Abstract
California uses a unique method to screen newborns for cystic fibrosis (CF) that includes gene scanning and DNA sequencing after only one California-40 cystic fibrosis transmembrane conductance regulator (CFTR) panel mutation has been identified in hypertrypsinogenemic specimens. Newborns found by sequencing to have one or more additional mutations or variants (including novel variants) in the CFTR gene are systematically followed, allowing for prospective assessment of the pathogenic potential of these variants. During the first 3 years of screening, 55 novel variants were identified. Six of these novel variants were discovered in five screen-negative participants and three were identified in multiple unrelated participants. Ten novel variants (c.2554_2555insT, p.F1107L, c.-152G>C, p.L323P, p.L32M, c.2883_2886dupGTCA, c.2349_2350insT, p.K114del, c.-602A>T, and c.2822delT) were associated with a CF phenotype (42% of participants were diagnosed at 4 to 25 months of age), whereas 26 were associated with CFTR-related metabolic syndrome to date. Associations with the remaining novel variants were confounded by the presence of other diseases or other mutations in cis or by inadequate follow-up. These findings have implications for how CF newborn screening and follow-up is conducted and will help guide which genotypes should, and which should not, be considered screen positive for CF in California and elsewhere.
Cystic fibrosis (CF; Online Mendelian Inheritance of Man no. 219700; http://www.ncbi.nlm.nih.gov/omim) is an autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR).1, 2, 3 As of January 1, 2013, >1900 variants in the CFTR gene have been identified (Cystic Fibrosis Mutation Database, http://www.genet.sickkids.on.ca/app, last accessed January 8, 2013). It is unknown exactly how many of these variants are disease causing because most have not been functionally characterized.4
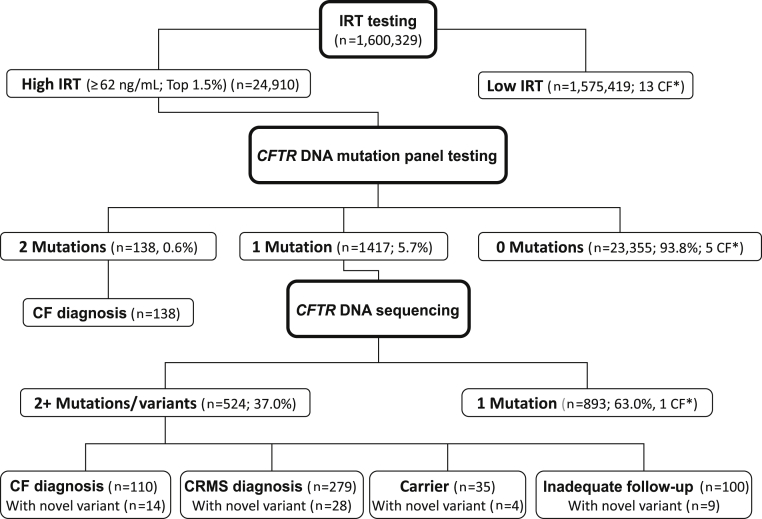
The algorithm that California has deployed to screen newborns for CF differs from the algorithms used by other screening programs. California's algorithm includes an additional DNA sequencing step for newborns found to have hypertrypsinogenemia and only one mutant allele from the California-40 CFTR mutation panel (Figure 1). The DNA sequencing step allows for the systematic identification, reporting, and referral of newborns positive for one panel mutation and one or more additional CFTR mutations, including novel variants. This offers the CF newborn screening (NBS) program the unique opportunity to prospectively assess, in a comprehensively genotyped population, the pathogenic potential of novel and other variants.
Figure 1.
California CF NBS algorithm. The number of newborns screened positive and negative at each step of the screening process is shown for July 16, 2007, through July 15, 2010. The asterisks indicate newborns with negative CF NBS results.
Herein, we determine the frequency and types of novel CFTR variants identified during the first 3 years of CF NBS in California. We describe the novel variants associated with CF disease manifestations and important haplotypes identified. Also included in this report is a presentation of our approach to the longitudinal follow-up of these infants, many of whom are initially asymptomatic, and our experience communicating to parents and primary care providers the complex implications of the genetic and clinical findings in these newborns.
Materials and Methods
Participant Selection and Definition of Novel Mutation
All the participants included in this report underwent an NBS test for CF in California between July 16, 2007, and July 15, 2010. Newborns were screened using the California method, which includes i) analysis of serum immunoreactive trypsinogen (IRT) levels using the AutoDELFIA neonatal IRT L kit (PerkinElmer, Waltham, MA) in all newborn blood spot specimens, ii) CFTR mutation panel [29-40 mutations (the mutations on the California panel were selected for the most part according to allelic frequencies found in a comprehensively genotyped group of California CF cases to achieve a ≥95% race/ethnicity-specific rate of CF case detection in black, white, and Hispanic individuals in California and include c.1585-1G>A, c.1680-1G>A, c.1973-1985del13insAGAAA, c.2175_2176insA, c.164 + 2T>A (removed on August 12, 2008), c.2988 + 1G>A, c.3717 + 12191C>T, c.3744delA, c.274-1G>A, c.489 + 1G>T, c.579 + 1G>T, p.A559T, p.F311del, p.F508del, p.I507del, p.G542X, p.G551D, p.G85E, p.H199Y, p.N1303K, p.R1066C, p.R1162X, p.R334W, p.R553X, p.S549N, p.W1089X, p.W1204X (c.3611G>A), p.W1282X, c.1153_1154insAT [added October 4, 2007], c.1923_1931del9insA, c.3140-26A>G, c.531delT, c.803delA, c.54-5940_273 + 10250del21kb, p.P205S, p.Q98R, p.R75X, p.S492F [added December 12, 2007], c.3659delC, p.G330X, p.W1204X [c.3612G>A] [added August 12, 2008] [Signature CF 2.0 ASR; Asuragen Inc., Austin, TX])] testing of specimens with IRT ≥62 ng/mL (highest 1.5%), iii) CFTR gene scanning and sequence analysis (Ambry Test: CF; Ambry Genetics, Aliso Viejo, CA) for specimens found to have only one mutation after CFTR mutation panel testing, and iv) referral to 1 of 15 pediatric CF care centers (CFCs) for sweat chloride (SC) testing and follow-up of all newborns with either two CFTR mutations detected during panel testing or one CFTR mutation detected during panel testing and one (or more) additional CFTR mutation and/or variant detected during sequencing. We defined a CFTR mutation/variant identified during sequencing to include i) any DNA sequence listed in the Cystic Fibrosis Mutation Database or published in the literature (except those documented to be benign polymorphisms, eg, p.M470V), ii) IVS8 Poly 5T of any TG tract length, and iii) any novel variant. A novel variant was defined as a previously unreported sequence change in the regions of the CFTR gene analyzed (see Gene Analysis) that was not listed in the Cystic Fibrosis Mutation Database or the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php, last accessed January 8, 2013), or elsewhere.
The study period covered by this paper was limited to the first 3 years of screening, allowing all newborns to have ≥24 months of potential follow-up. Individuals were included in the main study group if they had positive CF NBS results with one mutation detected during CFTR mutation panel testing and at least one novel variant detected during CFTR sequencing. Fifty-five individuals met the inclusion criteria. In addition, we included five infants with normal (negative) CF NBS results who subsequently presented to CFCs for diagnostic testing and evaluation and were found to carry at least one novel variant in the CFTR gene. Pediatric CFCs in California routinely report all persons diagnosed as having CF to the California Department of Public Health Genetic Disease Screening Program (CDPH GDSP) as part of quality assurance procedures.
To determine the frequency of the novel variants in a nondiseased population, a control group of 1613 asymptomatic individuals referred to Ambry Genetics for CF carrier screening between February 1, 2002, and December 31, 2011, was selected. Individuals were included in the control group if they had supporting documentation confirming that the indication for testing was CF carrier screening only (ovum or sperm donors or partners of known CF carriers) and not for suspicion of CFTR-related disease. Individuals meeting the control group inclusion criteria underwent CFTR gene sequence analysis, as described in Gene Analysis. In addition, at the time of manuscript preparation, all the variants were checked for reported frequencies in the Exome Variant Server [National Heart, Lung, and Blood Institute GO Exome Sequencing Project (ESP), Seattle, WA, http://evs.gs.washington.edu/EVS, last accessed April 29, 2013] and in 1000 Genomes5 and dbSNP.6
Data Collection and Diagnostic Criteria
For all the participants, race/ethnicity, SC measurements, diagnoses, and clinical follow-up data were collected from 15 California pediatric CFCs via the CDPH GDSP's secure online Screening Information System database. All the SC tests were performed by Cystic Fibrosis Foundation–accredited laboratories in accordance with the Clinical Laboratory and Standards Institute guidelines7 and the Cystic Fibrosis Foundation guidelines.8 Follow-up was conducted using minimum guidelines developed by the CFCs and the CDPH GDSP (CDPH NBS CF minimum guidelines, http://www.cdph.ca.gov/programs/nbs/Documents/NBS-CP-6-1-1(old3-15-1A)MinGuidelinesCF-2009.pdf, last accessed January 8, 2013). Data collected included clinical evaluation and laboratory testing designed to identify clinical symptoms and signs consistent with manifestations of CF disease. These included chronic sinopulmonary disease manifested by persistent colonization/infection with typical CF pathogens, chronic productive cough, persistent chest radiography abnormalities, airway obstruction manifested by wheezing and air trapping, and nasal polyps; gastrointestinal and nutritional abnormalities, including meconium ileus, distal intestinal obstruction, rectal prolapse, pancreatic insufficiency, failure to thrive, hypoproteinemia, and edema; and fluid and electrolyte abnormalities, such as acute salt depletion, dehydration, and pseudo-Bartter syndrome. CFTR-related metabolic syndrome (CRMS) was used to describe hypertrypsinogenemic infants with one or two previously described CFTR mutations, one or more novel CFTR variants, SC levels <60 mmol/L on one or more occasions, and no CF manifestations.9 DNA testing on the biological parents of the study participants with nonelevated SC values was offered to determine the cis/trans mutation phase. Individuals were diagnosed as CF carriers if all identified CFTR mutations and variants were inherited from a single parent. “Could not be determined” was used to describe participants who were never seen by a CFC, whose parents refused all follow-up, or who died before follow-up could be completed.
Laboratory Analysis
DNA Isolation
Genomic DNA was extracted from a newborn dried blood spot using the recommended protocol based on a procedure used at the National Institute for Standards and Technology.10
Gene Analysis
Genomic DNA was used in the Ambry Test: CF,11 which analyzes the CFTR gene by modified temporal temperature gradient electrophoresis analysis followed by dye terminator DNA sequencing of suspect regions. All the coding exons and the 5′ UTR promoter were analyzed, plus ≥20 bases 5′ and 3′ into each intervening sequence, as were select deep intronic mutations. Briefly, all exons and relevant intronic regions were amplified using PCR and proprietary primers. Before Ambry gel analysis, the PCR products were denatured and slowly cooled to allow for maximal heteroduplex formation. For a subset of CFTR regions, DNA was mixed with known wild-type DNA to facilitate detection of homozygous mutations. PCR products were then processed for temporal temperature gradient electrophoresis on DCode gels (Bio-Rad Laboratories, Hercules, CA). Regions indicating the presence of a mutation by temporal temperature gradient electrophoresis were then processed for dye terminator sequencing. Standard dye terminator cycle sequencing (Beckman Coulter, Fullerton, CA) was conducted, followed by analysis using a CEQ8000 capillary electrophoresis sequencer. Exons were always sequenced in both the sense and antisense directions to identify the exact nucleotide variation. Multiplex ligation-dependent probe amplification12 (MRC-Holland, Amsterdam, The Netherlands) was used to determine gross deletions or duplications when CF was suspected but no or one CFTR mutations were detected by sequencing. All reported variations follow the nomenclature based on GenBank entry NM_000492.3 and the Cystic Fibrosis Mutation Database. The interpretation for variants followed the recommended guidelines published by the American College of Medical Genetics.13
Data Analysis
Novel variants were described in terms of their gene locations and numbers. The percentage of CFTR sequencing screen positives with one or more novel variants was determined overall and by four race/ethnicity categories (non-Hispanic white, black, Hispanic, and multiple/other races). The mutation phase was determined via focused DNA sequencing of parents. Based on published diagnostic criteria,14 cis/trans mutation phase, overall genotype, locations and types of mutations, clinical features, and SC concentration, novel variants were categorized into four exclusive groups: i) expected to cause CF (only one panel mutation and one novel variant identified), ii) may or may not cause CF (more than one mutation/variant in cis with the novel variant or other comorbid condition), iii) in cis phase with known mutations, and iv) probably not causative of CF manifestations to date.
Results
During the study, 1,600,329 newborns were screened for CF in California (Figure 1). A total of 24,910 newborns had an IRT test value ≥62 ng/mL and went on to CFTR DNA mutation panel testing. Of these newborns, 23,355 (93.8%) had no CFTR mutations detected, 1417 (5.7%) had one panel mutation detected, and 138 (0.6%) had two panel mutations detected. Of the 1417 newborns with one panel mutation who had CFTR scanning and sequencing performed, 893 (63.0%) had no additional mutations/variants detected and 524 (37.0%) had one or more additional mutations/variants detected. A total of 266 CF cases were diagnosed during the study period, including 19 cases that were missed by screening, giving an estimated statewide minimum CF prevalence of 1 in 6016 newborns. Of the 19 screen-negative CF cases, 13 (68.4%) had an IRT level below the program cutoff level of 62 ng/mL, five (26.3%) were hypertrypsinogenemic but had no panel mutations identified, and one (5.3%) had a second CFTR mutation that was not identifiable by sequencing.
Of the 524 newborns with two or more CFTR variants detected by NBS, at least one of the additional mutations identified was a novel variant in 55 newborns (10%). This percentage varied by race/ethnicity: 6% of non-Hispanic white participants (11 of 189), 8% of black participants (3 of 38), 12% of Hispanic white participants (29 of 249), and 24% of participants of multiple or other races (12 of 49).
During the study period, 55 novel CFTR variants were identified in 60 participants. Table 1 describes the chromosomal locations and nucleotide changes for each novel variant: 58% (32 of 55) were located in exonic regions, 22% (12 of 55) in the promoter region, and 20% (11 of 55) in intronic regions. Only 1 of the 55 novel variants was detected in the 1613 control subjects. Six of the 55 novel variants were reported in ESP, one in 1000 Genomes5, and two in dbSNP,6 with none reported in multiple databases. The details and frequencies of these nine variants are provided in the remainder of the Results section. When not noted, the novel variant was not found in controls or reported in any of the previously mentioned databases.
Table 1.
Description of Novel CFTR Variants Identified in California, July 16, 2007, to July 15, 2010
| CFTR region | Nucleotide change | Predicted amino acid change | Type of mutation |
|---|---|---|---|
| Promoter | c.-983A>T | ||
| Promoter | c.-967T>C | ||
| Promoter | c.-837T>C | ||
| Promoter | c.-769A>G | ||
| Promoter | c.-730A>G | ||
| Promoter | c.-684G>A | ||
| Promoter | c.-635A>G | ||
| Promoter | c.-602A>T | ||
| Promoter | c.-510G>A | ||
| Promoter | c.-448A>G | ||
| Promoter | c.-288G>C | ||
| Promoter | c.-152G>C | ||
| Exon 1 | c.38C>G | p.S13C | Missense |
| Exon 2 | c.94C>A | p.L32M | Missense |
| Intron 2 | c.164 + 4T>A | ||
| Exon 3 | c.226T>C | p.C76R | Missense |
| Exon 4 | c.335A>G | p.D112G | Missense |
| Exon 4 | c.407T>C | p.L136P | Missense |
| Exon 4 | c.472_474delAAG | p.K114del | In-frame deletion |
| Intron 6 | c.744-15T>C | ||
| Exon 7 | c.745G>T | p.D249Y | Missense |
| Intron 7 | c.869 + 8G>T | ||
| Exon 8 | c.944T>C | p.F315S | Missense |
| Exon 8 | c.968T>C | p.L323P | Missense |
| Exon 8 | c.974A>G | p.Y325C | Missense |
| Exon 8 | c.1064C>T | p.P355L† | Missense |
| Exon 10 | c.1278delC | p.D426Efs∗16 (stop codon at 441) | Frameshift |
| Exon 11 | c.1479G>C | p.Q493H† | Missense |
| Exon 14 | c.1885A>G | p.T629A | Missense |
| Exon 14 | c.2153C>G | p.P718R | Missense |
| Exon 14 | c.2433G>T | p.R811S | Missense |
| Exon 14 | c.2349_2350insT | p.H784Sfs∗21 (stop codon at 804) | Frameshift |
| Intron 13 | c.1767-13T>G | ||
| Intron 14 | c.2490 + 14G>A | ||
| Intron 14 | c.2490 + 14G>T | ||
| Exon 15 | c.2554_2555insT | p.Y852Lfs∗44 (stop codon at 895) | Frameshift |
| Exon 15 | c.2510T>C | p.M837T | Missense |
| Exon 17 | c.2659A>C | p.T887P | Missense |
| Exon 17 | c.2883_2886dupGTCA | p.T963Vfs∗13 (stop codon at 975) | Frameshift |
| Exon 17 | c.2822delT | p.L941Qfs∗27 (stop codon at 967) | Frameshift |
| Exon 19 | c.3064G>A | p.V1022M | Missense |
| Exon 20 | c.3319T>C | p.F1107L | Missense |
| Intron 20 | c.3367 + 3A>C† | ||
| Exon 21 | c.3382A>G | p.R1128G | Missense |
| Exon 21 | c.3418A>T | p.M1140L | Missense |
| Exon 22 | c.3517G>A | p.G1173S | Missense |
| Exon 22 | c.3592G>A | p.V1198M | Missense |
| Intron 22 | c.3718-24G>A | ||
| Intron 23 | c.3963 + 6G>T | ||
| Exon 25 | c.3964G>C | p.V1322L | Missense |
| Exon 25 | c.4123C>A | p.H1375N | Missense |
| Intron 25 | c.4136 + 12A>G | ||
| Exon 26 | c.4186A>C | p.T1396P | Missense |
| Intron 26 | c.4243-5C>T | ||
| Exon 27 | c.4433C>G | p.T1478R | Missense |
A known mutation occurs at the same nucleotide position or codon.
Table 2 presents race/ethnicity, neonatal IRT level, the CFTR mutations and variants, and the diagnosis of the study participants. Table 3 presents SC concentrations and other clinical characteristics for these participants. Fifty novel CFTR variants were identified among the 55 participants with positive NBS results after DNA sequencing. The novel variant c.-448A>G was identified in three unrelated participants (No. 11, 43, and 44), novel variant c.744-15T>C was identified in two unrelated participants (No. 21 and 45), novel variant c.4243-5C>T was identified in two unrelated participants (No. 16 and 54), and novel variant p.K114del was identified in two siblings (No. 12 and 13). The remaining variants were identified in a single individual. Of these 55 participants with positive NBS results after CFTR sequencing, 42 (76%) carried one known mutation and one novel variant; 8 (14%) carried two known mutations and one novel variant; 2 (4%) carried one known mutation, a (TG)11-5T variant, and one novel variant; 1 (2%) carried one known mutation, a (TG)12-5T variant, and one novel variant; 1 (2%) carried two known mutations, a (TG)12-5T variant, and one novel variant; and 1 (2%) carried two known mutations, a (TG)11-5T variant, and one novel variant. No participants with positive CF NBS results were found to carry more than one novel variant.
Table 2.
Description of Study Participants Identified with one or more Novel CFTR Variant by Screening Test Result. California, July 16, 2007, to July 15, 2010
| Participant No. | Race/ethnicity | IRT (ng/mL) | Previously described CFTR variant/mutation 1 | Previously described CFTR variant/mutation 2 | Novel CFTR variant 1 | Novel CFTR variant 2 | Poly T tract | No. of parents receiving CFTR mutation testing | Diagnosis/status |
|---|---|---|---|---|---|---|---|---|---|
| Study participants with positive NBS results | |||||||||
| 1 | W, H | 83.5 | p.F508del∗ | c.2554_2555insT† | 7T/9T | 2 | CF | ||
| 2 | H | 527.0 | p.F508del | c.-877C>T | p.F1107L | 7T/9T | 0 | CF | |
| 3 | W | 86.5 | p.F508del | p.V562I† | c.-837T>C† | 5T†‡/9T | 1 | CF | |
| 4 | H | 222.3 | p.F508del | p.I556V | c.1278delC | NA | 0 | CF | |
| 5 | H, O | 93.5 | p.F508del∗ | c.-152G>C† | 7T/9T | 2 | CF | ||
| 6 | W, H, B, O | 95.4 | p.F508del∗ | p.L323P† | 5T†‡/9T | 2 | CF | ||
| 7 | H | 70.5 | p.F508del | p.L32M | 7T/9T | 0 | CF | ||
| 8 | W | 209.5 | p.F508del | c.2883_2886dupGTCA | 9T/9T | 0 | CF | ||
| 9 | H | 155.7 | p.F508del∗ | c.2349_2350insT | 7T/9T | 1 | CF | ||
| 10 | O | 146.8 | p.F508del∗ | c.3718-24G>A† | 5T†§/9T | 2 | CF | ||
| 11 | B | 99.4 | p.A559T∗ | p.L206W† | c.-448A>G∗ | 7T/9T | 2 | CF | |
| 12 | W, H | 90.3 | p.P205S | p.K114del | 7T/7T | 0 | CF | ||
| 13 | H | 69.7 | p.P205S | p.K114del | 7T/7T | 0 | CF | ||
| 14 | H | 82.9 | c.274-1G>A∗ | c.-602A>T† | 7T/7T | 2 | CF | ||
| 15 | W | 106.6 | p.F508del∗ | c.-461A>G† | c.-983A>T∗ | 7T/9T | 2 | CRMS | |
| 16 | W, B | 83.9 | p.F508del | c.4243-5C>T | 5T∗§/9T | 1 | CRMS | ||
| 17 | W | 81.5 | p.F508del∗ | p.I1027T∗ | p.Y325C | 7T/9T | 2 | CRMS | |
| 18 | H | 70.7 | p.F508del | c.-967T>C | 9T/9T | 0 | CRMS | ||
| 19 | W, H | 62.4 | p.F508del∗ | c.-635A>G | 7T/9T | 1 | CRMS | ||
| 20 | H | 65.4 | p.F508del† | c.2490 + 14G>T∗ | 7T/9T | 2 | CRMS | ||
| 21 | W | 69.3 | p.F508del∗ | c.744-15T>C† | 7T/9T | 2 | CRMS | ||
| 22 | W, H, O | 66.2 | p.F508del | p.D249Y | 7T/9T | 0 | CRMS | ||
| 23 | H | 94.8 | p.F508del | p.R811S | 7T/9T | 0 | CRMS | ||
| 24 | W | 75.8 | p.F508del∗ | p.H1375N† | 7T/9T | 2 | CRMS | ||
| 25 | H | 63.0 | p.F508del | p.L136P | 7T/9T | 0 | CRMS | ||
| 26 | W, O | 63.0 | p.F508del∗ | p.M1140L | 7T/9T | 1 | CRMS | ||
| 27 | W, O | 91.7 | p.F508del | p.V1198M | 9T/9T | 0 | CRMS | ||
| 28 | H | 69.3 | p.F508del† | c.1767-13T>G∗ | 7T/9T | 2 | CRMS | ||
| 29 | H | 108.8 | p.F508del | p.V1322L | 7T/9T | 0 | CRMS | ||
| 30 | H | 96.4 | p.F508del† | p.C76R∗ | 7T/9T | 2 | CRMS | ||
| 31 | H | 69.0 | c.3140-26A>G | c.-510G>A∗ | 7T/7T | 1 | CRMS | ||
| 32 | H | 100.2 | p.G542X | c.-684G>A∗ | 7T/9T | 1 | CRMS | ||
| 33 | H | 84.1 | c.1153_1154insAT∗ | c.-730A>G† | 7T/7T | 2 | CRMS | ||
| 34 | H | 62.9 | c.1973_1985del13insAGAAA∗ | p.D112G† | 7T/7T | 2 | CRMS | ||
| 35 | H | 116.7 | c.3744delA∗ | p.T887P | 7T/7T | 1 | CRMS | ||
| 36 | B | 73.3 | c.2988 + 1G>A | c.-288G>C | 7T/9T | 0 | CRMS | ||
| 37 | H | 93.5 | p.R75X | c.3367 + 3A>C | 7T/7T | 0 | CRMS | ||
| 38 | W, H | 81.4 | c.3717 + 12191C>T∗ | c.-769A>G† | 7T/7T | 2 | CRMS | ||
| 39 | W | 79.0 | c.3717 + 12191C>T† | p.R668C† | p.T1396P∗ | 7T/9T | 2 | CRMS | |
| 40 | H | 87.3 | c.274-1G>A | p.F315S | 7T/7T | 0 | CRMS | ||
| 41 | H | 79.7 | p.G542X | c.869 + 8G>T | 7T/9T | 0 | CRMS | ||
| 42 | O | 79.8 | p.R553X | p.T1478R | 7T/7T | 0 | CRMS | ||
| 43 | H | 70.5 | p.A559T∗ | c.-448A>G∗ | 7T/7T | 2 | Carrier | ||
| 44 | B | 76.2 | p.A559T∗ | c.-448A>G∗ | 7T/7T | 1 | Carrier | ||
| 45 | W, H | 69.2 | p.G85E∗ | c.744-15T>C∗ | 5T†‡/7T | 2 | Carrier | ||
| 46 | W | 69.1 | p.N1303K∗ | c.2490 + 14G>A∗ | 7T/9T | 1 | Carrier | ||
| 47 | W, O | 111.7 | p.F508del | c.3963 + 6G>T | 7T/9T | 0 | ND¶ | ||
| 48 | W | 80.1 | p.F508del | p.R1128G | 7T/9T | 0 | ND¶ | ||
| 49 | W | 80.6 | p.F508del | p.S13C | 7T/9T | 0 | ND¶ | ||
| 50 | H | 90.3 | c.274-1G>A | p.T629A | 7T/9T | 0 | ND¶ | ||
| 51 | W, B | 100.6 | p.F508del | p.P355L (c.1064C>T) | 7T/9T | 0 | ND¶ | ||
| 52 | H | 79.1 | p.F508del | p.Q493H (c.1479G>C) | 7T/9T | 0 | ND¶ | ||
| 53 | W, B | 64.8 | p.F508del | p.V1022M | 7T/9T | 0 | ND¶ | ||
| 54 | W | 74.7 | p.F508del | c.-887C>T | c.4243-5C>T | 7T/9T | 0 | ND‖ | |
| 55 | O | 136.6 | p.F508del | p.P718R | 7T/9T | 0 | ND¶ | ||
| Study participants with negative NBS results | |||||||||
| 56 | H | 276.7 | c.2822delT | c.2822delT | 7T/7T | 0 | CF | ||
| 57 | H | 179.2 | c.2822delT | c.2822delT | 7T/7T | 0 | CF | ||
| 58 | W | 15.6 | p.S1235R | c.-288G>C | 7T/9T | 0 | CF | ||
| 59 | H | 35.1 | c.164 + 4T>A∗ | p.G1173S∗ | Not done | 1 | Carrier | ||
| 60 | O | 20.2 | c.4136 + 12A>G∗ | p.M837T† | Not done | 2 | ND‖ | ||
B, black; H, Hispanic; NA, not available; ND, could not be determined; O, other or multiple races; W, white.
Confirmed by parental CFTR mutation testing to be on chromosome 1.
Confirmed by parental CFTR mutation testing to be on chromosome 2.
5T variant associated with (TG)11.
5T variant associated with (TG)12.
Inadequate follow-up.
Died before follow-up.
Table 3.
Clinical Characteristics of Study Participants Identified with One or More Novel CFTR Variants by Screening Test Result, California, July 16, 2007, to July 15, 2010
| Participant No. | Diagnosis/status | Age at diagnosis (months) | Age at last contact (months) | SC in mmol/L (age in months) | Positive culture for |
Lower respiratory tract infections | Failure to thrive or malnutrition | Abnormal chest X-ray | Fecal elastase (μg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| P. aeruginosa | S. aureus | |||||||||
| Study participants with positive NBS results | ||||||||||
| 1 | CF | 2 | 34 | Refused | No | Yes | Yes | No | Not done | <15 |
| 2 | CF | 2 | 50 | 108 (1) | No | Yes | Yes | Yes | No | <50 |
| 3 | CF | 15 | 15 | 8 (2), 14 (3) | Yes | Yes | Yes | Yes | 259 | |
| 4 | CF | 2 | 35 | 124 (2) | Yes | Yes | Yes | Yes | Yes | <50 |
| 5 | CF | 2 | 54 | 69 (2) | No | Yes | Yes | Yes | Yes | >500 |
| 6 | CF | 14 | 36 | 47 (10) | Yes | Yes | >500 | |||
| 7 | CF | 25 | 42 | 24 (3), 29 (6), 65 (12) | No | Yes | Yes | Yes | >500 | |
| 8 | CF | 2 | 32 | 92 (1) | No | No | Yes | No | No | <50 |
| 9 | CF | 3 | 30 | 123 (3) | Yes | Yes | No | Yes | No | 13 |
| 10 | CF | 21 | 34 | 54 (7), 50 (13), 32 (24) | Yes | Yes | Yes | No | Yes | >500 |
| 11 | CF | 2 | 41 | 101 (2), 87 (4), 65 (7), 70 (12) | Yes | Yes | Yes | Yes | Yes | 313 |
| 12 | CF | 1.5 | 44 | 84 (1) | Yes | Yes | Yes | No | Yes | >500 |
| 13 | CF | 24 | 34 | QNS (2, 3, 6, 12), 81 (38) | Yes | Yes | Yes | Yes | No | 324 |
| 14 | CF | 7 | 42 | 9 (2), 11 (6) | Yes | Yes | Yes | No | No | >500 |
| 15 | CRMS | 23 | 32 | 13 (2), 15 (6) | No | Yes | Yes | No | >500 | |
| 16 | CRMS | 12 | 12 | 8.5 (2), 10.6 (7) | No | No | No | No | No | 427 |
| 17 | CRMS | 16 | 16 | 14 (2), 25 (14) | No | Yes | No | 434 | ||
| 18 | CRMS | 17 | 17 | 16 (2), QNS (16) | Yes | Yes | No | Not done | ||
| 19 | CRMS | 9 | 9 | 10 (3), 14 (8) | No | No | No | No | >500 | |
| 20 | CRMS | 2 | 36 | 14 (2), 27 (7), 37 (12) | No | Yes | Yes | No | Yes | 363 |
| 21 | CRMS | 9 | 9 | 14 (2), 21 (6) | No | No | No | No | 396 | |
| 22 | CRMS | 7 | 32 | 14 (1), 16 (10) | No | Yes | Yes | Yes | No | 401 |
| 23 | CRMS | 21 | 50 | 17 (2), 10 (12), 18 (41) | No | No | No | No | Yes | >500 |
| 24 | CRMS | 10 | 39 | 11 (2), 6 (13) | No | Yes | No | No | No | >500 |
| 25 | CRMS | 3 | 3 | 9 (2) | No | No | No | No | >500 | |
| 26 | CRMS | 32 | 33 | 18 (2) | No | Yes | No | Yes | Yes | Not done |
| 27 | CRMS | 2 | 4 | 14 (2) | ||||||
| 28 | CRMS | 1 | 23 | QNS (2) | ||||||
| 29 | CRMS | 2 | 13 | 15 (2) | No | Yes | Yes | Yes | No | 426 |
| 30 | CRMS | 2 | 28 | 18 (3), 41 (10), 54 (13), 31 (19) | No | Yes | No | No | >500 | |
| 31 | CRMS | 4 | 37 | 7 (2), 7 (6), 13 (35) | No | Yes | Yes | No | Yes | 419 |
| 32 | CRMS | 12 | 52 | 11 (2), 10 (3), 7 (10), 12 (44) | No | Yes | Yes | No | Yes | >500 |
| 33 | CRMS | 12 | 54 | 11 (3), 10 (5), 11 (6), 18 (54) | No | Yes | No | No | No | 428 |
| 34 | CRMS | 4 | 15 | 21 (4), 13 (6), 16 (50) | No | Yes | No | Yes | 395 | |
| 35 | CRMS | 12 | 42 | 12 (3) | No | Yes | No | No | No | >500 |
| 36 | CRMS | 17 | 29 | 11 (2),11 (5) | No | Yes | No | Yes | No | 473 |
| 37 | CRMS | 12 | 24 | 14 (1.5), 12 (8), 17 (16), 15 (25) | No | No | No | No | Yes | Not done |
| 38 | CRMS | 2 | 5 | 6 (5) | >500 | |||||
| 39 | CRMS | 2 | 6 | 12 (2) | No | No | No | No | >500 | |
| 40 | CRMS | 16 | 14 (2), 23 (15) | No | Yes | No | No | Not done | 484 | |
| 41 | CRMS | 12 | 12 | 15 (5), 12 (8) | No | Yes | No | No | No | Not done |
| 42 | CRMS | 8 | 8 | 19 (2), 39 (8) | No | No | No | No | >500 | |
| 43 | Carrier | 3 | 3 | Not done | ||||||
| 44 | Carrier | 2 | 2 | 13 (1) | ||||||
| 45 | Carrier | 38 | 45 | 16 (1) | No | Yes | Yes | 421 | ||
| 46 | Carrier | 6 | 6 | 16 (3) | ||||||
| 47 | ND∗ | 7 | 7 (2), 20 (6) | |||||||
| 48 | ND∗ | 12 | 8 (2), 24 (10) | |||||||
| 49 | ND∗ | 13 | 13 (1), 15 (9) | |||||||
| 50 | ND∗ | 14 | 13 (4) | |||||||
| 51 | ND∗ | 12 | 17 (5) | |||||||
| 52 | ND∗ | Not done | ||||||||
| 53 | ND∗ | 13 | 15 (2) | |||||||
| 54 | ND† | <1 | Not done | |||||||
| 55 | ND∗ | 14 | 17 (2) | |||||||
| Study participants with negative NBS results | ||||||||||
| 56 | CF | <1 | 27 | 82 (1) | Yes | Yes | Yes | At birth only | Yes | Not done |
| 57 | CF | 4 | 40 | 94 (4) | Yes | Yes | Yes | Yes | Yes | <50 |
| 58 | CF | 12 | 43 | 10 (5) | No | Yes | Yes | >500 | ||
| 59 | Carrier | 5 | 5 | Not done | Not done | |||||
| 60 | ND† | <1 | Not done | Not done | ||||||
ND, could not be determined; QNS, quantity not sufficient.
Inadequate follow-up.
Died before follow-up.
During the 3-year study period, CFCs reported five infants with negative CF NBS results who were found to carry at least one novel CFTR variant (Table 2). All five infants were referred to CFCs for evaluation due to meconium ileus or other intestinal conditions, three of which were preterm births (range of gestational ages, 24 to 29 weeks). In total, six novel variants were identified among these participants, one of which (c.-288G>C) was also identified in the group with positive NBS results. Two of the participants (No. 59 and 60) were each found to carry two different novel variants, two (No. 56 and 57) were apparently homozygous for the same novel variant, and one (No. 58) carried one previously identified CFTR variant and one novel variant. Three of these participants (No. 56, 57, and 58) have been diagnosed as having CF, one (No. 60) died of genetic abnormalities unrelated to CF before SC testing could be performed, and one (No. 59) had CF ruled out after parent testing identified that both novel variants were inherited from one parent.
Of the 60 study participants identified, to date, 17 (28%) have been diagnosed as having CF, 28 (47%) have been classified as having CRMS, five (8%) have been identified as CFTR carriers, and 10 (17%) have had no diagnosis confirmed (owing to lack of follow-up or infant mortality).
Novel Variants of the Type Expected to Cause CF
Of the 55 novel variants identified during the study period, 10 (18%) were associated with elevated SC values (>60 mmol/L) or disease manifestations of CF. Of the 10 novel variants, 8 (80%) were located in exonic regions, two (20%) in the promoter region, and none in intronic regions of the CFTR gene. Of the 12 newborns carrying these 10 novel variants, 7 (58%) were diagnosed as having CF by age 3 months. The remaining 5 newborns (42%) were diagnosed between ages 4 and 25 months. Nine of these 12 participants (75%) were Hispanic, 1 (8%) was non-Hispanic white, and 2 (17%) were of other or mixed race.
c.2554_2555insT
After parental DNA testing, novel variant c.2554_2555insT and known variant c.164 + 28A>G were shown to be in trans with p.F508del in participant 1, who was diagnosed as having CF at 2 months of age. Although SC test results were not available to assist the diagnosis, the patient exhibited respiratory and gastrointestinal signs (fecal elastase level <15 μg/g) of CF during 34 months of follow-up. This participant has had recurrent Staphylococcus aureus infections, several episodes of sinusitis, and one mild pulmonary exacerbation. Because c.2554_2555insT results in a frameshift mutation (which is predicted to be deleterious) and c.164 + 28A>G has been reported in approximately 2% of African and African American populations5, 6 and has not been shown to be associated with CF based on unpublished California CF NBS program data, it is most likely that novel variant c.2554_2555insT contributed to the illness in this newborn.
p.F1107L
Although participant 2 did not receive parental DNA testing to confirm the phase of novel variant p.F1107L, the abnormal SC level (107 mmol/L) and symptoms of pancreatic insufficiency (including fecal elastase level <50 μg/g) indicate that, in addition to p.F508del, another mutation must be associated with CF. This participant was diagnosed as having CF at 2 months of age. Because c.-877C>T is thought to be a non–disease-causing polymorphism,15 p.F1107L is indicated as a possible CF-associated variant. p.F1107L was previously identified by Ambry Genetics in a set of twins who presented with rectal prolapse, elevated SC concentration, and a second deleterious mutation in trans with p.F1107L (S. Keiles, personal communication).
c.-152G>C
Participant 5 was diagnosed as having CF at 2 months of age after novel variant c.-152G>C was shown to be in trans with p.F508del via parental DNA testing. The participant had an abnormal SC level of 69 mmol/L. Fecal elastase levels at 2 months and 1 year of age were within normal limits. With 47 months of follow-up, the participant had recurrent S. aureus infection and abnormal pulmonary function. Participant 5 has also been diagnosed as having Prader-Willi syndrome and hypotonia with complications of sleep apnea requiring noninvasive ventilator support. Although gross deletions or duplications of the CFTR gene have not been ruled out, the mild CF symptoms suggest that these are unlikely and that c.-152G>C potentially contributes to this phenotype.
p.L323P
Novel variant p.L323P was identified in participant 6 with a borderline SC level (47 mmol/L) and positive respiratory cultures for Pseudomonas aeruginosa. Parental DNA testing confirmed that p.L323P and the (TG)11-5T variant were in trans with p.F508del; therefore, the individual effect of p.L323P on the phenotype is unclear. However, of 31 newborns with genotype p.F508del in trans with variant (TG)11-5T identified during the same period through the California CF NBS program, none had an SC level >26 mmol/L.16 Therefore, p.L323P alone or p.L323P in conjunction with (TG)11-5T is likely associated with the CF manifestations.
p.L32M
Participant 7 has a novel variant p.L32M and p.F508del; no other mutations or variants were identified, although gross duplications or deletions could not be ruled out because multiplex ligation-dependent probe amplification analysis was not conducted. A diagnosis of CF was made at 24 months of age because of an elevated SC level (65 mmol/L at 12 months of age), poor growth, and abnormal findings on chest radiography (bilateral peribronchial cuffing and increased interstitial markings). This child developed signs of steatorrhea at 24 months despite repeated measures of fecal elastase levels >500 μg/g. Considering the strong clinical evidence of malabsorption (presented as increased appetite, loose and frequent stools, iron deficiency anemia, and difficulties in gaining weight), pancreatic enzyme replacement therapy was initiated at 25 months. The enzyme replacement resulted in an improvement in BMI from the 26th to the 69th percentile in 1 year.
c.2883_2886dupGTCA
Novel variant c.2883_2886dupGTCA was identified with p.F508del in participant 8, who had an SC level of 92 mmol/L at 1 month of age. Based on the SC level and poor weight gain, a CF diagnosis was made at 2 months. With 22 months of follow-up, this participant has had respiratory specimens positive for Haemophilus influenzae and Candida sp. Although parental DNA testing was not available and deletions and duplications cannot be ruled out, this frameshift mutation is likely to be associated with disease.
c.2349_2350insT
Participant 9 was diagnosed as having CF at 3 months of age after having an SC level of 123 mmol/L, a diagnosis of failure to thrive in the first year of life (due in part to parental resistance to administration of prescribed medications, including enzymes), abnormal fecal elastase results (<6 μg/g), and positive cultures for P. aeruginosa and methicillin-resistant S. aureus. Because the only other CFTR mutation identified through sequencing was p.F508del, it is probable that c.2349_2350insT contributes to disease manifestations.
p.K114del
Two nontwin siblings with genotype p.P205S/p.K114del were identified [participants 12 and 13 (younger)]. Participant 12 was diagnosed as having CF at age 1½ months based on an SC level of 84 mmol/L. Fecal elastase levels at 2 months and 1 year of age were within normal limits. By 15 months of age, this participant had weight for length at the 10th percentile and required nutritional supplements to achieve adequate weight gain. Respiratory cultures at approximately 2 and 3 years of age have been positive for P. aeruginosa and S. aureus. Participant 13 had several unsuccessful attempts at SC testing and eventually had a positive SC test result at 3 years of age, with an SC level of 81 mmol/L. Participant 13 also experienced poor weight gain during infancy requiring supplements for recovery.
c.-602A>T
Although participant 14 had two normal SC levels (9 and 11 mmol/L), this patient was diagnosed as having CF owing to lower respiratory tract infections with positive cultures for P. aeruginosa and S. aureus chronically. During the first 2 years of life, this participant had persistently low levels of vitamins A and D, but after initiating supplementation, these levels rebounded to within the reference range. Focused DNA testing of both parents indicated that novel variant c.-602A>T and c.274-1G>A reside on opposite chromosomes; however, gross deletions or duplications in the CFTR gene have not been ruled out. Therefore, c.-602A>T potentially contributes to the CF phenotype.
c.2822delT
Although participant 56 had an elevated IRT level (276.7 ng/mL), this individual was deemed CF screen negative when no mutations were identified during panel testing. However, a family history of CF, a meconium ileus at birth, and an elevated SC level (82 mmol/L) resulted in this participant being diagnosed as having CF at younger than 1 month of age. Family history of CF included a sibling with CF who received no treatment and died at <1 year of age 15 years previously in Mexico. This participant required an ileostomy that was later reversed and total parenteral nutrition for 9 weeks in the hospital before successfully transitioning to oral feedings. The participant developed total parenteral nutrition–related cholestasis that subsequently resolved. A cardiac echocardiogram at 2 weeks revealed mild left pulmonary artery stenosis and an anomalous right coronary artery arising from the left coronary sinus. CFTR sequencing later revealed that this individual is apparently homozygous for novel variant c.2822delT. With 27 months of follow-up, this participant tested positive for P. aeruginosa and S. aureus. This participant also had other lower respiratory tract infections, abnormal chest X-ray findings, and steatorrhea.
Participant 57 had an elevated IRT level (179.2 ng/mL) and was later found to be apparently homozygous for c.2822delT. Multiplex ligation-dependent probe amplification of the CFTR gene showed no deletions or duplications. This participant was suspected of having CF due to meconium ileus at birth. An SC value of 94 mmol/L at 4 months, with steatorrhea and a very low fecal elastase level (<50 μg/g) resulted in a CF diagnosis. Through 40 months of follow-up, this participant has had several lower respiratory tract infections, including P. aeruginosa and S. aureus, and has had abnormal chest X-ray findings.
Novel Variants of the Type that May or May Not Cause CF
In the following four participants with CF, it was not possible to delineate the contribution of the novel variants because they were on the same allele with another CFTR mutation. Participant 3 was identified with novel variant c.-837T>C (found to be in cis with p.V562I after parent testing) and p.F508del on the opposite chromosome. Participant 4 carried p.F508del, p.I556V, and novel variant c.1278delC. Because parental DNA testing was unavailable, it is unclear which variant contributes to the CF phenotype in this child. It is surmised, however, that the frameshift mutation c.1278delC would be more deleterious than the missense mutation p.I556V. Novel variant c.3718-24G>A and the (TG)12-5T variant were shown to be in trans with p.F508del in participant 10, a patient of Asian East Indian descent. Participant 11 was identified with novel variant c.-448A>G in cis with p.A559T and in trans with p.L206W.
In unrelated participants 36 and 58, it was not possible to delineate the contribution of novel variant c.-288G>C to CF because of the existence of comorbid conditions. Participant 36, classified as having CRMS, exhibited failure to thrive symptoms that could be due to either the CFTR variant or Hirschsprung disease. Participant 58 had an IRT level (15.6 ng/mL) below the cutoff point for CF NBS but was a preterm infant with very low birth weight, intestinal perforation, and meconium ileus. Participant 58 was tentatively diagnosed as having CF at 12 months of age but also had developmental delays and bronchopulmonary disease. This individual died at 42 months of age with an unknown cause of death and no autopsy. It remains uncertain whether c.-288G>C is associated with CF. c.3718-24G>A was listed in ESP with a frequency of 1 in 7019 (0.01%).
Novel Variants in Cis with Known Mutations
Approximately half of the study participants received parental DNA testing: 33% (20 of 60) had two parents tested, 18% (11 of 60) had one parent tested, and 48% (29 of 60) received no parental mutation testing. In several instances, parental DNA testing revealed that novel variants were in cis with known mutations. Participants 11, 43, and 44 were all shown to carry novel variant c.-448A>G in cis with known mutation p.A559T. Participants 43 and 44 have been classified as CFTR carriers. Participant 11, who was diagnosed as having CF, also carries known mutation p.L206W (case described earlier). Participant 15, who was classified as having CRMS, was found to carry novel variant c.-983A>T in cis with p.F508del and in trans with c.-461A>G. Parental testing for participants 45, 46, and 59, all diagnosed as CFTR carriers, showed the following mutation pairs to be in cis: p.G85E with c.744-15T>C (novel), p.N1303K with c.2490 + 14G>A (novel), and c.164 + 4T>A (novel) with p.G1173S (novel), respectively. ESP reported the p.G1173S variant with a frequency of 1 in 10,757 (0.01%), and the c.-461A>G variant had a frequency of 2 in 2188 (0.09%) according to 1000 Genomes.5
Novel Variants Probably Not Causative of CF Manifestations to Date
Twenty-six participants carrying 26 of the 55 novel variants (47%) have not shown consistent manifestations of CF over time. Participants 16 to 35 and 37 to 42, with the following novel variants, have been classified as having CRMS to date: c.-967T>C, c.-769A>G, c.-730A>G, c.-684G>A, c.-635A>G, c.-510G>A, p.C76R, p.D112G, p.L136P, c.744-15T>C, p.D249Y, c.869 + 8G>T, p.F315S, p.Y325C, p.R811S, c.1767-13T>G, c.2490 + 14G>T, p.T887P, c.3367 + 3A>C, p.M1140L, p.V1198M, p.V1322L, p.H1375N, p.T1396P, c.4243-5C>T, and p.T1478R. For these variants, p.F315S was detected in 1613 control subjects one time. ESP reported p.T1396P with a frequency of 1 in 10,758 (0.01%), c.744-15T>C with a frequency of 1 in 10,681 (0.01%), and p.H1375N with a frequency of 2 in 10,758 (0.02%). The c.4243-5C>T variant was reported with a frequency of 17 in 1852 (0.91%) in black patients, making it likely to be a common polymorphism in this ethnic group.
A diagnosis could not be determined for 10 participants (No. 47 to 55 and 60) with the following novel variants owing to inadequate follow-up: p.S13C, p.P355L, p.Q493H, p.T629A, p.P718R, p.V1022M, p.R1128G, and c.3963 + 6G>T. Participants 54 and 60 died before a diagnosis could be made. The novel variants that these two participants carried were c.4136 + 12A>G with p.M837T (participant 60), and c.4243-5C>T (participant 54). In dbSNP,6 the p.P718R variant was reported with a frequency of 2 in 4492 (0.04%), and the p.V1022M variant was reported with a frequency of 1in 4550 (0.02%). For c.4243-5C>T, see the previous paragraph.
Discussion
During the first 3 years of CF NBS in California, novel variants were identified through testing by DNA sequencing in 3.9% of hypertrypsinogenemic newborns (55 of 1417) with only one mutation identified by the CA-40 panel. To date, ≥10 previously unidentified, CF-associated mutations were found, which represents ≥3.8% of the 266 CF cases identified by the California CF NBS program during the study period. Because the participants included in this analysis had fairly comprehensive CFTR gene analysis performed, the phenotypes reported herein have a low likelihood of being confounded by undetected CFTR mutations, although no sequencing technology is 100% accurate. However, the DNA sequencing technology routinely used to analyze the CFTR gene in this report could not detect the presence of gross deletions or duplications, which are estimated to occur at a frequency of 1.5% (S. Keiles, personal communication), with approximately one-third of those being c.54-5940_273 + 10250del21kb. Excluding c.54-5940_273 + 10250del21kb, which would be detected by the CA-40 mutation panel, the frequency of gross deletions or duplications would be predicted to be <1%. Although these changes could not be ruled out for all the participants, the likelihood of a study participant carrying a gross deletion or duplication is low.
All screened newborns in California who are found to have two known mutations or one known mutation and one or more variants [including novel variants (12)TG-5T and (13)TG-5T] are referred, usually between 2 and 8 weeks of age (mean, 36 days; median, 34 days; interquartile range, 26 to 42 days), to Cystic Fibrosis Foundation–accredited CFCs for SC testing and diagnostic follow-up. Those with elevated SC test values or CF manifestations are conclusively diagnosed as having CF and are followed up using current CF infant care guidelines.17, 18, 19 Those with lower SC values and no CF manifestations are evaluated using follow-up guidelines developed by the California CFCs and the CDPH GDSP, including testing for fecal elastase levels, growth parameters, and respiratory cultures (oropharyngeal, nasopharyngeal, or bronchoalveolar lavage, depending on the clinical circumstances). When evidence of CF manifestations is absent or inconclusive, genetic testing of parents (focused on the child's mutations/variants) at a DNA sequencing laboratory is recommended to determine the cis/trans phase of the mutations. When one parent is found to carry both mutations/variants (which are most likely on the same chromosome) and the other parent is found to carry none, the child is deemed an unaffected carrier and the family receives a letter from the CFC stating that no further follow-up in the CF clinic is required unless the child develops symptoms. When the mutations/variants are found to be inherited from more than one parent, the child is followed up for ≥1 year to monitor for the development of disease manifestations before making a diagnostic determination. Repeated SC testing is recommended every 3 to 6 months, growth is monitored, and respiratory cultures and other evaluations, including chest roentgenograms, may also be obtained, as clinically indicated. In addition, CFCs monitor vitamin A, E, and D levels as well as electrolytes and measurements of hepatic function in these infants. At CFCs, where it is available, infant pulmonary function testing is performed to evaluate pulmonary status as an added supportive variable.
Genetic counseling consultation is performed to supplement CFC visits. The consultation provides discussion of inheritance patterns, recurrence risk, etiology of CF and CFTR gene function. Counselors address the identified specific CFTR mutations/variants and the possible ambiguity of one mutation combined with novel variants. Recommendations are also made regarding parental testing to determine cis/trans phase, and carrier testing of family members is offered. Obtaining parent genetic testing proved difficult during the study period owing to financial constraints. Both parents were successfully tested in approximately one-third of the eligible participants in this study. However, the CDPH GDSP has taken steps to mitigate this problem by covering the cost of parent genetic testing as part of the CF NBS program and has seen an increase in parental testing requests filled.
The systematic review currently being conducted by the Clinical and Functional Translation of CFTR team (CFTR2, http://www.cftr2.org, last accessed January 8, 2013) to distinguish between CFTR mutations with, or with likely, clinical consequences is invaluable. However, with the continuing identification of novel CFTR mutations, conclusions from CFTR2 will necessarily remain delayed until a large enough number of persons with the mutation is identified over time.
The combination of the design of the California CF NBS program and the long-term follow-up by CFCs provides an important opportunity to describe the natural history of CFTR mutations, including novel variants, in a large and ethnically diverse cohort of hypertrypsinogenemic newborns. The information obtained provides evidence about the pathogenic potential of novel CFTR variants. This information will be used with other available information to define the mutations that should and should not be referred by the California CF NBS program in the future and to aid clinicians at large in making a CF diagnosis. The information obtained also shows that, by itself, an initial negative SC test result in the newborn's first few months cannot rule out CF and that longer-term clinical follow-up is necessary to make a conclusive diagnosis. Of the participants carrying novel variants and diagnosed as having CF reported herein, more than half (58%) were diagnosed by 3 months of age; the other 42% required follow-up of up to 2 years before a diagnosis of CF was confirmed.
These findings have implications for how CF NBS and CF diagnostic follow-up are being conducted currently in the United States and elsewhere and raise questions such as the following: Does comprehensive genotyping offer more prognostic value than SC testing? How long and rigorously should a child with novel genetic mutations but no disease manifestations be followed to be confident that CF can be ruled out? Who should pay for long-term clinical monitoring, testing, and evaluation? What negative consequences to the individuals identified with two or more CFTR mutations and their families will result, if any, from long-term follow-up by CFCs? Does the evidence that CF NBS does more good than harm for individuals with CF with elevated SC levels also hold true for those with nonelevated SC levels? It is our intention to continue to gather the information necessary to answer these questions and to more broadly assess the costs and benefits of systematically identifying and following up infants using the California CF NBS model.
Note Added in Proof
After the article was accepted, participant 1 (57 months of age) received an SC test result of 131 mmol/L, confirming the diagnosis of CF.
Acknowledgments
The Stanford Molecular Pathology Laboratory (Iris Schrijver, Director) conducted the California mutation panel testing during the study period under the guidance of George Helmer and supervision by Ajit Bhandal (California Genetic Disease Laboratory). Under the overall direction of Fred Lorey (Acting Chief of the California Genetic Disease Screening Program), Shellye Lessing and Tracey Bishop (Newborn Screening Program), Suzanne Young (Public Health Institute), and Charlene Sacramento (Sequoia Foundation) worked with CFCs to obtain up-to-date clinical information for this study. In addition to the listed authors from CFCs, the following members of the California CF NBS Consortium and CFC staff diligently evaluated the study participants: Nancy Wheeler-Dobrota (Loma Linda University Medical Center), Joanna Woodworth (Kaiser Permanente Los Angeles Medical Center), Myrza Perez and Marilyn Tsang (Sutter Memorial Hospital), Diana Dawson (University of California San Francisco Medical Center), Cheryl Meuter (Kaiser Permanente Northern California), Kim Franz (University of California Davis Medical Center), and Jill Edwards (Miller Children's Hospital).
Footnotes
This work was conducted as part of Newborn Screening Program evaluation and approved project number 12-06-0354 of the California Health and Human Agency Committee for the Protection of Human Subjects.
References
- 1.Rommens J.M., Iannuzzi M.C., Kerem B., Drumm M.L., Melmer G., Dean M., Rozmahel R., Cole J.L., Kennedy D., Hidaka N., Zsiga M., Buchwald M., Riordan J.R., Lap-Chee T., Collins F.S. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 2.Kerem B., Rommens J.M., Buchanan J.A., Markiewicz D., Cox T.K., Chakravarti A., Buchwald M., Tsui L.C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 3.Riordan J.R., Rommens J.M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.L., Drumm M.L., Iannuzzi M.C., Collins F.S., Lap-Chee T. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 4.Castellani C., Cuppens H., Macek M., Jr., Cassiman J.J., Kerem E., Durie P., Tullis E., Assael B.M., Bombieri C., Brown A., Casals T., Claustres M., Cutting G.R., Dequeker E., Dodge J., Doull I., Farrell P., Ferec C., Girodon E., Johannesson M., Kerem B., Knowles M., Munck A., Pignatti P.F., Radojkovic D., Rizzotti P., Schwarz M., Stuhrmann M., Tzetis M., Zielenski J., Elborn J.S. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179–196. doi: 10.1016/j.jcf.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A., 1000 Genomes Project Consortium An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherry S.T., Ward M.H., Kholodov M., Baker J., Phan L., Smigielski E.M., Sirotkin K. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute . ed 3. Clinical and Laboratory Standards Institute; Wayne, PA: 2009. Sweat Testing: Sample Collection and Quantitative Chloride Analysis: Approved Guideline. [Google Scholar]
- 8.LeGrys V.A., Yankaskas J.R., Quittell L.M., Marshall B.C., Mogayzel P.J., Jr. Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr. 2007;151:85–89. doi: 10.1016/j.jpeds.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Borowitz D., Parad R.B., Sharp J.K., Sabadosa K.A., Robinson K.A., Rock M.J., Farrell P.M., Sontag M.K., Rosenfeld M., Davis S.D., Marshall B.C., Accurso F.J. Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J Pediatr. 2009;155:S106–S116. doi: 10.1016/j.jpeds.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh P.S., Metzger D.A., Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- 11.Kammesheidt A., Kharrazi M., Graham S., Young S., Pearl M., Dunlop C., Keiles S. Comprehensive genetic analysis of the cystic fibrosis transmembrane conductance regulator from dried blood specimens: implications for newborn screening. Genet Med. 2006;8:557–562. doi: 10.1097/01.gim.0000237793.19868.97. [DOI] [PubMed] [Google Scholar]
- 12.Schouten J.P., McElgunn C.J., Waaijer R., Zwijnenburg D., Diepvens F., Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards C.S., Bale S., Bellissimo D.B., Das S., Grody W.W., Hegde M.R., Lyon E., Ward B.E. ACMG recommendations for standards for interpretation and reporting of sequence variations: revisions 2007. Genet Med. 2008;10:294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 14.Farrell P.M., Rosenstein B.J., White T.B., Accurso F.J., Castellani C., Cutting G.R., Durie P.R., Legrys V.A., Massie J., Parad R.B., Rock M.J., Campbell P.W., III Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherny SK, Keiles SB: The C.-755C>T variant in the CFTR gene most likely represents a non-disease causing polymorphic variant. Pediatric Pulmonology Supplement Special Issue: The 23rd Annual North American Cystic Fibrosis Conference Volume 44, Issue S32
- 16.Keiles S., Koepke R., Parad R., Kharrazi M. Impact of IVS8-(TG)m(T)n on IRT and sweat chloride levels in newborns identified by California CF newborn screening. J Cyst Fibros. 2012;11:257–260. doi: 10.1016/j.jcf.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Borowitz D., Robinson K.A., Rosenfeld M., Davis S.D., Sabadosa K.A., Spear S.L., Michel S.H., Parad R.B., White T.B., Farrell P.M., Marshall B.C., Accurso F.J. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155:S73–S93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson K.A., Saldanha I.J., McKoy N.A. Management of infants with cystic fibrosis: a summary of the evidence for the cystic fibrosis foundation working group on care of infants with cystic fibrosis. J Pediatr. 2009;155:S94–S105. doi: 10.1016/j.jpeds.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Sermet-Gaudelus I., Mayell S.J., Southern K.W. Guidelines on the early management of infants diagnosed with cystic fibrosis following newborn screening. J Cyst Fibros. 2010;9:323–329. doi: 10.1016/j.jcf.2010.04.008. [DOI] [PubMed] [Google Scholar]