ABSTRACT
Purpose: Following a first phase of trachoma mapping in Malawi with the Global Trachoma Mapping Project, we identified and mapped trachoma districts previously suspected to be non-endemic, although adjacent to districts with estimated trachoma prevalences indicating a public health problem.
Methods: We conducted population-based surveys in eight evaluation units (EUs) comprising eight districts in Malawi (total population 3,230,272). A 2-stage cluster random sampling design allowed us to select 30 households from each of 30 clusters per EU; all residents aged 1 year and older in selected households were examined for evidence of trachomatous inflammation–follicular (TF) and trachomatous trichiasis (TT).
Results: None of the eight EUs had a TF prevalence in 1–9-year-olds ≥10%, one district (Dedza) had a TF prevalence between 5.0% and 9.9%, and only one district (Karonga) had a trichiasis prevalence in adults ≥0.2%.
Conclusion: The prevalence of TF and TT in six of eight EUs surveyed was consistent with an original categorization of trachoma being unlikely to be a public health problem. In the absence of formal surveys, health management information system data and other locally available information about trachoma is likely to be useful in predicting areas where public health interventions against trachoma are required.
KEYWORDS: Blindness, epidemiology, Global Trachoma Mapping Project, Malawi, mapping, mHealth, neglected tropical diseases, prevalence, trachoma, trichiasis
Introduction
The clinical features of trachoma, an infectious and potentially blinding eye disease caused by the bacterium Chlamydia trachomatis, are fully described elsewhere 1 – 4 and are not the focus of this article. Previous findings by Kalua and colleagues 5 ,6 demonstrate that trachoma affects a substantial number of people in Malawi, although the problem is not as bad as it is in other endemic populations. 7
As part of the Global Trachoma Mapping Project (GTMP), all 17 suspected trachoma-endemic districts of Malawi were mapped between 2013 and 2014, and those confirmed as having trachoma as a public health problem have now started to implement the SAFE (Surgery, Antibiotics, Facial cleanliness, and Environmental improvement) strategy and are progressing well, with Malawi targeting to eliminate trachoma as a public health problem by 2019. A total of 11 remaining districts (five in the Northern Region, one in the Central Region, and five in the Southern Region), were not mapped in phase one of the GTMP in Malawi, based on data collected by the heath management information systems and other anecdotal information suggesting that trachoma was unlikely to represent a public health problem in these districts. Following completion of phase one mapping, however, Malawi Ministry of Health officials were able to consider trachoma prevalence in phase one districts and trachoma prevalence in bordering districts of Zambia, Tanzania, and Mozambique, and consensus was reached that mapping was needed in eight of the previously unsuspected districts (five Northern, one Central, and two Southern) of Malawi. The remaining three districts still lacked a rationale to undertake population-based prevalence surveys of trachoma.
With support from the GTMP, we set out to map these eight districts, in order to complete baseline trachoma mapping in Malawi.
Materials and methods
Approval was obtained from the National Health Sciences Research Committee of Malawi, and from the district health administrative offices. Approval for the GTMP in its entirety was obtained from the ethics committee of the London School of Hygiene & Tropical Medicine (reference 6319). Upon explanation of the purpose of the study, written informed consent was obtained from all subjects who participated in the study. Where the participant was a minor, informed consent was obtained from the head of household or guardian.
The study was a series of cross-sectional population-based surveys designed to obtain district level prevalence estimates of trachomatous inflammation–follicular (TF) in children aged 1–9 years, and trichiasis in persons aged 15 years and older. The study was conducted between May 2014 and July 2015. We used the GTMP standardized training package and methodologies, as described previously. 8 The Malawian team leader (KK), who was a GTMP Master Grader, conducted a 3-day field practice refresher course for graders, all of whom had been GTMP-certified in 2013. 5 ,6
Sample size
As described elsewhere, 8 the GTMP sample size in each evaluation unit is based on an expected TF prevalence in 1–9-year-olds of 10%, since this is the most critical threshold for programmatic decision making. Evaluation units in which the baseline TF prevalence in 1–9-year-olds is 10% or higher qualify for annual single-dose azithromycin treatment of the entire population for at least 3 years, whereas those with a TF prevalence in 1–9-year-olds <10% do not. According to the single population proportion for precision formula, if the expected TF prevalence is 10% and we wish to have 95% confidence of estimating the true prevalence with absolute precision of 3%, 384 children aged 1–9 years selected by simple random sampling would be required. Assuming a cluster size of 50 children, the design effect is estimated at 2.65 (based on previous trachoma prevalence surveys), so 1019 children are needed. Inflating this figure by a factor of 1.2 to account for nonresponse, we sampled a sufficient number of households in each evaluation unit for 1222 children aged 1–9 years to be resident therein. Using 30 clusters of 30 households each (based on the number of households that a team should be able to complete in a day), and assuming there are 1.4 Malawian children aged <9 years in each household, this sample size is achieved. Although we also aim to estimate trachomatous trichiasis (TT) prevalence in adults aged 15 years and older, sample sizes have been calculated based only on parameters relating to TF in children; the low prevalence of TT (nearly always <0.2% in adults except in the most hyper endemic areas) means that accurately estimating its prevalence requires substantially larger samples. Having determined the number of households required to recruit sufficient children to estimate TF prevalence as above, the sample of adults aged 15 years and older used for estimating TT prevalence is set as the adults living in those same households. We accept the loss of precision in the estimate of TT prevalence inherent in this approach.
Selection of clusters, households and individuals
In the first sampling stage, clusters were defined as villages, which in rural Malawi have a mean population of 1000–2000 residents. In each evaluation unit, a sampling frame of all villages was obtained from the district health office, and 30 were selected with probability proportional to size. In the second sampling stage, in each selected cluster, all head of households from the entire cluster were pre-listed by a community health care worker, and 30 of these were selected by matching the list with 30 pre-selected numbers using computer-generated random numbers. All residents of selected households aged 1 year and older were invited to participate.
Field methods
Each selected cluster was visited a few days in advance of the survey date by a community health worker (“Health Surveillance Assistant”) from the Ministry of Health; this cadre is normally responsible for disease surveillance and health promotion. Their role here was to brief the village chief and community members and organize the selected village household list to be used for random household selection by the survey team. Upon arrival in the villages, the survey team made a random selection of households. Teams then went house-to-house; after obtaining consent from the household head, global positioning system and water, sanitation and hygiene (WASH) data were collected, 8 and household members were enumerated and examined for signs of trachoma, using a 2.5× magnifying loupe (Binomag, Texas/Oklahoma, USA). Individuals found to have active trachoma were offered two tubes of 1% tetracycline eye ointment and adults with trichiasis were referred to the district hospital.
Quality control
The 10 teams were supervised by two supervisors, with each team being accompanied by a supervisor for one in every five field days. Supervisors were experienced ophthalmic clinical officers who had been trained and GTMP-certified as grader trainers and were part of the training team. In addition, at the completion of field work for each evaluation unit, investigators, supervisors and recorders met to discuss logistical challenges faced and suggest solutions and improvements.
Data management, analysis and reporting
Field data collection was performed using Android smartphones running the LINKS application system (Task Force for Global Health, Decatur, GA, USA). 9 Upon completion of field work each day, the recorder uploaded survey data from the Android smartphone over an encrypted connection to the GTMP secure server running in the Amazon.com Elastic Cloud (AWS EC2).
Results
Figure 1 is a map of all districts in Malawi. A total of 240 clusters from eight evaluation units (districts) were visited, and 27,765 residents examined, of whom 10,505 (37.8%) were children aged 1–9 years, 4092 (14.7%) were children aged 10–14 years, and 13,168 (47.4%) were adults aged over 15 years (Table 1).
Figure 1.
Map of Malawi showing regions and districts.
Table 1.
Sex distribution of examined individuals, Global Trachoma Mapping Project, Malawi phase two, 2014–2015.
| Sex | Total, n (%) | Children 1–9 years, n (%) | Adults 15+ years, n (%) |
|---|---|---|---|
| Male | 12,633 (45.50) | 5230 (49.79) | 5440 (41.31) |
| Female | 15,132 (54.50) | 5275 (50.11) | 7728 (58.69) |
| Total | 27,765 (100) | 10,505 (100) | 13,168 (100) |
The list of districts that were previously suspected not to have trachoma at levels indicative of a public health problem is shown in Table 2, and the data from the mapping exercise described here are shown in Table 3.
Table 2.
List of all districts in Malawi not mapped for trachoma prior to May 2014, and action taken, Global Trachoma Mapping Project, Malawi phase two, 2014–2015.
| Region | District | Populationa, N | Additional information received | Action taken |
|---|---|---|---|---|
| North | 1. Mzimba north | 261,332 | Adjacent to Zambia districts with TF >10% | Changed to suspected endemic and mapped |
| 2. Mzimba south | 572,079 | Borders Kasungu, which had TF >10% | Changed to suspected endemic and mapped | |
| 3. Nkhatabay and Likomab | 251,477 | Adjacent to Malawi district with TF >10% | Changed to suspected endemic and mapped | |
| 4. Rumphi | 197,200 | Adjacent to Zambia districts with TF >10% | Changed to suspected endemic and mapped | |
| 5. Karonga | 318,098 | Adjacent to Tanzania districts with TF >10% | Changed to suspected endemic and mapped | |
| 6. Chitipa | 208,815 | Adjacent to Tanzania districts with TF >10% | Changed to suspected endemic and mapped | |
| Central | 7. Dedza | 727,396 | Adjacent to Malawi and Mozambique districts with TF >10% | Changed to suspected endemic and mapped |
| South | 8. Mulanje | 612,699 | Adjacent to Mozambique districts with TF >10% | Changed to suspected endemic and mapped |
| 9. Blantyre | 1,040,652 | Not adjacent to any district in which trachoma is a public health problem | Suspected non-endemic; not mapped | |
| 10. Thyolo | 658,085 | Not adjacent to any district in which trachoma is a public health problem | Suspected non-endemic; not mapped | |
| 11. Chirazulu | 339,271 | Not adjacent to any district in which trachoma is a public health problem | Suspected non-endemic; not mapped |
aCalculated based on projections from 2008 census figures. 16
bLikoma is an island with a resident population of 11,859; it was mapped in an evaluation unit with Nkhatabay rather than as a separate evaluation unit, even though it is administratively recognized as a separate district.
TF, trachomatous inflammation–follicular.
Table 3.
Prevalences of trachomatous inflammation–follicular (TF) in 1–9-year-old children, and trichiasis in the whole population, Global Trachoma Mapping Project, Malawi phase two, 2014–2015.
| Region | District | TF in children 1–9 years, % (95% CI) | Trichiasis in population/1000, (95% CI) | Final status after mapping |
|---|---|---|---|---|
| North | 1. Mzimba north | 1.6 (0.3–3.2) | 0.9 (0.1–2.2) | Trachoma not a public health problem |
| 2. Mzimba south | 4.3 (2.4–6.8) | 0.7 (0.2–1.5) | Trachoma not a public health problem | |
| 3. Nkhatabay and Likomaa | 2.8 (1.1–4.6) | 0.7 (0.2–1.3) | Trachoma not a public health problem | |
| 4. Rumphi | 2.1 (1.1–3.3) | 0.6 (0.1–1.3) | Trachoma not a public health problem | |
| 5. Karonga | 4.1 (2.6–6.2) | 2.2 (1.2–3.3) | Trichiasis above threshold for elimination | |
| 6. Chitipa | 1.4 (0.3–2.7) | 0.0 (0.0–0.4) | Trachoma not a public health problem | |
| Central | 7. Dedza | 6.3 (4.5–8.6) | 0.0 (0.0–0.4) | TF above threshold for elimination |
| South | 8. Mulanje | 2.1 (0.8–3.9) | 0.0 (0.0–0.0) | Trachoma not a public health problem |
aLikoma is an island with a resident population of 11,859. It was mapped in an evaluation unit with Nkhatabay rather than as a separate evaluation unit, even though it is administratively recognized as a separate district.
Bold text: needs program intervention.
CI, confidence interval.
For TF, except for Dedza in the Central Region (which had a TF prevalence in 1–9-year-olds of 6.3%), all eight districts mapped in phase two had TF prevalences <5%, indicating that active trachoma was not present at levels suggesting a public health problem.
For trichiasis, in the phase two districts, only Karonga in the far north of the country had a prevalence in adults above the threshold (≥0.2%) defined as representing a public health problem. 10 ,11 Note that there was no justification for mapping of Blantyre, Thyolo, and Chirazulu, which therefore remain categorized as “suspected non-endemic” districts. Of 28 districts in Malawi, these are the only three that have now not been mapped.
Figure 2 is the updated baseline prevalence map for TF in Malawi, according to the most recent survey data, including those reported in the current manuscript. As can be readily seen, the TF prevalence in each district in Northern Malawi is below 5%.
Figure 2.
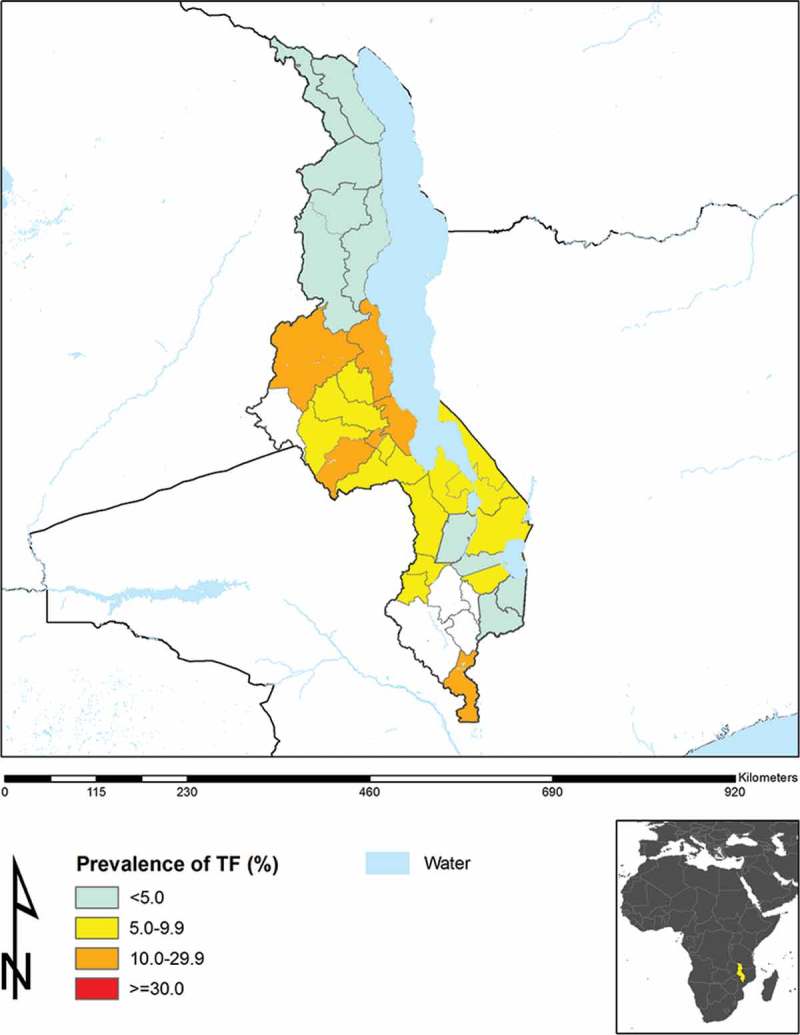
Prevalence of trachomatous inflammation–follicular (TF), Malawi.
Figure 3 shows the updated prevalence of trichiasis in Malawi, according to the most recent survey data, including those reported in the current manuscript. District-level prevalences of WASH access variables are not presented here, as these need much more detailed analysis, and will be considered separately.
Figure 3.
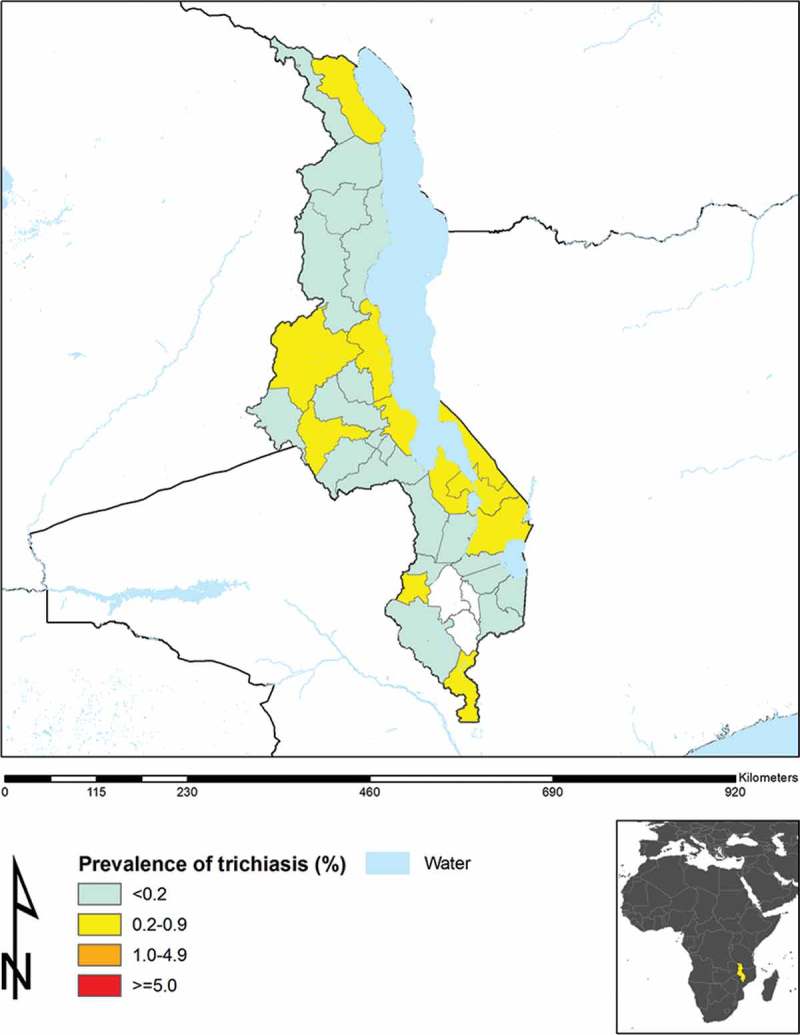
Prevalence of trichiasis, Malawi.
Discussion
Using the GTMP methodology, we set out to generate prevalence data on TF and trichiasis in districts that were previously suspected not to have trachoma as a public health problem, but which bordered districts in Malawi and adjacent countries that had relatively high burdens of trachoma. This has enabled us to complete the baseline trachoma map for Malawi, as part of the broader effort to finalize mapping of trachoma globally. 7 ,12 A more important domestic outcome is the contribution that these data will now make to guiding national policy and decision-making in regards to implementation of the SAFE strategy for trachoma elimination. Hearteningly, our data show that only two of the evaluation units mapped in phase two had either trichiasis or TF at levels warranting public health concern. One of those districts was Karonga, where the TF prevalence was <5% but the trichiasis prevalence was 0.2%. Karonga district borders Mbeya region of Tanzania, which has a moderately high prevalence of trichiasis. 19 It was initially hypothesized that perhaps the individuals with trichiasis seen in our Karonga survey were there as a result of migration of people with pre-existing cicatricial disease from across the shared border with Tanzania, rather than representing home-grown cases of trichiasis. However, some of the affected communities in Karonga are located quite some distance from the Tanzanian border, and travel between those communities and Tanzania is reportedly rare, seemingly making the issue of cross-border movement an unlikely explanation for the trichiasis cases that we found.
The only way to (circumstantially) confirm that trichiasis is likely to be trachomatous is to record the presence of trachomatous conjunctival scarring in the affected eye or eyes, because conjunctival scarring is a precursor to TT. Unfortunately, the presence or absence of conjunctival scarring was not recorded in the first six districts surveyed here; in the same way that these data have not formed part of the definition of “trachomatous trichiasis” in other trachoma surveys conducted in Malawi and elsewhere, as part of the GTMP and outside that effort. Data on the presence or absence of scarring were collected in the remaining two districts of Malawi (Dedza and Mulanje), but trichiasis was not frequently found there, so we are unable to say very much about the relationship between conjunctival scarring and trichiasis in Malawi.
If effort is expended to ensure that as many individuals with trichiasis as possible are identified and offered surgery, Karonga District may be able to reduce its trichiasis prevalence to less than 0.2% in adults within a short time, as the current backlog, estimated at 800 cases, is realistically not very high. One surgeon can comfortably operate on up to 10–20 cases per week, and could clear the entire backlog within 2 years. There is currently no justification for mass treatment with azithromycin in Karonga, nor for specific measures to implement the F and E components of the SAFE strategy for the purposes of fulfilling a trachoma elimination agenda. 13 – 15
Similar to nine evaluation units mapped as part of GTMP Malawi phase one, 5 Dedza in phase two had a TF prevalence in 1–9-year-olds between 5.0% and 9.9%. This was not too surprising, given that this district is bordered by Lilongwe West, Ntcheu, and Mangochi, each of which have estimated TF prevalences between 5.0% and 9.9%. 5 Recommendations for such districts have now changed; sub-district level mapping is no longer advocated. Instead, a single round of azithromycin mass drug administration is suggested, together with implementation of the F and E components of the SAFE strategy, followed by an early impact survey.
Of the three remaining unmapped districts (Blantyre, Thyolo, and Chirazulu), we believe we may say with some confidence that it is unlikely that they harbor trachoma at levels posing a threat to public health. Blantyre is a major commercial hub for Malawi and has a largely urban, affluent population, while Thyolo and Chirazulu have very cold weather and contain large farms where irrigation is practiced, so access to water is generally not a challenge. We therefore do not believe that an attempt to map trachoma in these remaining districts would be worthwhile from a resource allocation perspective. For countries that are still to complete baseline trachoma mapping, this study suggests that normal data collected using the routine heath management information systems, supplemented by other locally available clues, can inform decisions on whether and where mapping should be done.
Now that mapping is complete in Malawi and there are no new districts likely to require 3 years of interventions with SAFE prior to undertaking impact surveys, Malawi is in a much better position to accelerate its current control efforts and eliminate trachoma ahead of the year 2020 international goal. 16 ,17 It is left to the Ministry of Health and supporting partners in Malawi to ensure that all those currently at risk of trachoma blindness are attended to with the utmost urgency.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.
Funding Statement
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID) through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine, and is now a staff member of the World Health Organization (WHO). The views expressed in this article are the views of the authors alone and do not necessarily reflect the decisions, policies of views of WHO. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer reviewed press, or in preparation of the manuscript.
Acknowledgments
We thank the Ministry of Health for providing field staff, the District Health Management teams, the recorders and graders, the health surveillance assistants and community volunteers who took part in the survey, and the residents of surveyed communities. Thanks should also go to the European Foundation for Neglected Tropical diseases (EFINTD) for supporting KK’s salary.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID) through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine, and is now a staff member of the World Health Organization (WHO). The views expressed in this article are the views of the authors alone and do not necessarily reflect the decisions, policies of views of WHO. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer reviewed press, or in preparation of the manuscript.
References
- 1. Solomon AW, Peeling RW, Foster A, et al Diagnosis and assessment of trachoma. Clin Microbiol Rev 2004;17:982–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thylefors B, Dawson CR, Jones BR, et al. A simple system for the assessment of trachoma and its complications. Bull World Health Organ 1987;65:477–483. [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey R. rRNA-based tests for chlamydial infection in trachoma. Br J Ophthalmol 2007;91:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mabey DC, Forsey T, Treharne JD.. Serotypes of Chlamydia trachomatis in The Gambia. Lancet 1987;2:452. [DOI] [PubMed] [Google Scholar]
- 5. Kalua K, Phiri M, Kumwenda I, et al Baseline trachoma mapping in Malawi with the Global Trachoma Mapping Project (GTMP). Ophthalmic Epidemiol 2015;22:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalua K, Chirwa T, Kalilani L, et al. Prevalence and risk factors for trachoma in central and southern Malawi. One PLoS. 2010;5:e9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith JL, Flueckiger RM, Hooper PJ, et al. The geographical distribution and burden of trachoma in Africa. PLoS Negl Trop Dis 2013;7:e2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon AW, Pavluck AL, Courtright P, et al. The Global Trachoma Mapping Project: Methodology of a 34-country population-based study. Ophthalmic Epidemiol 2015;22:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pavluck A, Chu B, Mann Flueckiger R, et al. Electronic data capture tools for global health programs: evolution of LINKS, an Android-, web-based system. PLoS Negl Trop Dis 2014;8:e2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization Report of the 2nd global scientific meeting on trachoma, Geneva, 25–27 August, 2003. Geneva: World Health Organization, 2003. [Google Scholar]
- 11. World Health Organization Report of the 3rd global scientific meeting on trachoma, Johns Hopkins University, Baltimore, MD, 19–20 July 2010. Geneva: World Health Organization, 2010. [Google Scholar]
- 12. Smith JL, Haddad D, Polack S, et al. Mapping the global distribution of trachoma: why an updated atlas is needed. PLoS Negl Trop Dis 2011;5:e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. VH Hu, EM Harding-Esch, MJ Burton, et al. Epidemiology and control of trachoma: systematic review. Trop Med Int Health 2010;15:673–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuper H, Solomon AW, Buchan J, et al. A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis 2003;3:372–381. [DOI] [PubMed] [Google Scholar]
- 15. Emerson PM, Cairncross S, Bailey RL, et al. Review of the evidence base for the ‘F’ and ‘E’ components of the SAFE strategy for trachoma control. Trop Med Int Health 2000;5:515–527. [DOI] [PubMed] [Google Scholar]
- 16. International Coalition for Trachoma Control. The end in Sight: 2020 INSIGHT. Atlanta: International Coalition for Trachoma Control, 2011. [Google Scholar]
- 17. World Health Assembly Global Elimination of blinding trachoma. 51st World Health Assembly, Geneva, 16 May 1998, Resolution WHA51.11. Geneva: World Health Organization, 1998. [Google Scholar]
- 18. Malawi Housing and Population 2008 www.nsomalawi.mw census. National Statistical office, Zomba, Malawi, 2008.
- 19. Mwingira UJ, Kabona G, Kamugisha M, et al. Progress of trachoma mapping in mainland Tanzania: results of baseline surveys from 2012 to 2014. Ophthalmic Epidemiol 2016;23:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]


