Abstract
BACKGROUND
Nivolumab combined with ipilimumab resulted in longer progression-free survival and a higher objective response rate than ipilimumab alone in a phase 3 trial involving patients with advanced melanoma. We now report 3-year overall survival outcomes in this trial.
METHODS
We randomly assigned, in a 1:1:1 ratio, patients with previously untreated advanced melanoma to receive nivolumab at a dose of 1 mg per kilogram of body weight plus ipilimumab at a dose of 3 mg per kilogram every 3 weeks for four doses, followed by nivolumab at a dose of 3 mg per kilogram every 2 weeks; nivolumab at a dose of 3 mg per kilogram every 2 weeks plus placebo; or ipilimumab at a dose of 3 mg per kilogram every 3 weeks for four doses plus placebo, until progression, the occurrence of unacceptable toxic effects, or withdrawal of consent. Randomization was stratified according to programmed death ligand 1 (PD-L1) status, BRAF mutation status, and metastasis stage. The two primary end points were progression-free survival and overall survival in the nivolumab-plus-ipilimumab group and in the nivolumab group versus the ipilimumab group.
RESULTS
At a minimum follow-up of 36 months, the median overall survival had not been reached in the nivolumab-plus-ipilimumab group and was 37.6 months in the nivolumab group, as compared with 19.9 months in the ipilimumab group (hazard ratio for death with nivolumab plus ipilimumab vs. ipilimumab, 0.55 [P<0.001]; hazard ratio for death with nivolumab vs. ipilimumab, 0.65 [P<0.001]). The overall survival rate at 3 years was 58% in the nivolumab-plus-ipilimumab group and 52% in the nivolumab group, as compared with 34% in the ipilimumab group. The safety profile was unchanged from the initial report. Treatment-related adverse events of grade 3 or 4 occurred in 59% of the patients in the nivolumab-plus-ipilimumab group, in 21% of those in the nivolumab group, and in 28% of those in the ipilimumab group.
CONCLUSIONS
Among patients with advanced melanoma, significantly longer overall survival occurred with combination therapy with nivolumab plus ipilimumab or with nivolumab alone than with ipilimumab alone. (Funded by Bristol-Myers Squibb and others; CheckMate 067 ClinicalTrials.gov number, NCT01844505.)
The Treatment of Advanced Melanoma has improved considerably over the past 6 years. Ipilimumab, an anti–cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) fully human monoclonal antibody, was the first systemic therapy to show prolonged overall survival among patients with advanced melanoma in randomized, controlled, phase 3 trials.1,2 A pooled analysis of data from 12 studies of ipilimumab in advanced melanoma showed a 3-year rate of overall survival of 26% among previously untreated patients and survival up to 10 years among approximately 20% of all patients.3 The anti–programmed death 1 (PD-1) agents nivolumab and pembrolizumab have shown superior overall survival, progression-free survival, and objective response rate, with a better safety profile, than ipilimumab alone.4–6 In the phase 3 KEYNOTE-006 trial involving patients with advanced melanoma, the overall survival rate at 33 months was 50% in the pembrolizumab group, as compared with 39% in the ipilimumab group.6
Combination therapy with nivolumab plus ipilimumab has shown consistent efficacy in patients with advanced melanoma. At a follow-up of more than 33 months in a phase 1 dose-finding study, nivolumab plus ipilimumab resulted in an overall survival rate at 3 years of 68% among patients with advanced melanoma, regardless of whether they had received treatment previously.7 Results from the randomized phase 2 CheckMate 069 trial involving previously untreated patients8 showed a rate of overall survival at 2 years of 64% in the group that received combination therapy and 54% in the group that received ipilimumab alone.9 Previously reported data from the phase 3 CheckMate 067 trial involving patients with previously untreated advanced melanoma showed significantly longer progression-free survival and higher rates of objective response with nivolumab plus ipilimumab and with nivolumab alone than with ipilimumab alone.4,10 In this article, we provide the first analysis of 3-year overall survival data from the CheckMate 067 trial.
METHODS
PATIENTS
Adult patients with previously untreated, histologically confirmed stage III (unresectable) or stage IV melanoma, with known BRAF V600 mutation status, and with an Eastern Cooperative Oncology Group performance-status score of 0 or 1 (on a 5-point scale, with higher scores indicating greater disability) were included in the trial. The full list of eligibility criteria has been reported previously.4
TRIAL DESIGN AND TREATMENT
In this double-blind, phase 3 trial, patients were randomly assigned in a 1:1:1 ratio to receive one of the following regimens: nivolumab at a dose of 1 mg per kilogram of body weight every 3 weeks plus ipilimumab at a dose of 3 mg per kilogram every 3 weeks for four doses, followed by nivolumab at a dose of 3 mg per kilogram every 2 weeks; nivolumab at a dose of 3 mg per kilogram every 2 weeks (plus ipilimumab-matched placebo); or ipilimumab at a dose of 3 mg per kilogram every 3 weeks for four doses (plus nivolumab-matched placebo).4 Randomization was stratified according to BRAF mutation status, metastasis stage, and programmed cell death ligand 1 (PD-L1) status. Treatment was continued until disease progression, the development of unacceptable toxic events, or withdrawal of consent. Patients with clinical benefit and without substantial adverse events could be treated beyond progression according to the investigator’s decision.
The two primary end points were progression-free survival and overall survival, as compared between the nivolumab-plus-ipilimumab group or the nivolumab monotherapy group and the ipilimumab monotherapy group. Secondary end points were the assessment of the objective response rate; descriptive evaluations of overall survival, progression-free survival, and the objective response rate between the nivolumab-plus-ipilimumab group and the nivolumab monotherapy group; and the evaluation of tumor PD-L1 expression as a predictive biomarker for progression-free survival and overall survival.
ASSESSMENTS
Overall survival was defined as the time from randomization to death, progression-free survival as the time from randomization to the first documented disease progression or death (whichever occurred first), and the objective response rate as the proportion of patients with a best overall response of partial or complete response. Tumor response was assessed by the investigators according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, at 12 weeks after randomization and then every 12 weeks until progression or the discontinuation of treatment. Patients were followed for survival every 3 months until death, loss to follow-up, or withdrawal from the trial. Expression of PD-L1 on the surface of tumor cells was assessed in a central laboratory by means of immunohistochemical testing (Dako North America), as described previously.4 Adverse events and select adverse events (i.e., those with a potential immunologic cause) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
TRIAL OVERSIGHT
The protocol and amendments for this trial (available with the full text of this article at NEJM.org) were reviewed by the institutional review board at each trial site. The trial was conducted in accordance with the provisions of the Declaration of Helsinki and with Good Clinical Practice guidelines as defined by the International Conference on Harmonisation. All the patients provided written informed consent before enrollment.
The trial was designed by the senior academic authors and the sponsor, Bristol-Myers Squibb. Data were collected by the sponsor and analyzed in collaboration with the authors. The authors vouch for the accuracy and completeness of the analyses and data reported and also confirm adherence to the protocol. The initial manuscript was written in collaboration with the first author and last two authors, and all the authors contributed to subsequent drafts and provided final approval to submit the manuscript for publication. Professional medical writing and editorial assistance were paid for by the sponsor.
A data and safety monitoring committee provided oversight to assess the risk–benefit profile of nivolumab combined with ipilimumab. The data and safety monitoring committee recommended the continuation of the trial without modification after each of the six formal meetings that were held during the trial. The committee had a final meeting on November 22, 2016, after the database lock, because the trial met the primary end point regarding overall survival.
STATISTICAL ANALYSIS
Efficacy end points were based on the intention-to-treat population. We calculated that the enrollment of approximately 915 patients would be required for the analysis of the two primary end points of progression-free survival and overall survival at an alpha level of 0.01 and 0.04, respectively. Formal analyses of the two primary end points were conducted at different prespecified time points. The primary analysis of progression-free survival occurred after all the patients had 9 months of follow-up.4 The primary analysis of overall survival was to occur at 28 months of follow-up, with 644 deaths being anticipated to provide the trial with approximately 99% power to detect a hazard ratio of 0.65 for each nivolumab-containing group versus the ipilimumab group. The actual number of observed events at the primary analysis of overall survival (467 deaths) was lower than anticipated but still provided the trial with more than 95% power to detect a difference between each nivolumab-containing group and the ipilimumab group, with adjustment for multiple comparisons. Hochberg’s procedure11 was applied to address multiple comparisons and to control the overall type I error rate at 0.04 in the analysis of overall survival at 28 months; a Bonferroni adjustment was used for the two treatment comparisons, resulting in 98% confidence intervals for the comparisons in the primary analysis of overall survival. A descriptive update of overall survival, progression-free survival, and the objective response rate with confidence intervals reported at the 95% level (without formal hypothesis testing adjusted for multiple comparisons) was subsequently performed at a longer follow-up at 36 months. Confidence intervals for objective response rates were calculated with the use of the Clopper–Pearson method. The trial design was not powered for a comparison between the nivolumab-plus-ipilimumab group and the nivolumab group, but descriptive analyses without formal hypothesis testing were performed. Additional statistical methods are described in the Supplementary Appendix, available at NEJM.org.
RESULTS
PATIENTS AND TREATMENT
This trial was conducted at 137 sites in 21 countries, and 1296 patients were enrolled from July 2013 through March 2014. Of the 945 patients who underwent randomization (314 patients in the nivolumab-plus-ipilimumab group, 316 in the nivolumab group, and 315 in the ipilimumab group), 8 did not receive treatment (Fig. S1 in the Supplementary Appendix). The demographic and clinical characteristics of the patients, which have been reported previously,4 were generally balanced (Table S1 in the Supplementary Appendix).
At the database lock on May 24, 2017, all living patients had a minimum follow-up of 36 months. The median follow-up was 38.0 months in the nivolumab-plus-ipilimumab group, 35.7 months in the nivolumab group, and 18.6 months in the ipilimumab group. Patients who had been assigned to receive combination therapy received a median of 4 doses (range, 1 to 94) of nivolumab and 4 doses (range, 1 to 4) of ipilimumab, those assigned to the nivolumab group received a median of 15 doses (range, 1 to 94), and those assigned to the ipilimumab group received a median of 4 doses (range, 1 to 4). Patients who were treated beyond disease progression included 62 of 313 patients (20%) in the nivolumab-plus-ipilimumab group, 97 of 313 (31%) in the nivolumab group, and 108 of 311 (35%) in the ipilimumab group.
Subsequent systemic therapy was administered in 32% of the patients in the nivolumab-plus-ipilimumab group, 46% of those in the nivolumab group, and 63% of those in the ipilimumab group (Table S2 in the Supplementary Appendix). The most common subsequent systemic therapies were BRAF inhibitors in the nivolumab-plus-ipilimumab group (13% of patients), anti–CTLA-4 agents in the nivolumab group (28%), and anti–PD-1 agents in the ipilimumab group (43%). In an analysis that excluded patients who had died and had not received subsequent therapy, the median time to subsequent systemic therapy was not reached in the nivolumab-plus-ipilimumab group (258 patients) and was 25.5 months in nivolumab group (273 patients) and 8.1 months in the ipilimumab group (267 patients). The percentages of patients who were free from subsequent therapy at 3 years were 59% in the nivolumab-plus-ipilimumab group, 45% in the nivolumab group, and 20% in the ipilimumab group.
EFFICACY
In the updated analysis, patients in the two nivolumab-containing groups continued to have higher rates of objective response than those who received ipilimumab: the rate of objective response was 58% in the nivolumab-plus-ipilimumab group and 44% in the nivolumab group, as compared with 19% in the ipilimumab group (Table 1). The rate of complete response was 19% in the nivolumab-plus-ipilimumab group and 16% in the nivolumab group, as compared with 5% in the ipilimumab group. The median duration of response was not reached in either nivolumab-containing group and was 19.3 months in the ipilimumab group.
Table 1.
Response to Treatment.*
| Variable | Nivolumab plus Ipilimumab (N = 314) |
Nivolumab (N = 316) |
Ipilimumab (N = 315) |
|---|---|---|---|
| Best overall response — no. (%)† | |||
| Complete response | 61 (19) | 52 (16) | 16 (5) |
| Partial response | 122 (39) | 88 (28) | 43 (14) |
| Stable disease | 38 (12) | 31 (10) | 69 (22) |
| Progressive disease | 74 (24) | 121 (38) | 159 (50) |
| Unable to determine | 19 (6) | 24 (8) | 28 (9) |
| Objective response‡ | |||
| No. of patients with response | 183 | 140 | 59 |
| % of patients (95% CI) | 58 (53–64) | 44 (39–50) | 19 (15–24) |
| Estimated odds ratio (95% CI)§ | 6.46 (4.45–9.38) | 3.57 (2.48–5.15) | — |
| P value | <0.001 | <0.001 | — |
| Median duration of response (95% CI) — mo | NR | NR (36.3–NR) | 19.3 (8.3–NR) |
NR denotes not reached.
The best overall response was assessed by the investigator according to the Response Evaluation Criteria in Solid Tumors, version 1.1.
Data included patients with a complete response and those with a partial response. The calculation of the 95% confidence interval was based on the Clopper–Pearson method.
The comparison is with the ipilimumab group.
The median progression-free survival was 11.5 months (95% confidence interval [CI], 8.7 to 19.3) in the nivolumab-plus-ipilimumab group and 6.9 months (95% CI, 5.1 to 9.7) in the nivolumab group, as compared with 2.9 months (95% CI, 2.8 to 3.2) in the ipilimumab group (Fig. 1A). The hazard ratio for progression or death was 0.43 (95% CI, 0.35 to 0.52) with nivolumab plus ipilimumab versus ipilimumab (P<0.001) and was 0.55 (95% CI, 0.45 to 0.66) with nivolumab versus ipilimumab (P<0.001). The rate of progression-free survival at 3 years was 39% in the nivolumab-plus-ipilimumab group and 32% in the nivolumab group, as compared with 10% in the ipilimumab group. In a descriptive analysis, the hazard ratio for progression or death was 0.78 (95% CI, 0.64 to 0.96) with nivolumab plus ipilimumab versus nivolumab. Among patients whose disease progressed, 56 of 74 (76%) in the nivolumab-plus-ipilimumab group, 90 of 121 (74%) in the nivolumab group, and 120 of 159 (75%) in the ipilimumab group had at least one new lesion, including 8 of 74 patients (11%), 21 of 121 (17%), and 23 of 159 (14%), respectively, who had a new lesion in the central nervous system (Table S3 in the Supplementary Appendix).
Figure 1. Kaplan–Meier Estimates of Survival.

Panel A shows the Kaplan–Meier estimates of progression-free survival as assessed by the investigator. Patients were followed for a minimum of 36 months (dashed line). The median progression-free survival was 11.5 months (95% CI, 8.7 to 19.3) in the nivolumab-plus-ipilimumab group and 6.9 months (95% CI, 5.1 to 9.7) in the nivolumab group, as compared with 2.9 months (95% CI, 2.8 to 3.2) in the ipilimumab group. The rate of progression-free survival at 2 years was 43% in the nivolumab-plus-ipilimumab group and 37% in the nivolumab group, as compared with 12% in the ipilimumab group. The 3-year rate of progression-free survival was 39% in the nivolumab-plus-ipilimumab group and 32% in the nivolumab group, as compared with 10% in the ipilimumab group. Panel B shows the Kaplan–Meier estimates of overall survival. The median overall survival was not reached in the nivolumab-plus-ipilimumab group and was 37.6 months (95% CI, 29.1 to not reached) in the nivolumab group and 19.9 months (95% CI, 16.9 to 24.6) in the ipilimumab group. The overall survival rate at 2 years was 64% in the nivolumab-plus-ipilimumab group and 59% in the nivolumab group, as compared with 45% in the ipilimumab group. The 3-year rate of overall survival was 58% in the nivolumab-plus-ipilimumab group and 52% in the nivolumab group, as compared with 34% in the ipilimumab group. Symbols (tick marks, triangles, and circles) indicate censored data.
In the protocol-specified primary analysis of overall survival at a minimum follow-up of 28 months, in which the actual number of deaths was 467 (as compared with the 644 deaths anticipated), the two nivolumab-containing groups had significantly longer survival than the ipilimumab group. The rate of overall survival at 2 years was 64% in the nivolumab-plus-ipilimumab group and 59% in the nivolumab group, as compared with 45% in the ipilimumab group (hazard ratio for death with nivolumab plus ipilimumab vs. ipilimumab, 0.55 [98% CI, 0.42 to 0.72; P<0.001]; hazard ratio for death with nivolumab vs. ipilimumab, 0.63 [98% CI, 0.48 to 0.81; P<0.001]). In this 3-year analysis, the rate of overall survival at 3 years was 58% in the nivolumab-plus-ipilimumab group and 52% in the nivolumab group, as compared with 34% in the ipilimumab group. The median overall survival was not reached in the nivolumab-plus-ipilimumab group (95% CI, 38.2 months to not reached) but was reached in the nivolumab group (37.6 months; 95% CI, 29.1 to not reached) and the ipilimumab group (19.9 months; 95% CI, 16.9 to 24.6) (Fig. 1B). The hazard ratio for death with nivolumab plus ipilimumab versus ipilimumab was 0.55 (95% CI, 0.45 to 0.69; P<0.001) and with nivolumab versus ipilimumab was 0.65 (95% CI, 0.53 to 0.80; P<0.001). In a descriptive analysis, the hazard ratio for death with nivolumab plus ipilimumab versus nivolumab was 0.85 (95% CI, 0.68 to 1.07).
Numerically higher rates of progression-free survival and overall survival with the combination therapy over nivolumab monotherapy was seen in the majority of clinically relevant subgroups, including patients with BRAF mutations, those with stage M1c disease, and those with an elevated level of lactate dehydrogenase (Fig. 2). Survival outcomes favored the nivolumab-containing groups over the ipilimumab group in all the subgroups that were evaluated (Fig. S2 in the Supplementary Appendix).
Figure 2. (facing page). Subgroup Analysis of Overall Survival and Progression-free Survival at 3 Years.

Shown are descriptive subgroup analyses of overall survival and progression-free survival in prespecified subgroups of patients who received combination therapy with nivolumab plus ipilimumab (Nivo+Ipi), as compared with nivolumab monotherapy (Nivo). Results are expressed as unadjusted hazard ratios in the analyses of progression-free survival (hazard ratio for progression, relapse, or death) and overall survival (hazard ratio for death) among patients who received combination therapy with nivolumab plus ipilimumab, as compared with nivolumab monotherapy; the 3-year rates of overall survival and progression-free survival are also shown. Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability; a score of 0 indicates no symptoms, and 1 mild symptoms. LDH denotes lactate dehydrogenase, PD-L1 programmed death ligand 1, and ULN upper limit of the normal range.
In the two nivolumab-containing groups, the median overall survival was not reached among patients with BRAF mutations, and the rate of overall survival at 3 years was 68% in the nivolumab-plus-ipilimumab group and 56% in the nivolumab group (Fig. 3A). In a descriptive analysis, the hazard ratio for death with nivolumab plus ipilimumab versus nivolumab was 0.69 (95% CI, 0.44 to 1.07). Among patients without BRAF mutations, the median overall survival was reached in all three treatment groups (Fig. 3B).
Figure 3. Overall Survival among Patients with or without BRAF Mutations.
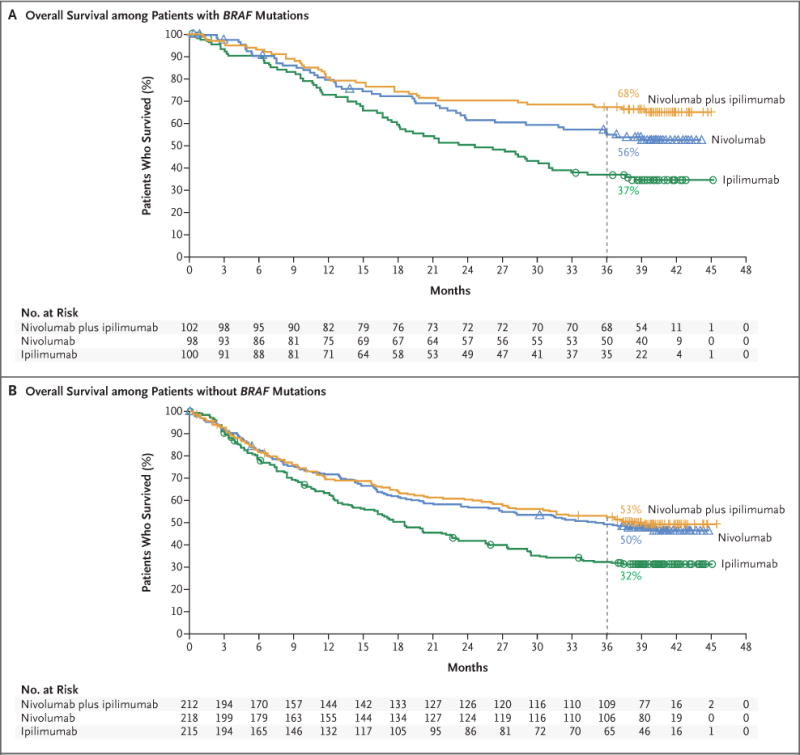
Panel A shows the Kaplan–Meier estimates of overall survival among patients with BRAF mutations. Patients were followed for a minimum of 36 months (dashed line). The median overall survival was not reached in either nivolumab-containing group and was 24.6 months (95% CI, 17.9 to 31.0) in the ipilimumab group. Symbols (tick marks, triangles, and circles) indicate censored data. Panel B shows the Kaplan–Meier estimates of overall survival among patients without BRAF mutations. The median overall survival was 39.1 months (95% CI, 27.6 to not reached) in the nivolumab-plus-ipilimumab group and 35.8 months (95% CI, 25.8 to not reached) in the nivolumab group, as compared with 18.5 months (95% CI, 14.1 to 22.7) in the ipilimumab group.
Additional analyses were performed to investigate efficacy according to the tumor PD-L1 expression level. Descriptive comparisons between the two nivolumab-containing groups suggest that as the data become more mature, better survival outcomes may be obtained with combination therapy than with monotherapy in patients with a lower tumor PD-L1 expression level (Fig. 2). However, overall survival was similar between the nivolumab-plus-ipilimumab group and the nivolumab group among patients with a tumor PD-L1 expression level of 1% or more or a level of 5% or more (Fig. 2, and Fig. S3 in the Supplementary Appendix). The overall response rate was higher in the nivolumab-plus-ipilimumab group than in the nivolumab group at each tumor PD-L1 expression level tested (Table S4 in the Supplementary Appendix). Time-dependent receiver-operating-characteristic (ROC) curves were generated for overall survival outcomes at 3 years in order to assess the PD-L1 expression level that was associated with the longest survival. Area-under-the-curve values, as compared with 0.5 (the line of no discrimination), were 0.56 (95% CI, 0.49 to 0.63) in the nivolumab-plus-ipilimumab group (P = 0.09) and 0.57 (95% CI, 0.50 to 0.63) in the nivolumab group (P = 0.04), which indicate that the level of tumor PD-L1 expression alone is a poor predictive biomarker of overall survival (Fig. S4 in the Supplementary Appendix).12,13
SAFETY
Adverse events that were considered by the investigators to be related to treatment were reported in 96% of the patients treated with combination therapy, 86% of those treated with nivolumab, and 86% of those treated with ipilimumab; adverse events of grade 3 or 4 occurred in 59%, 21%, and 28%, respectively (Table 2, and Table S5 in the Supplementary Appendix). Treatment-related adverse events of any grade that led to the discontinuation of therapy occurred more frequently with combination therapy than with either monotherapy (Table 2). Among 123 of 313 patients (39.3%) who received a median of three doses of combination therapy and who discontinued treatment owing to treatment-related adverse events, 67% were still alive at 3 years. Select adverse events (i.e., those with a potential immunologic cause) of any grade occurred with a similar frequency in each cohort, as previously reported for the respective group (Table S6 in the Supplementary Appendix).4 The most common select adverse events of any grade were skin-related events, which occurred in 62% of the patients who received combination therapy, in 46% of those who received nivolumab monotherapy, and in 56% of those who received ipilimumab monotherapy. The most common select adverse events of grade 3 or 4 were gastrointestinal events, which occurred in 15% of the patients who received combination therapy, in 4% of those who received nivolumab monotherapy, and in 12% of those who received ipilimumab monotherapy (specifically, diarrhea in 9%, 3%, and 6% of the patients, respectively). Except for endocrine adverse events that may have led to long-term hormone therapy, most treatment-related select adverse events of grade 3 or 4 resolved within 3 to 4 weeks (Table S7 in the Supplementary Appendix).
Table 2.
Treatment-Related Adverse Events.*
| Event | Nivolumab plus Ipilimumab (N = 313) |
Nivolumab (N = 313) |
Ipilimumab (N = 311) |
|||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| number of patients with event (percent) | ||||||
| Any treatment-related adverse event | 300 (96) | 184 (59) | 270 (86) | 67 (21) | 268 (86) | 86 (28) |
| Rash | 93 (30) | 10 (3) | 72 (23) | 1 (<1) | 68 (22) | 5 (2) |
| Pruritus | 112 (35) | 6 (2) | 67 (21) | 1 (<1) | 113 (36) | 1 (<1) |
| Vitiligo | 28 (9) | 0 | 29 (9) | 1 (<1) | 16 (5) | 0 |
| Maculopapular rash | 38 (12) | 6 (2) | 15 (5) | 2 (1) | 38 (12) | 1 (<1) |
| Fatigue | 119 (38) | 13 (4) | 114 (36) | 3 (1) | 89 (29) | 3 (1) |
| Asthenia | 30 (10) | 1 (<1) | 25 (8) | 1 (<1) | 17 (5) | 2 (1) |
| Pyrexia | 60 (19) | 2 (1) | 21 (7) | 0 | 21 (7) | 1 (<1) |
| Diarrhea | 142 (45) | 29 (9) | 67 (21) | 9 (3) | 105 (34) | 18 (6) |
| Nausea | 88 (28) | 7 (2) | 41 (13) | 0 | 51 (16) | 2 (1) |
| Vomiting | 48 (15) | 7 (2) | 22 (7) | 1 (<1) | 24 (8) | 1 (<1) |
| Abdominal pain | 26 (8) | 1 (<1) | 18 (6) | 0 | 28 (9) | 2 (1) |
| Colitis | 40 (13) | 26 (8) | 7 (2) | 3 (1) | 35 (11) | 24 (8) |
| Headache | 35 (11) | 2 (1) | 24 (8) | 0 | 25 (8) | 1 (<1) |
| Arthralgia | 43 (14) | 2 (1) | 31 (10) | 1 (<1) | 22 (7) | 0 |
| Increased lipase level | 44 (14) | 34 (11) | 27 (9) | 14 (4) | 18 (6) | 12 (4) |
| Increased amylase level | 26 (8) | 9 (3) | 20 (6) | 6 (2) | 15 (5) | 4 (1) |
| Increased aspartate aminotransferase level | 51 (16) | 19 (6) | 14 (4) | 3 (1) | 12 (4) | 2 (1) |
| Increased alanine aminotransferase level | 60 (19) | 27 (9) | 13 (4) | 4 (1) | 12 (4) | 5 (2) |
| Decreased weight | 19 (6) | 0 | 10 (3) | 0 | 4 (1) | 1 (<1) |
| Hypothyroidism | 53 (17) | 1 (<1) | 33 (11) | 0 | 14 (5) | 0 |
| Hyperthyroidism | 35 (11) | 3 (1) | 14 (4) | 0 | 3 (1) | 0 |
| Hypophysitis | 23 (7) | 5 (2) | 2 (1) | 1 (<1) | 12 (4) | 5 (2) |
| Decreased appetite | 60 (19) | 4 (1) | 36 (12) | 0 | 41 (13) | 1 (<1) |
| Cough | 25 (8) | 0 | 19 (6) | 2 (1) | 15 (5) | 0 |
| Dyspnea | 36 (12) | 3 (1) | 19 (6) | 1 (<1) | 12 (4) | 0 |
| Pneumonitis | 22 (7) | 3 (1) | 5 (2) | 1 (<1) | 5 (2) | 1 (<1) |
| Treatment-related adverse event leading to discontinuation | 123 (39) | 95 (30) | 37 (12) | 24 (8) | 49 (16) | 43 (14) |
Shown are treatment-related adverse events of any grade that occurred in more than 5% of the patients in any treatment group who had one or more treatment-related adverse events of grade 3 or 4. The relatedness of the adverse event to treatment was determined by the investigators. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Two deaths that were considered by the investigators to be related to a study drug occurred in the nivolumab group (neutropenia) and in the ipilimumab group (colonic perforation) within 100 days after the last dose of study drug; two additional deaths in the nivolumab-plus-ipilimumab group (one due to cardiac insufficiency and autoimmune myocarditis, and one due to liver necrosis) that were considered by the investigator to be related to a study drug were reported more than 100 days after the last dose of study drug.
As previously reported,4 two deaths related to a study drug (as assessed by the investigator) occurred within 100 days after the last dose of study drug: one death due to neutropenia occurred in the nivolumab group, and one due to colon perforation in the ipilimumab group. Two previously unreported deaths in the nivolumab-plus-ipilimumab group that were considered by the investigator to be related to a study drug were reported more than 100 days after the last dose of study drug. A 72-year-old man with a history of heart disease died on day 589 (433 days after receipt of the last dose) owing to cardiac insufficiency and autoimmune myocarditis, approximately 2 months after receiving a single dose of anti–PD-1 therapy outside the context of the trial, and a 69-year-old woman died on day 735 (234 days after receipt of the last dose) owing to liver necrosis, after she had grade 3 elevations in liver-enzyme levels.
DISCUSSION
This analysis from the CheckMate 067 trial showed that combination therapy with nivolumab plus ipilimumab and monotherapy with nivolumab resulted in significantly longer overall survival than ipilimumab monotherapy among patients with previously untreated advanced melanoma. The trial was not powered to compare the two nivolumab-containing groups directly; however, in descriptive analyses, the combination therapy resulted in a numerically higher rate of overall survival at 3 years than nivolumab alone. The median overall survival has now been reached in the nivolumab group but was still not reached in the nivolumab-plus-ipilimumab group after 36 months of follow-up. In a finding consistent with the initial report,4 the response rate remained numerically higher in the nivolumab-plus-ipilimumab group than in the nivolumab group.
Current evidence has shown that anti–PD-1 therapy produces survival outcomes superior to ipilimumab therapy. In the KEYNOTE-006 trial, at a median overall survival of 32.3 months (95% CI, 24.5 to not reached), the rate of overall survival at 33 months was 50% in the pembrolizumab group and 39% in the ipilimumab group.6 In the current analysis of the CheckMate 067 trial, the median overall survival in the nivolumab group was 37.6 months (95% CI, 29.1 to not reached), with 52% of the patients in the nivolumab group alive at 3 years, as compared with 34% of those in the ipilimumab group. In these two trials, patients whose disease had progressed during ipilimumab therapy could have received subsequent anti–PD-1 agents. These results indicate similar survival outcomes with regard to the use of anti–PD-1 agents as monotherapy. The survival rate at 3 years was 58% among patients in the nivolumab-plus-ipilimumab group. A much lower percent-age of patients in the nivolumab-plus-ipilimumab group than patients in the nivolumab group or the ipilimumab group had received a second-line therapy at 3 years, a finding that needs to be factored into cost considerations of these therapies.
Although the trial was not designed to compare the nivolumab-containing groups, the results regarding survival appeared to slightly favor combination therapy over monotherapy across clinically relevant subgroups, including subgroups of patients with a tumor PD-L1 expression level of less than 5% or less than 1%, with BRAF mutations, and with elevated lactate dehydrogenase levels. The results we observed according to BRAF mutation status showed 3-year rates of 68% for overall survival and 40% for progression-free survival among patients with BRAF mutations who received nivolumab plus ipilimumab. In the phase 3 COMBI-d trial involving patients with BRAF V600E or V600K–mutant metastatic melanoma, 3-year rates of 44% for overall survival and 22% for progression-free survival were reported with the combination of dabrafenib plus trametinib.14 To our knowledge, no trials have directly compared the combination of nivolumab plus ipilimumab with dabrafenib plus trametinib in patients with melanoma and BRAF mutations.
Combination therapy resulted in a higher rate of objective response than nivolumab alone regardless of the tumor PD-L1 expression level. Data from a phase 1b study, CA209-038, showed that tumor PD-L1 expression is up-regulated to a greater extent with combination treatment than with nivolumab alone, concomitant with a greater increase in interferon-γ, CXCL9, and CXCL10 expression in the tumor microenvironment.15 The ROC-curve analyses did not identify a threshold of tumor PD-L1 expression for the discrimination of a difference in overall survival, which suggests that the tumor PD-L1 expression level alone may not be a definitive predictive biomarker of outcomes in patients with advanced melanoma. In an analysis involving patients with a PD-L1 expression level of less than 1%, the separation of the overall survival curves between the nivolumab-containing groups suggests that this biomarker may be a starting point for discussions with patients and families regarding the risk–benefit profile of combination therapy. In patients with a tumor PD-L1 expression level of 1% or more, the use of nivolumab monotherapy may be warranted after a careful discussion of the risks and benefits of combination therapy.
As previously reported, the incidence of treatment-related adverse events was higher with combination therapy than with nivolumab or ipilimumab alone.4,10 The types and frequencies of events were consistent with previous results, and no new types of events occurred. Most immune-mediated adverse events resolved within 3 to 4 weeks when well-established safety guidelines (involving the use of immune-modulating agents as appropriate) were followed. These treatment guidelines require the close monitoring of immune-related adverse events with an experienced team of health care providers to care for the patients and to provide detailed education to patients. The discontinuation of therapy due to adverse events did not appear to compromise benefit with the combination therapy, because 67% of these patients were alive at 3 years despite having received three courses of treatment.
In conclusion, long-term survival outcomes occurred with nivolumab plus ipilimumab combination therapy and with nivolumab alone in patients with previously untreated advanced melanoma. Survival outcomes with either nivolumab-containing regimen were superior to those with ipilimumab alone.
Supplementary Material
Acknowledgments
Supported by Bristol-Myers Squibb, by a grant (P30CA008748, to Dr. Wolchok) from the National Cancer Institute, and by the NIHR Royal Marsden–Institute of Cancer Research Biomedical Research Centre (to Dr. Larkin).
We thank the patients and investigators who participated in the CheckMate 067 trial, and Ward A. Pedersen, Ph.D., Melissa Kirk, Ph.D., and Cara Hunsberger, M.S., of StemScientific for medical writing and editorial assistance with an earlier version of the manuscript.
APPENDIX
The authors’ full names and academic degrees are as follows: Jedd D. Wolchok, M.D., Ph.D., Vanna Chiarion-Sileni, M.D., Rene Gonzalez, M.D., Piotr Rutkowski, M.D., Ph.D., Jean-Jacques Grob, M.D., C. Lance Cowey, M.D., Christopher D. Lao, M.D., M.P.H., John Wagstaff, M.D., Dirk Schadendorf, M.D., Pier F. Ferrucci, M.D., Michael Smylie, M.D., Reinhard Dummer, M.D., Andrew Hill, M.D., David Hogg, M.D., John Haanen, M.D., Matteo S. Carlino, M.D., Oliver Bechter, M.D., Ph.D., Michele Maio, M.D., Ph.D., Ivan Marquez-Rodas, M.D., Ph.D., Massimo Guidoboni, M.D., Grant McArthur, M.D., Celeste Lebbé, M.D., Ph.D., Paolo A. Ascierto, M.D., Georgina V. Long, M.B., B.S., Ph.D., Jonathan Cebon, M.B., B.S., Ph.D., Jeffrey Sosman, M.D., Michael A. Postow, M.D., Margaret K. Callahan, M.D., Ph.D., Dana Walker, M.D., M.S.C.E., Linda Rollin, Ph.D., Rafia Bhore, Ph.D., F. Stephen Hodi, M.D., and James Larkin, F.R.C.P., Ph.D.
From the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York (J.D.W., M.A.P., M.K.C.); Oncology Institute of Veneto Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Padua (V.C.-S.), European Institute of Oncology, Milan (P.F.F.), Center for Immuno-Oncology, University Hospital of Siena, Istituto Toscano Tumori, Siena (M.M.), the Immunotherapy and Somatic Cell Therapy Unit, IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Meldola (M.G.), and Istituto Nazionale Tumori Fondazione Pascale, Naples (P.A.A.) — all in Italy; University of Colorado, Denver (R.G.); Maria Sklodowska-Curie Institute–Oncology Center, Warsaw, Poland (P.R.); Aix-Marseille University, Hôpital de la Timone, Marseille (J.-J.G.), and Assistance Publique–Hôpitaux de Paris, Dermatology and Centres d’Investigation Clinique, INSERM Unité 976, Hôpital Saint-Louis, Université Paris Diderot, Paris (C.L.) — both in France; Texas Oncology–Baylor Cancer Center, Dallas (C.L.C.); University of Michigan, Ann Arbor (C.D.L.); the College of Medicine, Swansea University, Swansea (J.W.), and Royal Marsden NHS Foundation Trust, London (J.L.) — both in the United Kingdom; the Department of Dermatology, University of Essen, Essen, and the German Cancer Consortium, Heidelberg — both in Germany (D.S.); Cross Cancer Institute, Edmonton, AB (M.S.), and Princess Margaret Cancer Centre, Toronto (D.H.) — both in Canada; Universitäts Spital, Zurich, Switzerland (R.D.); Tasman Oncology Research, Southport Gold Coast, QLD (A.H.), Crown Princess Mary Cancer Centre, Melanoma Institute Australia, University of Sydney (M.S.C.), and Melanoma Institute Australia, University of Sydney, and Royal North Shore and Mater Hospitals (G.V.L.), Sydney, and Peter MacCallum Cancer Centre (G.M.) and the Olivia Newton-John Cancer Research Institute, University of Melbourne (J.C.), Melbourne, VIC — all in Australia; Netherlands Cancer Institute, Amsterdam (J.H.); University Hospitals Leuven, KU Leuven, Leuven, Belgium (O.B.); General University Hospital Gregorio Marañón, Madrid (I.M.-R.); Northwestern University, Chicago (J.S.); Bristol-Myers Squibb, Princeton, NJ (D.W., L.R., R.B.); and the Dana–Farber Cancer Institute, Boston (F.S.H.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Schachter J, et al. Long-term outcomes in patients (pts) with ipilimumab (ipi)-naïve advanced melanoma in the phase 3 KEYNOTE-006 study who completed pembrolizumab (pembro) treatment. J Clin Oncol. 2017;15(Suppl: 35) abstract. [Google Scholar]
- 7.Sznol M, Callahan MK, Kluger H, et al. Updated survival, response and safety data in a phase 1 dose-finding study (CA209-004) of concurrent nivolumab (NIVO) and ipilimumab (IPI) in advanced melanoma; Presented at the Society for Melanoma Research Annual Meeting; San Francisco. November, 18–21, 2015; abstract. [Google Scholar]
- 8.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–68. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Overall survival results from a phase III trial of nivolumab combined with ipilimumab in treatment-naïve patients with advanced melanoma (CheckMate 067); Presented at the American Association for Cancer Research Annual Meeting; Washington, DC. April 1–5, 2017; abstract. [Google Scholar]
- 11.Hochberg Y. A sharper Bonferroni procedure for multiple significance testing. Biometrika. 1988;75:800–2. [Google Scholar]
- 12.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 13.Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12:132–9. doi: 10.1111/j.1365-2753.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 14.Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017 May 5; doi: 10.1093/annonc/mdx176. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribas A, Martin-Algarra S, Bhatia S, et al. Immunomodulatory effects of nivolumab and ipilimumab in combination or nivolumab monotherapy in advanced melanoma patients: CheckMate 038; Presented at the American Association for Cancer Research Annual Meeting; Washington, DC. April 1–5, 2017; abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


