ABSTRACT
Telomeres cap the ends of chromosomes and are crucial for genome stability. The p53 protein (TP53) is a vital tumor suppressor, activating the transcription of numerous genes in response to cell stress. We reported that direct binding of p53 at human subtelomeres corresponds with local transcription activation and enhanced telomere stability in the presence of DNA damage.
KEYWORDS: Chromatin, DNA-damage, eRNA, telomere, TERRA, TP53, yH2AX
Telomeres are composed of long stretches of repetitive DNA that are bound specifically by components of the shelterin protein complex. Shelterins regulate the length and overall structure of the telomere and prevent the ends of the chromosomes from being detected as DNA damage.7 Telomere dysfunction, whether induced by attrition through excessive cell divisions or by genotoxic stress, can induce cellular senescence and thereby inhibit tumor progression.8 Although telomeres possess features of heterochromatin they can produce telomere repeat-containing RNA (TERRA), which initiates in the adjacent subtelomere region.1 TERRA induction can result from DNA damage, and a requirement for p53 (TP53) has been reported.3
The p53 protein is a DNA-binding transcription factor with potent tumor suppressor properties.5 In response to cell stress, p53 protein accumulates and binds to degenerate consensus sequences throughout the genome, activating the transcription of many target genes involved in DNA damage repair, apoptosis, and other cellular processes.2 The tumor suppressor function of p53 is, however, not entirely attributable to its role in transcription activation, and indeed the majority of p53 binding sites are not associated with a target gene.4
Both telomeres and p53 play important roles in the maintenance of genome integrity and tumor suppression; however, direct involvement of p53 in telomere function has not been previously described. We recently reported the presence of non-canonical p53 response elements (REs) in the subtelomere region of human and mouse chromosomes. Binding of p53 to these sites stimulated local transcription and enhanced telomere stability in the presence of DNA damage.9
By combining p53 chip-seq data with the most recent build of the human subtelomere genome we identified 12 high-confidence binding sites in proximity to the telomere tract. These peaks correspond to a sequence element defined by RepeatMasker as MER31-int/(TG)n/LTR10C, a string of nucleotides largely restricted to the subtelomere that contain several consecutive degenerate p53 consensus half-sites with no spacers. The binding of p53 to several of these sites was confirmed by conventional chip-qPCR.
These subtelomeric p53 REs resemble enhancer elements in several different ways, consistent with recent reports on p53 function.6 When cloned upstream of a reporter, the 18q p53 RE confers transcription activation in a p53-dependent manner, with activity comparable to that of the MDM2 promoter. In stressed cells, p53 status correlates with induction of TERRA, local subtelomeric genes, and with a short transcript resembling an enhancer RNA (eRNA). Metadata analysis of public groSeq data reveals bidirectional transcription originating from the 18q RE induced by Nutlin-3a–activated p53. Finally, p53 accumulation at subtelomere REs is accompanied by local histone H3 acetylation at K27, a mark of active enhancers, and at K9, a modification associated with transcription activation.
Our data implicate a protective effect of p53 at the telomere in response to DNA damage. The phosphorylation of histone variant H2A.X (H2AFX) on serine 139 (referred to as γH2AX) is an early local event in response to DNA double-strand breaks. Accumulation of γH2AX at the subtelomere in response to etoposide is suppressed when p53 is present, as is global production of γH2AX. Cells expressing p53 are better able to produce colonies following DNA damage, and have telomeres that are more resilient. In cells lacking p53, DNA damage causes a rapid loss of telomere length and an increase in telomere-dysfunction foci. Finally, when the 18q p53 RE is removed using Crispr genome editing, p53 is prevented from binding, local transcription is not induced, and local γH2AX levels are increased in response to DNA damage.
Telomeres and the p53 protein are both critical for chromosomal stability, and it is now apparent there is a node of crosstalk between these 2 important mechanisms of tumor suppression. The direct binding of p53 to the subtelomere in response to DNA damage induces changes to local chromatin structure and transcription, which function to stabilize the telomere (Fig. 1). Further studies will be required to determine the exact mechanism, but we propose that p53 can recruit chromatin remodeling factors and DNA damage repair proteins directly to subtelomeres. The DNA damage-induced binding of p53 to chromosome fragile sites such as subtelomeres therefore enhances the ability of these regions to repair and replicate their DNA. In this way, p53 can directly enhance the stability of telomeres in response to DNA damage stress.
Figure 1.
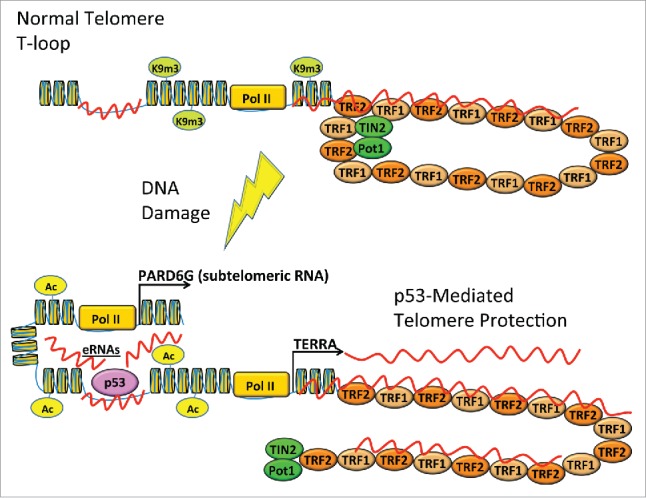
Local binding of p53 in response to DNA damage protects telomeres. Undamaged telomeres are heterochromatic and form T loop structures. When stressed by DNA damage, p53 (TP53) binds to unique response elements in the subtelomere and induces local histone acetylation and transcription. These changes prevent the accumulation of γH2AX (phospho-S139 H2A.X [H2AFX]) and stabilize the telomere tract.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Azzalin CM, Lingner J. Telomere functions grounding on TERRA firma. Trends Cell Biol 2015; 25:29–36; PMID:25257515; https://doi.org/ 10.1016/j.tcb.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 2.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 2014; 14:359–70; PMID:24739573; https://doi.org/ 10.1038/nrc3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caslini C, Connelly JA, Serna A, Broccoli D, Hess JL. MLL associates with telomeres and regulates telomeric repeat-containing RNA transcription. Mol Cell Biol 2009; 29:4519–26; PMID:19528237; https://doi.org/ 10.1128/MCB.00195-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang GS, Chen XA, Park B, Rhee HS, Li P, Han KH, Mishra T, Chan-Salis KY, Li Y, Hardison RC, Wang Y, Pugh BF. A comprehensive and high-resolution genome-wide response of p53 to stress. Cell Rep 2014; 8:514–27; PMID:25043190; https://doi.org/ 10.1016/j.celrep.2014.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane DP. Cancer. p53, guardian of the genome. Nature 1992; 358:15–6; PMID:1614522; https://doi.org/ 10.1038/358015a0 [DOI] [PubMed] [Google Scholar]
- 6.Melo CA, Drost J, Wijchers PJ, van de Werken H, de Wit E, Oude Vrielink JA, Elkon R, Melo SA, Leveille N, Kalluri R, et al.. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell 2013; 49:524–35; PMID:23273978; https://doi.org/ 10.1016/j.molcel.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 7.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet 2008; 42:301–34; PMID:18680434; https://doi.org/ 10.1146/annurev.genet.41.110306.130350 [DOI] [PubMed] [Google Scholar]
- 8.Suram A, Herbig U. The replicometer is broken: telomeres activate cellular senescence in response to genotoxic stresses. Aging Cell 2014; 13:780–6; PMID:25040628; https://doi.org/ 10.1111/acel.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tutton S, Azzam GA, Stong N, Vladimirova O, Wiedmer A, Monteith JA, Beishline K, Wang Z, Deng Z, Riethman H, McMahon SB, Murphy M, Lieberman PM.. Subtelomeric p53 binding prevents accumulation of DNA damage at human telomeres. EMBO J 2015; 35:193–207; PMID:26658110; https://doi.org/ 10.15252/embj.201490880. [DOI] [PMC free article] [PubMed] [Google Scholar]


