Abstract
Background/Aims
Fistula first is the general recommendation for all hemodialysis (HD) patients, but creating a mature arteriovenous fistula (AVF) can be challenging in elderly individuals. It is unclear if elderly incident HD patients derive a survival benefit from an AVF over an arteriovenous graft (AVG) or a tunneled catheter (TDC).
Methods
We examined the association of vascular access type (AVF, AVG, and TDC with and without a maturing AVF/AVG at dialysis transition) at hemodialysis initiation with all-cause, cardiovascular and infection-related mortality in 46,786 US veterans, using Cox models with adjustment for confounders. Effect modification by age was examined by examining associations in pre-specified age subgroups (<60, 60–<70, 70–<80 and ≥80 years old), and by including interaction terms.
Results
8,940 (19%) patients started HD with an AVF, 1,090 (3%) with an AVG, 8,262 (18%) with a TDC and a maturing AVF/AVG and 28,494 (61%) with a TDC without a maturing AVF/AVG. A total of 13,303 all-cause, 4,392 cardiovascular, and 1,058 infection-related deaths were observed in the first year after hemodialysis transition. Compared to patients with AVF, those with AVG and TDC with and without maturing AVF/AVG had incrementally higher overall risk of all-cause mortality and cardiovascular mortality. Only TDC use was associated with higher infection-associated mortality. These associations were not modified by age.
Conclusion
Although most of our patients consisted of male veterans and the results may not be generalized to the general population, the use of TDCs is associated with poor outcomes even in the most elderly incident hemodialysis patients.
Keywords: hemodialysis, mortality, chronic kidney disease, end-stage renal disease, vascular access
INTRODUCTION
The number of elderly patients with end-stage renal disease (ESRD) in the United States has been increasing in recent decades.[1] The National Institute on Aging reports that the United States population age 65 and over is expected to double in size within the next 25 years, and by 2030 more than 70 million people will be 65 years or older and more than 2.24 million patients will have ESRD.[1] Each year, more elderly patients transition to ESRD in the United States, the majority of whom are treated with in-center hemodialysis and require a vascular access, such as an arteriovenous fistula (AVF) or graft (AVG), or a tunneled central venous catheter (TDC).[2]
The type of vascular access for hemodialysis is one of the major factors known to be associated with mortality in ESRD patients. Clinical guidelines, including the Fistula First Breakthrough Initiative, endorse the use of AVF over AVG and TDC because it is associated with better survival and lower complications rates.[3–5] Despite higher initial successful function rates compared with AVFs and lower infection rates compared with TDCs, AVGs are the least used form of vascular access at hemodialysis initiation because they tend to require more interventions and are more likely to fail compared with AVFs.[4,6] On the other hand, multiple studies have shown that older patients are at greatest risk of having AVF maturation failure, potentially leaving them dependent on TDCs for vascular access.[7–9]
With these conflicting results about the different types of vascular access in elderly hemodialysis patients, it is unclear if elderly hemodialysis patients derive a survival benefit from an AVF over an AVG. Furthermore, knowledge is scarce about the association of access type with outcomes in the oldest hemodialysis patients (e.g. >80 years old), in whom it is possible that even a TDC could be equivalent to an AVF or AVG. We hypothesized that patients with advanced age and may not experience a survival benefit from an AVF over an AVG or a TDC during the immediate post-transition period to hemodialysis. Given that placement and maintenance are arguably more invasive for fistulas and grafts they take a longer time to mature, older individuals may not garner the same benefit from the “fistula first” approach as younger patients who have lower comorbidity levels and longer survival. Therefore, we investigated the association of vascular access type with all-cause and cause-specific patient mortality among four different age subgroups in a large national cohort of incident hemodialysis patients from the US Department of Veterans Administration (VA).
MATERIALS and METHODS
Cohort Definition
We analyzed data from the Transition of Care in Chronic Kidney Disease (TC-CKD) study, a retrospective cohort study examining US veterans transitioning to renal replacement therapy from October 1, 2007 through September 30, 2011.[10] A total of 52,172 US veterans were identified from the US Renal Data System (USRDS)[1] as the initial cohort. We used the vascular access type listed on the USRDS Patient and Medical Evidence Form 2728 to identify patients who initiated hemodialysis using either an AVF, an AVG, or a TDC with and without a maturing AVF or AVG (treated as separate categories). Patients with missing or unknown information about hemodialysis vascular access (n=4,909), those with missing age or age <18 years old (n=7), and those with no follow-up information (n=470) were excluded, resulting in a final analytical sample of 46,786 patients. Patients were categorized by their age at hemodialysis start into prespecified groups of <60, 60–<70, 70–<80 and ≥80 years old.
Data collection
Data from the USRDS Patient and Medical Evidence Form 2728 were used to determine baseline demographic characteristics at the time of dialysis initiation, laboratory variables prior to hemodialysis (estimated GFR, albumin and hemoglobin), and presence or absence of Nephrology subspecialty care received prior to hemodialysis. Information about comorbidities was extracted from the VA Inpatient and Outpatient Medical SAS Datasets, [11] using ICD-9-CM diagnostic and procedure codes and CPT codes, as well as from CMS Data files, as previously described.[12] Cardiovascular disease was defined as the presence of diagnostic codes for coronary artery disease, angina, myocardial infarction, or cerebrovascular disease. We calculated the Charlson Comorbidity Index score using the Deyo modification for administrative data sets, without including kidney disease.[13]
Statistical analysis
Data are presented as number (percent) for categorical variables and mean ± standard deviation or median (interquartile range [IQR]) as appropriate. Continuous variables were compared using ANOVA and categorical variables were compared with χ2 test. The association of various demographic and clinical characteristics with the type of vascular access was examined in multinomial logistic regression analyses.
The co-primary outcomes were all-cause, cardiovascular, and infection-related mortality during the first year after dialysis initiation. Information about all-cause mortality was obtained from the VA Vital Status Files, [14] and causes of death were obtained from the USRDS. We examined the association of vascular access type with outcomes during the first year following hemodialysis initiation using the Kaplan-Meier method and the log-rank test. Hazard ratios of all-cause mortality were calculated in unadjusted and multivariable adjusted Cox models. All multivariable models were adjusted based on a priori considerations for age, gender, race, comorbid conditions (history of diabetes mellitus, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic lung disease, liver disease and malignancies), and for the Charlson comorbidity index as an omnibus measure of illness. The effect of age on the association of vascular access type with the various outcomes was additionally examined by performing analyses in subgroups of patients categorized by their baseline age, and by including multiplicative interaction terms.
Of the variables included in the main multivariable model, data points were missing for race (<0.1%) and for comorbid conditions (11%). 41,578 patients (89%) had complete data for multivariable analysis; due to the relatively low proportion of missingness, missing data was not imputed.
In sensitivity analyses we examined the same associations over the first 6-month post-transition time period, and after additional adjustments for the last estimated glomerular filtration rate (eGFR), hemoglobin and serum albumin levels before transition to hemodialysis (obtained from USRDS Form 2728). Analyses were conducted using STATA MP Version 14 (STATA Corporation, College Station, TX). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
RESULTS
Overall, patients were 70±12 years old, 94% were male, 25% were African-American, and 58% had diabetes. 8,940 (19%) started hemodialysis with an AVF; 1,090 (2.3%) with an AVG; 8,262 (18%) with a TDC and a maturing AVF or AVG indicating prior attempts to create a permanent vascular access (of which 7,549 had a maturing AVF and 713 had a maturing AVG); and 28,494 (61%) with a TDC and no maturing AVF or AVG (indicating no attempts to create a permanent vascular access in the pre-dialysis time period). Patients’ baseline characteristics at the time of dialysis initiation by their initial vascular access type are presented in Table 1. Compared to patients with a mature AVF, patients with an AVG were more likely to be female and black, and to not have received Nephrology care during the pre-dialysis period. Compared to patients with a mature AVF, patients with a TDC and no maturing AVF/AVG had a higher prevalence of cardiovascular and chronic lung disease, and were substantially less likely to have received Nephrology care during prelude. Patients with a TDC who had a maturing AVF/AVG had similar or fewer comorbidities as those with a TDC and no maturing AVF/AVG, with the exception of diabetes mellitus, which was more common in the former group. Furthermore, the group with TDC and maturing AVF/AVG had a higher likelihood of receiving pre-dialysis Nephrology care compared to those with a TDC and no maturing AVF/AVG (although it was still lower compared to the group with a mature AVF).
Table 1.
Baseline characteristics of patients categorized according to the type of vascular access used to initiate hemodialysis
| AVF N=8,940) |
AVG N=1,090 |
TDC, maturing AVF/AVG N=8,262 |
TDC, no maturing AVF/AVG N=28,494 | P value | |
|---|---|---|---|---|---|
| Age (years) | 70.2±11.5 | 70.7±11.9 | 70.3±11.7 | 71.0±12.0 | <0.001 |
| Sex (male) | 8,582 (96) | 1,121 (91) | 7,797 (94) | 26,903 (94) | <0.001 |
| Race (black) | 2,148 (24) | 462 (37) | 2,073 (25) | 6,814 (24) | <0.001 |
| Ethnicity (Non-Hispanic) | 8,348 (93) | 1,168 (95) | 7,746 (94) | 28,861 (94) | 0.009 |
| Pre-dialysis Nephrology care | 8,187 (96) | 1,020 (87) | 6,101 (81) | 13,596 (56) | <0.001 |
| Diabetes mellitus | 5,277 (66) | 743 (67) | 5,138 (71) | 16,308 (64) | <0.001 |
| Myocardial infarction | 1,937 (24) | 284 (26) | 2,150 (30) | 7,964 (31) | <0.001 |
| Congestive heart failure | 3,984 (50) | 577 (52) | 4,406 (61) | 15,596 (61) | <0.001 |
| Peripheral vascular disease | 3,102 (39) | 441 (40) | 3,079 (42) | 10,327 (41) | 0.004 |
| Cerebrovascular disease | 2,360 (30) | 378 (34) | 2,348 (32) | 8,219 (32) | <0.001 |
| Dementia | 160 (2) | 60 (5) | 197 (3) | 850 (3) | <0.001 |
| Chronic lung disease | 3,079 (39) | 470 (43) | 3,282 (45) | 12,137 (48) | <0.001 |
| Liver disease | 819 (10) | 138 (13) | 819 (11) | 3,199 (13) | <0.001 |
| Malignancy | 1,965 (25) | 291 (26) | 1,749 (24) | 6,803 (27) | <0.001 |
| Last estimated GFR prior to dialysis (ml/minute/1.73 m2) | 11.5±4.14 | 12.0 ±4.4 | 11.7±4.7 | 12.4 ±5.4 | <0.001 |
| Charlson comorbidity index | 4.4±2.8 | 4.9±3.1 | 4.9±2.8 | 4.9±3.0 | <0.001 |
| Last serum albumin prior to dialysis (g/dl) | 3.4±0.60 | 3.3±0.63 | 3.2±0.63 | 3.0±0.67 | <0.001 |
| Last hemoglobin prior to dialysis (g/dl) | 10.3±1.5 | 10.1±1.5 | 9.9±1.5 | 9.9±1.6 | <0.001 |
Results presented as means ± standard deviations and number (percent).
AVF, arteriovenous fistula; AVG, arteriovenous graft; TDC, tunneled dialysis catheter
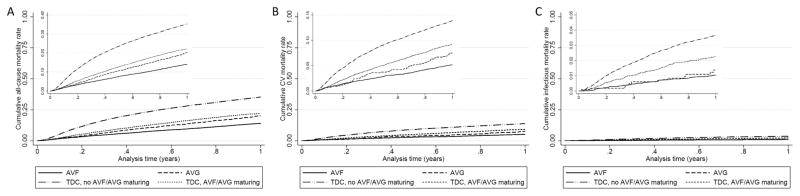
13,303 patients died (mortality rate 343/1000 patient-years, 95%CI: 338–350) during the first year after transition to hemodialysis. Figure 1 shows cumulative unadjusted event curves for overall all-cause (Panel A), cardiovascular (Panel B) and infection-related (Panel C) mortality, in patients grouped by their initial vascular access type, indicating incrementally higher risk of all-cause and cardiovascular mortality in patients with an AVG, a TDC and maturing AVF/AVG and a TDC without maturing AVF/AVG, compared to patients with a mature AVF. Table 2 shows the crude and multivariable adjusted hazard ratios of all-cause, cardiovascular and infection-related mortality associated with AVG and TDC (compared to AVF) in patient subgroups of various ages. All-cause mortality trended higher in patients with an AVG vs. AVF in all age groups; patients with a TDC and a maturing AVF/AVG experienced higher mortality than those with a mature AVF or AVG; and patients with a TDC and no maturing AVF/AVG experienced the worst mortality of all four groups in all age categories. The association of vascular access type with all-cause mortality was not significantly modified by age (p value for interaction=0.08). The associations of vascular access type with cardiovascular mortality were similar in nature and magnitude to those seen for all-cause mortality, with incremental risk associated with AVG, TDC with and without maturing AVF/AVG, and with no effect modification by age (p value for interaction=0.40) (Table 2). Compared to patients with AVF, the risk of infection-related mortality was not significantly higher in patients with AVG, although the number of events precluded assessment in some age groups. The risk of infection-related mortality was highest in patients with TDC and no maturing AVF/AVG (multivariable adjusted hazard ratios ranging from 2.07 to 8.92 in various age subgroups), followed by those with TDC and maturing AVF/AVG (multivariable adjusted hazard ratios ranging from 1.09 to 4.28 in various age subgroups), without significant effect modification by age (p value for interaction=0.45) (Table 2).
Figure 1.
Cumulative event curves for overall all-cause (Panel A), cardiovascular (Panel B) and infection-related (Panel C) mortality, in patients divided by their initial vascular access type.
Table 2.
Crude and multivariable adjusted hazard/subhazard ratios of all-cause, cardiovascular and infection-related mortality associated with AVG and TDC with and without maturing AVF/AVG (compared to AVF) in patient subgroups of various ages
| Age Group |
Mortality | Unadjusted HR/SHR (95% CI) | Adjusted HR/SHR (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVG (vs. AVF) |
P value | TDC, maturing AVF/AVG (vs. AVF) |
P value |
TDC, no maturing AVF/AVG (vs. AVF) |
P value |
AVG (vs. AVF) |
P value |
TDC, maturing AVF/AVG (vs. AVF) |
P value |
TDC, no maturing AVF/AVG (vs. AVF) |
P value |
||
| <60 | All-cause | 1.22 (0.74–2.00) | 0.4 | 1.77 (1.40–2.23) | <0.001 | 2.93 (2.41–3.55) | <0.001 | 1.33 (0.79–2.22) | 0.3 | 1.80 (1.40–2.33) | <0.001 | 2.76 (2.24–3.40) | <0.001 |
| CV | 1.42 (0.64–3.20) | 0.4 | 1.75 (1.17–2.62) | 0.006 | 2.36 (1.69–3.30) | <0.001 | 1.53 (0.64–3.68) | 0.3 | 1.92 (1.23–3.99) | 0.004 | 2.50 (1.72–3.65) | <0.001 | |
| Infection-related | - | - | 4.69 (1.56–13.94) | 0.005 | 7.98 (2.93–21.71) | <0.001 | - | - | 4.28 (1.19–15.36) | 0.026 | 8.92 (2.81–28.33) | <0.001 | |
| 60–<70 | All-cause | 1.68 (1.19–2.36) | 0.003 | 1.82 (1.54–2.15) | <0.001 | 3.19 (2.78–3.67) | <0.001 | 1.55 (1.08–2.21) | 0.016 | 1.80 (1.50–2-13) | <0.001 | 2.93 (2.53–3.39) | <0.001 |
| CV | 1.15 (0.56–2.22) | 0.7 | 1.83 (1.39–2.42) | <0.001 | 2.70 (2.13–3.40) | <0.001 | 1.10 (0.55–2.20) | 0.8 | 1.75 (1.31–2.32) | <0.001 | 2.37 (1.87–3.023) | <0.001 | |
| Infection-related | 1.51 (0.53–4.35) | 0.4 | 1.15 (0.66–2.00) | 0.6 | 2.23 (1.46–3.41) | <0.001 | 1.52 (0.52–4.42) | 0.4 | 1.09 (0.61–1.94) | 0.8 | 2.07 (1.33–3.22) | 0.001 | |
| 70–<80 | All-cause | 1.52 (1.20–1.94) | 0.001 | 1.64 (1.45–1.85) | <0.001 | 2.94 (2.61–3.41) | <0.001 | 1.54 (1.20–1.97) | 0.001 | 1.52 (1.34–1.73) | <0.001 | 2.70 (2.43–3.00) | <0.001 |
| CV | 1.92 (1.28–2.90) | 0.002 | 2.01 (1.61–2.50) | <0.001 | 3.30 (2.74–3.96) | <0.001 | 1.96 (1.29–2.97) | 0.002 | 1.80 (1.44–2.25) | <0.001 | 2.94 (2.44–3.55) | <0.001 | |
| Infection-related | 0.96 (0.22–4.15) | 0.9 | 3.55 (2.09–6.03) | <0.01 | 5.78 (3.60–9.31) | <0.001 | 1.06 (0.24–4.63) | 0.9 | 3.56 (2.03–6.20) | <0.001 | 5.73 (3.46–9.50) | <0.001 | |
| ≥80 | All-cause | 1.34 (1.06–1.69) | 0.012 | 1.57 (1.39–1.76) | <0.001 | 2.74 (2.49–3.01) | <0.001 | 1.33 (1.05–1.68) | 0.018 | 1.51 (1.34–1.70) | <0.001 | 2.56 (2.32–2.82) | <0.001 |
| CV | 1.13 (0.74–1.74) | 0.567 | 1.62 (1.32–1.99) | <0.001 | 2.77 (2.35–3.27) | <0.001 | 1.16 (0.75–1.79) | 0.5 | 1.51 (1.23–1.86) | <0.001 | 2.48 (2.10–2.94) | <0.001 | |
| Infection-related | 1.38 (0.58–3.31) | 0.5 | 1.98 (1.28–3.06) | 0.002 | 3.05 (2.12–4.40) | <0.001 | 1.27 (0.53–3.05) | 0.6 | 1.65 (1.06–2.56) | 0.027 | 2.58 (1.79–3.73) | <0.001 | |
Results are from unadjusted and multivariable adjusted Cox models (for all-cause mortality) and competing risk regression models (for cause-specific mortality). The number of events for infection-related deaths was too low in the <60 year old age group for reliable estimation of all sub-hazard ratios.
CV, cardiovascular; HR, hazard ratio; CI, confidence interval; AVG, arteriovenous graft; AVF, arteriovenous fistula; TDC, tunneled dialysis catheter
Results remained unchanged in sensitivity analyses examining outcomes occurring over the first 6 months following hemodialysis transition, and after additional adjustments for pre-transition eGFR, hemoglobin and serum albumin (data not shown).
DISCUSSION
In this large and nationally representative retrospective cohort study of incident hemodialysis patients, we compared the association of AVG and TDC (vs. AVF) with all-cause and cause-specific mortality in US veterans aged <60, 60–<70, 70–<80, and ≥80 years old. We found that using a TDC is associated with higher mortality in all age groups, the risk being highest for infection-related mortality. Patients with a TDC and no maturing AVF/AVG had higher mortality rates compared to patients with a TDC and a maturing AVF/AVG, but the latter had worse outcomes compared to patients with a mature AVF or AVG. Even though the risk of various outcomes associated with AVG vs. AVF was not statistically significantly different in all age groups, use of an AVG was associated with an overall modestly increased risk of all-cause and cardiovascular mortality, with no effect modification by age upon statistical testing.
Older age is often considered a challenge to the creation and use of vascular access. Given the complicated process of establishing a successful vascular access, clinicians are faced with difficult decisions regarding the choice of an optimal access in elderly CKD patients transitioning to hemodialysis, who face a high chance of having an access created but not used (due to either succumbing to death prior to reaching end stage renal disease, or due to primary maturation failure).[15,16] Recent studies showed a high rate of primary maturation failure in native AVFs, [15,17] yet there are no standard patient eligibility criteria to guide AVF placement. Currently, the primary and secondary fistula patency rate in the elderly at 1 year have ranged from 43% to 74% and 56% to 82%, respectively.[15] Older age has been associated with lower rates of fistula use which is attributable to a decrease in referral for AVFs as well as increased rates of failure to mature among those who do get a fistula.[5,8,17]
On the other hand, AVGs are the least used form of vascular access at hemodialysis initiation, presumably due to the lower long term patency rates and an increased association with morbidity and mortality.[4,6] The cumulative primary patency at 1 and 2 years was 81% and 65% in a study of 67 elderly patients over the age of 70 years, and the secondary patency was 65% and 58% at 1 and 2-years, respectively.[18] In another study the 1 and 2-year total patency rate among elderly patients with synthetic grafts was 44.2% and 38.6%.23 However, AVGs are considered viable options in patients with failed fistulas, exhausted, unsuitable, or damaged veins, or when there is late Nephrology referral and need for urgent cannulation with avoidance of central venous catheters.[19] Overall, AVF survival is no better than AVG when primary failures are included in access survival analyses.[19,20] In a study comparing access survival by access type among those >65 years old using data from the USRDS, [21] use of a AVF vs. AVG was not associated with increased patency among non-diabetic (OR 1.48, 95% CI 0.95–2.3) or diabetic elderly (OR 1.49, 95% CI 0.76–2.89). Placing an AVG first may in fact dramatically lengthen the proportion of the patient’s lifespan with freedom from catheter dependence and its potential complications.[19]
Similar to our study, in a previous observational study of 66,595 elderly (>67 years old) Medicare USRDS patients, the use of AVF as initial access for long-term dialysis therapy was associated with the best survival, followed by AVG and TDC use.[21] In another large analysis of patients >67 years old from USRDS, incident catheter use was associated with significantly higher long-term mortality compared to AVF use, but the association of AVG use with mortality was only significant in relatively younger patients (<80 years old), and was not present in patients ≥80 years old.[22] The seemingly contradictory results regarding the association of AVG with mortality in these studies may be caused by differences in sample size and analytical approaches, as the point estimates for the risk were fairly modest and similar in magnitude in these two studies.[4,22] Our study supports and extends these findings, by examining cause-specific mortality and emphasizing the major importance of infection-related deaths for TDC use, and by including a full spectrum of patient age and thus establishing that age is not a significant effect modifier of the associations between access type and mortality.
In our study TDC use was associated with the worst outcomes even when accounting for pre-dialysis attempts to create a permanent vascular access (AVF or AVG), although patients with such attempts experienced better outcomes compared to patients with no prior attempts. A recent study examining 2,300 incident hemodialysis patients from five Canadian dialysis centers also found that patients who do not undergo attempts to place pre-dialysis AVF had worse outcomes compared to those who had such attempts, independent of age, and suggested that the mortality associated with TDC use may not be caused by catheters, but by the underlying comorbid conditions necessitating catheter placement [23].
These results are difficult to compare to our findings, due to marked differences in sample size, in the nature of the two study populations (Canadian vs. US), and the different analytical approaches (we examined immediate post-dialysis mortality, as opposed to longer term mortality in the study by Quinn et al.). However, the fact that in our study even patients with TDC who had attempts at creating an AVF or AVG experienced significantly worse outcomes compared to patients with AVF, and the fact that associations were particularly strong with infection-related mortality suggests that a direct effect of catheter use on adverse outcomes cannot be ruled out. Findings similar to our study were also reported recently by Brown et al., who showed that patients initiating hemodialysis with a TDC after failed AVF placement had significantly lower all-cause mortality rates than the TDC-only group, but higher mortality than patients initiating dialysis using an AVF.[24]
Our study is notable for its large sample size of late-stage non-dialysis dependent CKD patients transitioning to dialysis, for representing the full age spectrum of such patients, and for being representative of veterans in the entire geographic United States. However, several limitations need to be acknowledged. This study was observational, and therefore susceptible to residual confounding and confounding by indication. Even though we adjusted for numerous available confounders, the possibility of residual confounding remains. This may be especially important in the case of patients who transitioned to dialysis using a TDC who have no maturing AVF/AVG, as catheters are the sole form of vascular access in patients who need emergent initiation of renal replacement therapy, a group that is known to experience worse outcomes.[25] Most of our patients consisted of male veterans; therefore, the results may not be generalized to other cohorts or the general population. We used administrative data for our study, which could be prone to a sampling bias and inaccurate measurement of the predictor variables.
In conclusion, an AVF at hemodialysis transition is associated with lower all-cause, cardiovascular and infection-related mortality across all age categories, including even the oldest incident hemodialysis patients. Using a TDC is associated with higher mortality in all age groups, even in patients with prior attempts to creating an AVF or an AVG. Our study thus suggests that a mature AVF at the time of hemodialysis transition is the preferable access even in all incident hemodialysis the most elderly patients. Further studies are needed to inform the field about the best strategies to increase AVF placement and maturation in those with advanced age. AVGs may offer an acceptable and in some cases even desirable alternative to AVFs in elderly individuals, due to their more predictable and shorter maturation, thus minimizing the duration of TDC exposure and potentially decreasing the potential risks associated with the latter.
Acknowledgments
This study is supported by grant 5U01DK102163 from the National Institute of Health (NIH) to KKZ and CPK, and by resources from the US Department of Veterans Affairs.
The data reported here have been supplied by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
CPK and KKZ are employees of the Department of Veterans affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government. The results of this paper have not been published previously in whole or part.
Footnotes
DISCLOSURES
None.
References
- 1.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67:Svii, S1–305. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vascular Access Work G. Clinical practice guidelines for vascular access. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;48(Suppl 1):S176–247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM. Associates of mortality and hospitalization in hemodialysis: potentially actionable laboratory variables and vascular access. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:79–90. doi: 10.1053/j.ajkd.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42:1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:475–491. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 6.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L. Cumulative patency of contemporary fistulas versus grafts (2000–2010) Clinical journal of the American Society of Nephrology : CJASN. 2013;8:810–818. doi: 10.2215/CJN.00730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monroy-Cuadros M, Yilmaz S, Salazar-Banuelos A, Doig C. Risk factors associated with patency loss of hemodialysis vascular access within 6 months. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1787–1792. doi: 10.2215/CJN.09441209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) Journal of the American Society of Nephrology : JASN. 2006;17:3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 9.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney international. 2003;63:346–352. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 10.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Jing J, Ravel VA, Soohoo M, Rhee CM, Streja E, Kalantar-Zadeh K, Kovesdy CP. Association of Slopes of Estimated Glomerular Filtration Rate With Post-End-Stage Renal Disease Mortality in Patients With Advanced Chronic Kidney Disease Transitioning to Dialysis. Mayo Clin Proc. 2016;91:196–207. doi: 10.1016/j.mayocp.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VIReC Research User Guide. VHA Medical SAS Inpatient Datasets FY2006–2007. 2007. [Google Scholar]
- 12.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, Molnar MZ, Kalantar-Zadeh K. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132:1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moist LM, Lok CE, Vachharajani TJ, Xi W, AlJaishi A, Polkinghorne KR, Vazquez M, Lee TC. Optimal hemodialysis vascular access in the elderly patient. Seminars in dialysis. 2012;25:640–648. doi: 10.1111/sdi.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Hare AM. Vascular access for hemodialysis in older adults: a “patient first” approach. Journal of the American Society of Nephrology : JASN. 2013;24:1187–1190. doi: 10.1681/ASN.2013050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, Canaud BJ, Pisoni RL. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:3219–3226. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staramos DN, Lazarides MK, Tzilalis VD, Ekonomou CS, Simopoulos CE, Dayantas JN. Patency of autologous and prosthetic arteriovenous fistulas in elderly patients. Eur J Surg. 2000;166:777–781. doi: 10.1080/110241500447407. [DOI] [PubMed] [Google Scholar]
- 19.Allon MLC. Dialysis fistula or graft: the role for randomized clinical trials. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:2348–2354. doi: 10.2215/CJN.06050710. [DOI] [PubMed] [Google Scholar]
- 20.Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. The journal of vascular access. 2008;9:231–235. [PubMed] [Google Scholar]
- 21.Chan MR, Sanchez RJ, Young HN, Yevzlin AS. Vascular access outcomes in the elderly hemodialysis population: A USRDS study. Seminars in dialysis. 2007;20:606–610. doi: 10.1111/j.1525-139X.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 22.DeSilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS. Fistula first is not always the best strategy for the elderly. Journal of the American Society of Nephrology : JASN. 2013;24:1297–1304. doi: 10.1681/ASN.2012060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn RR. The Effect of Predialysis Fistula Attempt on Risk of All-Cause and Access-Related Death. JASN. 2016 doi: 10.1681/ASN.2016020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS. The Survival Benefit of “Fistula First, Catheter Last” in Hemodialysis Is Primarily Due to Patient Factors. Journal of the American Society of Nephrology : JASN. 2017;28:645–652. doi: 10.1681/ASN.2016010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenzo V, Martn M, Rufino M, Hernandez D, Torres A, Ayus JC. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: an observational cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;43:999–1007. doi: 10.1053/j.ajkd.2004.02.012. [DOI] [PubMed] [Google Scholar]