Abstract
Progress in the beginning of the 21st century transformed our perception of complement from a blood-based antimicrobial system to a global regulator of immunity and tissue homeostasis. More recent years have witnessed remarkable advances regarding structure-function insights, mechanisms and locations of complement activation, thereby adding new layers of complexity in the biology of complement. This complexity is readily reflected by the multifaceted and contextual involvement of complement-driven networks in a wide range of inflammatory and neurodegenerative disorders and cancer. This Review provides an updated view of new and previously unanticipated functions of complement and how these impact immunity and disease pathogenesis.
The whole is greater than the sum of its parts
— Aristotle
Interest in complement recently resurged owing to increasing appreciation that this phylogenetically ancient system has broad and critical functions beyond those implied by its name. The complement cascade is triggered by distinct mechanisms (classical, lectin, or alternative) that converge at the third component (C3) and lead to generation of effectors that ‘complement’ the ability of antibodies and phagocytes to clear microbial intruders (via C3b opsonization), promote inflammation (via anaphylatoxins C3a and C5a), and lyse susceptible pathogens (via the C5b-9 membrane attack complex [MAC])1. In cooperation with other immune and physiological systems, complement integrates innate and adaptive immunity, mediates the clearance of immune complexes, cellular debris and apoptotic cells, contributes to normal tissue and organ development, and promotes tissue repair after injury1. The number of known complement-associated molecules has increased with our improved understanding of complement functions. Besides the classic components (C1-C9) of the cascade, the integrated complement system overall contains some 50 proteins. These form an elaborate network of fluid-phase, cell surface-associated, and intracellular proteins, which function as pattern-recognition molecules, proteases/convertases, regulators, and signaling receptors, collectively mediating immune surveillance and tissue homeostasis2,3.
New discoveries regarding the functional versatility of complement have raised the question how a small set of proteins can provide distinct, dynamic and context-tailored responses. Advances in biophysical methods, especially cryogenic electron microscopy4, have led to an increasingly refined picture not only of individual complement components but also of intricate complexes, such as the C3 convertases or the MAC5,6. We thus begin to understand their dynamic conversions and interactions, are able to map and predict the molecular and functional consequences of genetic alterations in complement proteins, and design and rationalize therapeutic strategies.
Recent studies have shown that complement is not an exclusively liver-derived intravascular and extracellular system. Complement components can be secreted locally by tissue-resident and infiltrating cells, while emerging evidence suggests the existence of an intracellular complement system with novel homeostatic and immune functions2. New insights into the mechanisms and locations of complement activation have revealed a new layer of complexity in the biology of complement and its impact on health and disease7,8. When dysregulated or overactivated due to host genetic or microbial virulence factors, complement can turn from a homeostatic to a pathological effector that drives various inflammatory disorders and cancer, which reflect the multifaceted nature of complement interactions3,9. This Review critically summarizes our updated view of hitherto unanticipated functions of complement and its pervasive role in immunity and disease.
Complement regulation of the host response
Complement has been historically linked to regulation of humoral adaptive immunity by virtue of its capacity to lower the activation threshold of B cells that engage antigens opsonized with C3b degradation products (iC3b, C3dg) via CR2 on the B cell co-receptor complex (CR2-CD19-CD81)1,10. Subsequently, complement was shown to also regulate cell-mediated adaptive immunity11. The stimulatory effects of complement on T cell activation could, in part, be attributed to C3a or C5a regulation of antigen-presenting cell (APC) maturation and function. However, findings that C3a and C5a could be produced locally at the APC-T cell interface, where reciprocal cognate interactions appeared to upregulate the expression of anaphylatoxin receptors in both partners, suggested that complement might have direct effects on the functional co-stimulation and differentiation of naive CD4+ T cells12.
The activation of complement anaphylatoxins may not necessarily occur in the extracellular milieu and recent studies have substantiated the existence of intracellular stores of complement components and receptors (e.g., anaphylatoxin receptors and their ligands; C3b, factor B, factor H), which perform novel homeostatic and surveillance functions within human T cells7,13,14. A cell-intrinsic and functionally active complement system was anticipated more than 30 years ago by pioneering studies showing that human lymphocytes synthesize various complement components, regulators, and receptors15,16. An intracellular complement system has been observed also in fibroblasts, endothelial and epithelial cells, although the mechanisms underlying its activation and regulation and its functional outcomes are largely unexplored17. A rapid and saturable recycling pathway has been identified for C3 in lymphocytes that allows for exchange between intracellular stores and extracellular sources18.
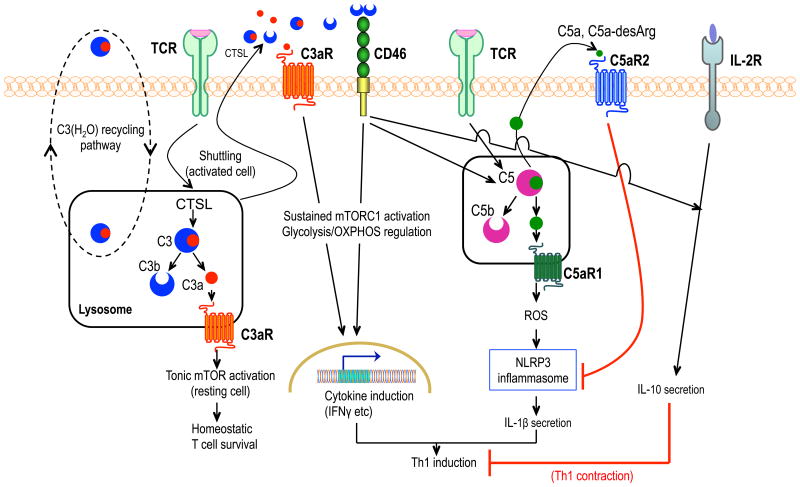
C3 can be activated tonically in human CD4+ T cells through cathepsin L cleavage into C3a and C3b. The tonically generated C3a activates its cognate receptor on lysosomes and this intracellular C3a-C3aR signaling pathway mediates homeostatic survival of T cells by maintaining low-level mTOR activity13(Fig.1). Resting CD4+ T cells do not display surface C3aR or cathepsin L. However, TCR activation and CD28 co-stimulation induces translocation of this intracellular C3 activation system (‘shuttling’) to the cell surface, where C3aR and CD46 can become activated, respectively, by C3a and C3b (Fig.1). These C3aR and CD46 signaling events upregulate growth factor receptors, cell proliferation, and secretion of predominantly TH1 but also TH17-type cytokines (interferon (IFN)-γ, TNF, IL-17) without significantly affecting TH2-type cytokines (IL-4, IL-5)13,19. Therefore, the location of complement receptor activation (intracellular vs. cell-surface) dictates the functional outcome (homeostatic survival vs. effector function).
Figure 1. Complement-mediated intracellular and autocrine regulation of CD4+ T cell activation.
Under steady-state conditions, C3(H2O) is recycling between the extracellular and intracellular space. The intracellular stores of C3(H2O) constitute a source of intracellular C3a. In resting CD4+ T cells, tonic intracellular C3a generation by cathepsin L (CTSL) cleavage activates the C3a receptor (C3aR) on lysosomes, leading to low-level mTOR activation for homeostatic T-cell survival. TCR activation with CD28 co-stimulation (not shown) causes translocation of this intracellular C3 activation system to the cell surface, where C3aR and CD46 are triggered, respectively, by C3a and C3b. The ensuing C3aR- and CD46-mediated signaling events stimulate sustained mTOR complex 1 (mTORC1) activation and reprogram glycolysis and oxidative phosphorylation in ways that support TH1 (IFN-γ) responses. In activated CD4+ T cells, CD46 costimulation triggers intracellular cleavage of C5 into C5a which induces C5aR1-dependent production of reactive oxygen species, in turn activating NLRP3 inflammasome-driven IL-1β secretion that sustains TH1 induction. IL-2R signaling together with autocrine CD46 activation induces IL-10 production, initiating transition to a TH1 contraction phase. This self-regulative activity is re-inforced by cell-surface C5aR2 signaling induced by increased levels of secreted C5a (or C5a-desArg) resulting from TH1 expansion; C5aR2 downregulates C5aR1-driven NLRP3 inflammasome activation.
Complement regulation of effector responses proceeds through metabolic reprogramming of CD4+ T cells. In activated human CD4+ T cells, autocrine stimulation of CD46 by C3b causes sustained mTORC1 activation and regulates glycolysis and oxidative phosphorylation in ways that promote induction of TH1 cells20(Fig.1). Whereas activated T cells from patients with severely reduced CD46 expression secrete significantly less IFN-γ than T cells from age- and sex-matched healthy donors, only T cells from a patient with complete lack of CD46 expression failed to produce IL-17, suggesting metabolic threshold differences involved in the induction of TH1- and TH17-type responses20. Overall, the combined signaling by CD46 and C3aR is strictly required for induction of IFN-γ-expressing TH1 cells and CD46-deficient individuals fail to elicit normal TH1 responses13,14,17. However, when the autocrine activation of CD46 occurs in combination with IL-2R signaling in TH1 cells (as when there is high concentration of local IL-2 produced during TH1 expansion), the resulting crosstalk induces IL-10 production and promotes transition to a self-regulative TH1 contraction phase21(Fig.1).
Human CD4+ T cells also contain intracellular stores of C5, although its origin (serum derived and/or intracellularly synthesized) is uncertain. TCR activation and CD46 costimulation trigger NLRP3 gene expression and intracellular activation of C5 via an unknown protease. The generated C5a induces intracellular C5aR1 activation (within an unspecified compartment), which is required for formation of reactive oxygen species and activation of NLRP3 inflammasome-driven IL-1β secretion14(Fig.1). This C5aR1-NLRP3 crosstalk preferentially induces IFN-γ production and TH1 (but not TH2 or TH17) differentiation. The finding that autocrine IL-1β promotes TH1 induction is surprising given that APC-derived IL-1β contributes to differentiation of TH17 cells22. The C5aR1-induced NLRP3 inflammasome activity is downregulated by cell-surface C5aR2, which is activated in an autocrine manner by secreted C5a14. It is currently unknown whether the underlying mechanism involves interference with C5aR1 activation or a novel C5aR2 inhibitory pathway for NLRP3 assembly. C5aR2 activation by secreted C5a (or C5adesArg), the levels of which accumulate during TH1 expansion, cooperates with CD46 and IL-2R in the transition to a TH1 contraction phase (Fig.1).
Additional complement-mediated mechanisms for inflammasome regulation were recently identified in different cell types. The ability of C5a-C5aR1 signaling to induce reactive oxygen species in mouse macrophages was associated with potentiation of NLRP3 inflammasome activation and IL-1β release23. In human monocytes, C3a-induced activation of cell-surface C3aR triggers the release of ATP into the extracellular space, thereby regulating P2X7 activation and synergizing with Toll-like receptor (TLR) signaling to activate the NLRP3 inflammasome. This results in monocyte secretion of IL-1β that in turn promotes production of IL-17 by human CD4+ T cells24. In human epithelial cells, the formation of sublytic MAC induces intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation and IL-1β release25. Similarly, sublytic C5b-9 deposition, but not C3a or C5a, activates NLRP3 inflammasome and IL-1β release in mouse dendritic cells (DCs)26. The membrane attack complex therefore appears to mimic the well-established effect of bacterial pore-forming toxins on NLRP3 inflammasome activation27. In contrast, C1q, which binds apoptotic cells and promotes their efferocytosis, suppresses NLRP3 inflammasome activation during apoptotic cell engulfment by human macrophages, thereby inhibiting their IL-1β release28.
These studies collectively show that complement has emerged as an important regulator of inflammasome activation in different cell types including lymphocytes, where optimally activated TH1 immunity requires the participation and cooperation of both C3- and C5-dependent pathways operating both intra- and extra-cellularly (Fig.1).
Complement in homeostatic immunity and microbial evasion
Microbial dysbiosis (quantitative, compositional, and functional alterations to the microbiome) is associated with the pathogenesis of polygenic and multifactorial conditions, including inflammatory, autoimmune, and metabolic diseases and cancer29. Mounting evidence suggests that complement can promote either homeostasis or dysbiosis in a contextual manner30-33. Apart from the well-characterized C3a- and C5a-induced cell modulation pathways, it was recently shown that the C4a activation fragment acts as an agonist for the protease-activated receptors-1 and -434. This novel function may not only connect complement to hemostasis and thrombosis (reviewed elsewhere - ref.35), but also suggests that what used to be thought of as the ‘third anaphylatoxin’ may actually endow complement with homeostatic activity given that protease-activated receptor-1 can mediate anti-inflammatory effects in endothelial cells36.
In the skin, complement was shown to modulate both the composition and diversity of the commensal microbiota, presumably in part through regulation of expression of antimicrobial peptides and inflammatory mediators33. Conversely, the commensal microbiota influences the basal cutaneous expression of complement genes, thus revealing a reciprocal relationship that maintains homeostatic host-microbiome interactions in the skin33. Perturbations of this homeostatic relationship promote skin inflammation. C3, which is hyperexpressed in human psoriatic epidermis, is also aberrantly upregulated in a mouse psoriasis model by calprotectin (S100A8-S100A9 complex) and drives the disease phenotype37. Complement also influences cutaneous wound healing, where C3–/– and C5ar1–/– mice exhibit an accelerated healing phenotype, suggesting that recruitment and activation of inflammatory cells to the wound might delay the healing process38.
Pathobionts (normally under-represented microorganisms that become abundant and pathogenic when homeostasis is disrupted) exploit inflammation to thrive and outcompete commensal symbionts39,40. A pathobiotic community, however, is faced with a survival conundrum: Whereas pathobionts need to evade immune clearance to persist, this cannot be achieved by suppressing the host response. This is because dysbiotic communities nutritionally rely on inflammatory tissue-breakdown molecules or other inflammatory by-products that selectively favor their metabolic activity39,40. In periodontitis, an oral dysbiotic inflammatory disease, complement is at the core of sophisticated immune subversion tactics, whereby bacteria uncouple immune clearance from the inflammatory response41. Porphyromonas gingivalis enzymatically cleaves C5 to generate high local concentrations of C5a for C5aR1 activation in neutrophils, which detect this bacterium through TLR2. This enables P. gingivalis to instigate C5aR1-TLR2 crosstalk that induces ubiquitination and proteasomal degradation of the TLR2 signaling adaptor MYD88, which is required for immune clearance of the bacteria41. Additionally, the C5aR1-TLR2 crosstalk triggers an alternate pathway wherein the TLR2 MYD88 adaptor-like protein (MAL) mediates phosphoinositide 3-kinase (PI3K) signaling, which inhibits RHOA GTPase-dependent actin polymerization and phagocytosis, while inducing a robust inflammatory response. These mechanisms promote the survival of P. gingivalis and bystander species in a nutritionally favourable inflammatory environment that is conducive for dysbiosis and disease development41,42.
In other settings, complement can suppress pathobionts. In a mouse model of Clostridium difficile-induced intestinal damage and systemic spreading of pathobionts, host resistance is mediated by IL-22, which in turn is dependent on complement32. Mechanistically, IL-22 is required for effective induction of C3 expression in the liver and intestine, which is required for efficient C3b opsonisation and phagocytosis of bacteria by neutrophils32. This study highlights a new functional connection between complement and IL-22 for host protection against systemic dissemination of pathobionts upon infection-induced damage to the intestinal barrier.
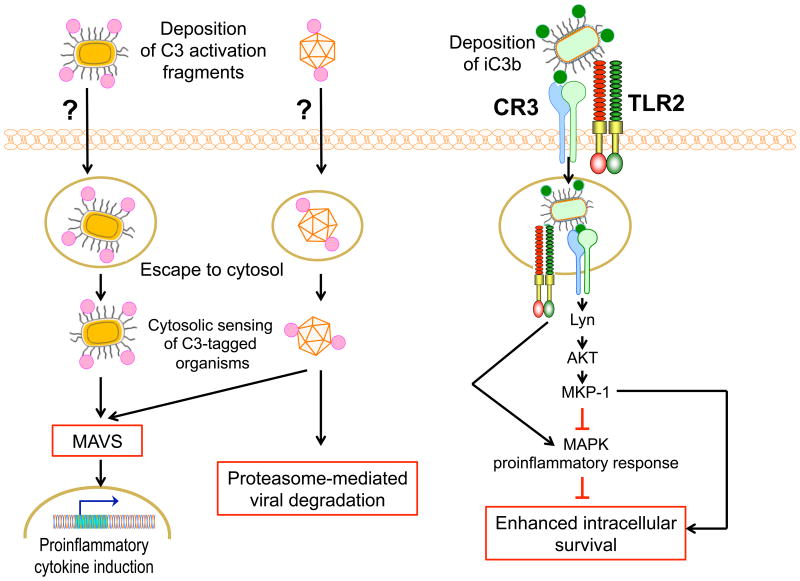
Another recent study has revealed a new C3-dependent anti-microbial mechanism that interferes with the replication of intracellular pathogens in the cytosol of non-immune cells after escaping from phagosomes43(Fig.2, left). Nonenveloped viruses and bacteria (e.g., adenovirus and Salmonella enterica serovar Typhimurium), which have been opsonized extracellularly with C3 cleavage fragments, can be sensed in the cytosol in a C3-dependent manner and trigger mitochondrial anti-viral signaling (MAVS). MAVS in turn induces pro-inflammatory cytokines through activation of several transcription factors (NF-κB, AP-1, and IFN regulatory factors-3 and -5). C3-coated viruses can be additionally restricted through proteasome-mediated viral degradation43. The identity of the opsonizing C3 split products and the uptake receptor(s) involved in these mechanisms have not been specified. Since different cell types contain intracellular C3 that may be cleaved into C3a and C3b13,18, the above-discussed cell autonomous immune mechanisms could potentially be triggered even by intracellular pathogens that have escaped extracellular C3b opsonization.
Figure 2. Differential intracellular fate of microbes opsonized by complement in the extracellular space.
(Left) Cytosolic sensing of C3-tagged microbes activates innate immunity. Certain intracellular pathogens can escape from phagosomes to replicate in the cytosol. However, nonenveloped viruses and bacteria, which were opsonized with C3 cleavage fragments in the extracellular space, can be sensed in the cytosol in a C3-dependent manner and trigger mitochondrial anti-viral signaling (MAVS) that leads to activation of innate immune responses. Cytosolic sensing of C3-opsonized viruses can additionally lead to proteasome-mediated viral degradation. The nature of the opsonizing C3 cleavage products and the uptake receptor(s) involved in this dual protective mechanism are uncertain. (Right) Immune evasion by intracellular pathogens dependent upon CR3 uptake. Several bacterial pathogens exploit CR3-mediated internalization into macrophages for enhanced intracellular persistence. For instance, CR3-mediated entry of F. tularensis induces CR3 signaling that activates Lyn kinase and AKT signaling, in turn upregulating MAP kinase phosphatase-1 (MKP-1) and thus attenuating MAP kinase-dependent pro-inflammatory responses downstream of TLR2.
The transmembrane protein V-set immunoglobulin domain containing 4 (VSIG4; aka CRIg) is a macrophage receptor for C3b and iC3b and can promote phagocytic killing of C3-opsonized pathogens44. Moreover VSIG4 was recently shown to function as a pattern-recognition receptor for lipoteichoic acid, thereby enabling Kuppfer cells to capture and eliminate circulating Gram-positive bacteria in the liver45. Another study has shown that VSIG4 can moreover induce autophagosome formation for eliminating C3-opsonized Listeria monocytogenes that has escaped from phagosomes46. This intracellular anti-evasion mechanism may not involve intracellular complement since the VSIG4 signal by itself (activated extracellularly by C3-opsonized bacteria or by agonistic antibody) is sufficient to induce autophagosome formation.
Despite the protective potential of C3-dependent cell autonomous immune mechanisms, an early study showed that the obligate intracellular parasite Leishmania major exploits C3 to enhance its intracellular survival47. In contrast to unopsonized parasites, C3-opsonized L. major could successfully establish parasitism in macrophages attributed to decreased activation of the respiratory burst47. Although the opsonizing C3 split products were not specified, it should be noted that leishmanolysin (gp63) cleaves attached C3b into iC3b. Differences in the outcomes of C3-dependent opsonophagocytosis might be attributed to several experimental variables, including the type of organisms, the use of non-immune cells vs. professional phagocytes, or the identity of complement receptors mediating pathogen entry. When bacterial pathogens enter macrophages via complement receptor-3 (CR3) (e.g., P. gingivalis, Francisella tularensis, and Bacillus anthracis spores), they enhance their intracellular persistence and capacity to cause disease in the mouse host8(Fig.2, right). The underlying mechanism was described in detail for F. tularensis, a facultative intracellular bacterium that can escape from the phagosome to replicate within the cytosol. The uptake of F. tularensis is promoted by iC3b opsonization and engagement of CR3, which activates Lyn kinase and AKT signaling, in turn increasing MAP kinase phosphatase-1 (MKP-1) expression and thereby limiting MAP kinase-dependent pro-inflammatory responses downstream of TLR248(Fig.2, right). These outcomes are consistent with the fact that CR3 mediates efferocytosis of iC3b-coated apoptotic cells, a non-phlogistic process that attenuates cellular activation including the respiratory burst1.
Besides exploiting specific complement-dependent pathways to their own advantage, pathogens have long been known to block complement activation in settings where complement could prevent infection. Mechanistically, pathogens can inhibit complement through mimicry or hijacking of complement regulatory proteins, proteolytic inactivation of key complement proteins, or secreting complement inhibitory molecules9,49. The list of evasive pathogens and their specific complement targets has greatly increased in recent years and apparently pathogen inhibition of complement can interfere with both innate and adaptive immunity9,50. Consequently, key virulence factors involved in complement evasion are currently considered as vaccine targets to promote pathogen susceptibility to complement-dependent host defenses9.
Complement in inflammatory diseases
The central role of complement in inflammatory circuits is reflected in its involvement in acute or chronic inflammatory diseases with prominent complement imbalance3,51. As innate immune senescence is growingly appreciated as a contributor to aging-related unresolved inflammation52, both the magnitude of complement responses and their spatiotemporal regulation can influence aging-related immunodegenerative phenotypes across different tissues. Recent findings have revealed involvement of complement-driven networks in chronic and aging-related inflammatory conditions ranging from cutaneous, renal and ocular to neuroinflammatory and neurodegenerative disorders3,33,53-56.
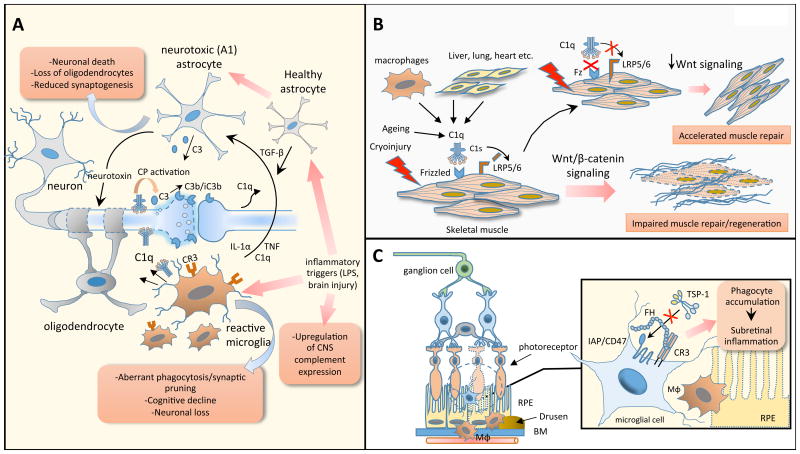
The central nervous system (CNS) deploys a distinct set of immune regulatory and effector pathways, including those mediated by locally produced complement components and resident phagocytes (i.e., microglia) to thwart both infectious and sterile inflammatory insults54. In neurodegenerative conditions, complement activation engages in a cross-talk between neuroinflammatory microglia and reactive astrocytes that fuels tissue destructive inflammation57(Fig.3a). Given that synaptic pruning in the developing mammalian brain temporally coincides with complement expression in neuronal and glial progenitors56,58, it was suggested that postnatal synaptic refinement may be influenced by innate immune pathways that can tag superfluous synaptic material for phagocytic clearance. Seminal studies revealed that C1q promotes synaptic refinement in the postnatal reticulogeniculate system59 and clearance of inappropriate synapses is mediated by CR3-expressing microglial cells that engulf C3b- or iC3b-coated synaptic surfaces59-61. This functional link between complement and developmentally regulated synaptic pruning was recapitulated in animal models of glaucoma, neurodegenerative disease and aging-related cognitive decline59,62,63.
Figure 3. Complement components implicated as drivers of chronic, non-resolving inflammation in neurodegenerative, aging-related and ocular pathologies.
(a) An aberrantly reactivated complement-microglial axis promotes neurodegenerative phenotypes in the CNS. A hallmark of several neurological disorders is the complement-driven polarization of microglia and astrocytes towards proinflammatory phenotypes (A1 astrocytes) which impair synaptogenesis and compromise neuronal survival, as depicted herein and detailed in the text. (b) C1q promotes aging-related inflammation and tissue injury. C1q triggers the canonical Wnt pathway, independently of complement activation, by binding to the Wnt receptor Frizzled (Fz) and promoting the C1s-dependent cleavage of the Wnt coreceptor, LDL receptor-related protein 6 (LRP6). C1q-dependent enhancement of Wnt signaling accelerates fibrotic changes and impairs tissue regenerative responses (e.g., muscle repair). (c) Factor H promotes subretinal inflammation in age-related macular degeneration (AMD). Under steady-state conditions, phagocytic cells are constantly eliminated in the subretinal space through a homeostatic mechanism involving the interaction of TSP-1 with integrin-associated CD47 on subretinal phagocytes. The AMD-associated FH variant (H402) potently inhibits the CD47-driven elimination of phagocytes, presumably by binding more effectively to CR3 than the non-risk isoform, thereby fueling chronic subretinal inflammation. Abbreviations: RPE, retinal pigmented epithelium; BM, Bruch's membrane; Mφ, macrophage; TSP-1, thrombospondin-1; IAP, integrin-associated protein.
Importantly, early synapse loss in the adult brain is a common hallmark preceding cognitive decline in many neurodegenerative disorders56. In these settings, a complement-microglial axis was shown to aberrantly reactivate developmental programs of synaptic pruning leading to disrupted neuronal connectivity and cognitive impairment56,64(Fig.3a). A detrimental role for C3 in promoting microglia-dependent synapse elimination was corroborated in aged C3–/– mice, which displayed attenuated synaptic pruning in the hippocampus, resulting in improved cognitive performance63. Furthermore, genetic deficiency of C3 or C3aR mitigated microglial-dependent synaptic loss and cognitive impairment in a murine model of neuroinvasive West Nile virus infection65. Importantly, enrichment of C1q and C3 in the cortex of patients with refractory epilepsy correlated with markers of aberrant microglial activity, suggesting that complement-driven loss of inhibitory synapses might account for the neuronal hyperexcitability observed in human epilepsy66.
AD, the leading cause of age-related dementia worldwide, is hallmarked by robust activation of astrocytes and microglia and complement deposition on amyloid-β plaques and tangles67,68. While early complement components (C1q, C3) have been associated with the phagocytic clearance of amyloid fibrils, terminal complement effectors (C5a, MAC) have been implicated in inflammatory neuronal damage54. Consistently, therapeutic targeting of C5aR1 ameliorated pathological indexes in different transgenic models of AD69,70. Moreover, neural synaptic loss, an early event in AD pathology preceding amyloid plaque deposition, was shown to require concerted activation of the classical pathway (CP) and CR3-mediated microglial activity71. Accordingly, C3-deficiency attenuated synapse loss and cognitive decline in a transgenic model of age-related AD pathology despite greater accumulation of cerebral Aβ plaques72. The latter finding argues for an essential role of complement in the phagocytic clearance of Aβ fibrils in the early stages of AD, while C3-dependent pathways appear to exacerbate AD neurodegeneration with age. This dual role of complement in neurodegeneration has wide therapeutic implications.
Augmented Wnt–β-catenin signaling is known to contribute to accelerated aging in mice73. C1q was recently shown to promote aging-related impairment of muscle regeneration by driving the C1s-dependent cleavage of LRP6, a Wnt co-receptor expressed on myocytes74(Fig.3b). Notably, C1q-dependent upregulation of Wnt signaling enhanced age-related fibrotic changes in the skeletal muscle of mice which could be reversed by genetic ablation of C1q or inhibition of C1s74. Since aberrant Wnt signaling is implicated in cancer and other degenerative diseases75, complement components may promote Wnt-dependent degenerative phenotypes during aging. In support of this notion, C1q was shown to promote hypertension-induced arterial remodeling through enhanced β-catenin signaling and aberrant proliferation of vascular smooth muscle cells76. Interestingly, complement downmodulates the expression of Klotho, a transmembrane co-receptor for FGF signaling that exerts pleiotropic anti-aging functions in various tissues77. Consistently, early downregulation of Klotho in a porcine model of kidney I/R injury was reversed after complement inhibition by C1-INH77.
Age-related macular degeneration (AMD) is a prevalent ocular inflammatory disease and the primary cause of blindness among the elderly worldwide53. Genome-wide association studies have identified polymorphisms in FH as key predisposing factors of AMD78,79. Certain genetic variants are associated with complement dysregulation in the subretinal space and likely contribute to unresolved inflammation, leading to irreversible loss of photoreceptors53. A recent study has dissected how the FH variant (H402Y) might promote inflammatory damage in AMD. FH binding to CR3 was shown to restrain thrombospondin-1 (TSP-1) activation of the integrin-associated CD47 receptor, which is required for homeostatic elimination of subretinal phagocytes (Fig.3c)80. Notably, the AMD-associated FH variant H402Y potentiates this inhibitory effect resulting in pathologic subretinal accumulation of phagocytes.
Complement also has an impact on diverse pathologies involving low-grade, chronic inflammation3. A study using mice deficient in C5 and downstream components implicated complement in osteoarthritis, an articular cartilage-degrading disease associated with chronic, low-grade inflammation in synovial joints81. Although the connection of complement with another chronic bone loss disorder, periodontitis, was earlier suggested by correlative clinical studies, new interventional studies in non-human primates demonstrated a causative link between C3 activation and inflammatory periodontal bone loss82,83. Furthermore, complement was recently incriminated in Gaucher disease (GD), a genetically driven lysosomal storage disorder associated with dysregulated glycolipid metabolism in various tissues84. GD is associated with chronic inflammation marked by a pronounced autoimmune response to intracellularly accumulated glucosylceramide. Robust clinical evidence and data from animal studies implicated C5a-C5aR1 signaling in a vicious cycle that fuels chronic inflammation in GD through C5aR1-driven glycolipid accumulation and modulation of glucosylceramide-specific autoantibody generation84. Finally, the kidneys are particularly susceptible to damage induced by complement-mediated inflammation, which appears to drive various renal disorders (reviewed in refs.3,55).
Collectively the above-discussed studies identify complement as a key contributor to pathologic inflammatory processes, including aging-related diseases. Regarding the latter, an emerging concept is that innate immune pathways that are homeostatically important in early development are aberrantly reactivated in an aging immune system.
Role of complement in cancer
The past decade has uncovered fundamental molecular pathways linking chronic inflammation and cancer85,86. Besides genetic and epigenetic modifications that may evoke unrestrained proliferation and death resistance of cancer cells, the tumor microenvironment is now acknowledged as key promoter of tumorigenesis by triggering and supporting local processes of inflammation, angiogenesis and metastasis87(Fig.4). Given the extensive crosstalk of complement with TLRs, inflammasomes, and GPCRs, which determine the activation status of innate and adaptive immune cells14,88,89, imbalanced complement activation in the tumor microenvironment may modulate local immunity resulting in inflammation and suppression of anti-tumor responses90.
Figure 4. Role of complement in cancer.
A complex interplay between complement effector proteins, tumor cells, and innate and adaptive immune cells in the tumor microenvironment determines tumor progression. Imbalanced complement activation in the tumor microenvironment triggers the release of proinflammatory cytokines by both tumor cells and tumor-infiltrating immune cells, such as macrophages, dendritic cells, and neutrophils, as well as immunosuppressive cytokines and reactive oxygen and nitrogen species by myeloid-derived suppressor cells (MDSCs). Local inflammation suppresses activation of effector T cells and creates a favorable environment for tumor growth. MDSCs also exert potent immunosuppressive effects that inhibit anti-tumor CD8+ T cell responses. Complement activation products, particularly C5a, promotes angiogenesis, thus facilitating tumor cell migration and adjacent tissue invasion and metastasis. Complement activation also triggers the accumulation of pro-tumorigenic neutrophils within solid tumors potentiating their procoagulant responses by releasing neutrophil extracellular traps (NETs).
Complement was shown to regulate the differentiation and recruitment of myeloid-derived suppressor cells (MDSCs) to tumor sites. MSDCs, in turn, establish an immunosuppressive environment that restrains anti-tumor T cell responses thereby allowing cancer development91,92. Studies in animal models of melanoma, intestinal, cervical and lung cancer showed that C3aR- and C5aR1-mediated pathways foster a pro-tumorigenic environment via activation and polarization of innate immune cells, release of pro-tumorigenic factors and suppression of effector T cells90,93-98. In spontaneous intestinal tumorigenesis in mice with mutation in the adenomatous polyposis coli gene, complement activation was detected locally in the intestine and also systemically upon consumption of high-fat diet94. In this model, imbalanced complement activation resulted in increased levels of pro-inflammatory cytokines and angiogenic factors, activation of the AKT-NF-κB pathway and development of intestinal polyps94. A recent study provided further support linking complement-induced inflammation and cancer by showing that the long pentraxin PTX3 suppresses mesenchymal and epithelial carcinogenesis by regulating complement-dependent chemokine production and recruitment of tumor-promoting macrophages99.
Regarding potential mechanisms of complement activation in cancer, recent studies demonstrated antibody-independent binding of C1q to phospholipids on the surface of tumor cells and generation of C5a via direct cleavage of C5 by tumor proteases100,101. Dysregulated complement activation with increased levels of C3a, C4a or C5a in the circulation has been reported in clinical settings such as hematological and solid tissue cancers90. Further, syngeneic, xenograft and genetic models of tumorigenesis in rodents indicate that imbalanced complement activation usually occurs in the tumor stroma, specifically along the vasculature, where complement activation is associated with angiogenesis and tumor growth92,94,102,103.
Complement also promotes tumor cell motility and invasiveness through induction of metalloproteinases from tumor cells, degradation of extracellular matrix and increased expression of stress fibers and filopodia104-106. C3aR signaling, for instance, is implicated in the dissemination of cancer to the nervous system by disrupting the blood-cerebrospinal fluid barrier107. Additionally, triggering of C3aR-, C5aR1- or MAC-mediated signaling activates PI3K-AKT pathways that culminate in cell proliferation108,109.
Genetic or pharmacological blockade of C3, C3aR or C5aR1 in various cancer models has consistently resulted in inhibition of tumorigenesis90. The combined blockade of complement and PD-L1, an immune checkpoint molecule that downregulates T cell responses, attenuated tumorigenesis in models of melanoma and lung cancer more efficiently than each individual therapy100,110. These findings open promising avenues for exploring complement inhibition in combination with immunotherapies tailored to reactivate the patients' own anti-cancer immunity.
Complement has also been associated with protective cytotoxic activity for tumor destruction111. Moreover, various monoclonal antibodies used in the clinic for cancer treatment function to activate complement on the surface of tumor cells, thereby enhancing their killing and elimination112. Apparently, complement should be tightly regulated to maintain tissue homeostasis. In this regard, mice injected with tumor cells expressing high levels of C5a (proinflammatory environment) show decreased numbers of effector T cells and increased tumorigenesis relative to counterparts injected with tumors producing low C5a amounts (environment closer to physiological)113. Consistently, complement activation along the tumor vasculature modulates endothelial function in a manner that enables efficient recruitment of effector T cells for tumor elimination in a model of cervical cancer114.
The above-discussed studies indicate that complement plays a key role in determining tumor growth by regulating inflammation, T cell immunity, vascularization, as well as proliferation, migration and invasiveness of tumor cells. However, complement may also stimulate protective anti-tumor responses when acting in concert with certain cancer therapies.
Molecular base of complement's functional diversity
Recent technological advances have enabled new insights into structure-function relationships of complement components, such as the elucidation of immunoglobulin-triggered complement activation via the CP. By employing an elegant liposomal surface model and a combination of structural techniques, it was demonstrated that, upon binding to antigen-covered surfaces, IgG molecules arrange in a hexameric form with a distinct conformational arrangement that facilitates binding and activation of the C1 complex115. This model not only provides a molecular explanation about the inability of soluble IgG to activate the CP, but also enable the rational design of engineered IgG variants with increased propensity to form hexamers115,116; these ‘hexabodies’ may have broad implications for complement-mediated antibody therapy in cancer, infectious or autoimmune diseases116,117.
Other studies provided molecular insight into the assembly, function and regulation of the alternative pathway C3 convertase (C3bBb)5. An intricate mechanism driven by conformational changes and tiered interactions enables instant opsonic reactivity while restricting the response to immediate sites of activation118(Fig. 5). For instance, both FB and FD circulate as mature enzymes but do not mediate cleavage activity in solution. Only upon interaction with C3b on target surfaces will FB reveal a hidden binding area for FD. Subsequently, FD docks via an area outside the active site (‘exosite’) and displaces an inhibitory loop to permit cleavage of FB that releases Ba and forms C3bBb118. Once the convertase is assembled, C3b is critical for bridging between the C3 substrate and the active site of the convertase to enable activation of C3. Finally, the transient nature of the C3bBb complex and the short-lived surface reactivity of the newly-formed C3b largely prevent bystander cell opsonization118. Emerging molecular knowledge may also elucidate disease implications and therapeutic mechanisms; for example, the anti-FD antibody lampalizumab, a therapeutic candidate for AMD treatment, binds to the FD exosite and impairs convertase assembly119, whereas the compstatin family of C3 inhibitors prevents the binding of C3 to the convertase120 .
Figure 5. Molecular mechanisms of C3-mediated complement activation, amplification, and effector generation.
After initial activation of C3, via the classical (CP) or lectin pathways (LP), or other triggers, C3b is deposited on the cell surface and interacts with factor B (FB). The resulting proconvertase (C3bB) is subsequently activated by factor D (FD) to generate the alternative pathway C3 convertase, C3bBb. In the absence of regulators, an amplification loop driven by C3b deposition and convertase formation feeds into the terminal effector pathway by activating C5 and generating the C5a anaphylatoxin and the membrane attack complex (MAC). On host cells, regulators of complement activation (RCA) destabilize the C3 convertase and enable the binding of factor I (FI) to C3b, leading to its degradation to iC3b and C3dg. Opsonins resulting from this breakdown pathway interact with different complement receptors (CR) and exert distinct effector functions as indicated. The following Protein Data Bank (www.rcsb.org) structures were used: 2A73, 2I07, 4HW5, 1C3D, 2WIN, 2XWJ, 2XWB, 5O32, 2OK5, 1DSU, 2XRC, 3CU7, 1KJS, 4A5W. A hypothetical iC3b model was prepared using PDB 1C2D and 2A74. FH1-4 from PDB 2WII was used to visualized RCAs. The MAC was derived from the Electron Microscopy Data Bank (http://www.ebi.ac.uk/pdbe/emdb; EMD-3135).
The structural characterization of various regulators of complement activation (RCA) revealed a common binding mode among FH, CD35, CD46, CD55, and viral homologs, in which subtle differences in domain composition and C3b interaction determine the functional preference for decay acceleration and cofactor activity121. Whereas the former is mediated by steric interference with Bb of the convertase, the RCAs' cofactor activity is provided by their ability to form a joint binding site with C3b, which binds FI and orients the catalytic site to enable up to three cleavages resulting in degradation to iC3b or C3dg122-124(Fig. 5). Importantly, analysis of the binding interfaces between C3b, RCAs, FB/Bb and/or FI fostered our understanding why certain polymorphisms or mutations may result in gain- or loss-of-function profiles that contribute to diseases such as AMD, aHUS or C3G.
The breakdown of C3b to iC3b and C3dg drives a dynamic process during which the unfolding of domains eliminates binding sites for activators and regulators concomitantly allowing access to receptors such as CR2 and CR35,123,125. The molecular analysis of the interaction of the integrin receptors CR3 and CR4 with their C3-derived ligands revealed differential binding profiles with only CR3, but not CR4, recognizing C3dg as a distinct ligand126-128. In contrast, structural insights into the anaphylatoxin receptors (C3aR, C5aR1, C5aR2) have remained limited, which is not surprising given their challenging seven-transmembrane structure. The recent surge of interest in and characterization of other G-protein-coupled receptors revives the hope that the molecular secrets of the anaphylatoxin receptors are revealed in the near future. Whereas we meanwhile gained detailed pictures of the MAC as the second terminal pathway effector (Fig. 5), the mechanisms that lead to C5 convertase formation and provide selectivity for activating C5 over C3 have remained elusive.
Future perspectives
The new mechanisms and functions attributed to complement have transformed our perception of it from a blood-based innate antimicrobial system to a global regulator of immunity and host-microbiome homeostasis. We now know that complement regulates T-cell function not only though paracrine effects (by cytokines released from complement-modulated APCs) but also through T-cell intrinsic effects mediated by complement receptors on or within T cells. A formidable challenge for future research is to obtain enhanced mechanistic knowledge into how intracellular complement is activated and regulated (or dysregulated) and how such knowledge could be harnessed therapeutically. Moreover, a better understanding of the mechanisms that trigger, sustain and amplify complement activation in the tumor microenvironment would facilitate targeted modulation of complement to control carcinogenesis. There are few frontiers remaining in the elucidation of the complement cascade and, given the steady progress on methods and specific inhibitors, it is expected that residual mysteries such as the nature of the C5 convertase can soon be resolved. The true power of emerging structure-function insights therefore lies in deciphering the complement crosstalk with other pathways and in the rational development of improved therapeutics. In this context, our increased understanding of complement's involvement in chronic and aging-related inflammatory pathologies is expected to shed more light on crosstalk mechanisms and to guide the comprehensive selection of optimal targets of therapeutic intervention in a disease-tailored fashion.
Acknowledgments
This work was supported by grants from the US NIH (AI068730, AI030040, DE021685, DE015254, DE026152) and by funding from the European Community's Seventh Framework Programme, under grant agreement number 602699 (DIREKT). Given the broad scope of this review and the limited space for references, we often refer to specialized review articles rather than primary literature; we therefore want to thank all our colleagues who are not specifically cited for their contributions and their understanding.
Footnotes
Competing interests statement: J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors (including third-generation compstatin analogs such as AMY-101). J.D.L., G.H, and D.R. are inventors of patents or patent applications that describe the use of complement inhibitors for therapeutic purposes, some of which are developed by Amyndas Pharmaceuticals. J.D.L. is also the inventor of the compstatin technology licensed to Apellis Pharmaceuticals (i.e., 4(1MeW)7W/POT-4/APL-1 and PEGylated derivatives). The other authors declare no competing interest.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbore G, Kemper C, Kolev M. Intracellular complement - the complosome - in immune cell regulation. Mol Immunol. 2017;89:2–9. doi: 10.1016/j.molimm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12:383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmlund D, Le SN, Elmlund H. High-resolution cryo-EM: the nuts and bolts. Curr Opin Struct Biol. 2017;46:1–6. doi: 10.1016/j.sbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3 - The “Swiss Army Knife” of innate immunity and host defense. Immunol Rev. 2016;274:33–58. doi: 10.1111/imr.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatz-Jakobsen JA, Pedersen DV, Andersen GR. Structural insight into proteolytic activation and regulation of the complement system. Immunol Rev. 2016;274:59–73. doi: 10.1111/imr.12465. [DOI] [PubMed] [Google Scholar]
- 7.Liszewski MK, Elvington M, Kulkarni HS, Atkinson JP. Complement's hidden arsenal: New insights and novel functions inside the cell. Mol Immunol. 2017;84:2–9. doi: 10.1016/j.molimm.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Lambris JD. More than complementing Tolls: complement-Toll-like receptor synergy and crosstalk in innate immunity and inflammation. Immunol Rev. 2016;274:233–244. doi: 10.1111/imr.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovingh ES, van den Broek B, Jongerius I. Hijacking Complement Regulatory Proteins for Bacterial Immune Evasion. Front Microbiol. 2016;7:2004. doi: 10.3389/fmicb.2016.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 11.Kaya Z, et al. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol. 2001;2:739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- 12.Strainic MG, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liszewski MK, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbore G, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. This study has shown that complement and the NLRP3 inflammasome, two systems traditionally associated with innate immunity, can also operate within T cells forming a C5aR1-NLRP3 axis that guides the differentiation of CD4+ T cells to the Th1 subset. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambris JD, Dobson NJ, Ross GD. Release of endogenous C3b inactivator from lymphocytes in response to triggering membrane receptors for beta 1H globulin. J Exp Med. 1980;152:1625–1644. doi: 10.1084/jem.152.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundsmo JS. The leukocyte complement system. Fed Proc. 1982;41:3094–3098. References 15 and 16 involve old but pioneering studies describing the synthesis of complement components and regulators by human lymphocytes, whose intracellular complement system has only now started to be functionally appreciated. [PubMed] [Google Scholar]
- 17.Freeley S, Kemper C, Le Friec G. The “ins and outs” of complement-driven immune responses. Immunol Rev. 2016;274:16–32. doi: 10.1111/imr.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elvington M, Liszewski MK, Bertram P, Kulkarni HS, Atkinson JP. A C3(H20) recycling pathway is a component of the intracellular complement system. J Clin Invest. 2017;127:970–981. doi: 10.1172/JCI89412. This investigation has identified a C3-recycling pathway between the extracellular and intracellular compartments, thus uncovering a new source of the intracellular complement components. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolev M, Kemper C. Keeping It All Going-Complement Meets Metabolism. Front Immunol. 2017;8:1. doi: 10.3389/fimmu.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolev M, et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity. 2015;42:1033–1047. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardone J, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 23.Khameneh HJ, et al. C5a regulates IL-1β production and leukocyte recruitment in a murine model of monosodium urate crystal-induced peritonitis. Front Pharmacol. 2017;8:10. doi: 10.3389/fphar.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asgari E, et al. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 25.Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 26.Laudisi F, et al. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1b release. J Immunol. 2013;191:1006–1010. doi: 10.4049/jimmunol.1300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Benoit ME, Clarke EV, Morgado P, Fraser DA, Tenner AJ. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J Immunol. 2012;188:5682–5693. doi: 10.4049/jimmunol.1103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 30.Hajishengallis G, Abe T, Maekawa T, Hajishengallis E, Lambris JD. Role of complement in host-microbe homeostasis of the periodontium. Semin Immunol. 2013;25:65–72. doi: 10.1016/j.smim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa M, et al. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity. 2014;41:620–632. doi: 10.1016/j.immuni.2014.09.010. This study has integrated IL-22-mediated immunity and complement by showing that IL-22 regulation of C3 leads to host protection from pathobionts that enter the systemic circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chehoud C, et al. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc Natl Acad Sci U S A. 2013;110:15061–15066. doi: 10.1073/pnas.1307855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HB, Ricklin D, Lambris JD. Complement activation fragment C4a, a novel untethered agonist, mediates effector functions by binding to PAR1 and PAR4. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1707364114. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekdahl KN, et al. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol Rev. 2016;274:245–269. doi: 10.1111/imr.12471. [DOI] [PubMed] [Google Scholar]
- 36.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 37.Schonthaler HB, et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity. 2013;39:1171–1181. doi: 10.1016/j.immuni.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Rafail S, et al. Complement deficiency promotes cutaneous wound healing in mice. J Immunol. 2015;194:1285–1291. doi: 10.4049/jimmunol.1402354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winter SE, Baumler AJ. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes. 2014;5:71–73. doi: 10.4161/gmic.27129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maekawa T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. This study has shown that dysbiotic bacteria manipulate complement-TLR crosstalk to inhibit bactericidal mechanisms while promoting a nutritionally favorable inflammatory response; the uncoupling of immune bacterial clearance from inflammation perpetuates dysbiosis and inflammatory disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajishengallis G, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345:1256070. doi: 10.1126/science.1256070. This study has revealed new intracellular antimicrobial functions of extracellularly activated complement: After escaping from phagosomes, C3-opsonized nonenveloped viruses and bacteria can be sensed in the cytosol in a C3-dependent manner leading to innate immune signaling and proteasome-mediated viral degradation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helmy KY, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Zeng Z, et al. CRIg functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe. 2016;20:99–106. doi: 10.1016/j.chom.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Kim KH, et al. Extracellular stimulation of VSIG4/complement receptor Ig suppresses intracellular bacterial infection by inducing autophagy. Autophagy. 2016;12:1647–1659. doi: 10.1080/15548627.2016.1196314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosser DM, Edelson PJ. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature. 1987;327:329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- 48.Dai S, Rajaram MV, Curry HM, Leander R, Schlesinger LS. Fine tuning inflammation at the front door: macrophage complement receptor 3-mediates phagocytosis and immune suppression for Francisella tularensis. PLoS Pathog. 2013;9:e1003114. doi: 10.1371/journal.ppat.1003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett KM, Rooijakkers SHM, Gorham RD. Let's Tie the Knot: Marriage of Complement and Adaptive Immunity in Pathogen Evasion, for Better or Worse. Front Microbiol. 2017;8:89. doi: 10.3389/fmicb.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Lookeren Campagne M, Strauss EC, Yaspan BL. Age-related macular degeneration: Complement in action. Immunobiology. 2016;221:733–739. doi: 10.1016/j.imbio.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Brennan FH, Lee JD, Ruitenberg MJ, Woodruff TM. Therapeutic targeting of complement to modify disease course and improve outcomes in neurological conditions. Semin Immunol. 2016;28:292–308. doi: 10.1016/j.smim.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Thurman JM. Complement in kidney disease: core curriculum 2015. Am J Kidney Dis. 2015;65:156–168. doi: 10.1053/j.ajkd.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 57.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. This paper provides mechanistic evidence for a pathogenic crosstalk between complement and reactive microglia in the CNS. It reveals a key role of complement in the induction of A1 astrocytes, which mediate neurotoxic effects in a number of neurodegenerative diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawksworth OA, Coulthard LG, Woodruff TM. Complement in the fundamental processes of the cell. Mol Immunol. 2017;84:17–25. doi: 10.1016/j.molimm.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. This work identified complement proteins C1q and C3 as key contributors of synaptic refinement and neuronal connectivity in the CNS. It is the first report implicating complement-triggered pathways in the regulation of developmental synaptic pruning, while prompting the question whether this phenomenon is recapitulated in neurological diseases associated with aberrant synapse loss. [DOI] [PubMed] [Google Scholar]
- 60.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Howell GR, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Q, et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci. 2015;35:13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whalley K. Neurodegenerative disease: Complement mediates pathological pruning. Nat Rev Neurosci. 2016;17:336. doi: 10.1038/nrn.2016.52. [DOI] [PubMed] [Google Scholar]
- 65.Vasek MJ, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wyatt SK, Witt T, Barbaro NM, Cohen-Gadol AA, Brewster AL. Enhanced classical complement pathway activation and altered phagocytosis signaling molecules in human epilepsy. Exp Neurol. 2017;295:184–193. doi: 10.1016/j.expneurol.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 68.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ager RR, et al. Microglial C5aR (CD88) expression correlates with amyloid-beta deposition in murine models of Alzheimer's disease. J Neurochem. 2010;113:389–401. doi: 10.1111/j.1471-4159.2010.06595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fonseca MI, et al. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. This paper establishes a link between early neurodenerative pathways in Alzheimer's disease and aberrant reactivation of complement-mediated synaptic pruning programs in the CNS. It supports the notion that early synapse loss is a hallmark of cognitive decline in Alzheimer's disease that actually precedes the appearance of pathogenic amyloid plaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi Q, et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med. 2017;9:eaaf6295. doi: 10.1126/scitranslmed.aaf6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 74.Naito AT, et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. This is the first report implicating complement C1q in aging-related processes, providing wider implications for a role of complement in subverted tissue homeostasis (impaired tissue repair) during ageing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 76.Sumida T, et al. Complement C1q-induced activation of beta-catenin signalling causes hypertensive arterial remodelling. Nat Commun. 2015;6:6241. doi: 10.1038/ncomms7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castellano G, et al. Complement modulation of anti-aging factor klotho in ischemia/reperfusion injury and delayed graft function. Am J Transplant. 2016;16:325–333. doi: 10.1111/ajt.13415. [DOI] [PubMed] [Google Scholar]
- 78.Hageman GS, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haines JL, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 80.Calippe B, et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity. 2017;46:261–272. doi: 10.1016/j.immuni.2017.01.006. This study offers a new perspective to the increasingly complex pathogenic role of complement in AMD, ascribing to the AMD-predisposing FH variant H402 a novel role in perpetuating subretinal inflammation through impaired phagocyte clearance from the subretinal space. [DOI] [PubMed] [Google Scholar]
- 81.Wang Q, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maekawa T, et al. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maekawa T, et al. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J Clin Periodontol. 2016;43:238–249. doi: 10.1111/jcpe.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pandey MK, et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature. 2017;543:108–112. doi: 10.1038/nature21368. [DOI] [PubMed] [Google Scholar]
- 85.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 86.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Hajishengallis G, et al. Complement inhibition in pre-clinical models of periodontitis and prospects for clinical application. Semin Immunol. 2016;28:285–291. doi: 10.1016/j.smim.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kemper C, Kohl J. Novel roles for complement receptors in T cell regulation and beyond. Mol Immunol. 2013;56:181–190. doi: 10.1016/j.molimm.2013.05.223. [DOI] [PubMed] [Google Scholar]
- 90.Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.97. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsieh CC, et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121:1760–1768. doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Markiewski MM, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corrales L, et al. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189:4674–4683. doi: 10.4049/jimmunol.1201654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doerner SK, et al. High-fat diet-induced complement activation mediates intestinal inflammation and neoplasia, independent of obesity. Mol Cancer Res. 2016;14:953–965. doi: 10.1158/1541-7786.MCR-16-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guglietta S, et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun. 2016;7:11037. doi: 10.1038/ncomms11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nabizadeh JA, et al. The complement C3a receptor contributes to melanoma tumorigenesis by inhibiting neutrophil and CD4+ T cell responses. J Immunol. 2016;196:4783–4792. doi: 10.4049/jimmunol.1600210. [DOI] [PubMed] [Google Scholar]
- 97.Ning C, et al. Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1beta/IL-17A axis. Mucosal Immunol. 2015;8:1275–1284. doi: 10.1038/mi.2015.18. [DOI] [PubMed] [Google Scholar]
- 98.Vadrevu SK, et al. Complement c5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Res. 2014;74:3454–3465. doi: 10.1158/0008-5472.CAN-14-0157. [DOI] [PubMed] [Google Scholar]
- 99.Bonavita E, et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. This study has uncovered a novel role for PTX3 as suppressor of tumorigenesis via regulationof complement and inflammatory responses. [DOI] [PubMed] [Google Scholar]
- 100.Ajona D, et al. A Combined PD-1/C5a Blockade Synergistically Protects against Lung Cancer Growth and Metastasis. Cancer Discov. 2017;7:694–703. doi: 10.1158/2159-8290.CD-16-1184. [DOI] [PubMed] [Google Scholar]
- 101.Bulla R, et al. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat Commun. 2016;7:10346. doi: 10.1038/ncomms10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ajona D, et al. Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer. J Natl Cancer Inst. 2013;105:1385–1393. doi: 10.1093/jnci/djt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nunez-Cruz S, et al. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14:994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaida T, et al. C5a receptor (CD88) promotes motility and invasiveness of gastric cancer by activating RhoA. Oncotarget. 2016;7:84798–84809. doi: 10.18632/oncotarget.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abdelbaset-Ismail A, et al. Activation of the complement cascade enhances motility of leukemic cells by downregulating expression of HO-1. Leukemia. 2017;31:446–458. doi: 10.1038/leu.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han X, Zha H, Yang F, Guo B, Zhu B. Tumor-Derived Tissue Factor Aberrantly Activates Complement and Facilitates Lung Tumor Progression via Recruitment of Myeloid-Derived Suppressor Cells. Int J Mol Sci. 2017;18:22. doi: 10.3390/ijms18010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boire A, et al. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell. 2017;168:1101–1113. doi: 10.1016/j.cell.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho MS, et al. Complement Component 3 Is Regulated by TWIST1 and Mediates Epithelial-Mesenchymal Transition. J Immunol. 2016;196:1412–1418. doi: 10.4049/jimmunol.1501886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Towner LD, Wheat RA, Hughes TR, Morgan BP. Complement Membrane Attack and Tumorigenesis: A systems biology approach. J Biol Chem. 2016;291:14927–14938. doi: 10.1074/jbc.M115.708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, et al. Autocrine Complement Inhibits IL10-Dependent T-cell-Mediated Antitumor Immunity to Promote Tumor Progression. Cancer Discov. 2016;6:1022–1035. doi: 10.1158/2159-8290.CD-15-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Surace L, et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity. 2015;42:767–777. doi: 10.1016/j.immuni.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 112.Taylor RP, Lindorfer MA. Cytotoxic mechanisms of immunotherapy: Harnessing complement in the action of anti-tumor monoclonal antibodies. Semin Immunol. 2016;28:309–316. doi: 10.1016/j.smim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 113.Gunn L, et al. Opposing roles for complement component C5a in tumor progression and the tumor microenvironment. J Immunol. 2012;189:2985–2994. doi: 10.4049/jimmunol.1200846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Facciabene A, et al. Local endothelial complement activation reverses endothelial quiescence, enabling T-cell homing, and tumor control during T-cell immunotherapy. Oncoimmunology. 2017;6:e1326442. doi: 10.1080/2162402X.2017.1326442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Diebolder CA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–1263. doi: 10.1126/science.1248943. This seminal study utilized a panel of structural and biochemical methods to provide an unprecedented molecular insight into classical pathway initiation by IgG hexamers, with implications for our understanding of complement activation and the design of therapeutic antibodies alike. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Jong RN, et al. A Novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol. 2016;14:e1002344. doi: 10.1371/journal.pbio.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cook EM, et al. Antibodies that efficiently form hexamers upon antigen binding can induce complement-dependent cytotoxicity under complement-limiting conditions. J Immunol. 2016;197:1762–1775. doi: 10.4049/jimmunol.1600648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Forneris F, et al. Structures of C3b in complex with factors B and D give insight into complement convertase formation. Science. 2010;330:1816–1820. doi: 10.1126/science.1195821. By describing the tiered interaction between C3b, FB, and FD, this elegant study builds an important base to explain the intrinsic and extrinsic control of complement activation and amplification as mediated by the alternative pathway C3 convertase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katschke KJ, Jr, et al. Inhibiting alternative pathway complement activation by targeting the factor D exosite. J Biol Chem. 2012;287:12886–12892. doi: 10.1074/jbc.M112.345082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mastellos DC, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest. 2015;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Forneris F, et al. Regulators of complement activity mediate inhibitory mechanisms through a common C3b-binding mode. EMBO J. 2016;35:1133–1149. doi: 10.15252/embj.201593673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roversi P, et al. Structural basis for complement factor I control and its disease-associated sequence polymorphisms. Proc Natl Acad Sci U S A. 2011;108:12839–12844. doi: 10.1073/pnas.1102167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xue X, et al. Regulator-dependent mechanisms of C3b processing by factor I allow differentiation of immune responses. Nat Mol Struct Biol. 2017;24:643–651. doi: 10.1038/nsmb.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu J, et al. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 2009;10:728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papanastasiou M, et al. Structural Implications for the Formation and Function of the Complement Effector Protein iC3b. J Immunol. 2017;198:3326–3335. doi: 10.4049/jimmunol.1601864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van den Elsen JM, Isenman DE. A crystal structure of the complex between human complement receptor 2 and its ligand C3d. Science. 2011;332:608–611. doi: 10.1126/science.1201954. [DOI] [PubMed] [Google Scholar]
- 127.Xu S, Wang J, Wang JH, Springer TA. Distinct recognition of complement iC3b by integrins alphaXbeta2 and alphaMbeta2. Proc Natl Acad Sci U S A. 2017;114:3403–3408. doi: 10.1073/pnas.1620881114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bajic G, Yatime L, Sim RB, Vorup-Jensen T, Andersen GR. Structural insight on the recognition of surface-bound opsonins by the integrin I domain of complement receptor 3. Proc Natl Acad Sci U S A. 2013;110:16426–16431. doi: 10.1073/pnas.1311261110. [DOI] [PMC free article] [PubMed] [Google Scholar]