ABSTRACT
TFEB is a master regulator for transcription of genes involved in autophagy, lysosome and mitochondrial biogenesis. Activity of TFEB is inhibited upon its phosphorylation. STUB1, a chaperone-dependent E3 ubiquitin ligase, modulates TFEB activity by preferentially targeting inactive phosphorylated TFEB for degradation by the ubiquitin proteasome pathway. Thus, the ubiquitin-proteasome pathway participates in regulating autophagy and lysosomal functions by regulating the activity of TFEB.
KEYWORDS: Autophagy, proteasome, ubiquitin, CHIP, TFEB, STUB1
TFEB (Transcription Factor EB) is a master regulator of autophagosome, lysosome, and mitochondrial biogenesis. Phosphorylation of TFEB by mammalian target of rapamycin (mTOR) inactivates it and sequesters it in the cytoplasm. TFEB transcriptional activity is executed by the non-phosphorylated form upon translocation to the nucleus.1-3 Cellular TFEB can be dephosphorylated by the phosphatase calcineurin catalytic subunit isoform beta (PPP3Cβ)4. However, the overall mechanisms by which TFEB levels in the cell are regulated are not well understood. Our recent study revealed some of the mechanisms of TFEB turnover and how they might influence its activity.5
STIP1 homology and U-Box containing protein 1 (STUB1) is a chaperone-dependent E3 ubiquitin ligase that promotes ubiquitin-mediated protein degradation and aids in cellular stress recovery.6 Our study on mice deficient in STUB1 led to the surprising observation that cells from these mice exhibited reduced autophagy and mitochondrial biogenesis. Additional studies revealed that STUB1 deficiency led to accumulation of TFEB but with a paradoxical reduction in TFEB activity. Further, cellular overexpression of STUB1 led to reduction in TFEB levels but an increase in TFEB activity. To explain this paradox, we conducted detailed mechanistic studies. These studies elucidated that STUB1 preferentially interacted with and ubiquitinated phosphorylated TFEB and targeted it for proteasomal degradation. By targeting the inactive phosphorylated TFEB for degradation, STUB1 facilitated the dimerization and nuclear translocation of the non-phosphoryated TFEB leading to increase in overall TFEB activity. In contrast, in STUB1-deficient cells, phosphorylated TFEB is not efficiently degraded. Accumulation of phosphorylated TFEB exerts a dominant negative effect by forming inactive heterodimers with non-phosphorylated forms. TFEB activity was evaluated by measuring proliferator-activated receptor coactivator 1α promoter (PGC1α) activity, transcription of autophagy and lysosomal genes, and TFEB nuclear translocation.
Importantly, we found that induction of TFEB activity by starvation or mTOR inhibition led to a marked increase in the interaction between TFEB and STUB1. These experiments suggested that phospho TFEB reduces TFEB activity by forming heterodimers with non-phosphorylated TFEB; leading to reduced TFEB translocation to the nucleus and that STUB1 regulates TFEB activity by modulating the level of phosphorylated TFEB through proteasomal degradation.
PGC1α levels, mitochondrial number, and mitochondrial oxygen consumption were reduced in STUB1-deficient cells. Overexpression of constitutively active mutants of TFEB (S142A/S211A-TFEB) into STUB1-deficient cells rescued autophagy and mitochondrial function, as evidenced by increased formation of microtubule-associated protein 1A/1B-light chain 3 (LC3) type-II and ATP production. These findings confirmed that the inhibition of autophagy and mitochondrial function observed in STUB1-deficient cells was due to reduced TFEB activity. These data also suggested that deficiency of STUB1 and a consequent reduction of TFEB activity would reduce the ability of cells to adapt to stress due to failure to increase energy stores or to induce autophagic-lysosomal stress responses. In support of this concept, we found that stub1−/− mice exhibited near complete neonatal lethality.
Proteasomal and autophagy systems are two major cellular degradation systems that play essential roles in maintaining proteostasis. Our finding that the STUB1 mediates the proteasomal degradation of the autophagy regulator TFEB has provided new insight into the interaction between these two degradation systems.
Overall, we revealed that STUB1 plays a role in maintaining the homeostasis of the autophagy-lysosome pathway and mitochondrial biogenesis by modulating phosphorylated TFEB. The diagram in Fig. 1 illustrates a proposed model of how STUB1 regulates TFEB activity. At resting state, activated mTOR phosphorylates TFEB, resulting in a low baseline TFEB activity. Upon stress, such as in starvation and/or mTOR inhibition, there is enhanced interaction between STUB1 and phosphorylated TFEB. The latter is ubiqutinated by STUB1 and is targeted for proteasomal degradation. Non-phosphorylated TFEB translocates to the nucleus and upregulate genes of the autophagy-lysosomal and mitochondrial pathways as well as TFEB itself. The results of this study suggest that targeting phosphorylated TFEB for degradation is an important mechanism to enhance TFEB activity. TFEB is active as a dimer.7 In the absence of phosphorylated TFEB, there is increased formation of active non-phosporylated homodimers of TFEB. One important implication for this cellular strategy of degrading inactive TFEB is the need to re-synthesize new TFEB. In support of this hypothesis, it has been shown that TFEB promotes its own transcription.8 In STUB1 deficient cells, phosphorylated TFEB is not efficiently degraded. Accumulating phosphorylated TFEB is inactive and it further reduces TFEB activity by forming heterodimers with nonphosphorylated TFEB, leading to reduced TFEB translocation to the nucleus. Reduced TFEB activity, in STUB1 deficient cells, leads to inhibition of autophagy-lysosome pathway and mitochondrial biogenesis. Taken together, our study suggests that the mechanism of degrading inactive phosphorylated TFEB and re-synthesizing new TFEB is an important cellular mechanism to cope with cellular stress conditions requiring increased autophagic-lysosomal and mitochondrial biogenesis.
Figure 1.
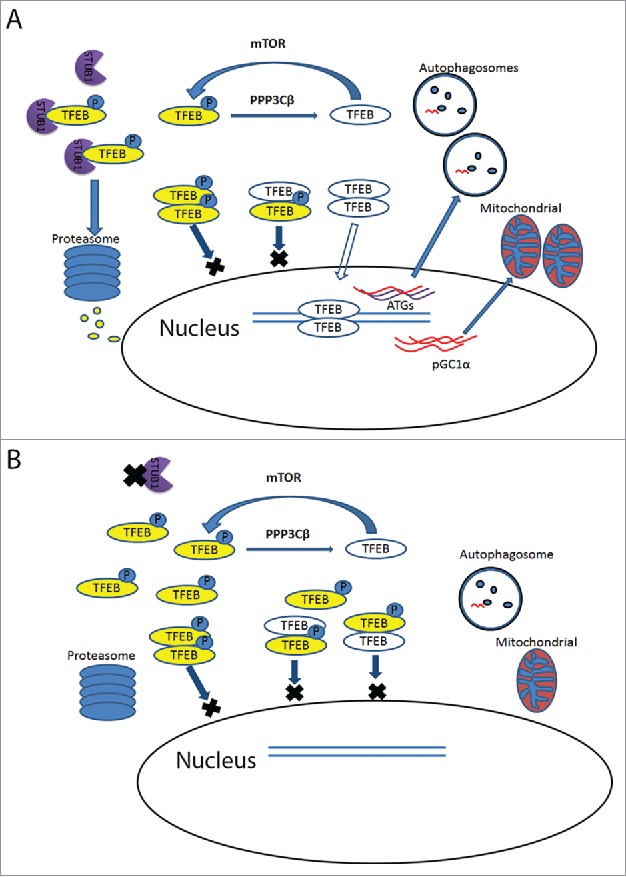
Mechanisms of TFEB regulation by STUB1. A. TFEB is phosphorylated by mTOR and dephosphorylated by PPP3Cβ. STUB1 interacts preferentially with the phosphorylated form of TFEB, ubiquitinates it and target it for proteasomal degradation. Degradation of the phosphorylated TFEB increases the proportion of non-phosphorylated TFEB dimers, which translocate to the nucleus and exerts its effect in increasing autophagosome, lysosome and mitochondrial biogenesis. B. STUB1 deficiency causes accumulation of the cytoplasmic phosphorylated TFEB. Phosphorylated TFEB is inactive, as it cannot translocate to the nucleus. More importantly, by forming heterodimers with nonphosphorylated TFEB, it exerts a dominant negative effect on TFEB activity. Reduced TFEB activity leads to inhibition of autophagy-lysosome pathway and mitochondrial biogenesis.
TFEB upregulates the expression of key genes involved in folding, proteostasis and lysosomal trafficking pathways. Further, activation of TFEB attenuates lysosomal storage pathology both in vitro and in vivo.9 Due to TFEB's ability to regulate autophagy, TFEB is now considered a therapeutic target in diseases associated with deficiencies in the autophagic-lysosomal pathways.10 In neurodegenerative diseases, activation of TFEB has been found to improve autophagic-lysosomal function and ameliorate neurodegeneration, by improving behavioral deficits10. Given its critical role in the modification of TFEB's activity, our work suggests that STUB1 may be a new potential therapeutic target in these diseases.
References
- 1.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, et al.. A gene network regulating lysosomal biogenesis and function. Science. 2009. July 24;325(59):473-7. [DOI] [PubMed] [Google Scholar]
- 2.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al.. TFEB links autophagy to lysosomal biogenesis. Science 2011;332:1429-33. doi: 10.1126/science.1204592. PMID:21617040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, et al.. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012 Mar 7; 31(5):1095-108. doi: 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, et al.. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 2015; 17:288-99. doi: 10.1038/ncb3114. PMID:25720963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sha Y, Rao L, Settembre C, Ballabio A, Eissa NT. STUB1 regulates TFEB-induced autophagy-lysosome pathway. EMBO J. 2017 Jul 28;pii: e201796699 [Epub ahead of print]. doi: 10.15252/embj.201796699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meacham GC, Patterson C, Zhang W, Younger JMCyr DM The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100-5. doi: 10.1038/35050509. PMID:11146634 [DOI] [PubMed] [Google Scholar]
- 7.Fisher DE, Carr CS, Parent LA, Sharp PA. TFEB has DNA-binding and oligomerization properties of a unique helix-loop-helix/leucine-zipper family. Genes Dev 1991;5:2342-52. doi: 10.1101/gad.5.12a.2342. PMID:1748288 [DOI] [PubMed] [Google Scholar]
- 8.Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Klisch TJ, et al.. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013 Jun;15(6):647-58. doi: 10.1038/ncb2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, et al.. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011 Sep 13;21(3):421-30. doi: 10.1016/j.devcel.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martini-Stoica H, Xu Y, Ballabio A, Zheng H. The Autophagy-Lysosomal Pathway in Neurodegeneration: A TFEB Perspective. Trends Neurosci. 2016 Apr;39(4):221-34. doi: 10.1016/j.tins.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


