ABSTRACT
The class III PI 3-kinase, VPS34 forms distinct complexes essential for cargo sorting and membrane trafficking in endocytosis as well as for autophagosome nucleation and maturation. We used integrative structural biology approach to provide insights into the conformational dynamics of the complex and mechanisms that regulate VPS34 activity at the membrane.
KEYWORDS: Autophagy, lipid kinase, protein dynamics, autophagy activators, VPS34
Autophagy is a highly regulated process of degradation and recycling of cellular components and pathogens, resulting in the management of biosynthetic precursors and allowing cells to temporarily overcome adverse conditions.1 The principal process in autophagy is the formation of a double-membrane organelle, the autophagosome, that encapsulates cellular components targeted for degradation. Autophagosome biogenesis consists of several sequential steps: initiation, nucleation, elongation and maturation of the double membrane – resulting in sequestration of cargo molecules and transport to the vacuole or lysosome for degradation. The classic initiator of autophagy is nutrient starvation. Starvation induces autophagy through deactivation of the nutrient sensing mechanistic target of rapamycin complex 1 (mTORC1). Inhibition of mTORC1 results in Unc-51 like autophagy activating kinase 1 (ULK1) activation, which in turn promotes recruitment of the class III phosphatidylinositol 3-kinase complex I (PI3KC3-C1) to the endoplasmic reticulum (ER). Activated PI3KC3-C1 generates the lipid phosphatidylinositol 3-phosphate PtdIns(3)P, which is responsible for recruitment of the downstream set of autophagy-regulating proteins to nucleate the isolation membrane in the proximity of cargo.2
In mammalian cells there are 2 different tetrameric PI3KC3 complexes, PI3KC3-C1 and PI3KC3-C2 involved at different stages of autophagy.3,4 The autophagy specific PI3KC3-C1 is involved in autophagosome formation and includes the core complex (phosphoinositide 3-kinase regulatory subunit 4 (also known as p150 or VPS15), phosphatidylinositol 3-kinase catalytic subunit type 3 (also known as PI3KC3 or VPS34) and Bcl2-interacting protein 1 (BECN1)) and Beclin 1-associated autophagy-related key regulator (also known as BARKOR or ATG14). PI3KC3-C2 is involved in autophagosome and endosome maturation and includes the core complex and UV radiation resistance-associated gene protein (UVRAG). VPS34 is a class III phosphatidylinositol 3-kinase at the heart of the PI3KC3 complexes, directly responsible for the production of the key lipid PtdIns(3)P 5,6
PI3KC3 complexes have been linked to human aging processes and a wide range of illnesses, including cancer and neurodegenerative diseases.7 BECN1 has special status in oncology since it was shown to be a haploinsufficient tumor suppressor gene that is monoallelically deleted in 40–75% of cases of human breast, ovarian, and prostate cancer.8 The recognition that autophagy may prevent the occurrence or delay the progression of certain diseases provides the primary rationale for the development of novel pharmacologic agents that can be potentially used as specific inhibitors and activators of the complexes. In the recent years, we have witnessed significant efforts to develop specific VPS34 inhibitors for use in the treatment of tumors. On the other hand, autophagy-inducing drugs are also currently undergoing rapid development as a potential approach to safe treatment of a wide range of diseases including neurodegeneration, cancer, atherosclerosis, diabetes, intracellular bacterial infections and others.9 Given these essential roles in the autophagy processes and disease pathology, it is important to gain a deeper understanding of how this complex is regulated. We used a hybrid structural biology approach combining electron microscopy, biochemistry and structural proteomics to characterize VPS15 and VPS34 kinase domain dynamics and their role in regulation of the autophagic PI3KC3 complex.10
To understand the structure and dynamics of PI3KC3-C1 and the VPS34 lipid kinase domain, we reconstituted the whole human PI3KC3-C1 complex and analyzed it by negative stain electron microscopy.5 We found that PI3KC3-C1 samples several distinctive conformational states. The most prominent state corresponds to the previously characterized “classic” conformation. In this state, the PI3KC3 complex displays V-shaped architecture, with the short arm of the complex containing the tightly interacting kinase domains of VPS34 and VPS15, whereas the other arm includes long coiled-coils and C-terminal domains of Beclin1 and Atg14 and VPS15 WD40 domain. This direct interaction between the VPS34 and VPS15 kinase domains appeared to us to be the best candidate for the mechanism how VPS15 regulates the kinase activity of VPS34. In agreement with this hypothesis we found that VPS34 lipid kinase domain can dissociate from VPS15 kinase and completely dislodge from the rest of the complex. In this dislodged state VPS34 kinase domain is not sterically restrained by the rest of the complex and appears to exist in multitude of conformations. To rule out negative staining artifacts and assess VPS34 conformational dynamics in solution, we performed chemical crosslinking coupled with mass spectrometry. We found long-range contacts that are consistent with VPS34 kinase domain dissociating from VPS15 and sampling a wide range of positions. These multiple interactions revealed by our crosslinking imply that VPS34 kinase domain is highly dynamic in solution and provide a validation of the observations of dynamics from EM.
Having found that VPS34 kinase domain can adapt different conformational states in solution, we went on to characterize its activity at the membrane. VPS34 activity was assessed alone and in the context of fully assembled complex. We found that PI3P production by isolated VPS34 reached only a small fraction of the level achieved by the fully assembled complex.10 Therefore, VPS34 association with rest of the complex appears to be essential for its function by enabling its recruitment to the membrane. This finding suggests that fully assembled complexes should be targeted for development of novel pharmacologic agents that could potentially serve as specific activators of VPS34. To understand how different conformational states of VPS34 kinase domain contribute to the complex activity, we engineered a construct that “leashed” the C-terminus of VPS34 to the N-terminus of VPS15 via a flexible linker.10 The principal idea behind this experiment was to prevent the VPS34 catalytic domain from dislodging from the complex, thereby keeping it in the sterically restrained state. This was confirmed by EM analysis that revealed absence of dislodged conformations in the leashed construct while preserving close interaction with VPS15 kinase domain. We have then assessed VPS34 lipid kinase activity in vitro. PI3P formation by leashed complex was completely inactive as compared with the fully assembled unleashed complex. This could be recapitulated in in vivo Pho8Δ60 processing activity assay where VPS34-VPS15 fusion resulted in a strong autophagy defect. These observations provide detailed insights into conformational changes required to reach optimal catalytic activity upon PI3KC3 complex membrane binding.
Together, the EM structure of the PI3KC3 complex, conformational dynamics analysis, lipid kinase activity and in vivo data allowed us to propose a model of how VPS34 complex assembly and conformational dynamics regulate its activity at the membrane (Fig. 1). To provide the optimal interaction with the lipid substrate, VPS34 must undergo VPS15-dependent assembly into the full complex. This ensures that VPS34 activity is only possible upon correct membrane targeting and so prevents nonspecific lipid phosphorylation elsewhere in the cell. Upon complex assembly and membrane binding, VPS34 catalytic activity is further sterically regulated by the interaction between the VPS34 and the VPS15 kinase domains. Our data show the central role of VPS15 in both positive and negative regulation of PI3KC3. In considering how this information might affect activator design, factors that promote assembly of the VPS15-VPS34 and BECN1-ATG14 subcomplexes with one another, yet disfavor VPS15-VPS34 kinase domain interactions, might serve as autophagy inducers.
Figure 1.
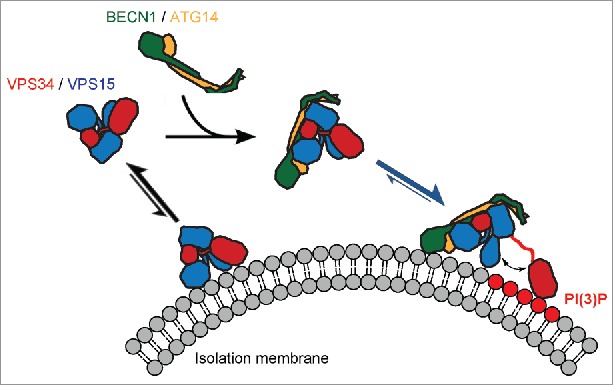
Potential mechanism for VPS34 complex assembly and activation at the membrane. Subunits are colored as follows: phosphatidylinositol 3-kinase catalytic subunit type 3 (VPS34), red; phosphoinositide 3-kinase regulatory subunit 4 (VPS15), blue; Bcl2-interacting protein 1 (BECN1), green; Beclin 1-associated autophagy-related key regulator (ATG14), orange. Phosphorylated phosphatidylinositol headgroups PtdIns(3)P are highlighted in red. Arrows indicate chemical equilibria. The scheme shows that the VPS34:VPS15 and BECN1:ATG14 subcomplexes are stable independently, however the VPS34:VPS15 subcomplex has low kinase activity and a low affinity for the membrane in the absence of the BECN1:ATG14 complex. Assembly into the 4-subunit PI3KC3-C1 complex is mediated by the HEAT and WD40 domains of the VPS15 subunit and their contacts with the BECN1:ATG14 subcomplex. This increases the affinity for the membrane and primes the complex for activation. The final step required for full activation is the dislodging of the VPS34 catalytic domain from the rest of the complex such that it engages with the membrane-bound substrate with optimal geometry and is freed from potentially inhibitory contacts with the VPS15 kinase domain.
Funding details
This research was supported by the NIH under grants GM051487 and GM111730. J.H.H. is a founder of Kinacticor.
References
- 1.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181(3):497–510. doi: 10.1083/jcb.200712064. PMID:18443221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurley JH, Young LN, Mechanisms of Autophagy Initiation. Annu Rev Biochem. 2017;86:225–244. doi: 10.1146/annurev-biochem-061516-044820. PMID:28301741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backer JM. The intricate regulation and complex functions of the Class III phosphoinositide 3-kinase Vps34. Biochem J. 2016;473:2251–71. doi: 10.1042/BCJ20160170. PMID:27470591 [DOI] [PubMed] [Google Scholar]
- 4.Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–30. doi: 10.1083/jcb.152.3.519. PMID:11157979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baskaran S, Carlson LA, Stjepanovic G, Young LN, Kim DJ, Grob P, Stanley RE, Nogales E, Hurley JH. Architecture and Dynamics of the Autophagic Phosphatidylinostol 3-Kinase Complex. eLife. 2014;3:1–19. doi: 10.7554/eLife.05115. PMID:25490155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rostislavleva K, Soler N, Ohashi Y, Zhang L, Pardon E, Burke JE, Masson GR, Johnson C, Steyaert J, Ktistakis NT, et al., Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science. 2015;350(6257):1–11. doi: 10.1126/science.aac7365. PMID:26450213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, et al., Autophagy in malignant transformation and cancer progression. Embo J. 2015;34(7):856–80. doi: 10.15252/embj.201490784. PMID:25712477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–6. [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G., Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16(7):487–511. doi: 10.1038/nrd.2017.22. PMID:28529316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stjepanovic G, Baskaran S, Lin MG, Hurley JH. VPS34 kinase domain dynamics regulate the autophagic PI 3-kinase complex. Mol Cell. 2017;67(3):528–34. doi: 10.1016/j.molcel.2017.07.003. PMID:28757208 [DOI] [PMC free article] [PubMed] [Google Scholar]


