The American Naturalist. 1992;139:S125–S53. [Google Scholar]
- 21.Mutumi GL, Jacobs DS, Winker H. Sensory drive mediated by climatic gradients partially explains divergence in acoustic signals in two horseshoe bat species, Rhinolophus swinnyi and Rhinolophus simulator. PloS one. 2016;11(1):e0148053 doi: 10.1371/journal.pone.0148053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe RS. Geographic variation: a synthesis of cause, data, pattern and congruence in relation to subspecies, multivariate analysis and phylogenesis. Italian Journal of Zoology. 1987;54(1):3–11. [Google Scholar]
- 23.Tobias JA, Aben J, Brumfield RT, Derryberry EP, Halfwerk W, Slabbekoorn H, et al. Song divergence by sensory drive in Amazonian birds. Evolution. 2010;64(10):2820–39. doi: 10.1111/j.1558-5646.2010.01067.x [DOI] [PubMed] [Google Scholar]
- 24.Schnitzler H-U, Moss CF, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends in Ecology & Evolution. 2003;18(8):386–94. [Google Scholar]
- 25.Thomas JA, Moss CF, Vater M. Echolocation in bats and dolphins. Chicago: University of Chicago Press; 2004. [Google Scholar]
- 26.Knörnschild M, Jung K, Nagy M, Metz M, Kalko E. Bat echolocation calls facilitate social communication. Proceedings of the Royal Society of London B: Biological Sciences. 2012;279(1748):4827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuchmann M, Puechmaille SJ, Siemers BM. Horseshoe bats recognise the sex of conspecifics from their echolocation calls. Acta Chiropterologica. 2012;14(1):161–6. [Google Scholar]
- 28.Finger NM, Bastian A, Jacobs DS. To seek or speak? Dual function of an acoustic signal limits its versatility in communication. Animal Behaviour. 2017;127:135–52. http://dx.doi.org/10.1016/j.anbehav.2017.03.005. [Google Scholar]
- 29.Dechmann DKN, Wikelski M, van Noordwijk HJ, Voigt CC, Voigt-Heucke SL. Metabolic costs of bat echolocation in a non-foraging context support a role in communication. Frontiers in Physiology. 2013; 4: 66 doi: 10.3389/fphys.2013.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas DW, Bell GP, Fenton MB. Variation in echolocation call frequencies recorded from North American vespertilionid bats: a cautionary note. Journal of Mammalogy. 1987;68(4):842–7. [Google Scholar]
- 31.Odendaal LJ, Jacobs DS. Morphological correlates of echolocation frequency in the endemic Cape horseshoe bat, Rhinolophus capensis (Chiroptera: Rhinolophidae). Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2011;197(5):435–46. doi: 10.1007/s00359-010-0601-x [DOI] [PubMed] [Google Scholar]
- 32.Odendaal LJ, Jacobs DS, Bishop JM. Sensory trait variation in an echolocating bat suggests roles for both selection and plasticity. BMC Evolutionary Biology. 2014;14(1):60 doi: 10.1186/1471-2148-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence BD, Simmons JA. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. The Journal of the Acoustical Society of America. 1982;71(3):585–90. [DOI] [PubMed] [Google Scholar]
- 34.Stilz W-P, Schnitzler H-U. Estimation of the acoustic range of bat echolocation for extended targets. The Journal of the Acoustical Society of America. 2012;132(3):1765–75. doi: 10.1121/1.4733537 [DOI] [PubMed] [Google Scholar]
- 35.Luo J, Koselj K, Zsebők S, Siemers BM, Goerlitz HR. Global warming alters sound transmission: differential impact on the prey detection ability of echolocating bats. Journal of The Royal Society Interface. 2014;11(91):20130961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snell-Rood EC. The effect of climate on acoustic signals: does atmospheric sound absorption matter for bird song and bat echolocation? The Journal of the Acoustical Society of America. 2012;131(2):1650–8. doi: 10.1121/1.3672695 [DOI] [PubMed] [Google Scholar]
- 37.Noack T. Das Quagga. Zool Gart Frankfurt. 1893;34:289–97. [Google Scholar]
- 38.Csorba G, Ujhelyi P, Thomas N. Horseshoe bats of the world:(Chiroptera: Rhinolophidae). Shropshire, UK: Alana books; 2003. [Google Scholar]
- 39.Jacobs DS, Barclay RMR, Walker MH. The allometry of echolocation call frequencies of insectivorous bats: why do some species deviate from the pattern? Oecologia. 2007;152(3):583–94. doi: 10.1007/s00442-007-0679-1 [DOI] [PubMed] [Google Scholar]
- 40.Benda P, Dietz C, Andreas M, Hotový J, Lučan RK, Maltby A, et al. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 6. Bats of Sinai (Egypt) with some taxonomic, ecological and echolocation data on that fauna. Acta Societatis Zoologicae Bohemicae. 2008;72(1–2):1–103. [Google Scholar]
- 41.Akaike H. Canonical correlation analysis of time series and the use of an information criterion, in: Raman K. Mehra and Dimitri G. Lainiotis, eds., System identification: Advances and case studies (Academic Press, New York, NY: ); 1976. [Google Scholar]
- 42.Sikes RS, Gannon WL. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy. 2011;92(1):235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aegerter J, Heritage S. The extent and frequency of European bat Lyssavirus (EBLV2) in Scotland as determined by surveillance testing of sero-prevalence and tissue sampling: Scottish Natural Heritage; 2005. [Google Scholar]
- 44.Kunz TH, Parsons S (eds). Ecological and behavioral methods for the study of bats. Baltimore, MD: Johns Hopkins University Press; 2009. [Google Scholar]
- 45.Nowak RM. Walker's mammals of the world. Baltimore and London: John Hopkins University Press; 1999. [Google Scholar]
- 46.Monadjem A, Taylor PJ, Cotterill W, Schoeman MC. Bats of southern and central Africa: a biogeographic and taxonomic synthesis. Johannesburg: Wits University Press Johannesburg; 2010. [Google Scholar]
- 47.Benda P, Vallo P. New look on the geographical variation in Rhinolophus clivosus with description of a new horseshoe bat species from Cyrenaica, Libya. Vespertilio. 2012;16:69–96. [Google Scholar]
- 48.Dool SE, Puechmaille SJ, Foley NM, Allegrini B, Bastian A, Mutumi GL, et al. Nuclear introns outperform mitochondrial DNA in inter-specific phylogenetic reconstruction: lessons from horseshoe bats (Rhinolophidae: Chiroptera). Molecular phylogenetics and evolution. 2016;97:196–212. doi: 10.1016/j.ympev.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 49.Neuweiler G. Foraging ecology and audition in echolocating bats. Trends in Ecology & Evolution. 1989;4(6):160–6. [DOI] [PubMed] [Google Scholar]
- 50.Fenton MB. Describing the echolocation calls and behaviour of bats. Acta Chiropterologica. 1999;1(2):127–36. [Google Scholar]
- 51.Fenton MB, Faure PA, Ratcliffe JM. Evolution of high duty cycle echolocation in bats. Journal of Experimental Biology. 2012;215(17):2935–44. [DOI] [PubMed] [Google Scholar]
- 52.Racey PA. Reproductive assessment in bats In: Kunz TH, editor. Ecological and Behavioural Methods for the Study of Bats. Washington DC: Smithsonian Institution Press; 1988. p. 31–45. [Google Scholar]
- 53.Anthony ELP. Age determination in bats Kunz TH, editor.Washington D.C: Smithsonian Institution Press; 1988. p.47–58 [Google Scholar]
- 54.Rughetti M, Toffoli R. Sex-specific seasonal change in body mass in two species of vespertilionid bats. Acta Chiropterologica. 2014;16(1):149–55. [Google Scholar]
- 55.Neuweiler G. Foraging, echolocation and audition in bats. Naturwissenschaften. 1984;71(9):446–55. [Google Scholar]
- 56.Schuller G, Suga N. Storage of Doppler-shift information in the echolocation system of the ‘CF-FM’ bat, Rhinolophus ferrumequinum. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1976;105(1):9–14. [Google Scholar]
- 57.Siemers BM, Beedholm K, Dietz C, Dietz I, Ivanova T. Is species identity, sex, age or individual quality conveyed by echolocation call frequency in European horseshoe bats? Acta Chiropterologica. 2005;7(2):259–74. [Google Scholar]
- 58.Holderied MW, Von Helversen O. Echolocation range and wingbeat period match in aerial-hawking bats. Proceedings of the Royal Society of London B: Biological Sciences. 2003;270(1530):2293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Körner C. The use of “altitude” in ecological research. Trends in ecology & evolution. 2007;22(11):569–74. [DOI] [PubMed] [Google Scholar]
- 60.Zuckerwar AJ. Handbook of the Speed of Sound in Real Gases (Volume 1) St Louis, Missouri, USA: Academic Press; 2002. [Google Scholar]
- 61.Gillam EH, McCracken GF, Westbrook JK, Lee Y-F, Jensen ML, Balsley BB. Bats aloft: variability in echolocation call structure at high altitudes. Behavioral Ecology and Sociobiology. 2009;64(1):69–79. [Google Scholar]
- 62.Bontadina F, Schofield H, Naef-Daenzer B. Radio-tracking reveals that lesser horseshoe bats (Rhinolophus hipposideros) forage in woodland. Journal of Zoology. 2002;258(3):281–90. [Google Scholar]
- 63.Goiti U, Aihartza JR, Garin I, Zabala J. Influence of habitat on the foraging behaviour of the Mediterranean horseshoe bat, Rhinolophus euryale. Acta Chiropterologica. 2003;5(1):75–84. [Google Scholar]
- 64.Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience. 2001;51(11):933–8. [Google Scholar]
- 65.Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. [Google Scholar]
- 66.Team RC. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria: URL. www.R-project.org/[Computer Software]. 2014. [Google Scholar]
- 67.Box GEP, Jenkins GM, Reinsel GC. Time series analysis, forcasting and control, volume 3rd edition. 3rd ed: Prentice Hall; 1994. [Google Scholar]
- 68.Pinheiro JC, Bates DM. Linear mixed-effects models: basic concepts and examples. Mixed-effects models in S and S-Plus. 2000:3–56. [Google Scholar]
- 69.Jiang T, Metzner W, You Y, Liu S, Lu G, Li S, et al. Variation in the resting frequency of Rhinolophus pusillus in Mainland China: effect of climate and implications for conservation. Journal of the Acoustical Society of America. 2010. 128(4):2204–11. doi: 10.1121/1.3478855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang T, You Y, Liu S, Lu G, Wang L, Wu H, et al. Factors affecting geographic variation in echolocation calls of the endemic Myotis davidii in China. Ethology. 2013;119(10):881–90. [Google Scholar]
- 71.Sun K, Luo L, Kimball RT, Wei X, Jin L, Jiang T, et al. Geographic variation in the acoustic traits of greater horseshoe bats: testing the importance of drift and ecological selection in evolutionary processes. PLoS One. 2013;8(8):e70368 doi: 10.1371/journal.pone.0070368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaverri G, Quirós OE. Variation in echolocation call frequencies in two species of free-tailed bats according to temperature and humidity. The Journal of the Acoustical Society of America. 2017;142(1):146–50. doi: 10.1121/1.4992029 [DOI] [PubMed] [Google Scholar]
- 73.Stoffberg S, Schoeman MC, Matthee CA. Correlated genetic and ecological diversification in a widespread southern African horseshoe bat. PloS One. 2012;7(2):e31946 doi: 10.1371/journal.pone.0031946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.James FC. Geographic size variation in birds and its relationship to climate. Ecology. 1970;51(3):365–90. [Google Scholar]
- 75.Jones G, editor Does echolocation constrain the evolution of body size in bats? Symposia of the Zoological Society of London; 1996: London: The Society, 1960–1999. [Google Scholar]
- 76.Maluleke T, Jacobs DS, Winker H. Environmental correlates of geographic divergence in a phenotypic trait: A case study using bat echolocation. Ecology and Evolution. 2017;7:7347–7361. doi: 10.1002/ece3.3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held R, et al. Regional climate projections Climate Change, 2007: The Physical Science Basis Contribution of Working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, University Press, Cambridge, Chapter 11, 2007. p. 847–940. [Google Scholar]
- 78.Russo D, Mucedda M, Bello M, Biscardi S, Pidinchedda E, Jones G. Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): a case of character displacement? Journal of Biogeography. 2007;34(12):2129–38. [Google Scholar]
- 79.Mutumi GL, Jacobs DS, Winker H. The relative contribution of drift and selection to phenotypic divergence: A test case using the horseshoe bats Rhinolophus simulator and Rhinolophus swinnyi. Ecology and Evolution. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
The top panels show the linear-mixed-effects model before correction for spatial autocorrelation. The bottom panels show the best model after all spatial autocorrelation structures with and without study sites as a random effect have been tested.
(TIF)
(TIF)
Data Availability Statement
All relevant data are available from figshare at: https://doi.org/10.25375/uct.5549944.v1.