Abstract
Background
Although bilateral prophylactic mastectomy (BPM) can reduce the risk of breast cancer, the decision to proceed surgically can have significant consequences and requires careful deliberation. To facilitate decision-making for women at high risk for breast carcinoma, the risks and benefits of BPM should be well-elucidated. We sought to determine the effects of BPM and immediate reconstruction on health-related quality of life outcomes among a multisite cohort of women at high risk for breast carcinoma.
Methods
Patient-reported outcome data were prospectively collected as part of the Mastectomy Reconstruction Outcomes Consortium Study. Data on a subgroup of 204 high-risk women who elected to have BPM and immediate reconstruction were evaluated. Baseline scores were compared with scores at one or two years after reconstruction.
Results
Satisfaction with breasts and psychosocial well-being were significantly higher at both one and two years (p<0.01). Anxiety was significantly lower at one or two years (p<0.01). However, physical well-being of the chest and upper body was significantly worse at one year (p<0.01).
Conclusion
Our results highlight the impact of BPM and immediate reconstruction on health-related quality of life outcomes in this setting. BPM and reconstruction can result in significant positive, lasting changes in a woman’s satisfaction with her breasts, as well as her psychosocial well-being. Furthermore, presurgery anxiety was significantly reduced by one year postreconstruction and remained reduced at two years. With this knowledge, women at high risk for breast carcinoma and their providers will be better equipped to make the best individualized treatment decisions.
Introduction
Women with no known risk factors for breast carcinoma have an estimated lifetime risk of developing breast cancer of approximately 12%. This means that one of every eight women in the general population will develop breast cancer during her lifetime.
In contrast, BRCA mutation carriers and women with a strong family history of breast carcinoma have an estimated lifetime risk of 45% to 67%.1–3 These high-risk women are advised to consider strategies aimed at reducing their risk of breast cancer, such as extensive and regular surveillance, chemoprevention, or surgical removal of both healthy breasts—known as bilateral prophylactic mastectomy (BPM).
Selecting the most appropriate risk-reducing option is not a straightforward task.4 The decision-making process must take into account not only the effect that each risk-reducing strategy has on cancer risk and survival but also the impact of each approach on overall quality of life.
BPM has proven to be the most effective option in reducing cancer risk. A recent meta-analysis suggests that BPM may decrease the risk of developing breast cancer by >90%.5,6 However, the irreversible approach of removing both healthy breasts is invasive and carries with it the potential for surgical complications. The long-term consequences for women who choose BPM, with or without reconstruction, may also include significant changes in body image as well as psychosocial, sexual, and physical well-being.
A recent systematic review attempted to collate the existing body of literature on patients’ perceptions of outcomes following BPM.7 This review suggests that, in general, patients report both high psychosocial well-being and favorable body image after BPM. Furthermore, the favorable outcomes do not change significantly over time. However, the studies in this review were limited by methodological issues: all 22 studies were observational, with the majority relying on ad hoc questionnaires without demonstrated reliability or validity. Additionally, a majority of these studies used generic questionnaires, such as the 36-Item Short Form Survey, which are not sufficiently sensitive to measure physical and mental changes in women undergoing BPM with or without reconstruction. Finally, the majority of studies evaluated outcomes at one or more times following surgery but failed to control for preoperative baseline measures of breast satisfaction and health-related quality of life. Without any baseline frame of reference for women with healthy breasts in this setting, it is difficult to put any postoperative data into context.
The purpose of this investigation was to evaluate the effects of BPM and immediate reconstruction on health-related quality of life in a multisite population of women at high risk for breast cancer. It is theorized that, with this knowledge, patients at high risk for breast carcinoma and their providers will be better equipped to make informed, individualized treatment decisions and set appropriate expectations for risk-reducing surgery.
Methods
Patient Recruitment
Patient-reported outcome data were prospectively collected as part of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study, a five-year, prospective, multicenter cohort study funded by the National Cancer Institute (NCI 1RO1CA152192). Fifty-seven plastic surgeons from 11 centers in the US (Michigan, New York, Illinois, Ohio, Massachusetts, Washington, D.C., Georgia, and Texas) and Canada (British Columbia and Manitoba) contributed patients undergoing breast reconstruction after mastectomy to the study. Institutional Review Board approval was obtained from all participating sites, and patients were consented in person by a research study assistant.
Eligibility Criteria
Women were eligible to participate in the MROC study if they were 18 years or older and undergoing first-time, immediate or delayed, bilateral or unilateral postmastectomy breast reconstruction for cancer treatment or prophylaxis. The choice of reconstructive procedure was based on patient and surgeon preference. The current investigation included a subgroup of MROC patients at high risk for breast cancer who underwent BPM and immediate reconstruction. For the analyses of one- and two-year outcomes, women who underwent immediate placement of a tissue expander (TE) but did not undergo an exchange to implant within 11 months of TE placement were excluded, as their one-year assessments will likely reflect the outcomes associated with the recent exchange. In addition, women who experienced reconstructive failure were excluded, as no patient-reported outcome data were collected from them once they experienced failure. Reconstructive failure was defined as the premature loss of a tissue expander or permanent implant resulting in absence of a breast mound.
Data Collection
Patients completed the patient-reported outcome measures (PROMs) preoperatively and at one and two years after surgery. The following PROMs were used: the BREAST-Q,8 the Numerical Pain Rating Scale (NPRS), the Short Form McGill Pain Questionnaire (SF-MPQ),9 the General Anxiety Disorder 7-Item (GAD-7) scale,10 the Patient Health Questionnaire–9 (PHQ-9),11 and the Patient-Reported Outcome Measurement Information System–29 (PROMIS-29).12 Patients were encouraged to complete the electronic questionnaires remotely; if they were unable to do so, a paper version was provided in clinic or by mail.
Patient-Reported Outcome Instruments
The BREAST-Q is a validated PROM that consists of independent scales measuring various aspects of outcomes following specific breast surgeries.8 The instrument was developed and validated with adherence to guidelines set by the Scientific Advisory Committee of the Medical Outcomes Trust (2002) and the US Food and Drug Administration. Four subscales of the BREAST-Q Reconstructive module—“satisfaction with breasts,” “psychosocial well-being,” “physical well-being,” and “sexual well-being”—were included in the analysis. Scores on the subscales were transformed using the Q-score to a number from 0 to 100, with higher numbers signifying better outcomes.
Pain was evaluated using the NPRS and the SF-MPQ. The NPRS asks patients to rate the “intensity” of their pain on a 0–10 numerical rating scale. The SF-MPQ provides an additional qualitative assessment of pain, distinct from the “intensity-alone” rating of the NPRS. More specifically, the sensory subscale of the SF-MPQ quantifies the sensory dimensions of pain experience, including its mechanical, spatial, and temporal characteristics, while the affective subscale provides a measure of the subjective unpleasantness or suffering associated with pain.
Anxiety was measured using the GAD-7, a seven-item scale shown to have good reliability and validity. Higher scores on the scale are strongly associated with functional impairment. Depressive symptoms were evaluated using the PHQ-9, a nine-item depression scale of the Patient Health Questionnaire used for screening, diagnosing, and monitoring symptoms over time. The nine items of the PHQ-9 are based directly on the nine diagnostic criteria for major depressive disorder in the DSM-IV.
Finally, the seven domains (anxiety, depression, physical function, fatigue, sleep disturbance, satisfaction with social role, and pain interference) of the PROMIS-29, a National Institute of Health–funded, validated PRO instrument, were administered.
Statistical Methods
Clinical and demographic characteristics of patients were summarized and are presented as counts (percentages) for categorical variables and medians (ranges) for continuous variables. Mean within-person changes of patient-reported outcome scores were calculated, with adjustment for clinical sites (hospitals). The within-person change was defined as an individual’s patient-reported outcome score at one or two years, minus that at baseline. To reduce potential bias from missing patient-reported outcomes at one or two years, mean within-person changes at each follow-up assessment time were weighted by the inverse of the probability of nonmissing response. The probability of response was estimated using a separate logistic regression model fit for each outcome measure, with nonmissing response status as the dependent variable and baseline patient characteristics and baseline values for the outcome variables from all eligible study participants as predictors. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC); statistical significance was set at 0.05.
Results
In total, 217 women who underwent bilateral prophylactic mastectomy and immediate reconstruction past their one-year follow-up assessment time were identified (Supplemental Figure 1). From this cohort, outcomes analyses excluded 4 women who received a TE procedure but did not undergo an exchange to implant within 11 months of TE placement and 9 women who experienced reconstructive failure. Thus, the remaining 204 women were considered eligible for the one-year outcomes analysis, and similarly 149 women were considered eligible for two-year outcomes analyses, as they had reached two years of follow-up and did not experience reconstruction failure (Table 1).
Table 1.
Patient sample size by procedure type, postoperative time, and failure status
| Procedure type | One year postoperative | Two years postoperative | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Not failed | Failed | Total | Not failed | Failed | Total | |
| Single-stage implant | 18 | 3 | 21 | 12 | 3 | 15 |
| Tissue expander/implant | 133 | 4 | 137 | 99 | 2 | 101 |
| Autologous | 53 | 2 | 55 | 38 | 2 | 40 |
|
| ||||||
| Total | 204 | 9 | 213 | 149 | 7 | 156 |
Of the 204 women in the initial cohort, 133 (64.3%) had TE/implant reconstruction, 18 (9.9%) had single-stage implant reconstruction, and 55 (25.8%) had autologous reconstruction (Table 1). Clinical and demographic characteristics of the cohort of women eligible for one-year outcomes analyses are summarized in Table 2. In this cohort of patients, 60% had simple mastectomies, and 40% had nipple-sparing mastectomies. The majority of patients were white (94.6%) with no comorbidities (84.3%). The median age was 41.0 years, and the median BMI was 24.9 kg/m2.
Table 2.
Baseline clinical and demographic characteristics of the cohort of women eligible for one-year outcomes analyses (N=204)
| Characteristic | Patients |
|---|---|
| Age, years, median (range) | 41.0 (43.0) |
| BMI, kg/m2, median (range) | 24.9 (41.0) |
| Race | |
| White | 192 (94.6) |
| Black | 4 (2.0) |
| Other | 7 (3.5) |
| Ethnicity | |
| Hispanic or Latino | 8 (4.0) |
| Not Hispanic or Latino | 192 (96.0) |
| Education | |
| High school or less | 10 (4.9) |
| Some college | 26 (12.8) |
| College degree with or without some graduate work | 105 (51.7) |
| Master or doctoral degree | 62 (30.5) |
| Household annual income | |
| <$50,000 | 20 (10.1) |
| $50,000 to $99,000 | 72 (36.2) |
| $100,000 or more | 107 (53.8) |
| Marital status | |
| Married or partnered | 167 (82.7) |
| Not married or partnered | 35 (17.3) |
| Employment status | |
| Full-time employed (including student) | 130 (64.4) |
| Part-time employed | 24 (11.9) |
| Unemployed | 48 (23.8) |
| Type of mastectomy | |
| Nipple sparing | 80 (39.2) |
| Simple or modified radical mastectomy | 124 (60.8) |
| Type of reconstruction | |
| Tissue expander/implant | 133 (65.2) |
| Silicone | 127 (62.3) |
| Saline | 6 (2.9) |
| Single-stage implant | 18 (8.8) |
| Silicone | 16 (7.8) |
| Saline | 2 (1.0) |
| Autologous tissue | 53 (26.0) |
| TRAM | 11 (5.4) |
| DIEP | 30 (14.7) |
| SIEA | 4 (2.0) |
| Mixed | 8 (3.9) |
| Charlson Comorbidity Index | |
| 0 | 172 (84.3) |
| 1 | 29 (14.2) |
| ≥2 | 3 (1.5) |
| Smoking status | |
| Never | 146 (73.0) |
| Previous | 51 (25.5) |
| Current | 3 (1.5) |
| Complication | |
| None | 166 (81.4) |
| Minor only | 19 (9.3) |
| Major with or without minor | 19 (9.3) |
Data are presented as no. (%), unless otherwise noted.
BMI, body mass index; DIEP, deep inferior epigastric perforator; SIEA, superficial inferior epigastric artery; TRAM, transverse rectus abdominis muscle.
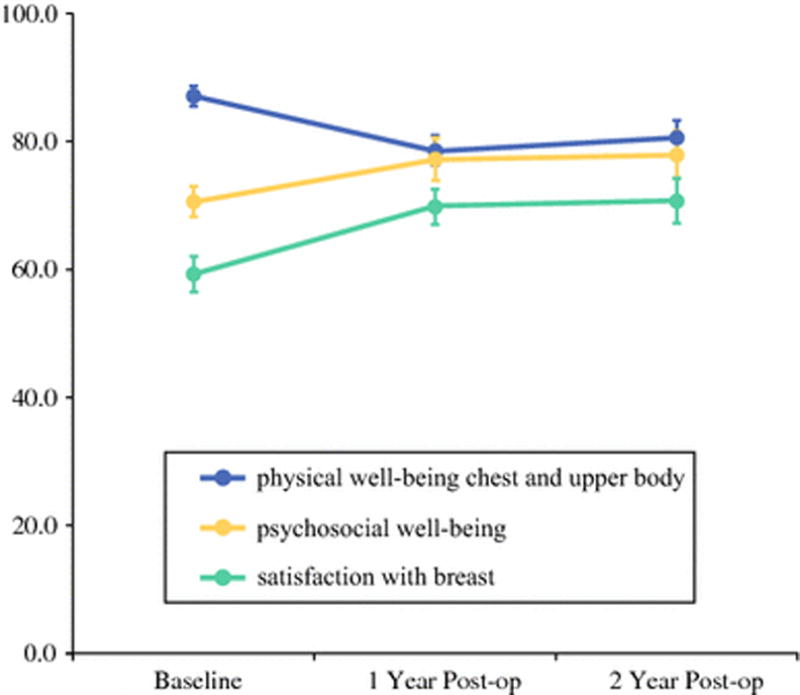
Mean within-person changes in patient-reported outcome scores, after adjusting for missingness, between baseline and one year and between baseline and two years, are summarized in Table 3. At both one and two years, patients experienced significantly higher satisfaction with their breasts (year-one mean difference, 9.13 [p=0.001]; year-two mean difference, 10.71 [p=0.001]) (Figure 1) and higher psychosocial well-being (year-one mean difference, 5.96 [p=0.003]; year-two mean difference, 7.9 [p=0.003]), compared with baseline. Patients’ anxiety levels, which were measured using both the GAD-7 and the PROMIS-29, were significantly lower at one and two years than at baseline. Sexual well-being was restored to baseline levels at one year and remained stable.
Table 3.
Within-person change of patient-reported outcomes between baseline and postoperative year one and two
| Patient-reported Outcome (Range)1 | Baseline Mean (SD) | Baseline vs. Year 1 (N=204) |
Baseline vs. Year 2 (N=149) |
||
|---|---|---|---|---|---|
|
| |||||
| Mean difference2 (95% CI) |
p | Mean difference2 (95% CI) |
p | ||
| BREAST-Q | |||||
| Satisfaction with breasts (0–100) | 59.3 (20.5) | 9.1 (5.4, 12.9) | 0.001 | 10.7 (5.6, 15.8) | 0.001 |
| Psychological well-being (0–100) | 70.6 (17.2) | 6.0 (2.6, 9.3) | 0.003 | 7.9 (3.5, 12.4) | 0.003 |
| Physical well-being (0–100) | 87.1 (12.0) | −8.6 (−12.3, −5.0) | 0.001 | −5.1 (−10.9, 0.8) | 0.079 |
| Sexual well-being (0–100) | 58.0 (20.9) | −1.1 (−4.7, 2.5) | 0.504 | −1.2 (−6.5, 4.0) | 0.601 |
| PHQ-9 total score (0–27) | 3.2 (3.4) | −0.4 (−1.2, 0.3) | 0.197 | −0.5 (−2.5, 1.6) | 0.626 |
| GAD-7 total score (0–21) | 4.5 (4.5) | −2.2 (−2.9, −1.5) | <.001 | −2.3 (−3.5, −1.2) | 0.002 |
| PROMIS-29 | |||||
| Anxiety (40.3–81.6) | 56.4 (9.1) | −10.0 (−12.2, −7.8) | <.001 | −8.1 (−10.9, −5.3) | <.001 |
| Depression (41.0–79.4) | 46.7 (7.0) | −1.5 (−2.9, −0.2) | 0.033 | −1.1 (−3.1, 0.8) | 0.219 |
| Fatigue (33.7–75.8) | 46.9 (9.5) | −0.8 (−2.6, 1.0) | 0.353 | −0.1 (−4.0, 3.7) | 0.941 |
| Pain interference (41.6–75.6) | 43.6 (5.1) | 1.2 (−0.5, 3.0) | 0.135 | 1.3 (−0.4, 3.0) | 0.121 |
| Physical function (22.9–56.9) | 54.9 (5.2) | −1.6 (−3.6, 0.3) | 0.083 | −0.5 (−1.7, 0.8) | 0.416 |
| Satisfaction with participation | |||||
| in social roles (29.0–64.1) | 55.5 (8.6) | 0.0 (−1.7, 1.6) | 0.946 | −0.9 (−3.4, 1.6) | 0.420 |
| Sleep disturbance (32.0–73.3) | 51.7 (3.9) | 0.6 (−0.6, 1.7) | 0.289 | 0.2 (−1.2, 1.5) | 0.780 |
| SF-MPQ | |||||
| Sensory (0–33) | 1.2 (2.4) | 1.2 (0.1, 2.3) | 0.040 | 1.0 (−0.5, 2.4) | 0.164 |
| Affective (0–12) | 0.8 (1.4) | −0.2 (−0.6, 0.1) | 0.145 | −0.4 (−1.3, 0.4) | 0.245 |
| NPRS (0–10) | 0.4 (1.2) | 0.4 (−0.1, 0.8) | 0.084 | 0.3 (−0.2, 0.7) | 0.215 |
Abbreviations: CI, confidence interval; GAD-7, General Anxiety Disorder 7-Item scale; NPRS, Numerical Pain Rating Scale; PHQ-9, Patient Health Questionnaire–9; PROMIS-29, Patient-Reported Outcome Measurement Information System–29; SF-MPQ, Short Form McGill Pain Questionnaire.
For the BREAST-Q, higher score indicates better outcome; for the PHQ-9, GAD-7, SF-MPQ, and NPRS, higher score indicates worse outcome; for the PROMIS-29, higher score indicates better outcome if the concept is positively worded (e.g., physical function) and worse outcome if the concept is negatively worded (e.g., anxiety).
Difference is defined as the value of PRO at postoperative 1 or 2 years minus that of baseline.
Figure 1.
Overall mean of BREAST-Q scores
In contrast, patients’ physical well-being of the chest and upper body, as measured using the Breast-Q, was significantly worse at one and two years, compared with baseline (year-one mean difference, −8.64 [p=0.001]; year-two mean difference, −5.09 [p=0.079]). In addition, pain levels, which were measured using the SF-MPQ sensory scale, were higher at one year (mean difference, 1.17 [p=0.04]). There were no significant differences in the SF-MPQ affective scales after surgery, compared with baseline.
Discussion
The performance of BPM in women at high risk for breast carcinoma has increased 12% per year during the last decade.13 It has been hypothesized that heightened awareness of genetic breast cancer, increased use of genetic testing, and improvements in postmastectomy reconstruction techniques have contributed to the higher rates of BPM.
When considering BPM, patients should be informed not only of the impact that prophylactic surgery has on cancer incidence and survival but also of the expected health-related quality of life outcomes. Ultimately, the decision to proceed with risk-reducing surgery should be driven by how the risk-to-benefit profile of the approach matches the patient’s values and health preferences.
A recent qualitative evaluation found that the majority of women undergoing prophylactic mastectomy were dissatisfied with their decision-making process, stating that their need for information was not adequately met.14 This underscores the need for high-quality data regarding how the performance of BPM and reconstruction in women at high risk for breast cancer affects their quality of life.
The results of this investigation suggest that high-risk women experience significant improvements in body image (satisfaction with breasts) and psychosocial well-being at one and two years after BPM and successful completion of postmastectomy reconstruction. Our findings also indicate that, for women who successfully undergo these procedures, general anxiety is significantly reduced following surgery. However, these benefits are not without costs: chest and upper body morbidity appears to worsen in these women postoperatively and remains affected at two years.
These results are generally similar to those found by Frost et al., who performed a cross-sectional study evaluating outcomes in 572 women at a mean of 14.5 years after BPM.15 Of their cohort, 93% completed postmastectomy reconstruction using implants, 5% underwent BPM alone, and 2% had unknown reconstruction status. The study used an ad hoc questionnaire consisting of single-item, ordinal scales to measure the effects of BPM on a range of psychosocial and social domains. Overall, 70% of women were “satisfied or very satisfied” with the procedure. Additionally, 74% reported a diminished level of emotional concern about developing breast cancer. In contrast to our current findings, however, only 16% of women in their series reported favorable effects on satisfaction with their body appearance, whereas 48% reported no change and 36% reported diminished or greatly diminished satisfaction with appearance. It may be that these findings by Frost et al., which represent longer-term outcomes, differ from the intermediate outcomes in the current investigation due to length of follow-up.
It is also noteworthy, however, that the ad hoc questionnaire used in their study was created using a compilation of nonvalidated, single-item scales and that they asked women to reflect on their experiences at 14.5 years from surgery. This methodologic approach may limit the confidence that can be placed in their findings. Additionally, Frost et al. recruited patients who had undergone BPM and reconstruction as early as 1960. Since then, decades of improvements to both the surgical techniques and the materials used in postmastectomy reconstruction have generally improved cosmetic outcomes. It may thus be hypothesized that, in general, patients’ perception of their overall appearance following postmastectomy reconstruction has improved, as significant strides have been made in the field of reconstructive surgery.
A Cochrane systematic review similarly reported that women who have BPM generally report satisfaction with their decision.16 Interestingly, cosmetic satisfaction after BPM was consistently less favorable. Data were derived from eight different sources, ranging from patient written responses to personal interviews to ad hoc questionnaires. Physical morbidity was generally defined as a return to the operating room for a perioperative or implant complication and did not address sensory morbidity or pain symptomatology after surgery.
Key strengths of this current investigation include the use of preoperative assessments, which provide a baseline with which to compare patients’ postoperative outcomes; the use of multiple, well-validated, reliable PROMs, including a breast reconstruction–specific survey instrument (the BREAST-Q); and the ability to reliably quantify the magnitude of effect that the surgical intervention may have on outcomes from a patient perspective. The study’s multisite design, which may have minimized the potential effects of an individual provider and/or institution, is a further strength.
The limitations of this study include the potential for volunteer bias. Our study sample contains only patients who elected to undergo risk-reducing surgery, thus constituting a self-selected population. Additionally, this study does not evaluate outcomes in women who chose BPM without reconstruction; thus, the role that reconstruction plays in the determination of postoperative satisfaction cannot be directly elucidated. Furthermore, although the MROC study has a multisite design, the majority of sites were based within larger academic centers, which may limit the generalizability of results to those in community practices and less urban locations. Similarly, these results represent only outcomes up to two years following surgery in relatively educated, higher-income, mostly white women who had BPM and successful reconstruction. It is not clear that these results can be generalized beyond this population and/or time frame. Finally, it has been suggested that satisfaction associated with BPM and reconstruction may be compromised by a postoperative complication. Future evaluation of the impact of these and other clinical and demographic variables on patients’ perception of outcomes following BPM and reconstruction is thus warranted.
Conclusions
The results of this study highlight the possible health-related quality of life benefits of BPM and immediate reconstruction for women at high risk for breast carcinoma. More specifically, this approach may provide positive, lasting changes in a woman’s satisfaction with her breasts as well as her psychosocial well-being. Furthermore, surgery can result in a significant reduction in anxiety during the postoperative period. Ultimately, this knowledge provides clinicians and patients alike with high-quality data to inform and improve their clinical decision-making process.
Supplementary Material
Supplemental Figure 1. Study flow diagram
Synopsis.
Knowledge of patients’ perceptions of outcomes following bilateral prophylactic mastectomy (BPM) will allow providers to set appropriate expectations for women considering risk-reducing surgery. The current study prospectively evaluated patient-reported outcome data in 204 women at high risk for breast cancer who elected to have BPM and immediate reconstruction. Our results demonstrate that BPM and reconstruction can result in significant positive, lasting changes in a woman’s satisfaction with her breasts, as well as her psychosocial well-being.
Acknowledgments
Financial support: This study was supported by NIH/NCI R01 CA152192 and, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748 (to C.M.M. and A.L.P.).
Footnotes
Disclosures: The BREAST-Q is owned by Memorial Sloan Kettering Cancer Center and University of British Columbia.
References
- 1.Giuliano AE, Boolbol S, Degnim A, et al. Society of Surgical Oncology: position statement on prophylactic mastectomy. Approved by the Society of Surgical Oncology Executive Council, March 2007. Ann Surg Oncol. 2007;14(9):2425–2427. doi: 10.1245/s10434-007-9447-z. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salhab M, Bismohun S, Mokbel K. Risk-reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Womens Health. 2010;10:28. doi: 10.1186/1472-6874-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, You R, Wang X, et al. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1465. [DOI] [PubMed] [Google Scholar]
- 6.Xue DQ, Qian C, Yang L, et al. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38(5):375–381. doi: 10.1016/j.ejso.2012.02.179. [DOI] [PubMed] [Google Scholar]
- 7.Razdan SN, Patel V, Jewell S, et al. Quality of life among patients after bilateral prophylactic mastectomy: a systematic review of patient-reported outcomes. Qual Life Res. 2016;25(6):1409–1421. doi: 10.1007/s11136-015-1181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 9.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 10.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinchcliff M, Beaumont JL, Thavarajah K, et al. Validity of two new patient-reported outcome measures in systemic sclerosis: Patient-Reported Outcomes Measurement Information System 29-item Health Profile and Functional Assessment of Chronic Illness Therapy-Dyspnea short form. Arthritis Care Res (Hoboken) 2011;63(11):1620–1628. doi: 10.1002/acr.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131(3):320e–326e. doi: 10.1097/PRS.0b013e31827cf576. [DOI] [PubMed] [Google Scholar]
- 14.Rolnick SJ, Altschuler A, Nekhlyudov L, et al. What women wish they knew before prophylactic mastectomy. Cancer Nurs. 2007;30(4):285–291. doi: 10.1097/01.NCC.0000281733.40856.c4. quiz 292–283. [DOI] [PubMed] [Google Scholar]
- 15.Frost MH, Schaid DJ, Sellers TA, et al. Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA. 2000;284(3):319–324. doi: 10.1001/jama.284.3.319. [DOI] [PubMed] [Google Scholar]
- 16.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010;(11):CD002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Study flow diagram


