Abstract
Astrocytes are abundant within mature neural circuits and are involved in brain disorders. Here, we summarise our current understanding of astrocytes and Huntington’s disease (HD) with a focus on correlative and causative dysfunctions of ion homeostasis, calcium signaling, and neurotransmitter clearance, as well as on the use of transplanted astrocytes to produce therapeutic benefit in mouse models of HD. Overall, the data suggest astrocyte dysfunction may be an important contributor to the onset and progression of some HD symptoms in mice. Additional exploration of astrocytes in HD mouse models and humans is needed and may provide new therapeutic opportunities to explore in conjunction with neuronal rescue and repair strategies.
Keywords: astrocyte, HD, GAT3, Kir4.1, calcium, GLT1, EAAT2, KCNJ10, GCaMP
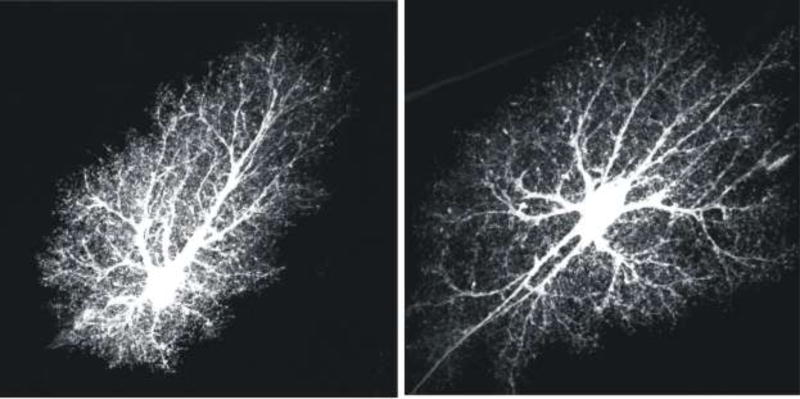
Astrocytes are critical components of neural circuits and are known to be involved in brain disorders [1, 2]. Astrocytes may represent about 40% of all brain cells and tile the nervous system [3]. In mice, cortical astrocytes are born soon after neurogenesis at around embryonic day 16, and following migration along radial glia they occupy well defined territories with properties and locations that may be tailored to the needs of nearby neurons [4]. Each individual mature astrocyte comprises a cell body, six or so major branches, a thick blood vessel-associated endfoot, and myriad finer branchlets and leaflets [3]. Although astrocytes are highly complex structures, they are not polarised like neurons and their complexity is largely equivalent throughout individual territories [5] that overlap very little in healthy rodent tissue [6] or in models of trauma [7]. It is these stereotypical features that give rise to “bushy” astrocyte appearances in healthy tissue (Figure 1). The loss of some of these anatomical features has been documented in disease [8], including in HD [9].
Figure 1. Bushy astrocyte morphology.
The images show hippocampal CA1 molecular layer protoplasmic astrocytes loaded with a fluorescent dye (Lucifer yellow). The images are from the Cell Centered Database [6, 123, 124] at the National Center for Microscopy and Imaging Research (http://ccdb.ucsd.edu/index.shtm) and have Accession #1066, 1063. One can observe a soma, several major branches and thousands of branchlets and leaflets, which are discussed in the text. These two images are reproduced from a published paper [3].
There has been progress in deciphering how astrocytes contribute to the nervous system since the first electrophysiological studies ~50 years ago [10, 11]. Established, proposed and debated roles for astrocytes include neurovascular coupling, stabilizing the blood brain barrier, regulation of synaptic function, ion homeostasis, neurotransmitter clearance, metabolic support of neurons, regulation of action potential waveforms, synaptic plasticity and regulation of behaviors such as sleep [3, 12–18]. In addition, it is now well established that astrocytes respond to all forms of injury, trauma and infection via a process termed astrogliosis [19].
Herein, we summarise recent work that is beginning to unravel how astrocyte dysfunction accompanies and contributes to neuronal deficits in Huntington’s disease model mice (Box 1). We do not consider other glia (e.g. microglia, oligodendrocytes, NG2 cells, Schwann cells, Bergmann glia, Müller cells), because these are beyond the scope of this review that concerns astrocytes. However, broad changes in glia are to be expected from human studies showing profound gene expression changes in HD: perhaps around 20% of all genes are altered [20]. In addition, there is currently a paucity of available data on these specific forms of glia in HD models. Furthermore, by necessity, we focus on recent aspects and seek to emphasize specific astrocyte dysfunctions rather than astrogliosis, which itself is a highly complex, often all-embracing general response [8] that could be secondary to neuronal changes in the context of HD mouse models [21]. We believe this distinction has value in order to form, test and ultimately prove or refute specific hypotheses. However, there has been recent progress in identifying reactive astrocytes classified as “A1” being potentially damaging in HD: the neurotoxic substance they release and A1 astrocytes themselves now also merit evaluation in HD [22].
Box 1: Commonly used HD mouse models where astrocyte assessments are available.
| Model (background) [key reference] |
Available strains (JAX stock #) |
Brief description | Known striatal astrocyte dysfunction |
|---|---|---|---|
|
| |||
| R6/2 (CBAxC57/BL6, C57/BL6) [113] | 002810 | Uses HD promoter - Human Exon 1 mHTT transgene. | Reduced Kir4.1 protein, function and K+ homeostasis [33] |
| 006494 | Reduced GLT1 mRNA and protein [33, 46, 53, 84] | ||
| 027419 | Impaired glutamate transport efficiency in striatum [46, 53, 81, 84, 93], contested in [39] | ||
| 027423 | Multiple strains with various CAG (Q) sizes exist. The 120Q line is widely used. | GAT3-mediated/GLT1-dependent GABA release impaired, resulting in reduced tonic GABA conductances [49] | |
| Increased extracellular [Gln] [84] | |||
| Decreased GS, altered GS immunoreactivity [46, 84] | |||
| Decreased astrocyte spontaneous Ca2+ signals [81] | |||
| Enhanced cortically-driven striatal astrocyte Ca2+ signals [81] | |||
| No overt astrogliosis at symptomatic ages [33, 113] | |||
| mHTT astrocyte inclusions [33] | |||
| Glial replacement with wild-type human glia slowed disease progression and increased survival [63]. | |||
|
| |||
| R6/1 [113] | 006471 | Uses HD promoter - Human Exon 1 mHTT transgene. | Reduced GLT1 protein [46] |
| CAG of ~ 120 is more stable than R6/2 | Decreased GS [46] | ||
|
| |||
| N171-82Q (C57/BL6) [119] | 003627 | Predominantly neuronal expression through PrP promoter – N terminal 171 mHTT transgene. | N171-82Q phenotype is exacerbated by crossing to [aphenotypic] |
| N208-98Q glial mHTT line [38] | |||
|
| |||
| YAC128 (C57/BL6, FVB/N) [120] | 027432 | Full length HTT YAC transgene | Impaired GLT1 function and impaired glutamate uptake from 3 months reported [50, 51], contested in [102] |
| 2 strains, CAG100 and 126 | |||
|
| |||
| BACHD (FVB/N) [121] | 008197 | Full length human HTT BAC transgene with 97 polyQ encoded by mixed CAGCAA - 2 strains | Glutamate uptake deficiency in the subthalamic nucleus [122] |
|
| |||
| zQ175 (C57/BL6J) [59, 86] | 027410 | Knock-in lines. Human HTT exon 1 sequence with an ~190 pure CAG tract replacing the mouse Htt exon 1. | Reduced Kir4.1 protein and function in Q175 homozygotes [33] |
| GLT1 loss [33, 86] | |||
| Impaired glutamate transport efficiency in striatum [93] | |||
| GAT3-mediated/GLT1-dependent GABA release impaired, resulting in reduced tonic GABA conductances [49] | |||
| Glutamate uptake deficiency in Q175 het STN [122] | |||
| Increased expression of GFAP, slight astrogliosis in striatum and cortex in Q175 homozygotes [75] | |||
Note to table: we have only highlighted mouse models for which astrocyte data are available. Additional models that have been assessed for neuronal properties are not listed. Key to table: GS = Glutamine synthetase. PrP = prion promoter. Gln = glutamine. Glu = glutamate. mHTT = mutant huntingtin.
Huntington’s disease (HD)
Huntington’s disease is a neurodegenerative disease characterized by progressive motor, psychiatric and cognitive dysfunction [23, 24], caused by an expansion of a polyglutamine-encoding CAG repeat (>36) in exon 1 of the huntingtin gene (HTT) [The Huntington’s Disease Collaborative Research Group, 25]. HD is primarily a basal ganglia disorder: cortico-striatal-thalamo-cortical neural circuits appear particularly affected [26] (Figure 2). Cortical thinning and basal ganglia degeneration are obvious (Figure 2). We have restricted our discussions to the striatum, because of the dearth of data exploring cortical astrocytes in HD mouse models.
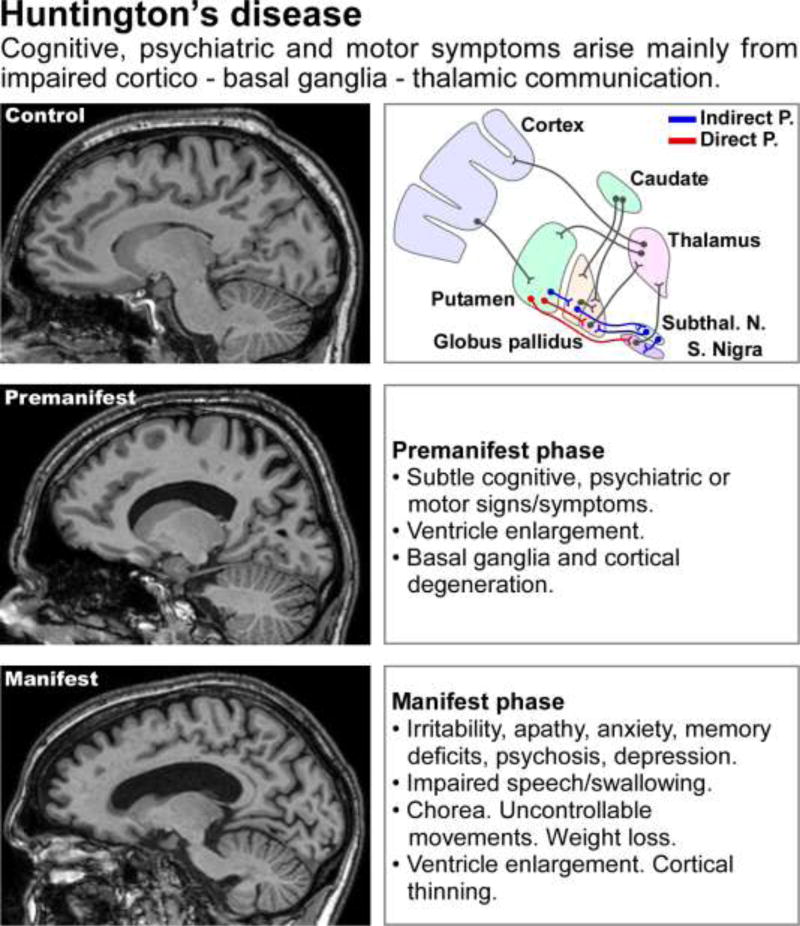
Figure 2. Imaging and clinical manifestations of Huntington’s disease.
Top row: MRI scan from a healthy control subject (left) and a scheme of the connectivity between cerebral cortex, basal ganglia and thalamus, indicating the indirect and direct pathways, the balance of which is thought to be altered in HD (right). Subthal. N. = subthalamic nuclei, S. Nigra = substantia nigra. Middle and bottom rows: MRI scans from a subject in the pre manifest stage of the disease (CAG length: 42; middle left) and a subject in the manifest stage of the disease (CAG length: 43; bottom left), and descriptions of the most common clinical findings at each stage (middle and bottom right). The MRI images are from the TRACK-HD study [23], courtesy of Dr. Sarah Tabrizi (University College of London).
The molecular and physiological role of normal HTT, or the known pathogenic mechanisms induced by mutant HTT (mHTT), have thus far failed to identify precise events that contribute to the subsequent stereotyped progression of the disease. Nonetheless, several general points merit discussion. There is profound loss of GABAergic medium spiny neurons (MSNs) in the caudate-putamen (the Corpus striatum) as well as of neurons in the Globus pallidus [27], in addition to heterogeneous grey and white matter loss, especially in cortical regions [reviewed in 28]. In accord, most effort has focused on the study of mHTT expression in, and on, neuronal function in the corticostriatal inputs and output MSNs. However, it is now clear from human imaging data [23] that HD related structural brain changes (Figure 2) are more widespread and heterogeneous than first appreciated [reviewed in 28]. Expression of HTT and mHTT is ubiquitous throughout the body: additional pathology (including peripheral pathology) is thus expected and known. However, the exquisite vulnerability of particular neural circuits and relative sparing of others remains largely unexplained. It remains to be clarified whether or not astrocyte dysfunction and loss of support of these circuits plays a significant role in the known patterns of pathology.
Astrocyte changes in humans with HD
A hallmark of HD neuronal pathology is the presence of intracellular mHTT aggregates. However, inclusions are also present in cortical and striatal astrocytes [29] and detected to the same extent as in neurons in adult-onset HD patient tissue [30], although neuronal inclusions are generally larger. Fibrillary astrocytosis, as assessed by microscopic analysis of cresyl violet stained sections within the Corpus striatum, is a classifying feature of HD: absent in Grade 0, but progressing from mild, to moderate and severe from Grade 1 – 4 [27, 31, 32]. At Grade 0, there is loss of neurons with no detectable astrocytosis [32]. Individual astrocytes imaged using GFAP immunostaining in HD brains display thicker processes and larger somata [9] than in control striatal tissue, where astrocytes were lightly labelled with GFAP and displayed a fine lace-like appearance, similar to healthy rodent astrocytes [33]. In Faideau et al, GFAP density markedly increased two-fold by Grades 2 and above [9]. In another study, increased GFAP immunoreactivity was noted in Grade 0–1 tissue and was confined to striatal striosomal rather than matrix regions [34]. In this section, we have used the terms astrocytosis and astrogliosis in accord with the original papers, but each term is poorly defined.
In the adult rodent forebrain, ~90% of glutamate uptake is via the high-affinity electrogenic transporter known as EAAT2 or GLT1 [35] (gene name Slc1A2), which is highly expressed in astrocytes. A consistent finding between multiple studies in human HD postmortem tissue is the loss of GLT1 [9, 29, 36]. The loss of GLT1 increased with HD severity, but was already substantially reduced by ~40% in Grade 0 specimens compared to healthy controls [9], making this the earliest astrocyte dysfunction detected in HD patients.
The aforementioned findings are from postmortem tissue in patients with a confirmed clinical diagnosis of HD, and it is important to reiterate that neuropathological HD Grade is not comparable to the clinical UHDRS rating of HD patients, which confusingly also uses a 0 – 4 scale [37]. Indeed, based on the firm clinical diagnosis of HD in postmortem Grade 0 patients, clinical symptoms are likely to precede astrocyte changes. Hence, to clarify if astrocyte dysfunction precedes, accompanies or follows that of neuronal pathology in human HD, and for the establishment of a relationship to clinical symptoms, improved in vivo imaging tools to assess astrocyte (dys)function need to be developed. Such work is highly relevant, because human and mouse astrocytes markedly differ.
Pathophysiology induced by expression of mHTT in astrocytes
Several pioneering rodent studies expressing mHTT specifically in astrocytes suggest that astrocyte dysfunction directly contributes to HD pathology. Heterologous expression of mHTT using lentiviruses in vivo reproduced many of the morphological features observed in human HD specimens, suggesting that the changes seen in HD patient astrocytes are cell-autonomous and perhaps not the result of neuronal damage [9]. Expression of mHTT in astrocytes resulted in death of co-cultured striatal neurons [29], and expression of a mHTT N-terminal fragment with either 98 or 160Qs in astrocytes in vivo using the human GFAP promoter lead to reduced GLT1 expression, astrogliosis, and HD-like pathology [9, 38, 39]. Loss of BDNF release from astrocytes has also been reported [40]. Behavioural alterations and premature death were observed in the hGFAP-Q160 expressing model [38, 41]. Further, in cynomologus monkeys, lentiviral overexpression of a N171-Q82 mHTT fragment triggered neuronal death and astrogliosis via activation of the JAK/STAT3 pathway [42]. However, these studies do not directly test if astrocyte physiology is altered in conventional mouse models of HD. Notably, transgenic mice using the GFAP promoter also target neuronal progenitors, some mature neurons and peripheral organs [43, 44]. Hence, an important task was to test the hypothesis that astrocyte physiology is altered in standard models of HD that, like the human disease, reflect mHTT expression in multiple cell types.
Neuronal changes with potential involvement of astrocytes in HD model mice
Rodent HD models have been invaluable to identify the earliest physiological changes that occur due to mHTT, and to clarify the sequence of primary and secondary pathological alterations that may occur. The plethora of available models include: transgenic mice overexpressing either full length, or fragment, mouse, human or hybrid mHTT under various promoters, and ‘knock-in’ mice expressing full length mHTT under control of the endogenous promoter. Box 1 summarizes astrocyte-related HD phenotypes found in popular and widely used mouse models.
The similarity of murine HD models to human HD is particularly obvious with regard to the progressive loss of GLT1 [33, 45–51] (Box 1). Also reminiscent of findings from HD post-mortem tissues, nuclear inclusions are present in astrocytes in HD mouse models [29, 30, 33]. Striatal astrocytes from R6/2 mice contained mHTT nuclear inclusions as early as 4–5 weeks of age [29], i.e. when the mice are just becoming symptomatic, but before detectable evidence of astrogliosis and overt tissue loss. These astrocytic inclusions are less abundant than in neurons at the same age, although the proportion of astrocytes showing inclusions increased progressively to 30–50% of S100β-positive astrocytes, while this proportion was much less in GFAP-positive astrocytes [29, 33]. Morphological analysis in humans and HD mice shows that there is neither glial cell death, nor strong evidence of reactive astrogliosis at early symptomatic ages when GLT1 loss occurs [30, 33].
Regarding a potential contribution of astrocytic dysfunction to neural dysfunction in HD, the first suggestion came from studies in awake behaving HD model mice during recording of cortical and striatal activity [47, 52]. Recording extracellular spike activity and local field potentials (LFPs) in R6/1, R6/2 and full-length (knock-in) mouse models, Rebec and colleagues discovered that signal processing in the HD striatum was altered. The HD-related changes included an increase in the mean firing frequency of units within the striatum, while coincident M1 and PFC cortical and striatal bursting, which measures the temporal overlap between bursting pairs, was reduced. The increased firing frequency deduced from LFP recording in the striatum of awake HD mice may have several causes: increased intrinsic excitability of MSNs, tonically elevated extracellular [K+] levels and a weakness of tonic inhibition via extrasynaptic GABA(A) receptors, among others. The impairment of cortical and striatal firing synchronicity also indicated reduced corticostriatal connectivity. Of particular interest, ceftriaxone administration to R6/2 mice (a beta-lactam antibiotic known to elevate GLT1) attenuated several HD behavioral phenotypes; paw clasping and twitching were reduced, while motor flexibility and open-field climbing were increased [53]. This was correlated with an improvement in striatal and cortical GLT1 levels [48], and increased striatal glutamate uptake, as measured by no-net-flux microdialysis [48, 53]. Although correction of the neurophysiological phenotypes were not explicitly evaluated, improvements in the behavioral phenotype implied a correction of the basal ganglia motor circuitry via GLT1 upregulation.
Many neurophysiological abnormalities in the HD corticostriatal connections and MSN excitability can be observed in acute slice preparations from HD models. HD MSNs show a progressive phenotype of emerging intrinsic hyperexcitability, whereby cells become modestly depolarized, exhibit reduced rheobase in response to current injection, and exhibit significantly higher membrane resistance around resting states, implying the loss of hyperpolarizing conductances that maintain quiescence. These findings are progressive and have been demonstrated in both R6/2 mice as early as 5–7 weeks [54–58] and in Q175 KI mice around 4 months of age [59–61]. It is likely that a loss of intrinsic MSN potassium conductances at least in part underlies this phenomenon; loss of inwardly rectifying potassium channels (Kirs) are likely contributors [56, 62] as they are largely responsible for maintaining Vm, and respective gene expression is known to be decreased [56, 62]. However, extracellular basal K+ levels measured in vivo in the striatum of symptomatic R6/2 mice are elevated by ~0.7–1.5 mM, which would also be expected to depolarize MSNs and contribute to this phenotype [33, 63].
Regarding corticostriatal connectivity, MSNs in symptomatic R6/2, YAC128 and Q175 mice also display a progressive impairment in responsiveness to cortical stimulation ex vivo, whereby equivalently sized cortically evoked EPSCs (relative to WT age-matched controls) can only be achieved with higher stimulus intensities [55, 59, 64, and reviewed in 65]. Multiple HD mouse models in addition show reduced frequency of mEPSCs and sEPSCs onto MSNs, indicating a paucity of striatal glutamatergic input, in R6/2 by 6–8 weeks [55], in Q175 het by 6 months [59, 60], and in YAC128 at 7 months [64]. In vivo, R6/2 mice show reduced glutamate release as measured by microdialysis in response to K+ stimulation by 6 weeks [52, 66]. These data point to an impairment in corticostriatal and/or thalamostriatal glutamatergic inputs.
Complex alterations in striatal GABAergic transmission are also apparent in HD mouse model striatum [58]. Unitary eIPSC amplitudes in MSNs in response to intrastriatal stimulation are reduced in R6/2 and Q175 mice, with a clear deficit in the probability of release, as indicated by a higher paired-pulse ratio, failure rate and coefficient of variation [67]. Pharmacological interrogation identified this suppression of synaptic GABA release as a glutamate- and endocannabinoid-dependent process [49, 67]. However, spontaneous action potential-dependent GABAergic synaptic activity is enhanced in these models [58, 67], likely due to the aberrant activity of MSNs and/or GABAergic interneuron populations [58]. There is also a noted decrease in tonic inhibition of HD MSNs, most likely due to reduced GAT3-mediated astrocytic release of GABA [49, 67].
In summary, the early neurophysiological changes within the striatum of HD models coincident with symptom onset are (i) increased intrinsic MSN excitability, (ii) increased [K+] and decreased tonic inhibition in the striatum, (iii) impairment of glutamatergic corticostriatal input, and (iv) altered GABAergic synaptic transmission. Given the proposed and established roles of astrocytes in the control of synapse stability, ion homeostasis, neurotransmitter clearance, synaptic modulation and plasticity, a role for mHTT-induced astrocyte dysfunction in contributing to the striatal circuit phenotypes seemed likely, and recent studies have confirmed this in some aspects.
Astrocyte K+ homeostasis and Ca2+ signaling in HD mouse models
K+ homeostasis
Astrocyte membrane conductance was decreased by loss of weakly inwardly rectifying K+ channels in astrocytes that express mHTT in HD mouse models [33]. Kir4.1 channels are astrocyte enriched (gene name KCNJ10), developmentally regulated, regionally variable in expression and involved in important functions, including bulk and local K+ homeostasis [17]. The importance of Kir4.1 channels is emphasized by studies of knock-out mice, which die three weeks after birth and display severe motor deficits, including jerky movements, loss of balance/posture, body tremors and frequent falls and rollovers [68, 69]. Some of these phenotypes are mediated by Kir4.1-expressing astrocytes [70, 71] although some are due to loss of Kir4.1 in oligodendrocytes [69]. Conditional Kir4.1 knock-out mice displayed deficits in K+ homeostasis in the brain [72]. Notably, humans with mutant Kir4.1 channels display Epilepsy, Ataxia, Sensorineural deafness and Tubolopathy – EAST syndrome [73], and Jack Russell terriers carrying Kir4.1 mutants suffer from juvenile-onset spinocerebellar ataxias [74]. Thus, it is clear that relatively simple changes in Kir4.1 function can produce profound neurological symptoms in mice, dogs and humans [17].
Reminiscent of the aforementioned Kir4.1 knock-out studies, evaluations of astrocytes in HD model mice (Box 1) revealed contributions of Kir4.1 channels to astrocyte membrane properties and basal K+ homeostasis [33]. However, it should be noted that further evaluations are needed to explore other mechanisms known to contribute to K+ homeostasis such as the Na+/K+ ATPase, gap junctions or other K+ channels [17]. There are several additional observations that are worth noting. At early stages, striatal astrocytes from symptomatic R6/2 mice displayed resting membrane potentials depolarized by a few millivolts and lower membrane conductances [33]. These electrophysiological differences between wild-type and R6/2 astrocytes could be accounted for by lower functional expression of Kir4.1 channels, and the alterations were recovered by AAV2/5 mediated delivery of Kir4.1 specifically to astrocytes [5, 33]. Extracellular basal K+ levels in the striatum at P60–80 were elevated by ~1.5 mM. This suggests that increased K+ may contribute to neuronal hyperexcitability phenotypes in HD model mice, a possibility that gains support from the empirical observations that increasing K+ concentrations in brain slices from healthy wild-type mice by ~1.5 mM reproduced some features of altered striatal medium spiny neuron (MSN) hyperexcitability observed in R6/2 mice [33]. The key experiments were also repeated in Q175 mice (Box 1) [33]. Recently, observations on changes in expression of Kir4.1 and GLT1 have been extended with next generation sequencing in HD model mice [62].
Perhaps the most interesting aspect of Tong et al. was the discovery that expression of Kir4.1-GFP in astrocytes also reversed some excitability MSN deficits observed in R6/2 mice, and that expression of Kir4.1-GFP within striatal astrocytes in vivo attenuated some HD–like motor deficits and prolonged survival in R6/2 mice [33]. Rescue of Kir4.1 function also appears effective after systemic AAV9 delivery to improving HD astrocyte phenotypes and a simple motor function in vivo [75]. These data provide proof-of-concept that correcting a key astrocyte homeostatic function may lead to general benefits that can be observed at the level of neural circuits and animal behavior. At a conceptual level, this adds credence to the hypothesis that astrocytes can be targeted for therapeutic benefit in complex multicellular diseases at their early stages [3]. Early stage dysfunctions may be critical to target, because later stage astrogliosis may also reflect external cues resulting from local damage [21]. More broadly, the data provide evidence for the hypothesis that some key aspects of altered MSN excitability in HD are secondary to astrocyte homeostasis dysfunction.
Ca2+ signaling
Intracellular Ca2+ signaling is an important aspect of astrocyte biology in brain slices, in vivo [12, 14] and during disease [76]. Astrocyte Ca2+ signaling was increased in mouse models of Alzheimer’s disease (AD) and stroke that are accompanied with strong astrogliosis [77–80]. In these settings, it was proposed that elevated astrocyte Ca2+ signaling may contribute to pathology. In contrast, little was known about striatal astrocyte Ca2+ signaling in HD model mice, prompting the exploration of the relationship between astrocyte Ca2+, Kir4.1 and GLT1 [81]. Astrocyte spontaneous Ca2+ signals were reduced in R6/2 mice, but evoked Ca2+ signals were increased in a manner that was dependent on GLT1, at ages when Kir4.1 and GLT1 deficits were noted [81]. The action potential-dependent evoked Ca2+ signals were mediated by astrocyte mGluR2/3 receptors and likely due to glutamate spill-over to distal sites where greater numbers of receptors were likely recruited (Figure 3). In accord, the lifetime of electrically-evoked glutamate release near the surface of astrocytes was prolonged in R6/2 relative to wild-type mice, likely reflecting loss of GLT1 function within astrocytes and reduced glutamate clearance [81]. Of note, the prolonged astrocyte iGluSnFR signals and elevated mGluR2/3-mediated evoked Ca2+ signals observed in R6/2 mice could be mimicked in wild-type mice by blocking GLT1, implying that reduced glutamate uptake may be the likely cause of these astrocyte dysfunctions in R6/2 mice. Some of the iGluSnFR and Ca2+ signal alterations observed in R6/2 mice could be rescued by astrocyte delivery of Kir4.1 [81]. Thus, it appears that Kir4.1 can set into effect a sequence of events that results in restoration of normal astrocyte ion and membrane potential gradients, recovery of GLT1 function, reduced perisynaptic glutamate levels as well as altered synaptic transmission and plasticity [82] in HD model mice. However, there was no evidence for elevated astrocyte glutamate release, which had been suggested based on cell-culture work [83].
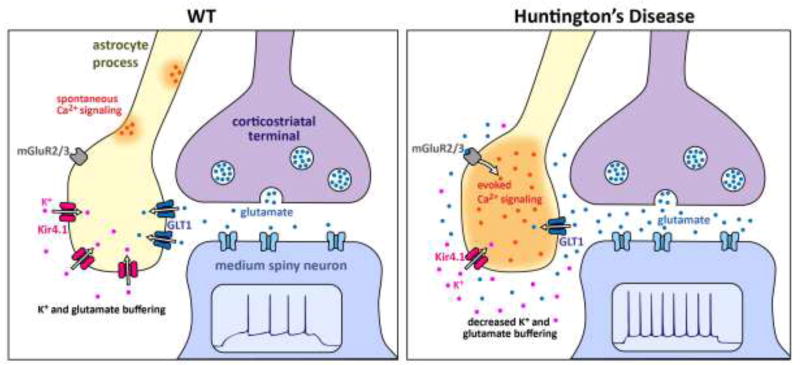
Figure 3. Schematic summary of the major Kir4.1, Ca2+ and GLT1 astrocyte changes observed in R6/2 mice at ~P70.
Only the key features are schematized and other differences between WT and HD mice are discussed in the text. In the WT striatum, normal expression levels of Kir4.1 and GLT1 maintain low levels of extracellular K+ and glutamate near synapses. As a result, electrical stimulation of cortico-striatal axons does not evoke robust mGluR2/3-mediated astrocyte Ca2+ signals. However, there are abundant spontaneous Ca2+ signals in striatal astrocytes. In HD mice, expression levels of Kir4.1 and GLT1 are reduced, and this has a number of observable consequences for astrocytes. Reduced GLT1 levels mean that astrocytes display robust mGluR2/3-mediated Ca2+ signals during stimulation of cortico-striatal axons. This gain of evoked Ca2+ signals is accompanied by a loss of spontaneous Ca2+ signals. The elevated glutamate and K+ levels in the extracellular space are proposed to contribute to increased excitability of MSNs. The cartoon is based on published work [33, 75, 81, 93].
Impaired glutamate uptake and possible consequences in HD mice
As mentioned, studies in humans and in several mouse models of HD consistently show a disease-related down-regulation of the gene encoding GLT1 (Slc1A2) [9, 29, 46, 51, 62, 84–87]. As HD is also characterized by a marked decrease in the density of glutamatergic synapses in the dorsal striatum [60, 88–90] the question arises as to what extent the observed reduction in striatal glutamate uptake may be an adaptation to the loss of synaptic glutamate release, or a risk factor to be eliminated by novel therapies of HD.
GLT1-related alterations in synaptic transmission would argue for the latter possibility, especially if the changes lead to significant loss in the corticostriatal coherence or the appearance of spontaneous firing at rest [47, 52]. While in normal mice glutamate transport is hardly ever overwhelmed [91], slowed glutamate uptake and spillover to perisynaptic sites are now being considered in some mechanisms of synaptic plasticity [82] and in neurological conditions, including HD. Recent experiments with the fluorescent glutamate sensor iGluSnFR illuminate the reduced glutamate clearance capacity of striatal astrocytes in R6/2 mice [81]. However, Raymond and colleagues [39] found no evidence for impaired clearance of synaptically released glutamate when analysing the glutamate transients elicited by local EFS in iGluSnFR-expressing MSNs [92].
Further evidence in support of HD-related changes in striatal glutamatergic synaptic transmission was provided by recording corticostriatal EPSCs in the absence of NMDA receptor blockers. In Q175 mice, EPSC decay was slowed [93], notably when the EPSC included NMDAR-mediated currents at membrane potentials of +50 mV and higher. This observation is in line with the results from normal mice and rats where pharmacological block of GLT1 with TBOA induced EPSC prolongation [94] and changes in the preferentially expressed type of plasticity [82]. It was suggested that GLT1 blockade results in the recruitment of a critical number of perisynaptic NMDA receptors to elicit non-Hebbian forms of plasticity at the expense of spike-time dependent plasticity (STDP). Finally, it was proposed that in HD, glutamate spillover to perisynaptic mGluR5 could account for the heterosynaptic CB1-dependent depression of synaptic GABA release, as observed in R6/2 and Q175 HD mice [67].
A very sensitive indicator of failing glutamate uptake is the level of nonsynaptic release of GABA from astrocytes [49]. It was found that astrocytic GAT3-mediated release of GABA contributes to the maintenance of a tonic GABAA receptor-mediated conductance (ITonic(GABA) in MSNs (Figure 4A). The driving force for the nonsynaptic GABA release through GAT3 is established by the [Na+] elevation at co-localized GLT1 clusters (see [95]), since pharmacological block of GLT1 reduces (ITonic(GABA) to the HD level (Figure 4B). We tentatively conclude that, in HD mice, glutamate uptake is insufficient as it leads to unwarranted alterations in glutamatergic and GABAergic synaptic transmission. Notably the loss of spike-time-dependent plasticity could contribute to the impaired corticostriatal control.
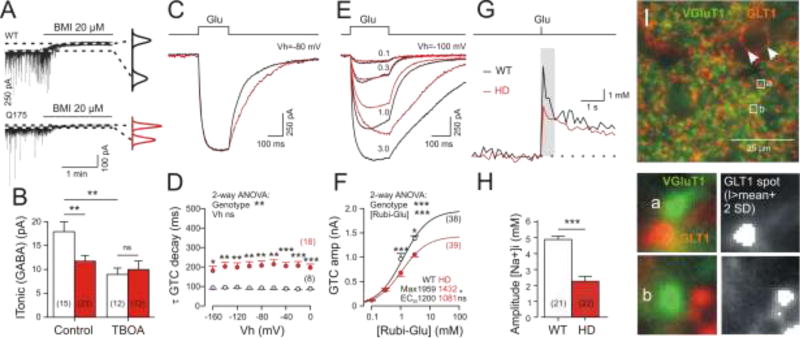
Figure 4. Altered function of GABA and glutamate transporters in HD model mice.
(A, B) Quantification of ITonic(GABA) from WT and HD MSNs [49]. Experiments in the presence of DNQX (10 µM), APV (50 µM), LY341495 (40 µM); no K+ channel block. Graphs on the right: Gaussian fits to all-points histograms derived from 30 s recording periods under control conditions and in the presence of BMI. The difference between the peaks of the fitting curves represents the amplitude of ITonic(GABA). Block of GLT1 with TBOA (100 µM) in WT reduces ITonic(GABA) to HD level. (C–H) Records and quantification of glutamate uptake activity in single sulphorhodamine-labelled astrocytes from the striatum of 1-year-old Q175 wild types and homozygotes [93]. (C–F) Experiments with somatic patch-clamp recording of glutamate transporter currents (GTCs) in the presence of carbenoxolone (100 µM) to block gap junctions and Ba2+ (100 uM) to block Kir4.1 channels. The latter reduces the possible impact of reduced driving forces (low Kir4.1 expression) in HD. Glutamate was applied by flash photolysis of Rubi-glutamate (3 mM). The stimulus covered the entire astrocyte. (C, D) HD-related prolongation of GTC decay. (E, F) Dose-response relationship to estimate the transport maximum and respective HD-related differences. (G) Average traces of [Na+]i from individual astrocytes in one wild-type and one HD section after loading of SBFI-AM into sulphorhodamine-labelled cells. The recordings were performed in the presence of the gap junction blocker carbenoxolone and the glutamate receptor blockers DNQX and MK101. The signals were acquired from the soma and proximal processes before and after optical uncaging of Rubi-glutamate (3 mM) within the same area. (H) Quantification of results. Numbers in brackets: number of cells (data from 9 sections from 3 animals per group). (I) View field from the dorsal striatum showing vGLUT1 (green) and GLT1 (red) immunofluorescence (Dvorzhak and Grantyn, unpublished). Clusters of GLT1 are found in co-localization with glutamatergic terminals (see boxed regions of interests at larger magnification below) and in contact with blood vessels (arrowheads). The GLT1 spots in co-localization with vGLUT1 terminals were delineated and evaluated by a threshold algorithm.
Additional studies are needed to evaluate if the observed changes in glutamate spillover are due to insufficient or inappropriate function of the transporter itself, or reflect changes in the basic properties of astrocytes, such as membrane depolarization and loss of high membrane conductance due to Kir4.1 deficiency. This question was addressed by recording glutamate transporter currents (GTC; Figure 4C–F) in single sulphorhodamine-labelled astrocytes [93]. Addition of Ba2+ to the recording solution decreased the impact of Kir4.1, thereby unmasking a functional deficit of GLT1 itself. In Q175 homozygous mice, the decrease in the GTC maxima amounted to about one third of the WT control. The deficit should be larger under conditions allowing for a combined effect of reduced GLT1 and Kir4.1 deficiency as is the case in the recordings of glutamate-induced [Na+]i transients in unperturbed WT and HD astrocytes (Figure 4G,H).
In addition to the impact of electrochemical driving force on GLT1 [96–98], the level of GLT1 transport would be expected to depend on the number of transporter molecules at synaptic sites, their distance from the sites of synaptic release, their transport rate, diffusion and a variable dependence on cAMP-mediated vesicular replenishment of transporter aggregates [99]. Astrocytes exhibiting the usual negative membrane potential at EK are assumed to restrict glutamate signalling to postsynaptic receptors [91], even though the synaptic glutamate concentration can increase by 5 orders of magnitude [89, 100, 101]. Nevertheless, excitotoxicity due to pathological activation of extrasynaptic NMDA receptors remains a frequently discussed, but mechanistically unclear consequence of GLT1 deficiency [102]. Considering the powerful glutamate clearance capacity still remaining in symptomatic HD (Figure 4F), one may wonder why astrocytic glutamate uptake does not cope with the levels of glutamate needed to activate the NR2B–containing extrasynaptic NMDA receptors (EC50 for L-glutamate ~3 µM [103]). However, if extracellular [K+] and intracellular [Na+] both increase to ~30 mM, an astrocyte depolarization of ~30 mV would suffice to silence glutamate uptake and to initiate a reverse mode of operation [104, 105]. Such extreme elevation of extracellular [K+] is indeed possible during states of spreading depression in cortical structures, but modelling of neurotransmission-induced [K+] transients and respective astrocyte membrane potentials predict much smaller changes [105].
Another basis for the excessive activation of NMDARs in HD could be a particularly low density of astrocytic glutamate transporters at extrasynaptic sites. It is already well known that GLT1 immunoreactivity is not evenly distributed [53, 106], but forms clusters adjacent to the areas of presynaptic vesicle accumulation (Figure 4I). Whether synapse loss in HD is associated with a change in the distribution of astrocytic glutamate transporters remains to be determined. Overall, there appears to be good evidence for GLT1-specific roles in HD pathology that add to the deficits imposed by altered K+ homeostasis [81, 93].
Cholesterol, astrocytes and HD
In the adult brain, astrocytes play a critical role in de novo synthesis of cholesterol, which regulates neuronal function. Dysregulation of cholesterol metabolism is observed in HD astrocytes, both in terms of gene expression and levels of various cholesterol intermediates, possibly via an undefined mechanism involving mHTT and the SREBPs transcription factors [107, 108]. A change in cholesterol levels have been inconsistently reported in HD models, although lower 24-hydroxy-cholesterol levels in plasma have been described in patients and in rodent models, supporting deficits in cholesterol metabolism [107, 109]. The pathogenic impact of cholesterol pathways in HD models is supported by a recent study showing that enhanced degradation of cholesterol in the striatum of R6/2 mice via AAVrh10 expression of CYP46A1 (the enzyme that converts cholesterol into 24-hydroxy-cholesterol) significantly modifies core features of the disease [110]. It remains to be determined how other metabolic astrocytic processes may be adversely influenced by the presence of mHTT.
Stem cell approaches in HD model mice
Notwithstanding the wealth of data pertaining to the nature of glial dysfunction in rodent models of HD, the relative role and importance of human glial pathology in HD has remained unclear. Furthermore, in vivo models for assessing the degree to which glial pathology might be necessary or sufficient for disease progression have been lacking, as has the ability to investigate HD glia with regards to their cell-autonomous pathology in live adults. This lack of understanding of the role of glial pathology in HD has reflected the lack of in vivo models that permit the separate interrogation of glial and neuronal functions in HD, particularly so in humans.
To address this fundamental gap in our knowledge, Benraiss and colleagues used human glial chimeric mice, in which neonatal implantation of human glial progenitor cells leads to substantial replacement of resident murine glia by their human counterparts [111, 112], to assess the role of human striatal glia in the pathogenesis of HD [63]. To that end, both the striatal neuronal physiology and behavior of immunodeficient mice xenografted at birth with mutant HD-expressing human hGPCs were assessed, and compared to that of mice engrafted with normal HTT hGPCs [63]. In this study, the motor behavior of mice engrafted with human embryonic stem cells (hESCs) HD-derived (48 CAG) GPCs was first contrasted to that of controls engrafted with hGPCs derived from an unaffected sibling line (18 CAG). The HD GPC-engrafted mice manifested impaired motor learning relative to control hGPC-engrafted mice, that was sustained and profound. Furthermore, mice then engrafted with human striatal astrocytes transduced to overexpress mutant HTT (73Q) exhibited sharply increased neuronal input resistance and excitability, relative to their controls engrafted with normal HTT (23 CAG)-transduced striatal glia.
Since striatal chimerization with HD-derived glia proved sufficient to recapitulate aspects of disease phenotype in normal mice, one might anticipate neonatal chimerization of HD mice with normal hESC-derived glia might delay or rescue aspects of disease phenotype. Using R6/2 transgenic HD mice as hosts [113], Benraiss and colleagues then found that the substantial replacement of diseased striatal glia with wild-type human glia indeed resulted in a slowing of disease progression and corresponding increment in survival in transplanted R6/2 mice. Of note, the glial donor cells were isolated on the basis of CD44, the hyaluronan receptor, so as to capture a more astrocyte-biased donor cell population [114]. This neonatal engraftment by CD44-defined glia yielded a transplant-associated fall in neuronal input resistance, and a corresponding drop in interstitial K+ in the R6/2 striatum, with an attendant rescue of the otherwise hyperexcitable phenotype of R6/2 striatal neurons [63]. This was accompanied not only by delayed disease progression and enhanced survival, but by improved cognitive and motor function on a variety of metrics, relative to untreated R6/2 mice. Normal glial progenitor cells and their derived astrocytes thus proved able to substantially delay and lessen the pathology of resident HD striatal neurons, and by so doing defined a substantially non-cell autonomous component to neuronal pathology in HD. Together, these studies indicate a critical role for glial pathology in the progression of HD. Going forward, they suggest the potential for glial cell replacement as a therapeutic strategy in Huntington’s disease, and more broadly, to other neurodegenerative diseases in which glial pathology might be causally contributory.
Concluding remarks
The last few years have witnessed the start of the beginning in our understanding of how astrocytes contribute to HD in mouse models and these studies signal the possibility that astrocytes may be exploited to derive therapeutic effect in HD. However, it is still necessary to determine the functions of normal HTT within astrocytes and it will be informative to overexpress and delete the disease causing mHTT only within astrocytes in the fully developed mature brain. These experiments are now feasible based on the availability of pan-astrocytic Aldh1l1-Cre/ERT2 mice [115], which better overcome the limitations of using Gfap-Cre lines that target only some astrocytes, but also neural progenitors, some neurons and some peripheral organs [43, 44]. In a complex disease such as HD, even modest expression in neurons would be a serious confounding factor and should be avoided at the outset when designing astrocyte studies. It is worth noting that healthy striatal astrocytes display low levels of GFAP [33, 113], which suggests that Gfap-Cre lines may be suboptimal in HD studies. Hence, the pursuit and availability of genetic tools to specifically and reliably study astrocytes will be important in explorations in the context of HD. We suggest that all molecular and cellular evaluations need to be performed in mature mice at ages relevant to the onset and progression of HD symptoms in disease models. Of course, analyses of human HD specimens is critical to place into context the data from mouse models. Furthermore, it remains to be determined if the changes in K+, glutamate and GABA homeostasis mediated by astrocytes in HD model mice are causative or correlative to disease. We suggest both scenarios are likely: it seems difficult to imagine how marked changes in extracellular K+, glutamate and GABA would not contribute causatively to neuronal dysfunction, as has been discussed in the preceding sections in detail. Additional mechanisms that warrant further study are astrocyte mitochondrial and nuclear alterations [116, 117].
The astrocyte studies in the last few years have important implications for therapeutic strategies in HD and suggest that approaches that target only neuronal disturbances are likely to be inadequate or suboptimal for restoring function. Strategies that target key mechanisms in neurons and astrocytes are likely to be most successful, because astrocytes and neurons function synergistically in the healthy brain and appear to have done so throughout the evolution of complex nervous systems [118]. We propose that restoring astrocyte-mediated homeostasis and normal synergistic relationships of neurons and astrocytes may provide new therapeutic strategies for brain disorders. This seems appropriate given that mHTT is found within neurons and astrocytes in humans with HD.
Trends box.
Astrocytes are involved in Huntington’s disease (HD)
Astrocyte dysfunctions contribute to HD pathophysiology in mice
Transplanted astrocytes produce therapeutic benefit in HD model mice
Targeting astrocyte dysfunction may provide new therapeutic targets in HD
Outstanding Questions Box.
Molecules
-
*
What is the molecular profile (RNA and protein) of striatal astrocytes in healthy tissue and at the various stages of disease progression in HD mouse models?
-
*
What is the molecular profile of astrocytes from humans with HD at various clinical stages?
-
*
Can selective Kir4.1 and GLT1 positive allosteric modulators be discovered and can they be used pharmacologically to mitigate the decrease of these proteins in HD?
-
*
What is the neurotoxic substance released from A1 reactive astrocytes [20] and does this contribute to HD?
Cells and circuits
-
*
How do astrocytes affect striatal synapses and circuits?
-
*
Do astrocytes interact with and “listen” to specific neural inputs?
-
*
Do striatal astrocytes release glutamate via reversal of transporters or via Ca2+-dependent exocytosis in adult brain?
-
*
Do astrocyte Ca2+ signals serve distinct functions in distinct circuits?
Mice
-
*
Can astrocyte biology be exploited to produce desirable effects in neural circuits associated with specific brain disorders?
-
*
Do altered astrocytes contribute to specific neurodegenerative diseases or do they have the same effect in all neurodegenerative disorders?
-
*
How do astrocytes contribute to dopaminergic control in intact mice?
-
*
Can astrocyte modifications prevent the loss of glutamatergic and GABAergic synapses?
Humans
-
*
Can paradigms be developed to evaluate if combined astrocyte and neuronal strategies are more effective than either one alone?
-
*
Can astrocytes be exploited for selective delivery of small molecules via intracellular gap-junctional transport specifically to synaptic sites?
-
*
Is it possible to design an astrocyte-specific PET probe to study astrocytes in humans as disease progresses and as treatments are administered?
Acknowledgments
Work in the Khakh lab was supported by CHDI and by NIH grants NS060677, MH099559 and MH104069. Grantyn lab: CHDI (A-7815), German Research Council (Exc 257/1) and Charité Research Funds (2016-040). Work in the Goldman lab was supported by CHDI, NIMH, NINDS, the Novo Nordisk Foundation, Lundbeck Foundation, Adelson Medical Research Foundation, Mathers Foundation, and ALS Association. The authors regret that many papers could not be cited (especially early pioneering studies), because of space limits and the requirement to focus on studies from the last five years.
Glossary
- Cre
short for cre recombinase, which catalyzes recombination between two LoxP sites in DNA
- Cre/ERT2
a type of tamoxifen inducible Cre
- GFAP
a type of astrocyte-enriched intermediate filament protein
- GLAST
a particular type of plasma membrane glutamate transporter found in astrocytes
- GLT1
a particular type of plasma membrane glutamate transporter strongly enriched in astrocytes
- GCaMP3
a type of genetically-encoded calcium indicator
- iGluSnFR
a type of genetically-encoded glutamate sensor
- HTT
huntingtin protein
- Kir4.1
a type of weakly inwardly rectifying potassium channel found in astrocytes
- mHTT
mutant huntingtin protein carrying disease causing glutamine expansion
- MSN
medium spiny neuron, frequently also called striatal projection neurons or spiny projection neurons
- UHDRS
Unified Huntington’s disease rating scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: All authors worked on subsections separately. BSK drafted the final version and all authors commented and agreed on it.
References
- 1.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol. 2014 Nov 20;7(1):a020362. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, Khakh EMHBS. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013;141:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faideau M, Kim J, Cormier K, Gilmore R, Welch M, Auregan G, Dufour N, Guillermier M, Brouillet E, Hantraye P, Déglon N, Ferrante RJ, Bonvento G. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington’s disease subjects. Hum Mol Genet. 2010;19:3053–3067. doi: 10.1093/hmg/ddq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuffler SW. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967;168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- 11.Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- 12.Shigetomi E, Patel S, Khakh BS. Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol. 2016;26:300–312. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci. 2014;15:327–335. doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 15.Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol. 2014;30:439–463. doi: 10.1146/annurev-cellbio-100913-013053. [DOI] [PubMed] [Google Scholar]
- 16.Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015 Feb 6;7(9):a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 2016;132:1–21. doi: 10.1007/s00401-016-1553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, Elliston LA, Hartog C, Goldstein DR, Thu D, Hollingsworth ZR, Collin F, Synek B, Holmans PA, Young AB, Wexler NS, Delorenzi M, Kooperberg C, Augood SJ, Faull RL, Olson JM, Jones L, Luthi-Carter R. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 2006;15:965–977. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 22.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 Jan 18; doi: 10.1038/nature21029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, Borowsky B, Landwehrmeyer B, Frost C, Johnson H, Craufurd D, Reilmann R, Stout JC, Langbehn DR, Investigators T-H. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet neurology. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 24.Biglan KM, Zhang Y, Long JD, Geschwind M, Kang GA, Killoran A, Lu W, McCusker E, Mills JA, Raymond LA, Testa C, Wojcieszek J, Paulsen JS P.-H.I.o.t.H.S. Group. Refining the diagnosis of Huntington disease: the PREDICT-HD study. Frontiers in aging neuroscience. 2013;5:12. doi: 10.3389/fnagi.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.T.H.s.D.C.R. Group, A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 26.Eidelberg D, Surmeier DJ. Brain networks in Huntington disease. J Clin Invest. 2011;121:484–492. doi: 10.1172/JCI45646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EPJ. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The Neuropathology of Huntington’s Disease. Curr Top Behav Neurosci. 2015;22:33–80. doi: 10.1007/7854_2014_354. [DOI] [PubMed] [Google Scholar]
- 29.Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen AH, van Hal M, Op den Kelder IC, Meier RT, de Ruiter AA, Schut MH, Smith DL, Grit C, Brouwer N, Kamphuis W, Boddeke HW, den Dunnen WF, van Roon WM, Bates GP, Hol EM, Reits EA. Frequency of nuclear mutant huntingtin inclusion formation in neurons and glia is cell-type-specific. Glia. 2017;65:50–61. doi: 10.1002/glia.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rub U, Vonsattel JP, Heinsen H, Korf HW. The Neuropathology of Huntington s disease: classical findings, recent developments and correlation to functional neuroanatomy. Adv Anat Embryol Cell Biol. 2015;217:1–146. [PubMed] [Google Scholar]
- 32.Myers RH, Vonsattel JP, Paskevich PA, Kiely DK, Stevens TJ, Cupples LA, Richardson EP, Jr, Bird ED. Decreased neuronal and increased oligodendroglial densities in Huntington’s disease caudate nucleus. J Neuropathol Exp Neurol. 1991;50:729–742. doi: 10.1097/00005072-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, Khakh BS. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedreen JC, Folstein SE. Early loss of neostriatal striosome neurons in Huntington’s disease. J Neuropathol Exp Neurol. 1995;54:105–120. doi: 10.1097/00005072-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Rothstein JD, dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 36.Arzberger T, Krampfl K, Leimgruber S, Weindl A. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington’s disease--an in situ hybridization study. J Neuropathol Exp Neurol. 1997;56:440–454. doi: 10.1097/00005072-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Group HS. Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 38.Bradford J, Shin JY, Roberts M, Wang CE, Sheng G, Li S, Li XJ. Mutant huntingtin in glial cells exacerbates neurological symptoms of Huntington disease mice. J Biol Chem. 2010;285:10653–10661. doi: 10.1074/jbc.M109.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons MP, Vanni MP, Woodard CL, Kang R, Murphy TH, Raymond LA. Real-time imaging of glutamate clearance reveals normal striatal uptake in Huntington disease mouse models. Nat. Commun. 2016 Apr 7;77:11251. doi: 10.1038/ncomms11251. 11210.11038/ncomms11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong Y, Zhao T, Li XJ, Li S. Mutant Huntingtin Impairs BDNF Release from Astrocytes by Disrupting Conversion of Rab3a–GTP into Rab3a–GDP. J Neurosci. 2016;36:8790–8801. doi: 10.1523/JNEUROSCI.0168-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc Natl Acad Sci U S A. 2009;106:22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben Haim L, Ceyzeriat K, Carrillo-de Sauvage MA, Aubry F, Auregan G, Guillermier M, Ruiz M, Petit F, Houitte D, Faivre E, Vandesquille M, Aron-Badin R, Dhenain M, Deglon N, Hantraye P, Brouillet E, Bonvento G, Escartin C. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J Neurosci. 2015;35:2817–2829. doi: 10.1523/JNEUROSCI.3516-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su M, Hu H, Lee Y, d’Azzo A, Messing A, Brenner M. Expression specificity of GFAP transgenes. Neurochem Res. 2004;29:2075–2093. doi: 10.1007/s11064-004-6881-1. [DOI] [PubMed] [Google Scholar]
- 44.Xie AX, Petravicz J, McCarthy KD. Molecular approaches for manipulating astrocytic signaling in vivo. Front Cell Neurosci. 2015 Apr 21;9:144. doi: 10.3389/fncel.2015.00144. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estrada-Sanchez AM, Montiel T, Segovia J, Massieu L. Glutamate toxicity in the striatum of the R6/2 Huntington’s disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol Dis. 2009;34:78–86. doi: 10.1016/j.nbd.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 46.Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- 47.Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington’s disease. J Neurophysiol. 2008;100:2205–2216. doi: 10.1152/jn.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wojtowicz AM, Dvorzhak A, Semtner M, Grantyn R. Reduced tonic inhibition in striatal output neurons from Huntington mice due to loss of astrocytic GABA release through GAT-3. Frontiers in neural circuits. 2013;7:188. doi: 10.3389/fncir.2013.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Huang K, Kang MH, Askew C, Kang R, Sanders SS, Wan J, Davis NG, Hayden MR. Palmitoylation and function of glial glutamate transporter-1 is reduced in the YAC128 mouse model of Huntington disease. Neurobiol Dis. 2010;40:207–215. doi: 10.1016/j.nbd.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Miller BR, Walker AG, Barton SJ, Rebec GV. Dysregulated Neuronal Activity Patterns Implicate Corticostriatal Circuit Dysfunction in Multiple Rodent Models of Huntington’s Disease. Front Syst Neurosci. 2011;5:26. doi: 10.3389/fnsys.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cepeda C, Cummings DM, Andre VM, Holley SM, Levine MS. Genetic mouse models of Huntington’s disease: focus on electrophysiological mechanisms. ASN Neuro. 2010;2:e00033. doi: 10.1042/AN20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mielcarek M, Landles C, Weiss A, Bradaia A, Seredenina T, Inuabasi L, Osborne GF, Wadel K, Touller C, Butler R, Robertson J, Franklin SA, Smith DL, Park L, Marks PA, Wanker EE, Olson EN, Luthi-Carter R, van der Putten H, Beaumont V, Bates GP. HDAC4 reduction: a novel therapeutic strategy to target cytoplasmic huntingtin and ameliorate neurodegeneration. PLoS Biol. 2013;11:e1001717. doi: 10.1371/journal.pbio.1001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington’s disease transgenic mice. J Neurophysiol. 2005;93:2565–2574. doi: 10.1152/jn.00791.2004. [DOI] [PubMed] [Google Scholar]
- 57.Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington’s disease transgenic mice. J Neurophysiol. 2001;86:2667–2677. doi: 10.1152/jn.2001.86.6.2667. [DOI] [PubMed] [Google Scholar]
- 58.Cepeda C, Galvan L, Holley SM, Rao SP, Andre VM, Botelho EP, Chen JY, Watson JB, Deisseroth K, Levine MS. Multiple sources of striatal inhibition are differentially affected in Huntington’s disease mouse models. J Neurosci. 2013;33:7393–7406. doi: 10.1523/JNEUROSCI.2137-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heikkinen T, Lehtimaki K, Vartiainen N, Puolivali J, Hendricks SJ, Glaser JR, Bradaia A, Wadel K, Touller C, Kontkanen O, Yrjanheikki JM, Buisson B, Howland D, Beaumont V, Munoz-Sanjuan I, Park LC. Characterization of neurophysiological and behavioral changes, MRI brain volumetry and 1H MRS in zQ175 knock-in mouse model of Huntington’s disease. PloS one. 2012;7:e50717. doi: 10.1371/journal.pone.0050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Indersmitten T, Tran CH, Cepeda C, Levine MS. Altered excitatory and inhibitory inputs to striatal medium-sized spiny neurons and cortical pyramidal neurons in the Q175 mouse model of Huntington’s disease. J Neurophysiol. 2015;113:2953–2966. doi: 10.1152/jn.01056.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beaumont V, Zhong S, Lin H, Xu W, Bradaia A, Steidl E, Gleyzes M, Wadel K, Buisson B, Padovan-Neto FE, Chakroborty S, Ward KM, Harms JF, Beltran J, Kwan M, Ghavami A, Haggkvist J, Toth M, Halldin C, Varrone A, Schaab C, Dybowski JN, Elschenbroich S, Lehtimaki K, Heikkinen T, Park L, Rosinski J, Mrzljak L, Lavery D, West AR, Schmidt CJ, Zaleska MM, Munoz-Sanjuan I. Phosphodiesterase 10A Inhibition Improves Cortico-Basal Ganglia Function in Huntington’s Disease Models. Neuron. 2016;92:1220–1237. doi: 10.1016/j.neuron.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 62.Langfelder P, Cantle JP, Chatzopoulou D, Wang N, Gao F, Al Ramahi I, Lu XH, Ramos EM, El Zein K, Zhao Y, Deverasetty S, Tebbe A, Schaab C, Lavery DJ, Howland D, Kwak S, Botas J, Aaronson JS, Rosinski J, Coppola G, Horvath S, Yang XW. Integrated genomics and proteomics define huntingtin CAG length-dependent networks in mice. Nat. Neurosci. 2016;19:623–633. doi: 10.1038/nn.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benraiss A, Wang S, Herrlinger S, Li X, Chandler-Militello D, Mauceri J, Burm H, Toner M, Osipovitch M, Xu Q, Wang F, Kang N, Kang J, Curtin P, Brunner D, Windrem M, Munoz-Sanjuan I, Nedergaard M, Goldman SA. Human glia can both induce and rescue aspects of phenotype in Huntington Disease. Nature Communications. 2016;7 doi: 10.1038/ncomms11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joshi PR, Wu NP, Andre VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, Bamford NS. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci. 2009;29:2414–2427. doi: 10.1523/JNEUROSCI.5687-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plotkin JL, Surmeier DJ. Corticostriatal synaptic adaptations in Huntington’s disease. Curr Opin Neurobiol. 2015;33C:53–62. doi: 10.1016/j.conb.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Traficante A, Riozzi B, Cannella M, Rampello L, Squitieri F, Battaglia G. Reduced activity of cortico-striatal fibres in the R6/2 mouse model of Huntington’s disease. Neuroreport. 2007;18:1997–2000. doi: 10.1097/WNR.0b013e3282f262ca. [DOI] [PubMed] [Google Scholar]
- 67.Dvorzhak A, Semtner M, Faber DS, Grantyn R. Tonic mGluR5/CB1-dependent suppression of inhibition as a pathophysiological hallmark in the striatum of mice carrying a mutant form of huntingtin. J Physiol. 2013;591:1145–1166. doi: 10.1113/jphysiol.2012.241018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21:5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seifert G, Hüttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhäuser C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci. 2009;29:7474–7488. doi: 10.1523/JNEUROSCI.3790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chever O, Djukic B, McCarthy KD, Amzica F. Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J Neurosci. 2010;30:15769–15777. doi: 10.1523/JNEUROSCI.2078-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdelhadi O, Iancu D, Stanescu H, Kleta R, Bockenhauer D. EAST syndrome: Clinical, pathophysiological, and genetic aspects of mutations in KCNJ10. Rare Dis. 2016 Jun 1;4(1):e1195043. doi: 10.1080/21675511.2016.1195043. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilliam D, O’Brien DP, Coates JR, Johnson GS, Johnson GC, Mhlanga-Mutangadura T, Hansen L, Taylor JF, Schnabel RD. A homozygous KCNJ10 mutation in Jack Russell Terriers and related breeds with spinocerebellar ataxia with myokymia, seizures, or both. J Vet Intern Med. 2014;28:871–877. doi: 10.1111/jvim.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vagner T, Dvorzhak A, Wojtowicz AM, Harms C, Grantyn R. Systemic application of AAV vectors targeting GFAP-expressing astrocytes in Z-Q175-KI Huntington’s disease mice. Mol. Cell. Neurosci. 2016;77:76–86. doi: 10.1016/j.mcn.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Nedergaard M, Rodríguez JJ, Verkhratsky A. Glial calcium and diseases of the nervous system. Cell Calcium. 2010;47:140–149. doi: 10.1016/j.ceca.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 77.Takano T, Han X, Deane R, Zlokovic B, Nedergaard M. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:40–50. doi: 10.1196/annals.1379.004. [DOI] [PubMed] [Google Scholar]
- 78.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delekate A, Füchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat Commun. 2014;5:5422. doi: 10.1038/ncomms6422. [DOI] [PubMed] [Google Scholar]
- 80.Rakers C, Petzold GC. Astrocytic calcium release mediates peri-infarct depolarizations in a rodent stroke model. J Clin Invest. 2016 Dec 19;:89354. doi: 10.1172/JCI89354. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang R, Diaz-Castro B, Tong X, Looger LL, Khakh BS. Dysfunctional calcium and glutamate signaling in striatal astrocytes from Huntington’s disease model mice. J Neurosci. 2016;36:3453–3470. doi: 10.1523/JNEUROSCI.3693-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valtcheva S, Venance L. Astrocytes gate Hebbian synaptic plasticity in the striatum. Nat Commun. 2016 Dec 20;7:13845. doi: 10.1038/ncomms13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee W, Reyes RC, Gottipati MK, Lewis K, Lesort M, Parpura V, Gray M. Enhanced Ca(2+)-dependent glutamate release from astrocytes of the BACHD Huntington’s disease mouse model. Neurobiol Dis. 2013;58:192–199. doi: 10.1016/j.nbd.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain. 2002;125:1908–1922. doi: 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- 85.Grewer C, Gameiro A, Rauen T. SLC1 glutamate transporters. Pflugers Arch. 2014;466:3–24. doi: 10.1007/s00424-013-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menalled LB, Kudwa AE, Miller S, Fitzpatrick J, Watson-Johnson J, Keating N, Ruiz M, Mushlin R, Alosio W, McConnell K, Connor D, Murphy C, Oakeshott S, Kwan M, Beltran J, Ghavami A, Brunner D, Park LC, Ramboz S, Howland D. Comprehensive Behavioral and Molecular Characterization of a New Knock-In Mouse Model of Huntington’s Disease: zQ175. PloS one. 2012;7(12):e49838. doi: 10.1371/journal.pone.0049838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meunier C, Merienne N, Jolle C, Deglon N, Pellerin L. Astrocytes are key but indirect contributors to the development of the symptomatology and pathophysiology of Huntington’s disease. Glia. 2016;64:1841–1856. doi: 10.1002/glia.23022. [DOI] [PubMed] [Google Scholar]
- 88.Deng YP, Wong T, Bricker-Anthony C, Deng B, Reiner A. Loss of corticostriatal and thalamostriatal synaptic terminals precedes striatal projection neuron pathology in heterozygous Q140 Huntington’s disease mice. Neurobiol. Dis. 2013;60:89–107. doi: 10.1016/j.nbd.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plotkin JL, Day M, Peterson JD, Xie Z, Kress GJ, Rafalovich I, Kondapalli J, Gertler TS, Flajolet M, Greengard P, Stavarache M, Kaplitt MG, Rosinski J, Chan CS, Surmeier DJ. Impaired TrkB receptor signaling underlies corticostriatal dysfunction in Huntington’s disease. Neuron. 2014;83:178–188. doi: 10.1016/j.neuron.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothe T, deliano M, Wojtowicz AM, Dvorzhak A, Harnack D, Paul S, Vagner T, Melnick I, Stark H, Grantyn R. Pathological gamma oscillations, impaired dopamine release, synapse loss and reduced dynamic range of unitary glutamatergic synaptic transmission in the striatum of hypokinetic Q175 Huntington mice. Neurosci. 2015;311:519–538. doi: 10.1016/j.neuroscience.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 91.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 92.Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen TW, Bargmann CI, Orger MB, Schreiter ER, Demb JB, Gan WB, Hires SA, Looger LL. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dvorzhak A, Vagner T, Grantyn R. Functional indicators of glutamate transport in single striatal astrocytes and the influence of Kir4.1 in normal and Huntington mice. J. Neurosci. 2016;16:4959–4975. doi: 10.1523/JNEUROSCI.0316-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beurrier C, Bonvento G, Kerkerian-Le Goff L, Gubellini P. Role of glutamate transporters in corticostriatal synaptic transmission. Neurosci. 2009;158:1608–1615. doi: 10.1016/j.neuroscience.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 95.Heja L, Nyitrai G, Kekesi O, Dobolyi A, Szabo P, Fiath R, Ulbert I, Pal-Szenthe B, Palkovits M, Kardos J. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol. 2012;10:26. doi: 10.1186/1741-7007-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 97.Jensen AA, Fahlke C, Bjorn-Yoshimoto WE, Bunch L. Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr. Opin. Pharmacol. 2015;20:116–123. doi: 10.1016/j.coph.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 98.Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev. 2013;93:1621–1657. doi: 10.1152/physrev.00007.2013. [DOI] [PubMed] [Google Scholar]
- 99.Li D, Herault K, Zylbersztejn K, Lauterbach MA, Guillon M, Oheim M, Ropert N. Astrocyte VAMP3 vesicles undergo Ca2+ -independent cycling and modulate glutamate transporter trafficking. J. Physiol. 2015;593:2807–2832. doi: 10.1113/JP270362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greget R, Pernot F, Bouteiller JM, Ghaderi V, Allam S, Keller AF, Ambert N, Legendre A, Sarmis M, Haeberle O, Faupel M, Bischoff S, Berger TW, Baudry M. Simulation of postsynaptic glutamate receptors reveals critical features of glutamatergic transmission. PLoS. One. 2011;6 doi: 10.1371/journal.pone.0028380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessler JP. Control of cleft glutamate concentration and glutamate spill-out by perisynaptic glia: uptake and diffusion barriers. PLoS. One. 2013;8 doi: 10.1371/journal.pone.0070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82:279–293. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 103.Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJ, Snyder JP, Traynelis SF. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D–containing N-methyl-D-aspartate glutamate receptors. Mol Pharmacol. 2007;72:907–920. doi: 10.1124/mol.107.037333. [DOI] [PubMed] [Google Scholar]
- 104.Enger R, Tang W, Vindedal GF, Jensen V, Johannes HP, Sprengel R, Looger LL, Nagelhus EA. Dynamics of Ionic Shifts in Cortical Spreading Depression. Cereb Cortex. 2015;25:4469–4476. doi: 10.1093/cercor/bhv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sibille J, Dao DK, Holcman D, Rouach N. The neuroglial potassium cycle during neurotransmission: role of Kir4.1 channels. PLoS Comput Biol. 2015;11:e1004137. doi: 10.1371/journal.pcbi.1004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Melone M, Bellesi M, Ducati A, Iacoangeli M, Conti F. Cellular and Synaptic Localization of EAAT2a in Human Cerebral Cortex. Front Neuroanat. 2011;4:151. doi: 10.3389/fnana.2010.00151. 110.3389/fnana.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valenza M, Cattaneo E. Emerging roles for cholesterol in Huntington’s disease. Trends Neurosci. 2011;34:474–486. doi: 10.1016/j.tins.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Valenza M, Marullo M, Di Paolo E, Cesana E, Zuccato C, Biella G, Cattaneo E. Disruption of astrocyte-neuron cholesterol cross talk affects neuronal function in Huntington’s disease. Cell Death Differ. 2015;22:690–702. doi: 10.1038/cdd.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leoni V, Mariotti C, Tabrizi SJ, Valenza M, Wild EJ, Henley SM, Hobbs NZ, Mandelli ML, Grisoli M, Björkhem I, Cattaneo E, Di Donato S. Plasma 24S–hydroxycholesterol and caudate MRI in pre-manifest and early Huntington’s disease. Brain. 2008;131:2851–2859. doi: 10.1093/brain/awn212. [DOI] [PubMed] [Google Scholar]
- 110.Boussicault L, Alves S, Lamazière A, Planques A, Heck N, Moumné L, Despres G, Bolte S, Hu A, Pagès C, Galvan L, Piguet F, Aubourg P, Cartier N, Caboche J, Betuing S. CYP46A1,the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain. 2016;139:953–970. doi: 10.1093/brain/awv384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, Goldman SA. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci. 2014;34:16153–16161. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S, Bates G. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 114.Liu Y, Han SW, Wu Y, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev. Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 115.Srinivasan R, Lu T-Y, Chai H, Xu J, Huang BS, Golshani P, Coppola G, Khakh BS. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, Geater C, Morozko E, Stocksdale J, Glatzer JC, Pham JT, Ahmed I, Peng Q, Wadhwa H, Pletnikova O, Troncoso JC, Duan W, Snyder SH, Ranum LP, Thompson LM, Lloyd TE, Ross CA, Rothstein JD. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron. 2017;94:93–107. doi: 10.1016/j.neuron.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Atherton JF, McIver EL, Mullen MR, Wokosin DL, Surmeier DJ, Bevan MD. Early dysfunction and progressive degeneration of the subthalamic nucleus in mouse models of Huntington’s disease. Elife. 2016 Dec 20;5:e21616. doi: 10.7554/eLife.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron. 2013;80:613–623. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- 120.Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simpson EM, Gutekunst CA, Leavitt BR, Hayden MR. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- 121.Gray M, Shirasaki DI, Cepeda C, Andre VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, Li XJ, Levine MS, Yang XW. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008;28:6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Atherton JF, McIver EL, Mullen MR, Wokosin DL, Surmeier DJ, Bevan MD. Early dysfunction and progressive degeneration of the subthalamic nucleus in mouse models of Huntington’s disease. Elife. 2016;5 doi: 10.7554/eLife.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martone ME, Gupta A, Wong M, Qian X, Sosinsky G, Ludaescher B, Ellisman MH. A cell centered database for electron tomographic data. J. Struct. Biology. 2002;138:145–155. doi: 10.1016/s1047-8477(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 124.Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]