Abstract
Objectives
Preterm delivery may predict increased risk of cardiovascular disease in mothers, providing opportunities for prevention. No study had examined whether gestation length within the term period predicts future CVD, and there are few data segregating spontaneous from medically indicated deliveries.
Study Design
We used proportional hazards models to predict CVD death by gestation length, adjusted for age, education, and delivery year, among 688,662 women with births from 1967–1998 in the Medical Birth Registry of Norway. Mothers were traced in the National Cause of Death Registry through 2009; there were 2,324 CVD deaths.
Results
Compared with women who delivered spontaneously at 39–41 weeks’ gestation, women who spontaneously delivered earlier had higher risks of CVD death. Hazard ratios (HR) = 1.9 at 22-31 weeks; 2.2 at 32-34 weeks; 1.6 at 35-36 weeks; and 1.4 at 37-38 weeks. Risks were higher among women with medically indicated deliveries (HR= 4.8 at 22-31 weeks; 2.7 at 32-34 weeks; 4.3 at 35-36 weeks; and 1.6 at 37-38 weeks compared with spontaneous deliveries at 39-41 weeks). Neither spontaneous nor indicated delivery after 41 weeks was associated with CVD mortality. Risks were highest with recurrent preterm pregnancies, and for women who delivered only one child - especially if that delivery was preterm.
Conclusions
Women who deliver spontaneously before 37 weeks had two-fold increased risk of CVD mortality compared with women who had delivered after 38 weeks. Even women with spontaneous deliveries at early term (37 -38 weeks) had a 41% elevated risk of CVD death compared with women delivering at 39-41 weeks.
Keywords: cardiovascular diseases, epidemiology, pregnancy, prevention, risk factors
Introduction
Common pregnancy complications, including preeclampsia, preterm delivery, gestational diabetes, and fetal growth restriction, are associated with elevated risks of future cardiovascular disease (CVD) in mothers.1 These associations may provide new insights into the development of cardiovascular disease and improve our understanding of the determinants of pregnancy complications.
Women who have delivered preterm have roughly twice the risk of CVD mortality.2–8 However, no study has examined the extent to which the association of gestation length with CVD varies within the term period, during which the vast majority of deliveries occur. Furthermore, with two exceptions,9–10 studies have not distinguished spontaneous from medically indicated deliveries. Finally, no study has reported the association of spontaneous preterm delivery with risk of stroke. We utilized linked Norwegian birth and mortality registries to quantify the extent to which the gestation length of spontaneous and indicated deliveries is associated with maternal risk of CVD mortality up to four decades after birth. We hypothesized that length of gestation would be inversely associated with CVD risk, especially for medically indicated deliveries. We examined recurrent preterm delivery, hypothesizing that women with recurrent preterm deliveries would be at higher CVD risk than women with both preterm and term deliveries.3 Finally, having observed previously that the CVD risk associated with preeclampsia was especially strong when a preeclamptic first birth was not followed by a later birth, we examined whether mortality patterns varied by whether the preterm birth was the last birth.11
Methods
This study was based on the Medical Birth Registry of Norway (MBRN), a registry of mandatory birth notifications completed by the attending clinician for all live births and stillbirths lasting at least 16 weeks in Norway since 1967. Gestation length was recorded as completed weeks from last menstrual period, as ultrasound dating was not recorded before 1999. We considered women with a first singleton live birth or stillbirth between 1967 and 1998. Births were linked to mothers by the national identification number. For most analyses, a woman’s first pregnancy was the exposure; to examine recurrent preterm birth, we considered first and second births. Of the 751,651 women with first singleton pregnancies, we excluded 46,261 (6.2%) whose records lacked gestational age and 978 (0.1%) missing birthweight. We excluded 1,241 women whose first pregnancies lasted <22 weeks and 5,604 women whose first pregnancies lasted >44 weeks. To exclude obviously misclassified gestational ages, we used gender-specific birthweight-for-gestational age Z-scores based on Norwegian standards to exclude 2,671 women where birth weights were more than 4 standard deviations higher or lower than the appropriate means.12 Finally, we excluded 6,226 women missing maternal education and 8 women whose deaths lacked dates. Sensitivity analyses including women missing data on education, employing missing indicator terms or assigning high or low education, yielded nearly identical results. For the analysis of second births, we applied the above exclusion criteria for missing and implausible gestation lengths to second births. The study population was 688,662 women for first births and 641,362 women for the analysis of first and second births.
Clinicians reported the ‘the start of delivery’ as spontaneous labor, induced labor or planned ceasarean delivery. In this analysis, induced labor and planned ceasarean delivery were considered ‘medically indicated’ delivery. Labor induced after spontaneous rupture of membranes was considered spontaneous. Augmentation of labor was recorded separately from induction of labor, and was not used to define delivery type. If amniotomy was listed as a means of labor induction, the delivery was classified as a medically indicated; amniotomy in the context of spontaneous ‘start of delivery’ was classified as spontaneous delivery. If spontaneous labor or rupture of membranes preceded a planned ceasarean section by less than eight hours, the delivery was considered spontaneous; if eight or more hours passed between spontaneous labor and the time of a planned ceasarean section, the delivery was classified as a planned ceasarean, and therefore medically indicated.
Preeclampsia was identified by the 8th revision of the international classification of disease (ICD), code 637, for births 1967-1998 and by ICD-10 codes O14-15 for births after 1998. A test of the accuracy of MBRN preeclampsia reports showed good predictive value: 86% of MBRN registry reports were validated by medical record review.13 Small-for-gestational age was defined as below the 10th percentile and large-for-gestational age as above the 90th percentile of birthweight for gestational age by Norwegian standards.14
Women were linked to the National Cause of Death Registry through 2009 via the national identification number. We identified CVD deaths by ICD codes (ICD8 from 1967 to 1985; ICD9 for 1986-1995; and ICD10 for 1996-2009). Deaths due to coronary heart disease (CHD, 410-414 for ICD8&9; I20-I25 for ICD10) and cerebrovascular disease (430-438 ICD8&9; I60-I69 ICD10) were considered as CVD deaths. We examined cardiovascular mortality as a whole as well as CHD and stroke separately. Forty records had both CHD and stroke listed as the cause of death, so that the total number of CVD deaths is less than the sum of the CHD and stroke deaths.
Each participant was followed from her first delivery between January 1, 1967 and December 31, 1998 until CVD death, other fatality, emigration or December 31, 2009. We used Kaplan Meier curves to estimate the survival time free of CVD death up to 40 years after first birth; the median follow-up was 24.8 years. We used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI), using years since first birth as the time axis. Spontaneous delivery at 39-41 completed weeks was the reference group for detailed analyses of gestation length and delivery type; however, to allow comparisons to less detailed estimates in the literature, where stated, we also provided statistics that use deliveries from 37-44 weeks (‘term’ deliveries) as the reference group. We adjusted for year of delivery (continuous), maternal age (continuous) and maternal education (less than high school, completed high school, university) at first birth. Multiplicative interaction was tested by modeling a cross-product interaction term between indication (yes/no) and gestation length categories. As a sensitivity analysis, we restricted to women whose births were uncomplicated by preeclampsia, fetal growth restriction or macrosomia (<10th or >90th percentile of birthweight for gestation length, respectively). We also performed sensitivity analyses to account for unmeasured confounding by smoking and obesity, using the bias correction method described by Lash and colleagues.15 Statistical analyses were performed with SPSS for Windows version 19.0 and bias correction with the website developed by Lash and colleagues for that purpose.15
This study complies with the Declaration of Helsinki and was approved by the review board of the Medical Birth Registry of Norway, the Regional ethics Committee, REK Vest, Norway.
Results
The demographic characteristics of the cohort are classified by length of first pregnancy in Table 1. Six percent of first births were preterm (<37 weeks). Eighty-five percent of first deliveries were spontaneous (85% of term and 81% of preterm births). As expected from trends in clinical practice, medically indicated deliveries were more prevalent in recent time periods and were more likely in the presence of preeclampsia. Women with medically indicated deliveries were slightly older and more educated (especially if the delivery was preterm).
Table 1.
Characteristics of first births by gestation length and indication status, Norwegian Medical Birth Registry.
| Spontaneous Deliveries | Medically Indicated Deliveries | |||||||
|---|---|---|---|---|---|---|---|---|
| Weeks gestation | 22–31 | 32–34 | 35–36 | 37–44 | 22–31 | 32–34 | 35–36 | 37–44 |
| N (%) | 5488 (0.9) | 8534 (1.5) | 19208 (3.3) | 550604 (94.3) | 1648 (1.6) | 2283 (2.2) | 3820 (3.6) | 97077 (92.6) |
| Mean (sd) | ||||||||
| Maternal age | 23.6 (5.2) | 23.6 (4.9) | 23.9 (4.7) | 23.9 (4.3) | 25.7 (5.4) | 25.7 (5.3) | 25.6 (5.1) | 25.0 (4.9) |
| Year of mother’s birth | 1956 (9) | 1957 (9) | 1957 (9) | 1957 (9) | 1961 (9) | 1961 (9) | 1960 (9) | 1958 (9) |
| Year of delivery | 1980 (9) | 1981 (10) | 1982 (10) | 1982 (9) | 1987 (8) | 1987 (9) | 1986 (9) | 1983 (9) |
| Offspring birthweight (g) | 1218 (534) | 2342 (597) | 2847 (535) | 3485 (467) | 1037 (496) | 1967 (632) | 2636 (637) | 3548 (541) |
| Percent | ||||||||
| Maternal education | ||||||||
| Less than high school | 56.4 | 53.3 | 51.2 | 46.4 | 43.6 | 42.8 | 42.2 | 44.0 |
| High school completed | 21.2 | 22.9 | 23.2 | 23.6 | 25.1 | 25.4 | 26.1 | 23.4 |
| University | 22.4 | 23.8 | 25.6 | 30.0 | 31.4 | 31.8 | 31.7 | 32.5 |
| Married | 73.4 | 76.0 | 78.9 | 82.3 | 81.7 | 83.4 | 85.1 | 84.2 |
| Preeclamptic | 5.5 | 7.0 | 5.3 | 2.2 | 32.0 | 35.9 | 32.5 | 10.7 |
Over a median 25 years of follow-up, there were 2,324 CVD deaths, including 1,182 deaths from CHD and 1,181 deaths from stroke. Figure 1 shows CVD mortality by gestation length through four decades after first birth. The cumulative risk of CVD was less than 3% in any group, consistent with the age of the cohort at the end of follow-up (median, interquartile range = 52, 45 to 59 years). The lowest CVD mortality was among women with spontaneous term deliveries, followed by indicated term, spontaneous preterm, and medically indicated preterm deliveries. The absolute risks of CVD mortality began to diverge within the first decade after birth and grew wider over four decades.
Figure 1.
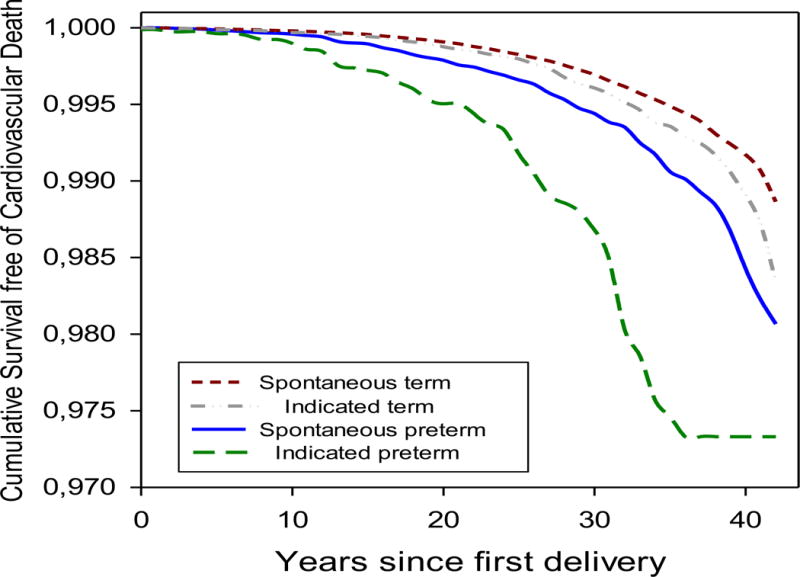
Cumulative survival free from cardiovascular disease mortality in the forty years after first delivery, by gestation length and spontaneous v. medically indicated delivery, Norwegian Medical Birth Registry.
Women whose first delivery was preterm had nearly double the risk of cardiovascular mortality compared with women who delivered at term (37-44 weeks) (HR 1.9, 95% CI, 1.7 to 2.2). Spontaneous preterm delivery was associated with an HR of 1.7 (95% CI, 1.5 to 2.0), and medically indicated preterm with an HR of 3.7 (95% CI, 2.9 to 4.8), both compared to spontaneous term delivery.
Figure 2 depicts the hazard ratios for CVD mortality for medically indicated and spontaneous first deliveries of varying gestation lengths, adjusted for maternal age, education, and year of delivery. All gestation lengths shorter than 39-41 weeks – including spontaneous term births at 37-38 weeks - were associated with elevated CVD mortality compared with spontaneous delivery at 39-41 weeks. Women with a history of medically indicated preterm delivery had 2.7- to 4.8-fold higher risks of CVD death, while women with a history of spontaneous preterm delivery had 1.6- to 2.2-fold higher risk of CVD death compared to women who had delivered spontaneously at 39-41 weeks (p=0.01 for interaction between gestation length categories and indicated delivery status). Deliveries after 41 completed weeks, whether spontaneous or indicated, predicted the same risk of CVD death as spontaneous deliveries at 39-41 weeks. There was no interaction between type of preterm delivery and maternal age at birth (<30 v. ≥30 years) in predicting CVD mortality (p=0.22).
Figure 2.
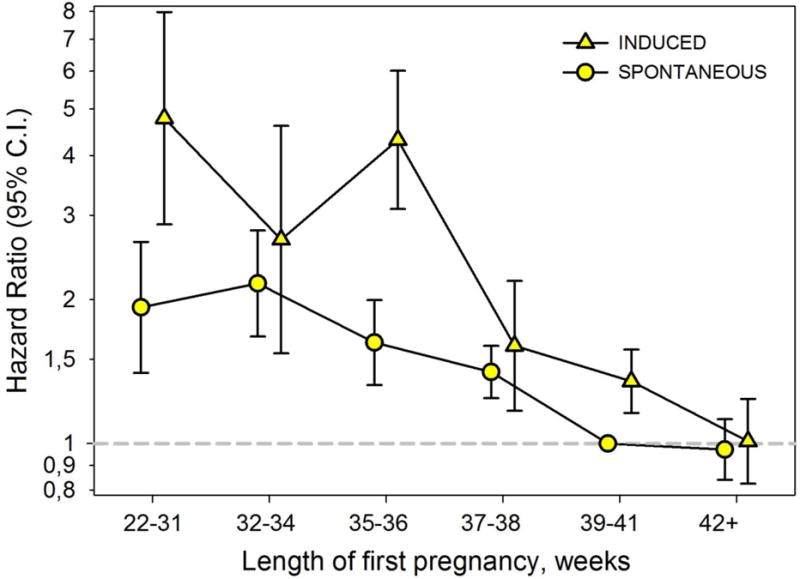
Hazard ratios (95% confidence intervals) for cardiovascular disease mortality by gestation length and indication status of first pregnancy, Norwegian Medical Birth Registry.
Women who delivered preterm spontaneously had twice the risk of CHD death (HR 2.1, 95% CI, 1.7 to 2.5) compared with women who delivered spontaneously at term (37-44 weeks). Table 2 shows the hazard ratios of CHD death by gestation length. Even early term spontaneous deliveries (37-38 weeks) predicted 49% (25%-78%) higher CHD mortality compared with later term spontaneous delivery (39-41 weeks) (Table 2). Although the hazard ratios for medically indicated delivery were higher than those for spontaneous delivery, the statistical test of interaction was not significant (p=0.30).
Table 2.
Age-adjusted and multivariate hazard ratios (95% confidence intervals, CI) for coronary heart disease and stroke mortality by gestation length and indication status, Norwegian Medical Birth Registry.
| Coronary Heart Disease | Stroke | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Women | Deaths n,(%) | Age-adjusted Hazard Ratio | Multivariate1 Hazard Ratio | 95% CI | Deaths n,(%) | Age-adjusted Hazard Ratio | Multivariate1 Hazard Ratio | 95% CI | |
| Spontaneous Deliveries | |||||||||
| 22–31 | 5488 | 23 (0.42) | 2.5 | 2.3 | 1.5 to 3.4 | 19 (0.35) | 1.9 | 1.8 | 1.1 to 2.8 |
| 32–34 | 8534 | 33 (0.39) | 2.5 | 2.4 | 1.7 to 3.4 | 29 (0.34) | 2.0 | 1.9 | 1.3 to 2.8 |
| 35–36 | 19208 | 62 (0.32) | 2.3 | 2.1 | 1.6 to 2.7 | 41 (0.21) | 1.3 | 1.3 | 0.9 to 1.7 |
| 37–38 | 71111 | 155 (0.22) | 1.6 | 1.5 | 1.3 to 1.8 | 161 (0.23) | 1.5 | 1.4 | 1.2 to 1.7 |
| 39–41 | 403028 | 573 (0.14) | 1.0 | 1.0 | reference | 640 (0.16) | 1.0 | 1.0 | reference |
| 42–44 | 76465 | 104 (0.14) | 1.0 | 1.0 | 0.8 to 1.2 | 116 (0.15) | 1.0 | 1.0 | 0.8 to 1.2 |
| Medically Indicated Deliveries | |||||||||
| 22–31 | 1648 | 7 (0.42) | 5.9 | 4.7 | 2.2 to 9.8 | 9 (0.55) | 5.9 | 5.4 | 2.8 to 10.4 |
| 32–34 | 2283 | 8 (0.35) | 4.4 | 3.4 | 1.7 to 6.9 | 5 (0.22) | 2.2 | 1.9 | 0.8 to 4.7 |
| 35–36 | 3820 | 25 (0.65) | 7.7 | 6.2 | 4.2 to 9.3 | 13 (0.34) | 3.3 | 2.9 | 1.7 to 5.1 |
| 37–38 | 12256 | 28 (0.23) | 2.8 | 2.3 | 1.6 to 3.4 | 14 (0.11) | 1.1 | 1.0 | 0.6 to 1.7 |
| 39–41 | 53094 | 112 (0.21) | 1.8 | 1.6 | 1.3 to 2.0 | 84 (0.16) | 1.2 | 1.1 | 0.9 to 1.4 |
| 42–44 | 31727 | 52 (0.16) | 1.1 | 1.1 | 0.8 to 1.5 | 50 (0.16) | 1.0 | 1.0 | 0.7 to 1.3 |
Adjusted for maternal age at delivery, year of delivery, and maternal education (less than high school, completed high school, university)
Associations of gestation length with stroke mortality were less consistent, although generally elevated for both spontaneous and medically indicated preterm births (Table 2). Overall, spontaneous preterm delivery was associated with an HR of 1.5 (95% CI, 1.2 to1.8), and medically indicated preterm delivery with an HR of 3.0 (95% CI, 2.0 to 4.3) compared with spontaneous delivery at 37-41 weeks. Women who delivered spontaneously at early term (37-38 weeks) had a 41% (19% to 68%) increased risk of stroke mortality compared with women delivering later in the term period (39-41 weeks). However, there was no evidence that medically indicated delivery predicted future stroke risk across the range of term deliveries. The association of gestation length with stroke was stronger for medically indicated than for spontaneous deliveries (interaction p=0.01).
Table 3 shows CVD mortality risk by preterm status in first and second births. Compared with women who bore at least two term infants, women who bore only one child had increased risk of CVD mortality, whether their first and only child was born at term (HR 2.0, 95% CI, 1.8 to 2.3) or preterm (HR 4.2, 95% CI, 3.4 to 5.1). Women with two consecutive preterm pregnancies had a three-fold increase in CVD risk (HR 3.3, 95% CI, 2.4 to 4.5). Women who had two deliveries and delivered preterm in only one of them had intermediate risks of CVD. The order of the term and preterm births did not seem to matter. There was no apparent interaction between these categories and maternal age at first birth (<30 v. ≥30 years) in predicting CVD mortality (p=0.19).
Table 3.
Multivariate hazard ratios (95% confidence intervals, CI) for cardiovascular death by preterm status of first and second deliveries, Norwegian Medical Birth Registry.
| First birth | Second birth | CVD deaths | Women | HR1 | 95% CI |
|---|---|---|---|---|---|
| Preterm | None | 108 | 7786 | 4.2 | 3.4 to 5.1 |
| Preterm | Preterm | 41 | 4819 | 3.3 | 2.4 to 4.5 |
| Preterm | Term | 98 | 25393 | 1.5 | 1.2 to 1.8 |
| Term | None | 621 | 96919 | 2.0 | 1.8 to 2.3 |
| Term | Preterm | 87 | 18038 | 1.8 | 1.4 to 2.2 |
| Term | Term | 1234 | 488407 | 1.0 | reference |
Adjusted for maternal age at delivery, year of delivery, and maternal education (less than high school, completed high school, university)
To examine spontaneous deliveries that were least likely to be complicated by preeclampsia or gestational diabetes, we excluded women with pregnancies complicated by preeclampsia, fetal growth restriction, or macrosomia (as an approximation to gestational diabetes, which was not systematically recorded during this period). Hazard ratios (95% CI) for CVD mortality associated with these spontaneous deliveries were: 2.1 (1.4 to 3.1) at 22-31 weeks; 1.9 (1.3 to 2.7) at 32-34 weeks; 1.4 (1.0 to 1.8) at 35-36 weeks; and 1.3 (1.1 to 1.5) for delivery at 37-38 weeks (early term delivery) compared with spontaneous delivery at 39-41 weeks. When we excluded women from the analysis of CVD mortality with any form of diabetes (chronic or gestational) at the time of pregnancy, we observed similar patterns of statistically significantly elevated risk of CVD mortality with a history of preterm delivery; hazard ratios for medically indicated deliveries were somewhat attenuated (data not shown).
We conducted sensitivity analyses to assess the degree of unmeasured confounding by smoking and obesity, which were not available from the MBRN during this period. Assuming prevalences of smoking (30%) and obesity (10%) typical of this time in Norway,16–17 the associations of these factors with preterm delivery (relative risks (RR) for spontaneous PTB of 1.3 for smoking and 0.8 for obesity) and CVD (RR of for CVD of 1.9 for smoking and 2.0 for obesity),8, 18–22 we derived a smoking-corrected estimate of 1.9 and an obesity-corrected estimate of 2.0 for the association of spontaneous PTB with CVD mortality. These adjusted estimates are less than a 10% departure from the crude RR of 2.0.
Comment
At a median 25 years after their first birth, women who had delivered preterm were twice as likely to have died from CVD, compared with women whose first pregnancy went to term. Within preterm deliveries, the HRs for CVD risk ranged from 1.6 to 2.2 for spontaneous deliveries, and 2.7 to 4.8 for medically indicated deliveries, depending on degree of prematurity. In particular, our study indicates for the first time that even the 12% of women who delivered early within the term period (37-38 weeks) had 41% (if spontaneous) to 60% (if indicated) increased risks of future CVD death compared with women who delivered spontaneously at 39-41 weeks.
This study also contributed new data with respect to recurrent preterm birth and preterm birth to women who bore only one child. Compared with women who bore two term infants during the study period, women who delivered two preterm infants were at a 3-fold elevated risk of CVD mortality. Interestingly, women who bore only one infant were also at increased CVD risk, especially if that child was preterm. This is consistent with the increased CVD mortality risk observed in this cohort among women whose last observed pregnancy was preeclamptic.11 This pattern suggests that pregnancy outcomes severe enough to discourage further pregnancy may mark especially high maternal CVD risk, that women with underlying chronic disease choose to bear fewer children, and/or that subfertility is correlated with shorter gestation and with future CVD risk.
Preterm delivery rates vary markedly by place and time.23 Six percent of singleton births in this Norwegian cohort delivered 1967-1998 were preterm. The prevalence of preterm delivery in singleton pregnancies in Norway dropped to 4.9% in 2011. In contrast, the prevalence of preterm delivery among singletons in the U.S.in 2011 was 10.1%.24 This variation in preterm delivery rates presumably reflects variation in the causes of preterm birth. This may be especially true for indicated deliveries, as both the practice standards for medical delivery and the prevalence of the indications for medical delivery vary by place and time. Our analysis shows large differences in CVD mortality predicted by indicated versus spontaneous preterm delivery; this likely reflects the particular mix of underlying indications for medical delivery. The associations might be quite different for more recent preterm deliveries, which are increasingly likely to occur in the context of CVD risk factors such as obesity and hypertension.25
Despite the presumed variation in causes of preterm birth, the reports from large registry studies consistently report relative risks of maternal CVD incidence and/or mortality associated with total preterm delivery ranging from 1.4 to 2.5.2–7, 26–27 Previous studies examining risk of stroke associated with a history of delivering preterm have reported relative risks of 1.9,5 1.9,28 and 1.1-2.4 (depending on degree of accompanying fetal growth restriction.)2 However, none of these studies distinguished between indicated and spontaneous delivery. We found that spontaneous preterm delivery predicted a nearly 50% increase in stroke mortality compared with spontaneous term delivery, while medically indicated preterm delivery predicted nearly a 3-fold increased stroke risk.
Two studies have considered total CVD in relation to spontaneous or medically indicated preterm deliveries. An Israeli study reported increased odds ratios of ‘complex cardiovascular events’ (largely CHD) for women with a history of ever having had a spontaneous preterm delivery compared with women who had delivered only term infants. Although they did not formally test interactions between delivery type and prematurity, the authors reported higher odds ratios of CVD after spontaneous preterm delivery (3.8, 95% CI, 2.2 to 6.6) than after induced preterm labor (2.6, 0.5 to 12.5).26 In Scotland, Hastie and colleagues reported that spontaneous preterm delivery had an HR of 2.1 for CHD mortality compared with spontaneous term delivery, which agrees closely with the HR of 2.0 for the same comparison in our cohort.7
The contrast between medically indicated and spontaneous preterm deliveries echoes previously reported differences between hypertensive and normotensive deliveries. Preeclampsia is a primary indication for medical delivery associated with a doubling of CVD risk in mothers.9–10 Compared with normotensive term births, preeclamptic preterm delivery is associated with relative risks as small as 1.8 for stroke,29 but as high as 4.5 for CHD6 and 8.0 for total CVD mortality.9 Preterm birth uncomplicated by preeclampsia has been associated with more modest relative risks of CVD, ranging from 1.2 to 3.0.3, 5, 30 In our dataset, spontaneous preterm delivery was associated with increased CVD mortality, whether or not the pregnancy was complicated by preeclampsia.
This dataset had several limitations, some of which reflect medical practices of the era. Gestational diabetes was not routinely screened until late in this period, so we were unable to account for possible confounding by gestational diabetes. However, gestational diabetes is not a strong risk factor for either medically indicated or spontaneous preterm delivery.31 Furthermore, we observed that women with spontaneous preterm births uncomplicated by preeclampsia, small-for-gestational age, and large-for-gestational age still had elevated CVD risk; this restriction would have eliminated many diabetic pregnancies. Finally, analysis excluding women with diabetes before pregnancy showed a similar pattern.
The MBRN did not start collecting data on smoking during pregnancy until 1998, after the births in this analysis had occurred. However, our senstivity analysis and at least three previous studies have shown through adjustment2 or sensitivity analysis3, 28 that smoking explains little of the association between preterm delivery and CVD mortality; this is consistent with the modest association of smoking with risk of preterm birth, estimated in meta-analysis as a relative risk of 1.3.18 As is typical of registries, there were no data available on CVD risk factors, such as body mass index. However, sensitivity analysis suggested that adjustment for obesity would have yielded less than a 10% change the association of spontaneous preterm delivery with CVD mortality. Finally, we were not able to validate the registry reports of gestation length or type of delivery against medical records. Even so, we could exclude obviously misclassified gestation lengths by using gestational-age and gender-specific birth weight distributions as a standard.
Our understanding of the risk factors linking CVD and length of gestation, especially spontaneously delivered pregnancies, is weak. Infection and inflammation play a large role in spontaneous preterm delivery.32 Several studies have found that maternal plasma C-reactive protein levels in pregnancy predict spontaneous preterm delivery,33–36 particularly if women also have high cholesterol or triglyceride levels.37 C-reactive protein levels predict elevated CVD risk.38–39 Although C-reactive protein levels have been associated with a history of eclampsia and indicated preterm delivery,7, 40 they were not associated with a history of spontaneous preterm delivery in a small study (n=30 cases) of women 1-29 years after pregnancy.7 The Avon Longitudinal Study of Parents and Children found no evidence of dyslipidemia, glucose intolerance, elevated blood pressure, or inflammation (including C-reactive protein) in a group of 139 women studied at a mean 18 years after preterm delivery.41
The various associations of preterm delivery, preeclampsia, gestational diabetes, and fetal growth restriction with CVD suggest that further research into their common causes may shed new light on the pathology and etiology of pregnancy complications as well as CVD. Knowledge generated in this arena is already changing clinical practice. For example, two organizations have recommended accelerated glucose screening among women with a history of gestational diabetes.42–43 The American College of Obstetricians and Gynecologists and the American Heart Association have recommended that preeclampsia be considered a cardiovascular risk factor.44–45 Our findings suggest that spontaneous preterm delivery may also merit attention as a CVD risk factor. A better understanding of the mechanisms linking gestation length of spontaneously delivered pregnancies with CVD may yield novel risk factors and opportunities for prevention of both preterm delivery and cardiovascular disease.
Condensation.
Gestation length of spontaneously delivered pregnancies, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women
Acknowledgments
none
Funding sources: This work was supported in part by the Intramural Program of the NIH, National Institute of Environmental Health Sciences. The authors’ institutions had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
The authors report no conflicts of interest.
This research was presented as a poster at the 45th Annual meeting of the Society for Epidemiologic Research, Minneapolis, MN, June 26-29, 2012.
References
- 1.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: An underused opportunity to improve women’s health? Epidem Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonamy A-KE, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: Effects of gestational age and fetal growth. Circulation. 2011;124:2839–46. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 3.Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol. 2010;20:604–09. doi: 10.1016/j.annepidem.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davey Smith G, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356:2066–67. doi: 10.1016/S0140-6736(00)03406-1. [DOI] [PubMed] [Google Scholar]
- 5.Irgens HU, Reisæter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. Br Med J. 2001;323:1213. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith G, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129 290 births. Lancet. 2001;357:2002–06. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 7.Hastie CE, Smith GC, Mackay DF, Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: Retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol. 2011;40:914–19. doi: 10.1093/ije/dyq270. [DOI] [PubMed] [Google Scholar]
- 8.Davey Smith G, Hyppönen E, Power C, Lawlor DA. Offspring birth weight and parental mortality: Prospective observational study and meta-analysis. Am J Epidemiol. 2007;166:160–69. doi: 10.1093/aje/kwm054. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Br Med j. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcdonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am Heart J. 2008;156:918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Skjaerven R, Wilcox AJ, Klungsøyr K, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: Prospective, population based cohort study. Br Med J. 2012;345 doi: 10.1136/bmj.e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skjærven R, Gjessing HK, Bakketeig LS. New standards for birth weight by gestational age using family data. Am J Obstet Gynecol. 2000;183:689–96. doi: 10.1067/mob.2000.106590. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen LC, Klungsøyr K, Roten LT, et al. Validity of the diagnosis of pre-eclampsia in the medical birth registry of norway. Acta Obstet Gynecol Scand. 2013 doi: 10.1111/aogs.12159. [DOI] [PubMed] [Google Scholar]
- 14.Skjaerven R, Gjessing HkBLs. Birthweight by gestational age in norway. Acta Obstet Gynecol Scand. 2000;79:440–49. [PubMed] [Google Scholar]
- 15.Lash TL, Fox MP, Fink AK. Applying quantitative bias analysis to epidemiologic data. New York, NY: Springer Science + Business Media; 2009. Unmeasured and unknown cofounders. [Google Scholar]
- 16.Health N.I.O.P. Facts about overweight and obesity in norway. 2011 [Google Scholar]
- 17.Barbara Forey JH. OUP WIoPMa. Sutton, UK: 2012. John Hamling, Alison Thornton, Peter Lee. International smoking statistics norway. [Google Scholar]
- 18.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182:465–72. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 20.Colditz GA, Bonita R, Stampfer MJ, et al. Cigarette smoking and risk of stroke in middle-aged women. N Engl J Med. 1988;318:937–41. doi: 10.1056/NEJM198804143181501. [DOI] [PubMed] [Google Scholar]
- 21.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–89. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Green A, Stampfer MJ, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317:1303–09. doi: 10.1056/NEJM198711193172102. [DOI] [PubMed] [Google Scholar]
- 23.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JA, Hamilton BE, Ventura SJ, Jk MM, Osterman M, Mathews T. Births: Final data for 2011. Natl Vital Stat Rep. 2013;62 [PubMed] [Google Scholar]
- 25.Siega-Riz AM, Laraia B. The implications of maternal overweight and obesity on the course of pregnancy and birth outcomes. Matern Child Health J. 2006;10:153–56. doi: 10.1007/s10995-006-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Davey Smith G, Hart C, Ferrell C, Upton M, Hole D, Hawthorne V, Watt G. Birth weight of offspring and subsequent cardiovascular mortality of the parents. Br Med J. 1997;315(7117):1189–93. doi: 10.1136/bmj.315.7117.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: A retrospective cohort study of 119,668 births. Am J Epidemiol. 2004;159:336–42. doi: 10.1093/aje/kwh064. [DOI] [PubMed] [Google Scholar]
- 29.Lykke J, Paidas M, Damm P, Triche E, Kuczynski E, Langhoff-Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type-ii diabetes in the mother. Br J Obstet Gynaecol. 2010;117:274–81. doi: 10.1111/j.1471-0528.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- 30.Catov JM, Newman AB, Roberts JM, et al. Preterm delivery and later maternal cardiovascular disease risk. Epidemiol. 2007;18:733–39. doi: 10.1097/EDE.0b013e3181567f96. [DOI] [PubMed] [Google Scholar]
- 31.Villar J, Papageorghiou AT, Knight HE, et al. The preterm birth syndrome: A prototype phenotypic classification. Am J Obstet Gynecol. 2012;206:119–23. doi: 10.1016/j.ajog.2011.10.866. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:021–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hvilsom GB, Thorsen P, Jeune B, Bakketeig LS. C-reactive protein: A serological marker for preterm delivery? Acta obstet gynecol scand. 2002;81:424–29. doi: 10.1034/j.1600-0412.2002.810509.x. [DOI] [PubMed] [Google Scholar]
- 34.Lohsoonthorn V, Qiu C, Williams MA. Maternal serum c-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin Biochem. 2007;40:330–35. doi: 10.1016/j.clinbiochem.2006.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moghaddam Banaem L, Mohamadi B, Asghari Jaafarabadi M, Aliyan Moghadam N. Maternal serum c-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. J Obstet Gynaecol Res. 2012;38:780–86. doi: 10.1111/j.1447-0756.2011.01804.x. [DOI] [PubMed] [Google Scholar]
- 36.Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma c-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162:1108–13. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166:1312–19. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 38.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 40.Hubel CA, Powers RW, Snaedal S, et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension. 2008;51:1499–505. doi: 10.1161/HYPERTENSIONAHA.108.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: The avon longitudinal study of parents and children. Circulation. 2012;125:1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Association’ A.A.D. Standards of medical care in diabetes-2008. Diabetes Care. 2008;31:S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 43.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care. 2007;30:S251–S60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 44.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: A guideline from the american heart association. Circulation. 2011;123:1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gynecology A.C.O.O.A. Hypertension in pregnancy: Executive summary. Obstet Gynecol. 2013;122:1122–31. doi: 10.097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]


