Abstract
Two randomized, placebo-controlled trials conducted in patients with nondialysis-dependent (NDD) chronic kidney disease (CKD), iron deficiency anemia, and normal or elevated serum phosphorus demonstrated that ferric citrate (FC) significantly increased hemoglobin and decreased serum phosphate concentrations. Pooling these trial results could provide a more robust evaluation of the safety and efficacy of FC in this population. We pooled results of a phase 2 (n = 149) and 3 trial (n = 233) of patients randomized and treated for up to 12 and 16 weeks, respectively. The starting dose in both trials was three 1-g (elemental iron 210 mg) tablets/day with food, up to 12 tablets/day. Doses were titrated in the phase 2 and 3 trials to lower serum phosphate concentrations to a target range (0.97–1.13 mmol/L) and to achieve a ≥10-g/L hemoglobin increase, respectively. Safety was assessed in all patients who received ≥1 dose of FC (n = 190) and placebo (n = 188). Treatment-emergent adverse events (AEs) were reported in 143 of 190 (75.3%) FC-treated and 116 of 188 (61.7%) placebo-treated patients; gastrointestinal AEs were the most frequent (94 [49.5%] vs. 52 [27.7%], respectively). Specific events reported in >5% of patients (FC vs. placebo, respectively) included discolored feces (41 [21.6%] vs. 0 [0.0%]), diarrhea (39 [20.5%] vs. 23 [12.2%]), constipation (35 [18.4%] vs. 19 [10.1%]), and nausea (18 [9.5%] vs. 8 [4.3%]). Twenty FC-treated (10.5%) and 21 placebo-treated patients (11.2%) experienced a serious AE. Two patients (1.1%) died in each group. A pooled efficacy assessment demonstrated a consistent hemoglobin rise and modest serum phosphate decline, with few excursions below the normal range. When used for treatment of patients with NDD-CKD, FC contributes to gastrointestinal AEs at higher rates than placebo, while simultaneously correcting two of the principal metabolic manifestations of CKD (iron deficiency anemia and relative hyperphosphatemia).
Introduction
Chronic kidney disease (CKD), as currently defined, is reported to be present in one in seven individuals in the United States (US) [1]. Although >650,000 patients require either dialysis or a functioning kidney transplant to sustain life, the vast majority of patients with CKD do not. A heightened risk of cardiovascular disease may be the most significant complication of moderate to advanced CKD [2,3]; other clinical manifestations can include impaired physical and cognitive function, frailty, protein energy wasting, fractures [4–7], and numerous laboratory abnormalities including iron deficiency and hyperphosphatemia. Iron deficiency [8–10] contributes to anemia in CKD [11], as well as to heart failure [12,13], central nervous system dysfunction, and diminished health-related quality of life in some patients [14,15]. Hyperphosphatemia can contribute to dystrophic calcification, secondary hyperparathyroidism, and other abnormalities of mineral metabolism [16].
The optimal management strategies for iron deficiency and phosphate retention in advanced, nondialysis-dependent CKD (NDD-CKD) are unknown. Ferric citrate was initially developed as a phosphate binder and received regulatory approval for the control of serum phosphorus in patients with dialysis-dependent CKD (DD-CKD) [17]. When prescribed for the treatment of hyperphosphatemia in DD-CKD, investigators observed that ferric citrate not only decreased serum phosphate concentrations but also increased transferrin saturation (TSAT) and serum ferritin concentrations, allowing patients to maintain hemoglobin concentrations on lower doses of erythropoiesis-stimulating agents (ESAs) and intravenous iron [18]. Given the expanding evidence base linking higher serum phosphate concentrations to adverse events (AEs) in individuals with impaired as well as normal or near-normal kidney function, we initially designed a phase 2 placebo-controlled trial (ClinicalTrials.gov identifier: NCT01736397) to examine the effects of ferric citrate among patients with NDD-CKD, iron deficiency anemia, and high-normal or elevated serum phosphate (“relative hyperphosphatemia”) [19]. Having observed favorable effects on hemoglobin, TSAT, and ferritin levels with ferric citrate dosing guided by serum phosphate concentrations, we designed a phase 3 placebo-controlled trial (NCT02268994) to examine the effects of ferric citrate among patients with iron deficiency anemia with dosing guided by hemoglobin concentrations [20]. Primary results of these trials have been previously published [20]. Herein, we pool results from both trials, primarily to obtain a more robust assessment of the safety of ferric citrate. Since both trials included similar populations, doses, and duration and captured the same laboratory-based outcomes, we also pool results to determine efficacy.
Materials and methods
Design of the phase 2 and 3 studies
The phase 2 study was a 12-week, randomized, multicenter, double-blind, placebo-controlled trial undertaken at 27 centers in the US, with the first patient visit in November 2012 and last patient visit in October 2013. The phase 3 study was a randomized, multicenter, double-blind, placebo-controlled trial comprised of a 16-week randomized period followed by an 8-week safety extension period and undertaken at 32 centers in the US, with the first patient visit in October 2014 and last patient visit in January 2016. Both trials were conducted in accordance with Title 21, US Code of Federal Regulations Parts 11, 50, 54, 56, and 312; the International Conference on Harmonisation guideline on Good Clinical Practices (E6, April 1996); the Declaration of Helsinki (Seoul, October 2008); and applicable local regulatory requirements and laws. All versions of the protocol implemented in each study were reviewed and approved by the Liberty Institutional Review Board before patient enrollment at each site. Institutional review board-approved written informed consent was obtained from each patient before performing any study assessments. Detailed methods for both trials have been previously published [19,20].
Study population
Patients in both trials had CKD stages 3–5 (estimated glomerular filtration rate <60 mL/min/1.73 m2) and were not receiving dialysis. In the phase 3 trial, patients had intolerance or an inadequate response to conventional oral iron formulations; this was not an eligibility criterion in the phase 2 trial. In the phase 2 trial, patients were required to have serum phosphate ≥1.29 and <1.94 mmol/L at baseline, whereas serum phosphate was required to be ≥1.13 mmol/L in the phase 3 trial. Inclusion criteria regarding iron deficiency anemia differed slightly otherwise: screening TSAT, ferritin, and hemoglobin concentrations were required to be <30%, <674 pmol/L, and 91–119 g/L, respectively, in the phase 2 trial; corresponding values were <25%, <449 pmol/L, and 90–115 g/L in the phase 3 trial. Phase 3 trial participants tended to have slightly more pronounced iron deficiency and anemia, although there was extensive overlap across the two trial samples vis-à-vis baseline serum phosphate, hemoglobin, TSAT, and ferritin. Neither intravenous iron nor ESAs were allowed during the 12-week or 16-week placebo-controlled treatment periods in the phase 2 and 3 trials, respectively. The starting dose in both trials was three 1-g (210 mg elemental iron) tablets/day with food, which could be increased to a maximum of 12 tablets/day. In the phase 2 trial, the dose was titrated to achieve a target serum phosphate concentration (0.97–1.13 mmol/L); in the phase 3 trial, the dose was titrated to achieve a ≥10-g/L increase in hemoglobin.
Analysis populations
The pooled safety analysis included all patients who received ≥1 dose of study drug (ferric citrate or placebo) and data from baseline until the end of the randomized period of each study (12 weeks for the phase 2 trial; 16 weeks for the phase 3 trial). The pooled efficacy analysis included all patients who were randomized, received ≥1 dose of study drug, and had ≥1 postbaseline laboratory value in the randomized period. Nine patients were enrolled in both trials; of these, three were randomized to the same treatment in both trials and only data from the phase 3 trial were included for these patients. Six patients were randomized to receive different treatments in the phase 2 and 3 trials, and these six patients contributed data from each trial.
Safety and efficacy end points
Key safety end points included all treatment-emergent AEs and serious AEs, as traditionally defined, at any point up to the end of the randomized period of each trial. Based on prior trials of ferric citrate in DD-CKD, we considered gastrointestinal AEs and ferritin ≥1573 pmol/L, TSAT ≥70%, and serum phosphate <0.65 mmol/L as outcomes of special interest.
Efficacy end points included those related to the treatment of iron deficiency anemia: the proportion of patients achieving a ≥10-g/L increase in hemoglobin at any time during the randomized study periods and mean changes in hemoglobin, TSAT, ferritin, and serum phosphate from baseline to week 12.
Statistical analysis
For safety data, we provide counts and proportions. Inference tests were not performed. For the analysis of discrete proportions, we used the Cochran-Mantel-Haenszel test stratified by trial to estimate the difference in the proportion of patients randomized to ferric citrate and placebo who achieved a ≥10-g/L increase in hemoglobin at any point up to the end of each trial. We performed a Breslow-Day test for homogeneity to assess whether the effect of treatment was similar in the phase 2 and 3 trials using a significance level of P = 0.10. We analyzed changes from baseline in laboratory values to all postbaseline time points during randomized treatment up to week 12 with a mixed model for repeated measures (MMRM) using restricted maximum likelihood with treatment (ferric citrate and placebo), trial (phase 2 and phase 3), visit, and treatment-by-visit interaction as fixed effects; the baseline variable as a covariate; and patient as a random effect. Finally, we added a term for trial-by-treatment interaction to the model to assess whether the effect of treatment differed by trial, which was tested at a significance level of P = 0.10. A compound symmetry covariance structure was used to model within-patient errors. Point estimates and 95% confidence intervals for the treatment differences at each assessment time, along with P values for the treatment comparisons, were obtained from the model. There was no imputation of missing data. We conducted all analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
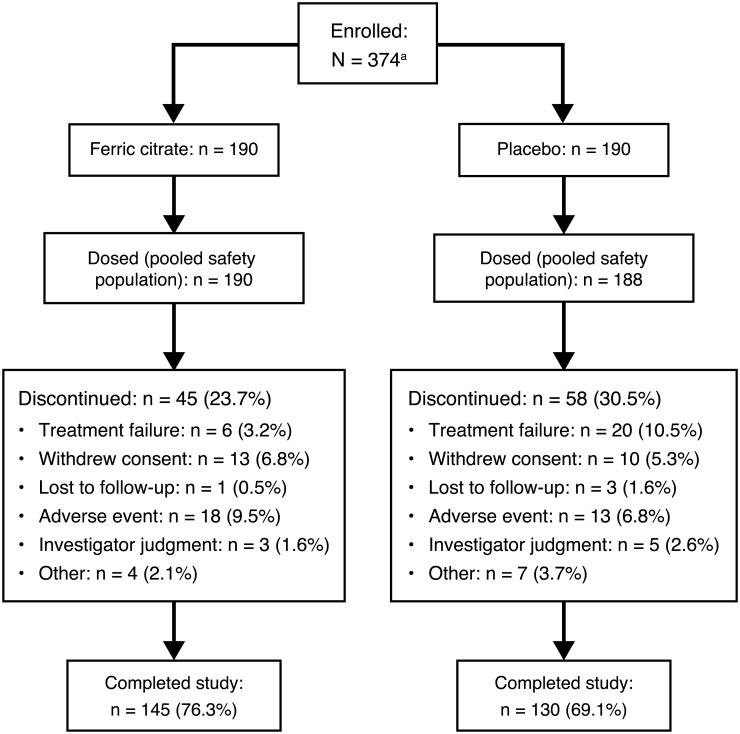
Fig 1 shows the disposition of patients from the two trials. The pooled safety population included 190 ferric citrate-treated and 188 placebo-treated patients; of these, 145 (76.3%) and 130 (69.1%) patients, respectively, completed the randomization period. Baseline characteristics of all participants are shown in Table 1.
Fig 1. CONSORT diagram.
aNine patients were enrolled in both studies; of these, three were randomized to the same treatment in both trials and only their data from the phase 3 trial were included, and six patients who were randomized to different treatments contributed data from each trial but were only counted once in the total number of enrolled subjects.
Table 1. Baseline characteristics (pooled data).
| Characteristic | Ferric citrate (n = 190) | Placebo (n = 188) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 65.5 (11.6) | 64.8 (13.3) |
| Min–max | 25–87 | 21–93 |
| Sex, n (%) | ||
| Female | 126 (66.3) | 115 (61.2) |
| Race, n (%) | ||
| White | 135 (71.1) | 137 (72.9) |
| Black | 54 (28.4) | 47 (25.0) |
| Other | 1 (0.5) | 4 (2.1) |
| Ethnicity, n (%) | ||
| Hispanic | 43 (22.6) | 45 (23.9) |
| Weight, kg | ||
| Mean (SD) | 92.6 (22.7) | 91.1 (24.1) |
| Min–max | 46.9–154.7 | 47.9–167.8 |
| Past medical history, n (%) | ||
| Diabetes mellitus | 132 (69.5) | 131 (69.7) |
| Heart failure | 40 (21.1) | 41 (21.8) |
| Ischemic heart disease | 53 (27.9) | 55 (29.3) |
| Current stage of CKD, n (%) | ||
| Stage 3 | 78 (41.1) | 87 (46.3) |
| Stage 4 | 88 (46.3) | 83 (44.1) |
| Stage 5 | 24 (12.6) | 18 (9.6) |
| eGFR, mL/min/1.73 m2 | ||
| Mean (SD) | 26.7 (12.5) | 26.8 (11.9) |
| Min–max | 6–66 | 8–63 |
| Hemoglobin, g/dL | ||
| Mean (SD) | 10.48 (0.76) | 10.47 (0.90) |
| Min–max | 8.5–12.4 | 8.4–14.7 |
| Transferrin saturation, % | ||
| Mean (SD) | 20.9 (6.8) | 20.3 (7.4) |
| Min–max | 6–50 | 4–45 |
| Ferritin, ng/mL | ||
| Mean (SD) | 98.0 (68.9) | 92.5 (68.5) |
| Min–max | 3–380 | 6–355 |
| Serum phosphate, mg/dL | ||
| Mean (SD) | 4.34 (0.81) | 4.33 (0.70) |
| Min–max | 2.8–8.7 | 2.8–6.9 |
| Bicarbonate, mmol/L | ||
| Mean (SD) | 21.0 (3.6) | 21.6 (3.6) |
| Min–max | 10–30 | 12–30 |
To convert g/dL to g/L for hemoglobin, multiply by 10. To convert ng/mL to pmol/L for ferritin, multiply by 2.247. To convert mg/dL to mmol/L for phosphate, multiply by 0.323.
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Treatment duration and dose
The mean (standard deviation [SD]) treatment durations in the ferric citrate and placebo groups were 12.8 (4.2) and 12.1 (4.9) weeks, respectively, with corresponding mean (SD) study drug doses of 5.4 (1.5) and 5.7 (1.7) g/day.
Safety
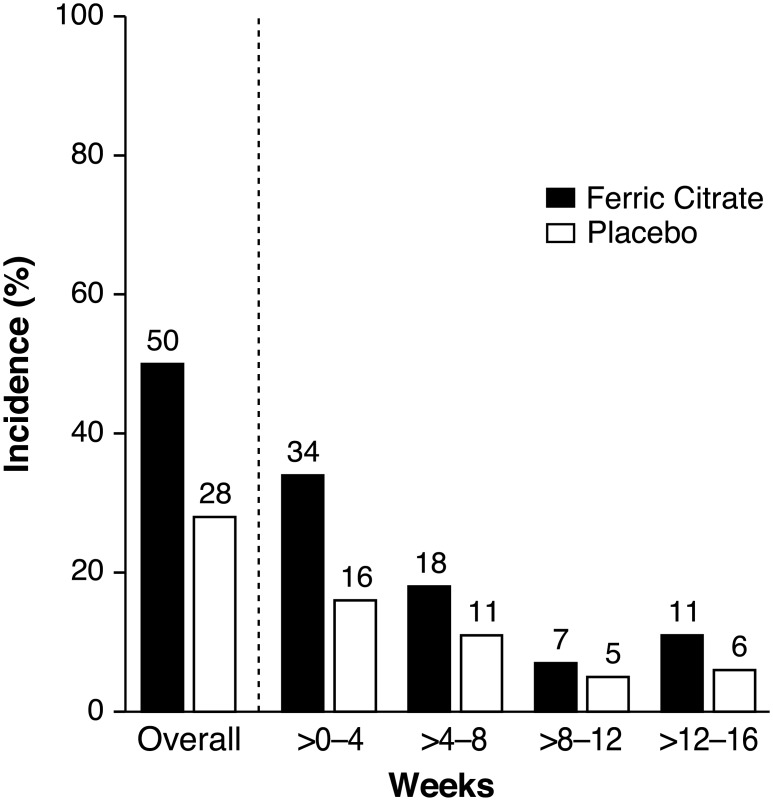
Treatment-emergent AEs were reported in 143 ferric citrate-treated (75.3%) and 116 placebo-treated patients (61.7%); gastrointestinal AEs were the most frequent (94 [49.5%] vs. 52 [27.7%], respectively). Ten ferric citrate-treated patients (5.3%) and two placebo-treated patients (1.1%) discontinued the study drug because of gastrointestinal AEs. Specific events reported in >5% of patients (ferric citrate vs. placebo, respectively) included discolored feces (41 [21.6%] vs. 0 [0.0%]), diarrhea (39 [20.5%] vs. 23 [12.2%]), constipation (35 [18.4%] vs. 19 [10.1%]), and nausea (18 [9.5%] vs. 8 [4.3%]). Twenty ferric citrate-treated patients (10.5%) and 21 placebo-treated patients (11.2%) experienced a serious AE; the highest proportion of serious AEs were cardiac disorders (3.7% vs. 2.7%, respectively) and infections and infestations (2.6% vs. 3.7%, respectively). Fig 2 describes gastrointestinal AEs by study week. Table 2 includes additional, less commonly observed gastrointestinal AEs. Two patients (1.1%) died in each treatment group. None of the serious AEs or deaths were deemed to be related to the study drug.
Fig 2. Gastrointestinal adverse events by study week.
Discontinuations because of gastrointestinal treatment-emergent adverse events during the randomized periods (ferric citrate [5.3%] vs. placebo [1.1%]).
Table 2. Gastrointestinal AEs observed in ≥2% of patients.
| Gastrointestinal AEs, n (%) | Ferric citrate (n = 190) | Placebo (n = 188) |
|---|---|---|
| Any AE (≥2% of patients) | 94 (49.5) | 52 (27.7) |
| Feces discolored | 41 (21.6) | 0 (0.0) |
| Diarrhea | 39 (20.5) | 23 (12.2) |
| Constipation | 35 (18.4) | 19 (10.1) |
| Nausea | 18 (9.5) | 8 (4.3) |
| Abdominal pain | 9 (4.7) | 3 (1.6) |
| Vomiting | 8 (4.2) | 8 (4.3) |
| Flatulence | 6 (3.2) | 3 (1.6) |
| Abdominal discomfort | 4 (2.1) | 1 (0.5) |
| Dyspepsia | 4 (2.1) | 1 (0.5) |
AE, adverse event.
Episodes of TSAT ≥70%, serum ferritin ≥1573 pmol/L, and serum phosphate <0.65 mmol/L were observed in 28 (14.9%), one (0.5%), and two (1.1%) ferric citrate-treated patients, respectively, compared with 0 (0.0%), 0 (0.0%), and 0 (0.0%) placebo-treated patients, respectively.
Efficacy
Iron deficiency anemia
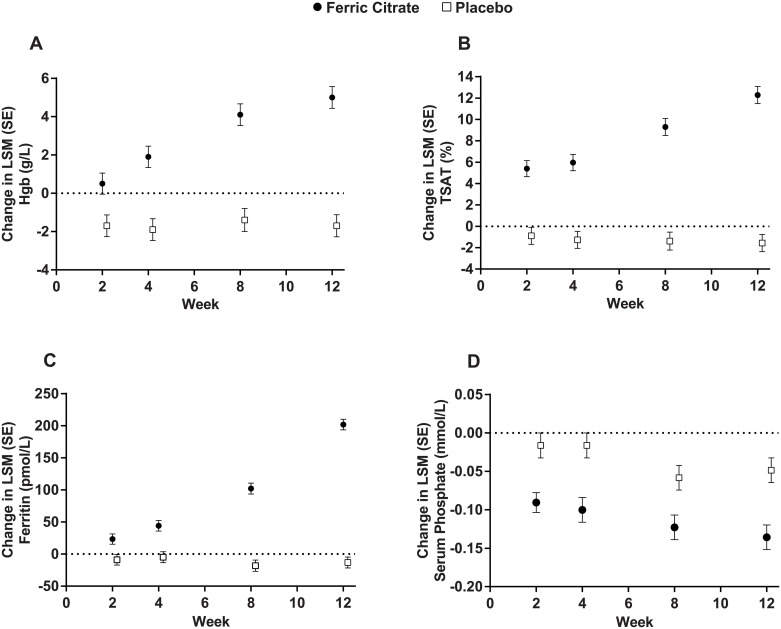
The proportion of patients who achieved a ≥10-g/L increase in hemoglobin from baseline at any time during the randomization period was higher among patients treated with ferric citrate compared with placebo (47.8% vs. 18.6%, respectively; difference of proportions, 28.7%; P < 0.001. Breslow-Day test for homogeneity indicated no differences across trials, P = 0.46. At week 12, the mean (SD) change in hemoglobin was 5.0 (0.6) g/L in the ferric citrate group versus –1.7 (0.6) g/L in the placebo group (mean difference, 6.7 g/L; P < 0.001). The mean (SD) change in TSAT was 12.3 (0.80) % in the ferric citrate group versus –1.6 (0.81) % in the placebo group (mean difference, 13.8%; P < 0.001). The mean (SD) change in ferritin was 210.8 (8.5) pmol/L in the ferric citrate group versus –13.3 (8.5) pmol/L in the placebo group (mean difference, 224.0 pmol/L; P < 0.001). Fig 3A–3C show pooled results for hemoglobin, TSAT, and ferritin over the 12-week randomized treatment period in the phase 2 trial and for the first 12 of 16 weeks of the randomized treatment period in the phase 3 trial. The trial-by-treatment interaction showed no treatment heterogeneity between trials for hemoglobin (P = 0.62) but suggested heterogeneity for TSAT and ferritin (P = 0.055 and P = 0.076, respectively). TSAT and ferritin increased in ferric citrate-treated patients in both trials, with more pronounced treatment effects observed in the phase 3 trial.
Fig 3. Pooled changes from baseline in (A) Hgb, (B) TSAT, (C) ferritin, and (D) serum phosphate over the 12-week randomized treatment period in the phase 2 trial and the first 12 of 16 weeks of the randomized treatment period in the phase 3 trial.
Changes were analyzed using mixed model for repeated measures with restricted maximum likelihood, with treatment (ferric citrate vs. placebo), study, visit, and treatment-by-visit interaction as fixed effects; baseline as a covariate; and patient as a random effect. Study-by-treatment interaction P value was obtained from the same model with the addition of the study-by-treatment interaction term. Hgb, hemoglobin; LSM, least-squares mean; SE, standard error; TSAT, transferrin saturation.
Phosphorus
At week 12, the mean (SD) change in serum phosphate was –0.14 (0.02) mmol/L in the ferric citrate group versus –0.05 (0.02) mmol/L in the placebo group (mean difference, –0.09 mmol/L; P < 0.001). There was a reduction in serum phosphate in ferric citrate-treated patients in both trials, with more pronounced treatment effects observed in the phase 2 trial (P < 0.001). Fig 3D shows pooled results for serum phosphate over the 12-week randomized treatment period in the phase 2 trial and for the first 12 of 16 weeks of the randomized treatment period in the phase 3 trial.
Discussion
This pooled analysis of two randomized, placebo-controlled trials, which comprises the largest dataset of patients with NDD-CKD stages 3–5 treated with ferric citrate to date, has enabled a comprehensive evaluation of potential AEs with ferric citrate, including hypophosphatemia, in patients with NDD-CKD with normal to high phosphate levels and iron deficiency anemia. Of note, no major or unexpected safety concerns were observed in this analysis. We also report a more complete and detailed evaluation of gastrointestinal AEs compared with prior publications of the individual trials. We show that gastrointestinal AEs including diarrhea, constipation, and nausea were relatively common, but few were classified as serious or resulted in hospitalization or discontinuation of medication. The frequency of AEs among placebo-treated patients in CKD trials is typically high, but the percentage of ferric citrate-treated patients who experienced AEs was greater by 14% for any treatment-emergent AE, 22% for any gastrointestinal AE, 8% for diarrhea, 8% for constipation, 5% for nausea, and 3% for abdominal pain. There have been no head-to-head comparisons of ferric citrate and other iron formulations. Bearing that in mind, the AEs for ferric citrate in the pooled dataset are similar to those in other clinical trials in which conventional oral iron formulations (e.g. ferrous sulfate, fumarate, and gluconate) have been used [21–23] and to clinical practice experience, where AEs (predominantly constipation) of conventional oral iron formulations are common [22]. The discontinuation rate due to gastrointestinal AEs in this trial (5.3%) was in line with that observed with other conventional oral iron formulations; however, it is difficult to make a direct comparison across trials because of variations in the methodology of AE reporting, particularly with respect to how discontinuations due to AEs were reported [24–26].
While these trials did not compare ferric citrate to conventional oral iron formulations or intravenous iron in a head-to-head manner, ferric citrate showed robust efficacy in light of the effects seen with conventional oral iron formulations in other trials in this patient population, although patients in the ferric citrate trials were less iron deficient at baseline [24,25,27,28]. Similarly, although ferric citrate has not been compared directly with other phosphate binders in this patient population, the achieved decrease in serum phosphate was larger than in another randomized, placebo-controlled trial in patients with CKD stages 3–5 in which calcium acetate, sevelamer carbonate, and lanthanum carbonate were compared [29]. Trials with head-to-head comparisons would enable more definitive conclusions to be made on the relative efficacy of ferric citrate. A trial comparing ferric citrate versus ferrous sulfate on iron parameters and hemoglobin in patients with CKD and iron deficiency is currently underway (NCT02888171).
It is noteworthy that the incidence of all gastrointestinal AEs declined over time despite an increase in the study medication dose. This phenomenon could be due to patients developing awareness of stool discoloration when using oral iron salts and/or adjusting to an increase in the number and frequency of bowel movements. These results should serve as a reminder to health care providers to inform patients starting ferric citrate of possible expectations regarding changes in stool color and consistency or frequency of bowel movements. Patients should also be reminded that, once on therapy, subsequent changes in stool color or character should prompt discussion with health care providers, so that other gastrointestinal issues (e.g. melena or hematochezia) are not masked.
Treatment with ferric citrate in this patient population produced a modest decline in serum phosphate that generally stayed within the normal range. It was reassuring that the incidence of hypophosphatemia was low (serum phosphate concentrations did not go lower than 1.6 mg/dL/0.09 mmol/L); however, it should be noted that patients with low-normal serum phosphate concentrations were excluded from participation. There remains some debate on the use of phosphate binders in patients with moderate to advanced CKD and normal or near-normal serum phosphate. A pilot 4-arm trial (calcium acetate, sevelamer carbonate, lanthanum carbonate and placebo) showed only modest reductions in serum phosphate and failed to attenuate vascular calcification [29]. In view of potential adverse skeletal and extraskeletal effects of prolonged hypophosphatemia, physicians may consider periodic monitoring of patients receiving ferric citrate for the treatment of iron deficiency anemia with low or low-normal serum phosphate concentrations, and severe nutritional vitamin D deficiency should be corrected. It is now recognized that 1,25-dihydroxy vitamin D deficiency in patients with NDD-CKD can be exacerbated by sustained increases in fibroblast growth factor 23 (FGF23), which directly suppress cytochrome P450 (CYP)27 (1-alpha-hydroxylase) and stimulate catabolic CYP24 (24-hydroxlase) [30–32]. Another interesting effect of ferric citrate is the reduction of FGF23 [19,20]. Elevated FGF23 in patients with CKD has been shown to be a risk factor for left ventricular hypertrophy and cardiovascular events, progressive loss of kidney function, and death [33–37].
A sizeable fraction of patients treated with ferric citrate experienced a transient increase in TSAT levels above 70%, although these elevated levels were not sustained. We believe that the transient increases in TSAT reflect the fact that the blood draws were not timed relative to drug intake. As described by Kobune et al., TSAT levels can rise above 60% within 2–3 hours after intake of an oral iron dose [38]. In future trials, it will be important to specify that laboratory draws for TSAT be obtained before daily dosing to minimize lability following oral ingestion of iron-containing compounds. As per the US Food and Drug Administration label, ferric citrate is contraindicated in patients with iron overload syndromes [17], and patients with a history of hemochromatosis were excluded from participating in these clinical trials.
Two additional studies examined the provision of ferric citrate in patients with NDD-CKD but were not similar enough to be included in the pooling: a Japanese trial of patients with hyperphosphatemia in which ferric citrate was used as a phosphate binder but in a lower dose and with concomitant use of intravenous iron and ESAs [39], and another small trial (NCT02128074) without a placebo control in which ferric citrate was given at a lower dose without food. The adverse event profiles in these studies were similar to that reported here [39].
This pooled analysis provides the most robust results available to date regarding the safety of ferric citrate when used for the treatment of either iron deficiency anemia or relative hyperphosphatemia in patients with NDD-CKD. Major limitations include the relatively small sample size and short duration of treatment. Also, women were overrepresented in the trials, most likely due to the higher prevalence of iron deficiency anemia in women than in men. Trial participants were more likely to be obese by Quételet’s (body mass) index than what might be expected for a typical population with iron deficiency anemia and normal or near normal kidney function, probably related to the higher prevalence of, and rates of progression with, CKD in the setting of obesity [1].
Ferric citrate is approved for the treatment of hyperphosphatemia in adult patients receiving dialysis and as an iron replacement product for the treatment of iron deficiency anemia in adult patients with chronic kidney disease not on dialysis. It will be important to assess longer-term safety end points in clinical practice and to determine its optimal use for maintenance therapy. The recently initiated COMPASS trial (NCT03236246) is designed to assess long-term safety and efficacy (over 48 weeks) of ferric citrate in patients with NDD-CKD and iron deficiency anemia.
Conclusions
In summary, results from a pooled analysis of two randomized, placebo-controlled trials of ferric citrate for iron deficiency anemia with or without relative hyperphosphatemia in patients with NDD-CKD show a modest increase in diarrhea, constipation, nausea, and other nonserious gastrointestinal signs and symptoms, with no major or unexpected safety signals. Beneficial effects on the treatment of iron deficiency anemia and bone and mineral metabolism were also observed. Longer-term trials and observational studies examining the effects of ferric citrate on these and other intermediate outcomes, as well as on mortality, cardiovascular events, and fractures, are warranted.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
Robin LeWinter, PhD, of Keryx Biopharmaceuticals, Inc and Robert Schupp, PharmD, CMPP, of inScience Communications, Springer Healthcare (New York, NY, USA), provided editorial support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
GMC has served as a consultant to Akebia, Amgen, Ardea, AstraZeneca, Bristol-Myers Squibb, Gilead, Keryx, and ZS Pharma, has ownership interest in Ardelyx, Durect, Outset, PuraCath Medical, Physiowave, and Thrasos, has received research support from Amgen, Janssen, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Heart, Lung, and Blood Institute, and has received honoraria from the American Society of Nephrology. GAB has served as a consultant for Akebia, Amgen, Ardelyx, AstraZeneca, Celgene, Daiichi Sankyo, Keryx, Relypsa, Sanfit, and ZS Pharma, has ownership interest in Ardelyx and Nephroceuticals, has received research support from Keryx, and has received honoraria from Akebia, Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Keryx, and Sanofi. JFN and KU are employees of Keryx and have ownership interest in Keryx. PEP has served as a consultant for Keryx, Relypsa, Sandoz, Vifor Pharma, and ZS Pharma, and has received honoraria from Keryx, Relypsa, Sandoz, and ZS Pharma. SF has served as a consultant for AstraZeneca, Keryx, Rockwell, and ZS Pharma, has received research funding from Amgen, AstraZeneca, Janssen, and ZS Pharma, and has received honoraria from AstraZeneca, Keryx, Rockwell, and ZS Pharma. The phase 2 and phase 3 clinical trials and the pooled analysis were supported by Keryx Biopharmaceuticals, Inc. Keryx provided support in the form of salaries for KU and JFN, both of whom contributed towards the design of the study and data analysis, as well as reviewing and editing of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. Robert Schupp, PharmD, CMPP, of inScience Communications, Springer Healthcare (New York, NY, USA), provided editorial support, funded by Keryx Biopharmaceuticals, Inc.
References
- 1.United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
- 2.Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031 . [DOI] [PubMed] [Google Scholar]
- 4.Hyun YY, Lee KB, Han SH, Kim YH, Kim YS, Lee SW, et al. Nutritional status in adults with predialysis chronic kidney disease: KNOW-CKD study. J Korean Med Sci. 2017;32(2):257–263. doi: 10.3346/jkms.2017.32.2.257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):280–335. [Google Scholar]
- 6.Naylor KL, Prior J, Garg AX, Berger C, Langsetmo L, Adachi JD, et al. Trabecular bone score and incident fragility fracture risk in adults with reduced kidney function. Clin J Am Soc Nephrol. 2016;11(11):2032–2040. doi: 10.2215/CJN.00720116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagels AA, Soderkvist BK, Medin C, Hylander B, Heiwe S. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes. 2012;10:71 doi: 10.1186/1477-7525-10-71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishbane S, Pollack S, Feldman HI, Joffe MM. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol. 2009;4(1):57–61. doi: 10.2215/CJN.01670408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotloib L, Silverberg D, Fudin R, Shostak A. Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol. 2006;19(2):161–167. . [PubMed] [Google Scholar]
- 10.Stancu S, Barsan L, Stanciu A, Mircescu G. Can the response to iron therapy be predicted in anemic nondialysis patients with chronic kidney disease? Clin J Am Soc Nephrol. 2010;5(3):409–416. doi: 10.2215/CJN.04280609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization; Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers 2001. [March 28, 2017]. http://apps.who.int/iris/bitstream/10665/66914/1/WHO_NHD_01.3.pdf?ua=1. [Google Scholar]
- 12.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28(1):53–61. . [DOI] [PubMed] [Google Scholar]
- 13.Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16(2):101–105. . [DOI] [PubMed] [Google Scholar]
- 14.McClung JP, Murray-Kolb LE. Iron nutrition and premenopausal women: effects of poor iron status on physical and neuropsychological performance. Annu Rev Nutr. 2013;33:271–288. doi: 10.1146/annurev-nutr-071812-161205 . [DOI] [PubMed] [Google Scholar]
- 15.Meroño O, Cladellas M, Ribas-Barquet N, Poveda P, Recasens L, Bazan V, et al. Iron deficiency is a determinant of functional capacity and health-related quality of life 30 days after an acute coronary syndrome. Rev Esp Cardiol (Engl Ed). 2017;70(5):363–370. doi: 10.1016/j.rec.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 16.Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol. 2016;11(6):1088–1100. doi: 10.2215/CJN.11901115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auryxia (ferric citrate) tablets: US prescribing information. Boston, MA: Keryx Biopharmaceuticals; 2016.
- 18.Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26(2):493–503. doi: 10.1681/ASN.2014020212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, et al. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3–5. Am J Kidney Dis. 2015;65(5):728–736. doi: 10.1053/j.ajkd.2014.10.014 . [DOI] [PubMed] [Google Scholar]
- 20.Fishbane S, Block GA, Loram L, Neylan J, Pergola PE, Uhlig K, et al. Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol. 2017;28(6):1851–1858. doi: 10.1681/ASN.2016101053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R, Kusek JW, Pappas MK. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015;88(4):905–914. doi: 10.1038/ki.2015.163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91(1):31–38. doi: 10.1002/ajh.24201 . [DOI] [PubMed] [Google Scholar]
- 23.Wingard RL, Parker RA, Ismail N, Hakim RM. Efficacy of oral iron therapy in patients receiving recombinant human erythropoietin. Am J Kidney Dis. 1995;25(3):433–439. . [DOI] [PubMed] [Google Scholar]
- 24.Macdougall IC, Bock AH, Carrera F, Eckardt KU, Gaillard C, Van Wyck D, et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29(11):2075–2084. doi: 10.1093/ndt/gfu201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qunibi WY, Martinez C, Smith M, Benjamin J, Mangione A, Roger SD. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599–1607. doi: 10.1093/ndt/gfq613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra PA, Bhandari S, Saxena S, Agarwal D, Wirtz G, Kletzmayr J, et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant. 2016;31(4):646–655. doi: 10.1093/ndt/gfv293 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal R, Rizkala AR, Bastani B, Kaskas MO, Leehey DJ, Besarab A. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol. 2006;26(5):445–454. doi: 10.1159/000096174 . [DOI] [PubMed] [Google Scholar]
- 28.Van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S; United States Iron Sucrose Clinical Trials Group. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68(6):2846–2856. doi: 10.1111/j.1523-1755.2005.00758.x . [DOI] [PubMed] [Google Scholar]
- 29.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai X, Miao D, Xiao S, Qiu D, St-Arnaud R, Petkovich M, et al. CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. J Clin Invest. 2016;126(2):667–680. doi: 10.1172/JCI81928 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052 . [DOI] [PubMed] [Google Scholar]
- 32.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82(7):737–747. doi: 10.1038/ki.2012.176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60(3):200–207. doi: 10.1016/j.jacc.2012.03.040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munoz Mendoza J, Isakova T, Cai X, Bayes LY, Faul C, Scialla JJ, et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 2017;91(3):711–719. doi: 10.1016/j.kint.2016.10.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, et al. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2–4. Clin J Am Soc Nephrol. 2014;9(6):1049–1058. doi: 10.2215/CJN.07870713 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobune M, Miyanishi K, Takada K, Kawano Y, Nagashima H, Kikuchi S, et al. Establishment of a simple test for iron absorption from the gastrointestinal tract. Int J Hematol. 2011;93(6):715–719. doi: 10.1007/s12185-011-0878-8 . [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, et al. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol. 2014;9(3):543–552. doi: 10.2215/CJN.05170513 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.