Abstract
Fields of forensics, transplantation, and paternity rely on human identity testing. Currently, this is accomplished through amplification of microsatellites followed by capillary electrophoresis. An alternative and theoretically better approach uses multiple single-nucleotide polymorphisms located within a small region of DNA, a method we initially developed using HLA-A and called haplotype counting. Herein, we validated seven additional polymorphic loci, sequenced a total of 45 individuals from three of the 1000 Genomes populations (15 from each), and determined the number of haplotypes, heterozygosity, and polymorphic information content for each locus. In addition, we developed a multiplex PCR that amplifies five of these loci simultaneously. Using this strategy with a small cohort of leukemic patients who underwent allogeneic bone marrow transplantation, we first attempted to define a threshold (0.26% recipient) by examining seven patients who tested all donor and did not relapse. Although this initial threshold will need to be confirmed in a larger cohort, we detected increased recipient DNA above this threshold 90 to 145 days earlier than microsatellite positivity, and 127 to 142 days before clinical relapse in four of eight patients (50%). Haplotype counting using these novel loci may be useful for ultrasensitive detection in fields such as bone marrow transplantation, solid organ transplant rejection, patient identification, and forensics.
Once reserved for treatment of lethal hematological malignancies in children and young adults, the indications for allogeneic bone marrow transplantation (BMT) are expanding and clinical trials now include less severe conditions, such as thalassemia and sickle cell disease.1, 2 Advances in conditioning regimens have resulted in decreased morbidity and mortality, thereby allowing this broader use. Chimerism testing at set intervals is a standard practice after allogeneic BMT because it is an effective method for detecting graft rejection or relapse of the original disease.
Testing for chimerism usually relies on microsatellites [or short tandem repeats (STRs)]. Most clinical laboratories use multiplex PCR-based kits originally developed for forensic purposes using Combined DNA Index System loci.3, 4, 5 Microsatellite analysis involves PCR amplification with fluorescently labeled primers, followed by capillary electrophoresis. Huisman et al6 (and references therein) previously documented that increasing host chimerism or stable mixed chimerism was associated with increased probability of relapse, and pointed to the need for a more sensitive assay. However, detection at ultrasensitive levels is not possible using STRs and capillary electrophoresis because the limit of detection for capillary electrophoresis analysis is approximately 3% to 5%. In addition, microsatellites are inherently inaccurate because the smaller fragment size allele's peak height exceeds the larger fragment size allele's peak height.
In 2000, Oliver et al7 reported that single-nucleotide polymorphisms (SNPs) could be used for chimerism testing, and this has also been reported by several other groups.8, 9, 10 However, using individual SNPs is less desirable because they are inherently less informative than microsatellites, which may have >10 alleles at a single locus. For example, we previously demonstrated that >20 SNPs would need to be screened to find a locus that is homozygous for one allele in a recipient and homozygous for the opposite allele in an unrelated donor with 95% certainty.7 Only four SNPs would need to be analyzed if heterozygous loci were included; however, more SNPs would need to be analyzed for related individuals.
Next-generation sequencing (NGS) has become widely used in both research and clinical settings. This is largely a result of its remarkable capabilities and decreasing cost. However, single base error rates are relatively high across most NGS platforms, ranging from approximately 0.1% to 1%.11 This precludes using NGS for ultrasensitive detection of individual SNPs. In 2014, we and another group demonstrated a solution to this problem using multiple SNPs on the same molecule, allowing one to circumvent the inherently high single base error rates.12, 13 We achieved a limit of detection of 1 in 10,000, providing that a sufficient number of genomes were tested. In addition to the HLA-A prototypic locus, we also identified 4349 additional polymorphic loci that contained at least nine SNPs within a 300-bp window using available variant calls from three populations (Africans, Asians, and Europeans) for the 1000 Genomes study.14
Herein, we experimentally validated eight of these loci (including HLA-A) by sequencing samples from 15 individuals from three different populations from 1000 Genomes study (total of 45 individuals). Using fully engrafted patients, we defined an initial threshold of 0.26% recipient in bone marrow, although this will need to be confirmed in a larger cohort. Furthermore, we demonstrate the ability to detect increased host DNA at an earlier time point than the conventional STR-based assay, preceding leukemic relapse. However, the validity of this approach will need to be confirmed in a larger study, and the clinical utility of a more sensitive assay and early relapse intervention will need to be demonstrated.
Materials and Methods
Sanger Sequencing
From 4349 polymorphic loci previously identified, we selected eight loci based on high informativity and their localization to separate chromosomes.12 Primers were designed and obtained (IDT, Coralville, IA). Five normal samples were PCR amplified and Sanger sequenced to confirm primer amplification and polymorphism (data not shown).
Whole Genome Sequencing Analysis and Alignments
We analyzed whole genome sequencing data at these eight loci from eight normal samples to identify initial number of haplotypes.15 For each sample, haplotype sequences of both alleles were determined. Haplotypes were named with respect to the gene in which they were located, followed by a number in ascending order. Number 1 was always assigned to the haplotype in the reference human genome (hg19). Polymorphic loci that were intergenic were named using the nearest gene. HLA-A and HLA-B haplotypes were assigned using the Major Histocompatibility Complex database (http://www.ncbi.nlm.nih.gov/projects/gv/mhc/main.fcgi?cmd=init, last accessed October 18, 2016), a publically accessible platform for DNA and clinical data related to the human major histocompatibility complex.
1000 Genomes Samples
Fifteen samples from three populations (CEU, Utah residents with Northern and Western European ancestry; JPT, Japanese in Tokyo; YRI, Yoruba in Ibadan and Nigeria) were selected from the 1000 Genomes study. The following DNA samples were obtained from the National Institute of General Medical Sciences Human Genetic Cell Repository at the Coriell Institute for Medical Research (Camden, NJ). CEU population samples: NA07048, NA07348, NA10852, NA10853, NA10854, NA10857, NA10861, NA10863, NA10864, NA10865, NA12335, NA12385, NA12455, NA12470, NA12708. JPT population samples: NA18939, NA18940, NA18941, NA18942, NA18943, NA18944, NA18945, NA18946, NA18947, NA18948, NA18949, NA18950, NA18951, NA18952, NA18953. YRI population samples: NA18486, NA18487, NA18488, NA18489, NA18498, NA18499, NA18501, NA18502, NA18504, NA18505, NA18507, NA18508, NA18510, NA18511, NA18516.
Monoplex PCR and Mixing
Each sample was PCR amplified at eight loci using barcode-containing primers (Table 1), containing Ion Torrent (Thermo Fisher, Waltham, MA) specific adaptors (A- and P1-adaptors) at their 5′ ends. All loci for a given sample were amplified using the same barcode. Each PCR contained 50 ng DNA, 200 nmol/L forward and reverse primers, in Platinum PCR SuperMix High Fidelity (Invitrogen, Carlsbad, CA) in a 50-μL total reaction volume. Cycling conditions were as follows: 94°C for 3 minutes, 30 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 68°C for 30 seconds, and a final hold at 4°C. Amplified products were pooled by mixing at equal ratios, and purified using QIAquick PCR Purification kit (Qiagen, Germantown, MD). Emulsion PCR, NGS, and mapping to hg19 were all performed per manufacturer's protocol (Ion Torrent). Data were analyzed using Integrated Genome Viewer version 2.3.37 (Broad Institute, Cambridge, MA).
Table 1.
Primer Sequences
| Chromosome | Locus | Forward primer∗ | Reverse primer† |
|---|---|---|---|
| 1 | TFB2M | 5′-TGGCATCTAGACATTTAAGAAAAGAA-3′ | 5′-TGCTGGAATTACAAGCGTGA-3′ |
| 4 | SORCS2 | 5′-CTGGATTCACAGCCCAAGAT-3′ | 5′-GTCTGCCTCAACCCTGACA-3′ |
| 6 | HLA-A | 5′-AGGACCTGCGCTCTTGGAC-3′ | 5′-CCAATTGTCTCCCCTCCTTG-3′ |
| 6 | HLA-B | 5′-GCCCAGGTCTCGGTCAGG-3′ | 5′-GAGAAGAGGGATCAGGACGA-3′ |
| 8 | CSMD1 | 5′-GAGGATAACAGTAATGGGAC-3′ | 5′-TGTGTCTTCACATGGACTTTCC-3′ |
| 13 | FARP1 | 5′-TCCCAAAGTGCTGGGATTAC-3′ | 5′-GCTGATGGGCACATTACAAA-3′ |
| 16 | MT4 | 5′-AAGGCAGTATTTTGGGCTTTC-3′ | 5′-GTCCAGGCTGGTCTTGAATC-3′ |
| 21 | TMPRSS15 | 5′-AAGCTGACTCGTGACCTGCT-3′ | 5′-GGAAAACAAGTCTTAGGCTTTGC-3′ |
All forward primers contained A-adaptor (Ion Torrent PGM), 5′-CCATCTCATCCCTGCGTGTCTCCGACTCAG-3′, followed by a unique IonTorrent barcode at the 5′ end, where the underlined TCAG is the library key that is used to differentiate library reads from internal control reads.
All reverse primers contained P1-adaptor (Ion Torrent PGM), 5′-CCTCTCTATGGGCAGTCGGTGAT-3′, at the 5′ end.
To compute the haplotype diversity into the selected regions, we considered each multi-SNP haplotype as an allele; for each region, we estimated the heterozygosity (H) and polymorphism information content (PIC) as measures of diversity. H is computed as follows:
| (1) |
for a marker with k alleles, pi is the frequency of the ith allele. H is the probability a given individual in that population is heterozygous at that marker locus.16 The H value is expressed as a continuous numerical value ranging from 0 (no heterozygosity) to nearly 1.0 (high diversity).
As a measure of haplotype diversity, we also calculated PIC, a method designed to select informative markers for linkage analysis.17 PIC is the probability at a given locus that it can be determined which parent transmitted a given allele to a child.18 PIC is computed as follows:
| (2) |
where pi is the population frequency of the ith of a alleles. As with H, PIC values range from 0 (no diversity) to 1 (high diversity).
As the comparison of the two equations shows, PIC value is always smaller than the heterozygosity; however, for loci characterized by large numbers of alleles with similar frequencies, the heterozygosity and PIC values are similar. Analytical scripts are provided in Supplemental Script S1.
Identification of Individuals from 1000 Genomes Data Using SNP Haplotypes
We performed a blinded analysis (M.D. and E.M.) to see if we could correctly identify the selected individuals in our NGS data within the 2504 individuals included in the 1000 Genomes phase 3 data (ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20130502, last accessed October 18, 2016). Among the 45 individuals, only 30 were included in the 1000 Genomes data release; the remaining 15 served as controls. The 30 sequenced samples included all of the JPT samples, all of the YRI samples except NA18487, and only sample NA07048 in the CEU population. The 15 control (nonsequenced) samples included NA18487 and the remaining 14 CEU samples.
We obtained the genetic data of the selected regions for all of the 2504 samples from the last updated version (October 2014) of 1000 Genomes. For each region, genetic variants of the two data sets were matched by physical position (National Center for Biotechnology Information Build 37) with those reported in 1000 Genomes data set. One hundred eight variants occurred in both data sets. Individual SNPs were used for identification because when the phased haplotypes were used, we were only able to match a subset of the 30 samples, because of allele dropout from low coverage or errors in phasing (data not shown). These errors in phasing were not unexpected given the 1000 Genomes phased haplotypes are determined using computational methods and represent the most likely haplotype versus experimental data. We matched individuals based on the percentage of concordance between the 1000 Genomes genotype data and our NGS calls. However, because of the relatively low coverage of 1000 Genomes data, we did not exclude a sample based on the lack of correspondence at any single SNP.
Automated Genotype Analysis
We developed a program to assign haplotypes from individuals. The software accepts Ion Torrent's raw reads in either bam (using samtools19 to interconvert formats) or fastq format. The software first size selects reads that are ≥ to 200 bp. Selected reads are then aligned to a haplotype allele database for each locus, using the BWA alignment algorithm.20 Aligned reads of specific haplotypes are then sorted and counted using SAMtools.19 All assigned haplotypes were visually confirmed using Integrated Genome Viewer.
Multiplex PCR
Five loci were selected to produce a multiplex PCR. All primer pairs within a given multiplex contained the same barcode sequence. Different barcodes were used for each of the eight multiplex reactions. Multiplex PCR was performed using the conditions reported above and products cleaned up using QIAquick PCR Purification kit (Qiagen), mixed at equal ratios and sequenced on the same 316 chip. The multiplex reaction selected for general use empirically provided the most balanced read number.
Acute Myeloid Leukemia Relapse and Nonrelapse Multiplex
A total of 15 bone marrow samples from patients who underwent allogeneic BMT were obtained from the Molecular Diagnostic Laboratory at the Johns Hopkins Hospital on an institutional review board–approved protocol. All 15 samples demonstrated complete remission with 100% donor DNA shown by STR analysis (Identifiler; Applied Biosystems, Carlsbad, CA). Among these 15 samples, eight were selected from patients who became host STR positive 53 to 145 days later and subsequently clinically relapsed (relapse group). The other seven samples remained fully engrafted and repeatedly tested all donor in subsequent testing (nonrelapse group). Each sample was prepared for sequencing as described above and sequenced on the Ion Torrent PGM.
Results
Approach
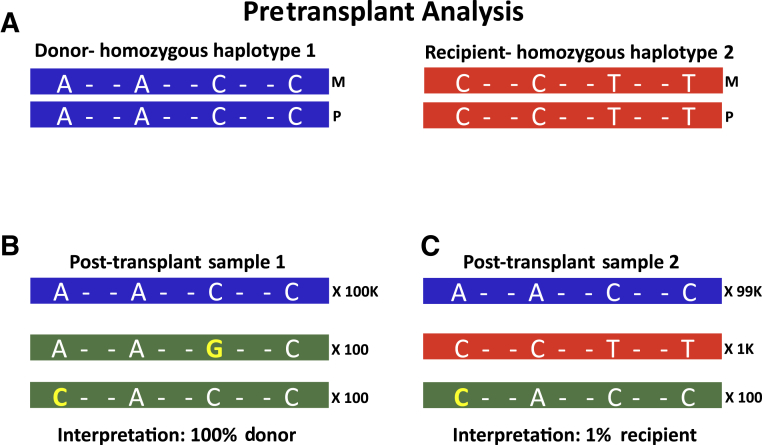
The approach of haplotype counting is demonstrated in Figure 1. Imagine a region in the human genome that contains four SNPs and two individuals: a donor who is homozygous for haplotype number 1 (A-A-C-C) and a recipient who is homozygous for haplotype number 2 (C-C-T-T) (Figure 1A). A post-transplant sample 1 with results shown in Figure 1B would be interpreted as 100% donor (100,000 reads), because the other 200 reads do not perfectly match either the donor or recipient haplotypes and are therefore interpreted as PCR error. A second post-transplant sample (Figure 1C) containing 99,000 reads that perfectly match the donor and 1000 reads that perfectly match the recipient haplotype would be interpreted as 1% recipient DNA. The 100 reads with cytosine at the first SNP position are interpreted as PCR error and excluded from the calculation.
Figure 1.
Haplotype counting approach. A: Shown are theoretical haplotypes (horizontal bars) containing four single-nucleotide polymorphisms (SNPs), where pretransplant analysis shows a donor (left) is homozygous for haplotype number 1 (AACC) and recipient (right) who is homozygous for haplotype number 2 (CCTT). B: Post-transplant sample where 100,000 reads (blue bar) perfectly match the donor (haplotype number 1), 100 reads with guanine at the third SNP position, and 100 reads with cytosine at the first SNP position (green bars). The 200 reads with a single base change can be attributed to PCR error because they do not perfectly match either donor or recipient haplotypes. As such, this sample would be interpreted as 100% donor. C: Another post-transplant sample that contains 99,000 reads (blue bar) that perfectly match the donor haplotype, and 1000 reads (red bar) that perfectly match the recipient haplotype, and so are interpreted as real. The 100 reads (green bar) with a single cytosine are PCR errors because they cannot be attributed to either donor or recipient haplotype. Accordingly, this sample would be interpreted as containing 1% recipient DNA.
Loci Selection and Validation
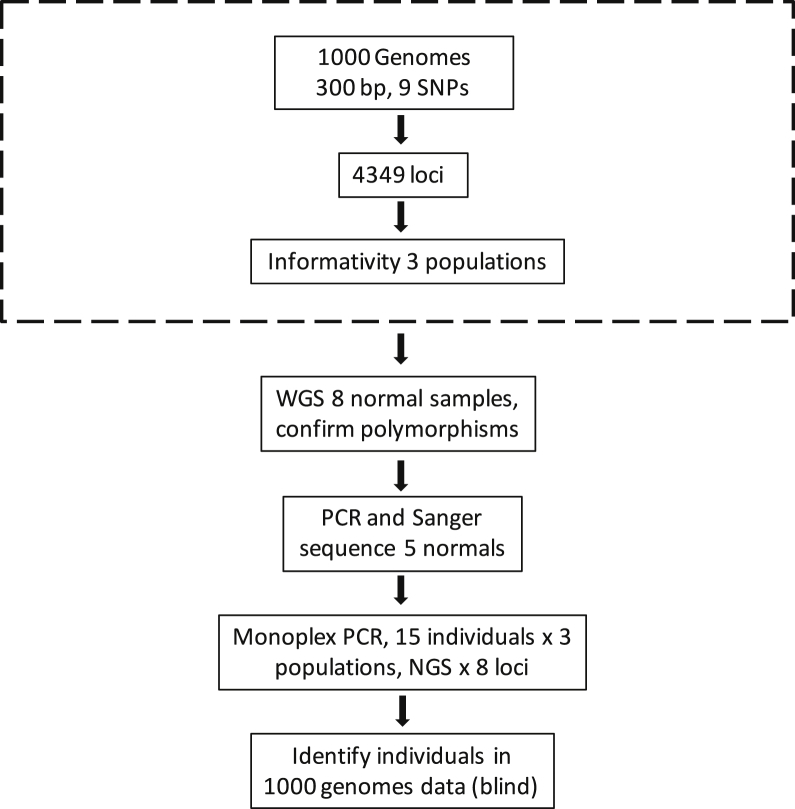
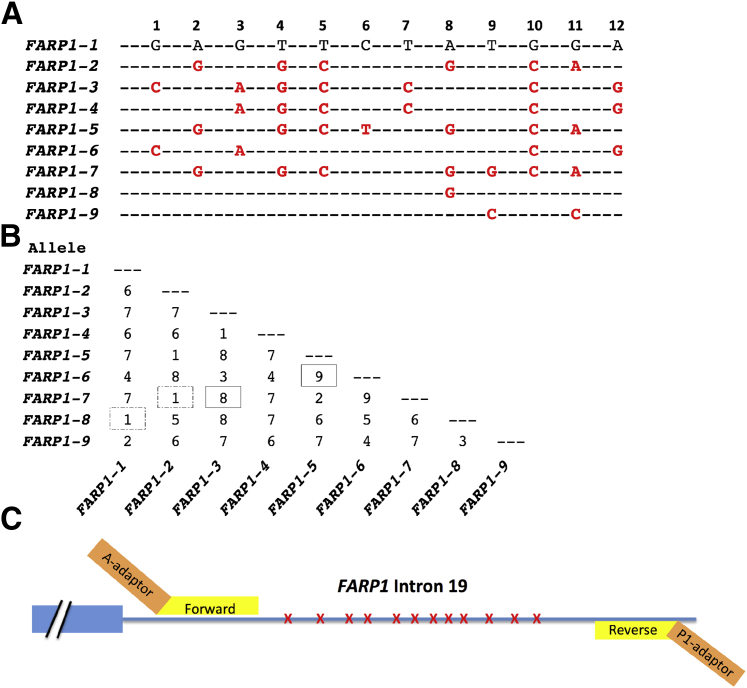
The overall strategy of these experiments is shown in Figure 2.12 From the 4349 previously identified polymorphic loci, we selected eight loci based on high informativity scores and residing on different chromosomes (primers in Table 1). We included both HLA loci because the haplotypes are mostly known and the loci are highly informative. We then initially validated these eight loci in silico using germline whole genome sequencing data.15 We assigned haplotypes sequentially, generated alignments for all detected haplotypes, and tabulated the number of distinct haplotypes. These experimentally confirmed haplotypes are hereafter simply referred to as haplotypes. An example using the FARP1 locus is shown in Figure 3A (alignments for all eight loci are presented in Supplemental Figures S1–S8). Comparing the number of SNPs that differ between any two haplotype combinations are shown for the FARP1 locus in a 2 × 2 table (Figure 3B). Because of the potential for cross talk between haplotypes (because of NGS errors), combinations that vary by only one or two SNPs are not used.12 Most combinations at this locus differ by three or more SNPs (30/36, 83%), whereas only six combinations vary by 1 or 2 SNPs (6/36, 17%). The 2 × 2 tables, including their frequencies in the three populations studied, for all eight loci are presented in Supplemental Table S1. Primer placement in intron 19 of the FARP1 locus is shown in Figure 3C, where the FARP1 primers and the Ion Torrent adapters are shown.
Figure 2.
Overall strategy. Previously, using the 1000 Genomes data and a window of 300 bp that contained at least nine single-nucleotide polymorphisms (SNPs), we identified 4349 polymorphic loci (dashed box).12 Polymorphisms at the eight selected loci were confirmed in eight whole genome sequencing (WGS) normal samples. Primers and polymorphism were initially confirmed by PCR and Sanger sequencing. Total of 45 individuals from three populations (15 individuals each) were PCR amplified and sequenced. Using sequenced data in a blind manner (M.D. and E.M.), we identified 30/30 sequenced samples, and none of the 15 control samples. NGS, next-generation sequencing.
Figure 3.
Polymorphic region of the FARP1 locus. A: Shown are nine different haplotypes observed in FARP1 locus. Only single-nucleotide polymorphisms (SNPs) that differ from reference haplotype (FARP1-1) are shown (red, boldfaced). B: Number of SNP differences between FARP1 haplotypes. Some haplotype combinations are more informative, such as FARP1-5 and FARP1-6 or FARP1-3 and FARP1-7 (boxes). Others have fewer discriminating SNPs, such as FARP1-1 and FARP1-2 or FARP1-2 and FARP1-7 (dashed boxes). C: Intron 19 of FARP1 (blue line) containing 12 SNPs (red Xs) and two FARP1 PCR primers (yellow). The PCR primers are tailed on their 5′ ends with sequencing adaptors (orange).
Observed Haplotypes in Three Populations
To experimentally confirm the relevance of these new loci, we obtained DNA from some of the 1000 Genomes populations (Coriell Institute for Medical Research). We then assigned haplotypes for 45 individuals (15 individuals per population) at eight loci in three populations (CEU, JPT, and YRI). The number of haplotypes ranged from seven for TMPRSS15 to 21 for MT4 (Table 2). Because the variation across the three populations was generally similar (Supplemental Tables S2 and S3), the diversity is reported collectively for all 45 samples (Table 2). All of the eight loci showed high diversity (mean H, 0.81; PIC, 0.80) with the FARP1 locus as the least diverse region (H, 0.73; PIC, 0.72) and MT4 as the most diverse region (H, 0.90; PIC, 0.90). The estimates of diversity by population (Supplemental Table S2) showed a common trend for most of the regions where the JPT population generally showed the lowest values (lower diversity), and the African population generally showed the highest diversity. As an example among the various loci, HLA-A had a slightly higher heterozygosity in the YRI (Nigeria) population.
Table 2.
Summary of All Loci from 45 Individuals from Three Populations
| Chromosome | Locus | Location∗ | Alleles |
Heterozygosity |
PIC |
|||
|---|---|---|---|---|---|---|---|---|
| Total unique | Average† | All | Average‡ | All | Average‡ | |||
| 1 | TFB2M | Intron | 10 | 5 | 0.81 | 0.72 | 0.80 | 0.69 |
| 4 | SORCS2 | Intron | 13 | 6.3 | 0.77 | 0.71 | 0.75 | 0.67 |
| 6 | HLA-A | Junction | 17 | 8.7 | 0.86 | 0.76 | 0.86 | 0.75 |
| 6 | HLA-B | Junction | 12 | 8.7 | 0.85 | 0.82 | 0.85 | 0.81 |
| 8 | CSMD1 | Intron | 10 | 6 | 0.79 | 0.73 | 0.77 | 0.71 |
| 13 | FARP1 | Intron | 9 | 5 | 0.73 | 0.71 | 0.72 | 0.70 |
| 16 | MT4 | Intergenic | 21 | 10 | 0.90 | 0.85 | 0.90 | 0.85 |
| 21 | TMPRSS15 | Intergenic | 7 | 4.3 | 0.79 | 0.66 | 0.78 | 0.63 |
PIC, polymorphism information content.
Junction indicates that the amplicon contains part intron and part exon. For polymorphic regions between genes (intergenic) the locus of the nearest gene is designated.
Average number of haplotypes across all three populations in the 45 samples.
Average heterozygosity and PIC among the 45 samples by population.
Identification of 1000 Genomes Samples Using Genotypes from the Eight Loci
Once all single marker SNPs were assigned for each individual at each locus, the data were deidentified. Of the 45 samples, sequencing data were available in the 1000 Genomes database for 30 samples (Materials and Methods). The remaining 15 samples were used as negative controls.
Applying this method, we were able to correctly identify 30/30 sequenced samples, and showed an average of 105.8 matching variants (range, 98 to 108) of the 108 total SNPs tested. As expected, we were not able to identify a matching sample for the remaining 15 samples that were not part of the 2504 sequenced samples of 1000 Genome phase 3 project; for these controls, the average number of matching variants was 87.3 (range, 82 to 94). This method allowed us to identify one individual among >2500 by using genetic data of 108 SNPs located in high diversity regions of the genome.
We also identified three haplotypes that occurred only once in the 45 samples, and these could be considered rare haplotypes. Two of these samples (NA18488 and NA18486) contained single haplotypes (SORCS2-8 and MT4-15, respectively) that were only present once, whereas a third sample (NA18504) contained two haplotypes (MT4-10 and MT4-13) where the combination of them was present in only that single sample. However, these individual haplotypes were present in additional samples in the 1000 Genomes database. We therefore probed the 1000 Genomes phased data for the frequency of each of the experimentally documented haplotypes and found three haplotypes that were present only once among three separate loci (SORCS2-8, CSMD1-7, FARP1-5) (Supplemental Table S4). This may be an overestimate of the number of rare haplotypes because of allele dropout and phasing errors.
Detection of Earlier Leukemic Relapse
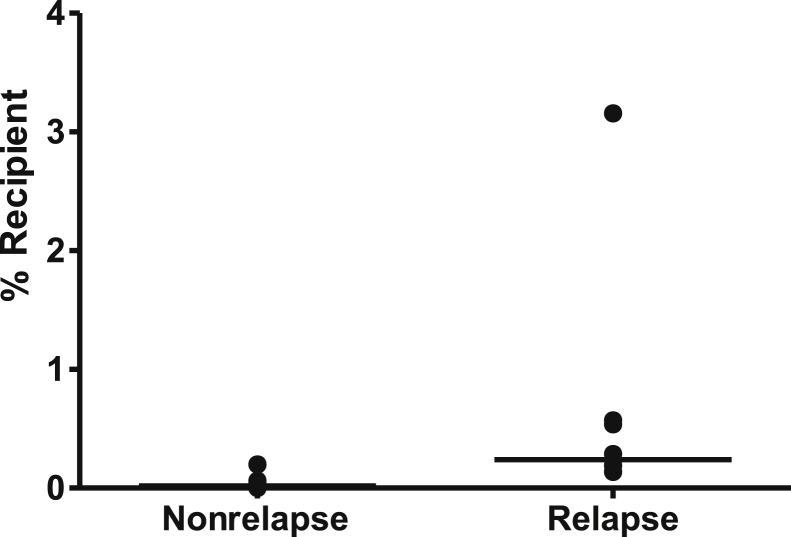
We next wanted to test whether such an assay might be more useful than STR-based engraftment testing. We selected eight patients (relapse patients) who ultimately relapsed clinically, and identified when the STR analysis first demonstrated detectable host DNA. We then selected the sample (which tested all donor) that immediately preceded the first sample showing increasing host DNA by STRs (average, 124 days earlier). We reasoned that this prerelapse sample might demonstrate a level of recipient DNA that can be detected with the new assay at a level below that detected by capillary electrophoresis (<3% to 5%). We also selected seven recipients' control bone marrow samples (nonrelapse) that tested as all donor using the STR assay and remained all donor for at least 18 months after that sample (median, 721 days all donor). Using HLA-A–based haplotype counting, we determined the percentage recipient DNA (Table 3) and compared the two groups (Figure 4). The relapse group showed a higher percentage recipient (mean, 0.65%; median, 0.24%; SD, 1.03%) than the seven nonrelapse group samples (mean, 0.05%; median, 0.02%; SD, 0.069%; P = 0.0019, Mann-Whitney rank sum test). We then calculated a threshold for the nonrelapse samples of 0.257 (mean plus 3 SDs). Using this cutoff, four of eight (BME1, BME4, BME26, and BME29) relapsed samples were above this threshold (mean, 1.14%; median, 0.55%). Respectively, the timing of these positive samples was 90, 130, 145, and 130 days before the first host-positive STR-based test and 130, 127, 142, and 130 days before clinical relapse.
Table 3.
Percentage Recipient in Nonrelapse Versus Relapse Patients
| Variable | Patient ID | Disease | Days post-transplant∗ | Days pre-STR relapse† | Days preclinical relapse‡ | % Recipient |
|---|---|---|---|---|---|---|
| Nonrelapse patients | BME10 | AML | 150 | NR | NR | 0.02 |
| BME11 | AML | 180 | NR | NR | 0.20 | |
| BME14 | AML | 360 | NR | NR | 0.01 | |
| BME18 | MDS/AML | 365 | NR | NR | 0.02 | |
| BME19 | AML | 423 | NR | NR | 0.05 | |
| BME20 | MDS | 378 | NR | NR | 0.00 | |
| BME25 | ALL | 182 | NR | NR | 0.06 | |
| Relapse patients | BME1 | Multiple myeloma | 56 | 90 | 130 | 0.57§ |
| BME4 | Acute erythroid leukemia | 60 | 130 | 127 | 0.29§ | |
| BME16 | AML | 60 | 111 | 147 | 0.19 | |
| BME26 | ALL | 179 | 145 | 142 | 0.54§ | |
| BME27 | AML | 56 | 131 | 172 | 0.14 | |
| BME29 | AML | 94 | 130 | 130 | 3.16§ | |
| BME30 | AML | 63 | 53 | 116 | 0.14 | |
| BME31 | Multiple myeloma | 141 | 129 | 0 | 0.19 |
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; NR, no relapse; STR, short tandem repeat.
Indicates the number of days after transplant (day 0) when the sample was collected.
Days between the collected sample used in this study until the patient tested positive using STRs.
Days between the collected sample used in this study until the patient relapsed clinically.
Samples above threshold.
Figure 4.
Host DNA detected in a series of nonrelapse and before relapse bone marrow samples. Using the nonrelapse samples, a threshold of 0.257 (mean plus 3 SDs) was generated. Samples from recipients who subsequently relapsed were significantly higher. Horizontal bars indicate median values (nonrelapse median, 0.020% recipient; relapse median, 0.24% recipient). SEM was 0.026 for nonrelapse group and 0.363 for the relapse group. P value between the groups was 0.0019 (Mann-Whitney rank sum test).
Multiplex PCR
Sensitive detection requires that a sufficient number of input genomes are analyzed, and that they are sequenced to an adequate depth of coverage.21 For example, a limit of detection of 1:1000 cannot be achieved if only 1 ng of DNA is analyzed, because 1000 pg are equivalent to only 167 genomes, based on 6 pg of DNA per diploid cell. In addition, donors and recipients can carry identical haplotypes at a particular locus, making that locus noninformative. Accordingly, it is desirable to analyze multiple loci simultaneously. To determine the best multiplex PCR, we tested five primer pairs in eight different combinations from the eight possible loci. We selected the one multiplex reaction that empirically had the most evenly distributed read counts among the loci. We selected four samples (0.3% to 3.2% recipient-specific DNA) from patients who eventually relapsed, and tested them using the five loci multiplex reaction in triplicate (Table 4). Three of the four samples were informative for four or five loci; however, one sample (BME26) was only informative for HLA-A. The precision was good for a given locus for the same sample. For example, for the HLA-A locus, the means and SD for the triplicate samples were 0.570% ± 0.042%, 0.287% ± 0.017%, 0.537% ± 0.102%, and 3.157% ± 1.523% for samples BME1 to BME29, respectively. However, the variation between loci for a given sample was higher than desirable and will need additional refinement of primer placement and bioinformatics (Table 4).
Table 4.
Multiplex Haplotype Counting: Percentage Recipient
| Patient ID | Locus |
Mean | ||||
|---|---|---|---|---|---|---|
| FARP1 | HLA-A | HLA-B | MT4 | TMPRSS15 | ||
| BME1 | 0.09 | 0.57 | 1.64 | – | 1.98 | 1.07 |
| BME4 | 1.13 | 0.29 | 0.65 | 0.65 | 0.38 | 0.62 |
| BME26 | – | 0.54 | – | – | – | – |
| BME29 | 0.83 | 3.16 | 1.55 | 0.25 | 0.39 | 1.24 |
All values are percentages. Dashes represent loci that were not informative.
Discussion
Previously, we reported that haplotype counting could be used for ultrasensitive detection of human DNA mixes.12 We demonstrated this using the HLA-A locus, where we could reliably detect 1 in 10,000 (0.01%), and identified 4349 additional polymorphic loci.12 Herein, we experimentally confirmed eight loci (including HLA-A) to be highly diverse (SNP dense) and assigned haplotypes by sequencing a total of 45 individuals from three of the populations from the 1000 Genomes study. We demonstrated the ability to multiplex PCR five of these loci, critical for samples with limited DNA. We defined an initial threshold percentage recipient for fully engrafted patients, although this will need to be confirmed in a larger cohort of patients. Finally, we present data that haplotype counting can detect increased host DNA (likely indicating subsequent relapse) earlier than STR-based testing.
Detecting relapse earlier may allow one to intervene while the malignant clone is theoretically smaller. It may be possible with this method to define what a normal percentage recipient is in the peripheral blood for a transplant recipient who has been fully engrafted and is known to remain so. In our limited data set, we set a cutoff in bone marrow from nonrelapse patients of the mean plus 3 SDs of 0.257% (Table 3). Alternatively, such a baseline threshold might be patient specific and will need to be defined for that patient as a function of time. Among the nonrelapse samples, only one lacked evidence of any recipient DNA, and the other six samples had a low level of positivity (mean, 0.06%). It is interesting to speculate about what host cells might still be present in a fully engrafted patient, such as plasma cells, histiocytes, or nonhematopoietic stromal cells (eg, osteoclasts, osteoblasts, or endothelial cells). As discussed, one advantage of this approach is the ability to multiplex multiple loci because a sample might be limited. Finally, this approach might be less expensive than microsatellites because of the ability to analyze multiple samples on a single NGS chip; however, this assumes that the laboratory has a sufficient volume of samples that allow multiple samples can be run on a single chip. The turn-around time may be somewhat longer because the assay requires approximately three times as much instrument time, and the proposed assay would require greater expertise of the personnel running this test compared to an STR/capillary electrophoresis–based test.
A fundamental disadvantage of some chimerism analyses is that they are not leukemia cell specific and the diagnosis of relapse needs to be supported by clinical relapse using morphology, flow cytometry, and other clinical findings. This includes Y-chromosome–based testing, SNPs, STRs, and the current work reported herein. We and others have used leukemia-specific markers, such as Flt3, DNMT3A, IDH, and NPM1.22, 23, 24 It is possible that it could be improved by testing CD34 sorted cell populations, possibly including peripheral blood.
With haploidentical BMT, leukemic cells can evade graft-versus-leukemia effect by eliminating the mismatched HLA allele.25, 26, 27 The multiplex PCR-based haplotype counting might be an ideal way to determine the ratios of the HLA haplotypes in the context of the percentage engraftment. The non-HLA loci could be used to calculate the percentage engraftment, and the loss of the mismatched HLA locus would be detected by a reduction in the percentage recipient at the HLA loci relative to the non-HLA loci.
In addition to allogeneic BMT, chimerism testing can be useful in solid organ transplantation. Along with transplantation of an organ or limb, some of the lymphoid tissue may be transferred by cell migration from the donor organ, thus generating chimerism in the patient.28 Transplanting donor marrow intentionally along with the solid organ can facilitate organ and limb tolerance.29, 30 Haplotype counting could be used to quantify the circulating cell-free DNA of donor origin seen in the noninvasive diagnosis of heart transplant rejection.31, 32 This method can also be used to study large numbers of cells in parallel to identify rare cells. For example, Beaudet and colleagues enrich for fetal cells from pregnant mothers' peripheral blood and pick individual cells for analysis. After whole genome amplification, they test individual cells to identify those that are fetal rather than maternal (Art Beaudet, unpublished data).
Haplotype counting may have applications in forensic testing. During the past 20 years, the field of forensics has relied on microsatellites to identify suspects and human remains,33, 34 although some have proposed using SNPs for this purpose.13, 35 Using haplotype analyses, one could theoretically allow identification of suspects in a mixture of human DNAs, so-called multisuspect cases. For example, a mixed sample containing homozygous haplotypes present at 89%, 10%, and 1%. If a series of haplotypes at different loci were all present at the 10% level, they would likely be from the same individual; however, the number of samples and the SNP frequencies that allow sufficient differentiation of individuals remains to be determined experimentally.36
We found only one allele for three phased haplotypes in the 1000 Genomes database, suggesting that these are ultrarare haplotypes. One can imagine that whole genome sequencing of a crime scene sample could identify a series of ultrarare haplotypes that could be used in combination to screen for and identify a single living individual.
Finally, haplotype counting can be used for patient identification. As the use of NGS continues to expand in the clinical laboratory, individual patients may have more than one NGS-based test. A locus (such as HLA-A) to confirm identity could be included in all such tests, which could also be used to confirm the lack of contamination with another sample. This could be especially useful for patients enrolled in clinical trials. Others have demonstrated that single SNPs, which coincidentally reside in sequenced regions, can be used to detect high levels of contamination.37 Given the frequency of clinical sample mix ups, estimated at 0.3% to 0.7%,38, 39 it is conceivable that at some time in the future, the cost of NGS will decrease and technology will mature enough that every tissue sample, or perhaps even every blood test, will include a locus to confirm patient identity.
In summary, we validated eight loci that can be used for haplotype counting. We developed a multiplex PCR that uses five of these loci, and demonstrate that this approach can identify increased host DNA (indicative of leukemic relapse) earlier than conventional STR analysis. Additional work will need to confirm these findings in a larger sample set and determine whether earlier detection of relapse is clinically actionable.
Acknowledgments
We thank Drs. Dwight Oliver, John Isaacs, Maria Bettinotti, Annette Jackson, Sarah Wheelan, and Jonathan Pevsner, in addition to Nate Brennen, Heather Wick, Don Freed, and Brian Iglehart for helpful discussions; and the individuals who generously contributed their samples to the 1000 Genomes Project.
Footnotes
Supported in part by the Institute for Clinical and Translational Research Accelerated Translational Incubator Pilot Program (J.R.E.), The Sol Goldman Pancreatic Cancer Research Center (J.R.E.), the Stringer Foundation (J.R.E.), and the Rolfe Pancreatic Cancer Foundation (J.R.E.).
Disclosures: A provisional patent has been applied for WO 2016/022641, and if awarded and licensed, J.R.E. may receive royalty payments.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.jmoldx.2017.01.005.
Supplemental Data
Alignment of haplotypes, TFB2M. Alignment of the 10 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 8. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, SORCS2. Alignment of the 13 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 14. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, HLA-A. Alignment of the 17 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 22. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, HLA-B. Alignment of the 12 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 33. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, CSMD1. Alignment of the 10 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 15. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, FARP1. Alignment of the nine alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 13. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, MT4. Alignment of the 21 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 12. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, TMPRSS15. Alignment of the seven alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 13. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
References
- 1.Brodsky R.A., Luznik L., Bolanos-Meade J., Leffell M.S., Jones R.J., Fuchs E.J. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42:523–527. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolanos-Meade J., Fuchs E.J., Luznik L., Lanzkron S.M., Gamper C.J., Jones R.J., Brodsky R.A. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scharf S.J., Smith A.G., Hansen J.A., McFarland C., Erlich H.A. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85:1954–1963. [PubMed] [Google Scholar]
- 4.Thiede C., Florek M., Bornhauser M., Ritter M., Mohr B., Brendel C., Ehninger G., Neubauer A. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999;23:1055–1060. doi: 10.1038/sj.bmt.1701779. [DOI] [PubMed] [Google Scholar]
- 5.Thiede C., Bornhauser M., Ehninger G. Evaluation of STR informativity for chimerism testing: comparative analysis of 27 STR systems in 203 matched related donor recipient pairs. Leukemia. 2004;18:248–254. doi: 10.1038/sj.leu.2403212. [DOI] [PubMed] [Google Scholar]
- 6.Huisman C., de Weger R.A., de Vries L., Tilanus M.G., Verdonck L.F. Chimerism analysis within 6 months of allogeneic stem cell transplantation predicts relapse in acute myeloid leukemia. Bone Marrow Transplant. 2007;39:285–291. doi: 10.1038/sj.bmt.1705582. [DOI] [PubMed] [Google Scholar]
- 7.Oliver D.H., Thompson R.E., Griffin C.A., Eshleman J.R. Use of single nucleotide polymorphisms (SNP) and real-time polymerase chain reaction for bone marrow engraftment analysis. J Mol Diagn. 2000;2:202–208. doi: 10.1016/S1525-1578(10)60638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochberg E.P., Miklos D.B., Neuberg D., Eichner D.A., McLaughlin S.F., Mattes-Ritz A., Alyea E.P., Antin J.H., Soiffer R.J., Ritz J. A novel rapid single nucleotide polymorphism (SNP)-based method for assessment of hematopoietic chimerism after allogeneic stem cell transplantation. Blood. 2003;101:363–369. doi: 10.1182/blood-2002-05-1365. [DOI] [PubMed] [Google Scholar]
- 9.Gineikiene E., Stoskus M., Griskevicius L. Single nucleotide polymorphism-based system improves the applicability of quantitative PCR for chimerism monitoring. J Mol Diagn. 2009;11:66–74. doi: 10.2353/jmoldx.2009.080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koldehoff M., Steckel N.K., Hlinka M., Beelen D.W., Elmaagacli A.H. Quantitative analysis of chimerism after allogeneic stem cell transplantation by real-time polymerase chain reaction with single nucleotide polymorphisms, standard tandem repeats, and Y-chromosome-specific sequences. Am J Hematol. 2006;81:735–746. doi: 10.1002/ajh.20693. [DOI] [PubMed] [Google Scholar]
- 11.Quail M.A., Smith M., Coupland P., Otto T.D., Harris S.R., Connor T.R., Bertoni A., Swerdlow H.P., Gu Y. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics. 2012;13:341. doi: 10.1186/1471-2164-13-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debeljak M., Freed D.N., Welch J.A., Haley L., Beierl K., Iglehart B.S., Pallavajjala A., Gocke C.D., Leffell M.S., Lin M.T., Pevsner J., Wheelan S.J., Eshleman J.R. Haplotype counting by next-generation sequencing for ultrasensitive human DNA detection. J Mol Diagn. 2014;16:495–503. doi: 10.1016/j.jmoldx.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidd K.K., Pakstis A.J., Speed W.C., Lagace R., Chang J., Wootton S., Haigh E., Kidd J.R. Current sequencing technology makes microhaplotypes a powerful new type of genetic marker for forensics. Forensic Sci Int Genet. 2014;12:215–224. doi: 10.1016/j.fsigen.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Sudmant P.H., Rausch T., Gardner E.J., Handsaker R.E., Abyzov A., Huddleston J., 1000 Genomes Project Consortium An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris A.L., Kamiyama H., Makohon-Moore A., Pallavajjala A., Morsberger L.A., Lee K., Batista D., Iacobuzio-Donahue C.A., Lin M.T., Klein A.P., Hruban R.H., Wheelan S.J., Eshleman J.R. Transflip mutations produce deletions in pancreatic cancer. Genes Chromosomes Cancer. 2015;54:472–481. doi: 10.1002/gcc.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott J. ed 3. Johns Hopkins University Press; Baltimore: 1999. Analysis of Human Genetic Linkage. [Google Scholar]
- 17.Botstein D., White R.L., Skolnick M., Davis R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X., Elston R.C. Linkage information content of polymorphic genetic markers. Hum Hered. 1999;49:112–118. doi: 10.1159/000022855. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genomes Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin M.T., Mosier S.L., Thiess M., Beierl K.F., Debeljak M., Tseng L.H., Chen G., Yegnasubramanian S., Ho H., Cope L., Wheelan S.J., Gocke C.D., Eshleman J.R. Clinical validation of KRAS, BRAF, and EGFR mutation detection using next-generation sequencing. Am J Clin Pathol. 2014;141:856–866. doi: 10.1309/AJCPMWGWGO34EGOD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunwald M.R., Tseng L.H., Lin M.T., Pratz K.W., Eshleman J.R., Levis M.J., Gocke C.D. Improved FLT3 internal tandem duplication PCR assay predicts outcome after allogeneic transplant for acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20:1989–1995. doi: 10.1016/j.bbmt.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brambati C., Galbiati S., Xue E., Toffalori C., Crucitti L., Greco R., Sala E., Crippa A., Chiesa L., Soriani N., Mazzi B., Tresoldi C., Stanghellini M.T., Peccatori J., Carrabba M.G., Bernardi M., Ferrari M., Lampasona V., Ciceri F., Vago L. Droplet digital polymerase chain reaction for DNMT3A and IDH1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101:e157–e161. doi: 10.3324/haematol.2015.135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shayegi N., Kramer M., Bornhauser M., Schaich M., Schetelig J., Platzbecker U., Rollig C., Heiderich C., Landt O., Ehninger G., Thiede C., Study Alliance Leukemia The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood. 2013;122:83–92. doi: 10.1182/blood-2012-10-461749. [DOI] [PubMed] [Google Scholar]
- 25.Vago L., Perna S.K., Zanussi M., Mazzi B., Barlassina C., Stanghellini M.T., Perrelli N.F., Cosentino C., Torri F., Angius A., Forno B., Casucci M., Bernardi M., Peccatori J., Corti C., Bondanza A., Ferrari M., Rossini S., Roncarolo M.G., Bordignon C., Bonini C., Ciceri F., Fleischhauer K. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 26.Villalobos I.B., Takahashi Y., Akatsuka Y., Muramatsu H., Nishio N., Hama A., Yagasaki H., Saji H., Kato M., Ogawa S., Kojima S. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood. 2010;115:3158–3161. doi: 10.1182/blood-2009-11-254284. [DOI] [PubMed] [Google Scholar]
- 27.Lin M.T., Tseng L.H., Beierl K., Harada S., Hafez M.J., Eshleman J.R., Gocke C.D. Analysis of hematopoietic stem cell transplant engraftment: use of loss or gain of microsatellite alleles to identify residual hematopoietic malignancy. Diagn Mol Pathol. 2011;20:194–202. doi: 10.1097/PDM.0b013e31821dac16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl T.E., Demetris A.J., Trucco M., Murase N., Ricordi C., Ildstad S., Ramos H., Todo S., Tzakis A., Fung J.J., Nalesnik M., Zeevi A., Rudert W.A., Kocova M. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeberger S., Gorantla V.S., Brandacher G., Zeevi A., Demetris A.J., Lunz J.G., Metes D.M., Donnenberg A.D., Shores J.T., Dimartini A.F., Kiss J.E., Imbriglia J.E., Azari K., Goitz R.J., Manders E.K., Nguyen V.T., Cooney D.S., Wachtman G.S., Keith J.D., Fletcher D.R., Macedo C., Planinsic R., Losee J.E., Shapiro R., Starzl T.E., Lee W.P. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. 2013;257:345–351. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim Z., Cooney D.S., Shores J.T., Sacks J.M., Wimmers E.G., Bonawitz S.C., Gordon C., Ruben D., Schneeberger S., Lee W.P., Brandacher G. A modified heterotopic swine hind limb transplant model for translational vascularized composite allotransplantation (VCA) research. J Vis Exp. 2013 [Internet];80:50475. doi: 10.3791/50475. doi: 10.3791/50475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vlaminck I., Valantine H.A., Snyder T.M., Strehl C., Cohen G., Luikart H., Neff N.F., Okamoto J., Bernstein D., Weisshaar D., Quake S.R., Khush K.K. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6:241ra77. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grskovic M., Hiller D.J., Eubank L.A., Sninsky J.J., Christopherson C., Collins J.P., Thompson K., Song M., Wang Y.S., Ross D., Nelles M.J., Yee J.P., Wilber J.C., Crespo-Leiro M.G., Scott S.L., Woodward R.N. Validation of a clinical-grade assay to measure donor-derived cell-free DNA in solid organ transplant recipients. J Mol Diagn. 2016;18:890–902. doi: 10.1016/j.jmoldx.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt C., Benecke M. Five cases of forensic short tandem repeat DNA typing. Electrophoresis. 1997;18:690–694. doi: 10.1002/elps.1150180506. [DOI] [PubMed] [Google Scholar]
- 34.Nurit B., Anat G., Michal S., Lilach F., Maya F. Evaluating the prevalence of DNA mixtures found in fingernail samples from victims and suspects in homicide cases. Forensic Sci Int Genet. 2011;5:532–537. doi: 10.1016/j.fsigen.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Seo S.B., King J.L., Warshauer D.H., Davis C.P., Ge J., Budowle B. Single nucleotide polymorphism typing with massively parallel sequencing for human identification. Int J Legal Med. 2013;127:1079–1086. doi: 10.1007/s00414-013-0879-7. [DOI] [PubMed] [Google Scholar]
- 36.Voskoboinik L., Darvasi A. Forensic identification of an individual in complex DNA mixtures. Forensic Sci Int Genet. 2011;5:428–435. doi: 10.1016/j.fsigen.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Sehn J.K., Spencer D.H., Pfeifer J.D., Bredemeyer A.J., Cottrell C.E., Abel H.J., Duncavage E.J. Occult specimen contamination in routine clinical next-generation sequencing testing. Am J Clin Pathol. 2015;144:667–674. doi: 10.1309/AJCPR88WDJJLDMBN. [DOI] [PubMed] [Google Scholar]
- 38.Marberger M., McConnell J.D., Fowler I., Andriole G.L., Bostwick D.G., Somerville M.C., Rittmaster R.S. Biopsy misidentification identified by DNA profiling in a large multicenter trial. J Clin Oncol. 2011;29:1744–1749. doi: 10.1200/JCO.2010.32.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeifer J.D., Liu J. Rate of occult specimen provenance complications in routine clinical practice. Am J Clin Pathol. 2013;139:93–100. doi: 10.1309/AJCP50WEZHWIFCIV. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of haplotypes, TFB2M. Alignment of the 10 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 8. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, SORCS2. Alignment of the 13 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 14. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, HLA-A. Alignment of the 17 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 22. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, HLA-B. Alignment of the 12 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 33. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, CSMD1. Alignment of the 10 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 15. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, FARP1. Alignment of the nine alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 13. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, MT4. Alignment of the 21 alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 12. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.
Alignment of haplotypes, TMPRSS15. Alignment of the seven alleles is shown. Single-nucleotide polymorphisms are in red text and numbered from 1 to 13. A dash for a given position indicates that this base matches the reference (allele 1) base (ie, only those bases that differ from the reference are shown in red). Forward and reverse primers are highlighted in gray.