Abstract
The US Food and Drug Administration (FDA) approved vemurafenib to treat patients with metastatic melanoma harboring the BRAF c.1799T>A (p.V600E) mutation. However, a subset of melanomas harbor non-p.V600E BRAF mutations, and these data are of potential importance regarding the efficacy of current targeted therapies. To better understand the BRAF mutation profile in melanomas, we retrospectively analyzed data from 1112 primary and metastatic melanomas at our institution. The cohort included nonacral cutaneous (n = 774), acral (n = 111), mucosal (n = 26), uveal (n = 23), leptomeningeal (n = 1), and metastatic melanomas of unknown primary site (n = 177). BRAF mutation hotspot regions in exons 11 and 15 were analyzed by pyrosequencing or with the primer extension MassARRAY system. A total of 499 (44.9%) specimens exhibited BRAF mutations, involving exon 15 [497 (99.6%)] or exon 11 [2 (0.4%)]. p.V600E was detected in 376 (75.4%) cases; the remaining 123 (24.6%) cases exhibited non-p.V600E mutations, of which p.V600K was most frequent [86 (17.2%)]. BRAF mutations were more frequent in nonacral cutaneous (51.4%) than acral melanomas [18 (16.2%)] (P < 0.001); however, there was no significant difference among cutaneous histological subtypes. All mucosal, uveal, and leptomeningeal melanomas were BRAF wild type (WT). The high frequency of non-p.V600E BRAF mutations in melanoma has important implications because the FDA-approved companion diagnostic test for p.V600E detects some but not all non-p.V600E mutations. However, the therapeutic efficacy of vemurafenib is not well established in these lesions.
The most frequent BRAF mutation in melanoma is a substitution at the second position of codon 600 of exon 15 (GTG>GAG), c.1799T>A, that results in an amino acid change from valine (V) to glutamic acid (E) (p.V600E). In the initial study by Davies et al,1 19 of 20 cases tested had the p.V600E mutation and the single non-p.V600E mutation was p.V600D (GTG>GAT). Subsequently, other authors have described variant mutations involving codon 600, including p.V600K (GTG>AAG), the so-called p.V600E2 (GTG>GAA), p.V600D, and p.V600R (GTG>AGG/CGG) in relatively small patient cohorts.2 Here we use p.V600E only to refer to the GTG>GAG change, and not to other nucleotide changes that may result in glutamic acid at this position, such as GTG>GAA.
These advances in the molecular characterization of melanoma have led to the development of therapeutic BRAF inhibitors (eg, vemurafenib) that target the constitutively active mutant BRAF protein. In recently published phase 1 and phase 2 clinical trials, vemurafenib therapy showed unprecedented response rates in patients with advanced-stage, BRAF-mutated melanoma.3, 4 Moreover, in a phase 3 trial, vemurafenib therapy resulted in improved overall and progression-free survival, compared with dacarbazine, in patients with previously untreated metastatic melanoma with BRAF mutation.5 In 2011, vemurafenib was approved by the US Food and Drug Administration (FDA) to treat patients with advanced stage melanoma who harbor the BRAF p.V600E mutation; also approved was a companion diagnostic test, the cobas 4800 BRAF V600 mutation test (Roche Molecular Diagnostics, Branchburg, NJ), designed to detect the BRAF c.1799T>A (p.V600E) mutation from formalin-fixed, paraffin-embedded tissue samples in a qualitative manner (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm268241.htm, last accessed January 2, 2013).
Several reports, however, have suggested that non-p.V600E BRAF mutations are more common in melanoma than was initially appreciated, and that some of these tumors, particularly those harboring a p.V600K mutation, may also respond to vemurafenib therapy.2, 5, 6, 7, 8, 9 Furthermore, variable cross-reactivity of the cobas 4800 BRAF V600 mutation test with non-p.V600E BRAF mutations has been reported.7, 8 We therefore sought to achieve a better understanding of the frequency and spectrum of mutations in hotspot regions of BRAF mutations in various types of melanoma. Here we report our experience testing for BRAF mutations involving exons 11 and 15 in a large number of different subtypes of melanoma in a clinical setting.
Materials and Methods
We retrospectively analyzed the results of clinical BRAF mutation testing for patients with melanoma over a 4-year period (January 1, 2008, to August 31, 2011) in the Clinical Laboratory Improvement Amendments (CLIA)–certified Molecular Diagnostics Laboratory of the Division of Pathology and Laboratory Medicine at The University of Texas MD Anderson Cancer Center. During this time period, known hotspots for mutations in exons 11 and 15 of BRAF were tested routinely. A subset of this cohort was published in 2012.10
Genomic DNA was isolated from formalin-fixed, paraffin-embedded tissue sections using a PicoPure DNA extraction kit (Life Technologies, Carlsbad, CA). Microscopic glass slide tissue sections were used. A representative H&E-stained section for each sample was examined by a pathologist on service, who circled the area of interest and estimated the tumor burden therein. If tumor cells represented ≥20%, manual dissection of the corresponding area from additional unstained sections was performed; if <20%, then laser capture microdissection was used to isolate tumor cells. Analysis for BRAF mutations involving hotspot regions in exons 11 and 15 was performed using PCR-based conventional DNA pyrosequencing or using matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) (Sequenom MassARRAY; Sequenom, San Diego, CA). The pyrosequencing assay, previously developed in our laboratory,11 covers mutational hotspots in exons 11 and 15 with an allelic analytical sensitivity of 6.25%. Our nine-well multiplex DNA-based primer extension MALDI-TOF MS assay was designed to assess single nucleotide polymorphisms (SNPs) involving 82 hotspot regions of 11 genes frequently mutated in cancer, including BRAF, with an allelic analytical sensitivity of 10%.
After initial optimization of the pyrosequencing assay using the A375 human melanoma cell line (ATCC, Manassas, VA), derived from a melanoma that harbors an exon 15 homozygous p.V600E BRAF mutation, clinical validation was conducted using 20 melanoma samples. Pyrosequencing detected p.V600E BRAF mutation in 5 of the 20 samples (25%) and BRAF WT in the remaining 15 samples (75%). Pyrosequencing showed 100% concordance with conventional bidirectional Sanger sequencing. Thus, using Sanger sequencing as the gold standard and applying the Wilson score method for confidence interval (CI) estimation, the detection sensitivity and specificity of pyrosequencing were 100% (5/5; 95% CI = 56.6% to 100%) and 100% (15/15; 95% CI = 79.6% to 100%), respectively. For validation of the MALDI-TOF assay, parallel testing with pyrosequencing of 145 specimens, including melanomas, was performed with 99.3% (144/145) concordance between both assays. In 41/145 specimens, a BRAF mutation was detected by both MALDI-TOF assay and pyrosequencing. WT BRAF detected in the remaining 104 specimens by MALDI-TOF assay was confirmed by pyrosequencing in all specimens except one. This specimen, which was negative by MALDI-TOF assay, was low positive for p.V600E BRAF by pyrosequencing. Using pyrosequencing as the gold standard, the sensitivity and specificity of the MALDI-TOF assay using the Wilson score method were 97.6% (41/42; 95% CI = 87.7% to 99.6%) and 100% (103/103; 95% CI = 96.4% to 100%), respectively.
Amplification and extension primers designed by our laboratory were ordered from Integrated DNA Technologies (Coralville, IA) to cover codons 464 (base 2), 466 (bases 1 and 2), 469 (base 1), 594 (base 2), 597 (base 2), 600 (bases 1, 2, and 3), and 601 (bases 1 and 3). Primer sequences for both pyrosequencing and MALDI-TOF MS tests are listed in Table 1. PCR amplification primers for pyrosequencing were tagged with M13 sequence to allow for Sanger sequencing using M13 primers. MALDI-TOF MS spectrograms were analyzed using Typer software version 4.0 (Sequenom).
Table 1.
Primer Sequences for PCR-Based Pyrosequencing and MALDI-TOF Mass Spectrometry Tests Used to Interrogate BRAF Exons 11 and 15 in Melanoma
| Primer sequences | |
|---|---|
| PCR amplification primer sequences for pyrosequencing | |
| BRAF-11 Fwd | 5′-M13-TCCTGTATCCCTCTCAGGCATAAGGTAA-3′ |
| BRAF-11 Rev | 5′-biotin-M13-CGAACAGTGAATATTCCTTTGAT-3′ |
| BRAF-15 Fwd | 5′-M13-CATAATGCTTGCTCTGATAGGA-3′ |
| BRAF-15 Rev | 5′-biotin-M13-GGCCAAAAATTTAATCAGTGGA-3′ |
| Sequencing primer sequences for pyrosequencing | |
| BRAF-11 | 5′-TTGGATCTGGATCATTT-3′ |
| BRAF-15 | 5′-GAAGACCTCACAGTAAAAATA-3′ |
| PCR amplification primer sequences for MALDI-TOF mass spectrometry | ||
|---|---|---|
| PCR primer 1 | PCR primer 2 | |
| BRAF G464 | 5′-ACGTTGGATGTGGGCAGATTACAGTGGGAC-3′ | 5′-ACGTTGGATGACTTACCATGCCACTTTCCC-3′ |
| BRAF G466 | 5′-ACGTTGGATGTGGGCAGATTACAGTGGGAC-3′ | 5′-ACGTTGGATGACTTACCATGCCACTTTCCC-3′ |
| BRAF G466R | 5′-ACGTTGGATGACTTACCATGCCACTTTCCC-3′ | 5′-ACGTTGGATGACTTACCATGCCACTTTCCC-3′ |
| BRAF G469 | 5′-ACGTTGGATGACTTACCATGCCACTTTCCC-3′ | 5′-ACGTTGGATGTGGGCAGATTACAGTGGGAC-3′ |
| BRAF D594 | 5′-ACGTTGGATGCCACTCCATCGAGATTTCAC-3′ | 5′-ACGTTGGATGTCTTCATGAAGACCTCACAG-3′ |
| BRAF L597R | 5′-ACGTTGGATGGACCCACTCCATCGAGATTT-3′ | 5′-ACGTTGGATGTCTTCATGAAGACCTCACAG-3′ |
| BRAF V600-1 | 5′-ACGTTGGATGATGGGACCCACTCCATCGAG-3′ | 5′-ACGTTGGATGTCTTCATGAAGACCTCACAG-3′ |
| BRAF V600-2 | 5′-ACGTTGGATGTGATGGGACCCACTCCATCG-3′ | 5′-ACGTTGGATGTCTTCATGAAGACCTCACAG-3′ |
| BRAF V600-3 | 5′-ACGTTGGATGATGGATCCAGACAACTGTTC-3′ | 5′-ACGTTGGATGATAGGTGATTTTGGTCTAGC-3′ |
| BRAF K601E | 5′-ACGTTGGATGATGGATCCAGACAACTGTTC-3′ | 5′-ACGTTGGATGATGGATCCAGACAACTGTTC-3′ |
| BRAF K601N | 5′-ACGTTGGATGGGTGATTTTGGTCTAGCTAC-3′ | 5′-ACGTTGGATGATGGATCCAGACAACTGTTC-3′ |
| Extension primers for MALDI-TOF mass spectrometry | |
|---|---|
| BRAF G464 | 5′-CAGTGGGACAAAGAATTG-3′ |
| BRAF G466R | 5′-TGGGACAAAGAATTGGATCT-3′ |
| BRAF G469 | 5′-CCACTTTCCCTTGTAGACTGTTC-3′ |
| BRAF D594 | 5′-ACTGTAGCTAGACCAAAA-3′ |
| BRAF L597R | 5′-CGAGATTTCACTGTAGCT-3′ |
| BRAF V600-1 | 5′-CACTCCATCGAGATTTCA-3′ |
| BRAF V600-2 | 5′-TGGGACCCACTCCATCGAGATTTC-3′ |
| BRAF V600-3 | 5′-CCCACTCCATCGAGATTT-3′ |
| BRAF K601E | 5′-CCCACTCCATCGAGATT-3′ |
| BRAF K601N | 5′-TGATTTTGGTCTAGCTACAGTGAA-3′ |
All assays were run in duplicate and included negative, positive, sensitivity, and reagent controls. For pyrosequencing, the HL-60 human promyelocytic leukemia cell line, negative for BRAF exon 11 and 15 mutations, was used as a negative control; for a sensitivity control, DNA from the A375 cell line was diluted into HL-60 (ATCC) DNA at 1:16. For MALDI-TOF MS, the HEL92.1.7 human erythroleukemia cell line (Sigma-Aldrich, St. Louis, MO), which is negative for all SNPs present in the panel, was used as a negative control; for a sensitivity control, a 5 ng/μL mix of the H2122 cell line (ATCC) (positive for KRAS c.34G>T, p.Gly12Cys, and for MET c.1124A>G, p.Asn375Ser) and HEL92.1.7 cell line was used.
Statistical analysis using Pearson’s χ2 test with Yates’ continuity correction was performed using statistical software R version 2.10.0.
Results
A total of 1112 biopsy specimens of primary or metastatic melanoma were tested for BRAF mutations involving hotspot regions of exons 11 and 15 during the study time period. Pyrosequencing was used to test 1103 cases; the remaining 109 cases were analyzed using MALDI-TOF MS, because this latter assay recently replaced pyrosequencing as the primary assay for clinical BRAF mutation analysis at our laboratory.
Specimens in the study sample included melanomas arising at various sites: 774 nonacral cutaneous (151 primary and 623 metastatic), 111 acral (62 primary and 49 metastatic), 26 mucosal (4 primary and 22 metastatic), 23 uveal (1 primary and 22 metastatic), and 1 primary leptomeningeal, along with 177 metastatic melanomas of unknown primary. BRAF mutations were present in 44.9% (499/1112) of all melanoma cases, of which 99.6% (497/499) involved exon 15 and 0.4% (2/499) involved exon 11 (Table 2). For specimens with a known primary site, only cutaneous melanomas showed BRAF mutation: 51.4% (398/774) of nonacral and 16.2% (18/111) of acral cases (P < 0.001). For the metastatic melanomas of unknown primary site, 46.9% (83/177) harbored BRAF mutations. All primary and metastatic mucosal (26) and uveal (23) cases, as well as the single leptomeningeal case, were WT for BRAF.
Table 2.
Frequency of BRAF Mutations Involving Exons 11 and 15 in Melanoma by Site
| Melanoma site | Exon 11 | Exon 15 | Wild type | Total |
|---|---|---|---|---|
| Nonacral cutaneous | ||||
| Primary | 2 | 72 | 77 | 151 |
| Metastatic | 324 | 299 | 623 | |
| Acral | ||||
| Primary | 7 | 55 | 62 | |
| Metastatic | 11 | 38 | 49 | |
| Mucosal | ||||
| Primary | 4 | 4 | ||
| Metastatic | 22 | 22 | ||
| Uveal | ||||
| Primary | 1 | 1 | ||
| Metastatic | 22 | 22 | ||
| Leptomeningeal | 1 | 1 | ||
| Metastatic, of unknown primary | 83 | 94 | 177 | |
| Total | 2 | 497 | 613 | 1112 |
Of the 151 primary nonacral cutaneous melanomas, 102 were classified pathologically into well-described subtypes (Table 3): 65.8% (25/38) of superficial spreading type melanoma were BRAF mutated, compared with 54.6% (24/44) for nodular melanoma, and 20% (4/20) for lentigo maligna melanoma. Another 49 cases were not classified pathologically; of these, 42.9% (21) were BRAF mutated.
Table 3.
Frequency of BRAF Mutations in Nonacral Primary Cutaneous Melanoma by Histopathologic Subtype
| Melanoma subtype | Exon 15 | Exon 11 | Wild type | Total | Mutated (%) |
|---|---|---|---|---|---|
| Invasive, unclassified | 21 | 0 | 28 | 49 | 43 |
| Nodular | 24 | 0 | 20 | 44 | 55 |
| Superficial spreading | 25 | 0 | 13 | 38 | 66 |
| Lentigo maligna melanoma | 2 | 2 | 16 | 20 | 20 |
| Total | 72 | 2 | 77 | 151 | 52 |
The mutation profile of BRAF exons 11 and 15 in the 499 BRAF-mutated melanoma samples is summarized in Table 4. All codon 600 GTG>AAG and GTG>GAA mutations, as well as non–codon 600 mutations detected by pyrosequencing, were confirmed by Sanger sequencing. The c.1799T>A (p.V600E) mutation was detected in 376 (75.4%) cases, whereas 86 (17.2%) showed c.1798_1799delinsAA (p.V600K). Other non-p.V600E mutations involving codon 600 were rare, detected in only 23 (4.6%) cases: 13 (2.6%) c.1798_1799delinsAG (p.V600R), 7 (1.4%) c.1799_1800delinsAA (the so-called p.V600E2); 2 (0.4%) c.1799_1800delinsAT (p.V600D), and 1 (0.2%) c.1798_1799delinsCG (p.V600R). Rare mutations involving codons 594, 597, and 601 of exon 15 were also detected. The 2 (0.4%) mutations detected in exon 11 were c.1397G>A (p.G466E) and c.1406G>A (p.G469E). Interestingly, both of the exon 11 mutations occurred in lentigo maligna melanoma (Table 3).
Table 4.
Profile of BRAF Mutations in 499 Cutaneous Melanomas, Including Primary and Metastatic Acral and Nonacral Lesions
| Codon | Nucleotide change | Coding DNA change | Amino acid change∗ | Cases no. (%) |
|---|---|---|---|---|
| Exon 11 | ||||
| 466 | GGAGAA | c.1397G>A | p.G466E | 1 (0.2) |
| 469 | GGA>GAA | c.1406G>A | p.G469E | 1 (0.2) |
| Exon 15 | ||||
| 594 | GAT>GCT | c.1781A>C | p.D594A | 1 (0.2) |
| 597 | CTA>TCA | c.1789-1790delinsTC | p.L597S | 3 (0.6) |
| 597 | CTA>CAA | c.1790T>A | p.L597Q | 1 (0.2) |
| 597 | CTA>CGA | c.1790T>G | p.L597R | 1 (0.2) |
| 600 | GTG>GAG | c.1799T>A | p.V600E | 376 (75.4) |
| 600 | GTG>GAA | c.1799_1800delinsAA | p.V600E2 | 7 (1.4) |
| 600 | GTG>AAG | c.1798_1799delinsAA | p.V600K | 86 (17.2) |
| 600 | GTG>AGG | c.1798_1799delinsAG | p.V600R | 13 (2.6) |
| 600 | GTG>CGG | c.1798_1799delinsCG | p.V600R | 1 (0.2) |
| 600 | GTG>GAT | c.1799_1800delinsAT | p.V600D | 2 (0.4) |
| 601 | AAA>GAA | c.1801A>G | p.K601E | 5 (1.0) |
| 601 | AAA>AAT | c.1803A>T | p.K601N | 1 (0.2) |
We reserve p.V600E for the GTG>GAG change, and not to other nucleotide changes that may result in glutamic acid at this position. Thus, the GTG>GAA change is described as p.V600E2.
Discussion
Others have shown that the mitogen-activated protein kinase (MAPK) pathway is constitutively activated in a substantial subset of cutaneous melanomas, usually as a result of mutually exclusive mutations involving either BRAF or NRAS.12, 13, 14 Most BRAF mutations in cancer involve exons 11 and 15, and in melanoma, the p.V600E is by far the most commonly reported abnormality. Non-p.V600E mutations, however, appear to be more frequent than was initially thought.2, 9 The largest single cohort of patients analyzed for BRAF mutations in the literature to date is 302 patients, and most studies have assessed fewer than 100 patients.15, 16 We undertook the present study because of the increasing clinical use of selective BRAF inhibitors, including vemurafenib and more recently dabrafenib (GSK2118436, which, at present, is in clinical trials), to target the activated p.V600E mutant form of BRAF kinase.17 We realized that a more comprehensive review of BRAF mutation data in melanomas would be helpful.
Our data show that approximately 25% of BRAF mutations in melanoma are of non-p.V600E type. Most (70%) non-p.V600E BRAF mutations were p.V600K, with an overall incidence of 17.2%. Other non-p.V600E mutations involving codon 600 (p.V600R, p.V600E2, and p.V600D) occurred rarely (4.6%), which is in keeping with other reports.7, 8 We also detected rare exon 15 BRAF mutations occurring at codons 594, 597, and 601, as well as two exon 11 mutations (p.G466E and p.G469E) of unknown clinical significance in melanoma. Both of the exon 11 mutations occurred in lentigo maligna melanoma, but whether this has any biological or clinical significance is uncertain.
In this series, we used tumor site as a surrogate for sun exposure, as other authors have done.18 Based on the primary tumor site, one can estimate the frequency of BRAF mutation. Overall, mutations were significantly more frequent in nonacral cutaneous melanoma (51.3%) than in acral melanoma (16.2%) (P < 0.001), which is in keeping with other reports.15, 18 Interestingly, BRAF mutations were seen twice as frequently in metastatic lesions arising from an acral primary tumor (22.4%) than in the primary acral tumors themselves (11.3%). This difference between metastatic and primary tumors was not observed in cutaneous, nonacral melanomas (52.0% and 49.0%, respectively). Our results also confirm that BRAF mutations are rare to absent in melanomas that arise from mucosal sites and the uvea, as others have reported, and reiterate the suggestion of differences in pathogenesis between melanomas that arise at different sites.15, 19 These data further suggest that the yield of clinical testing for BRAF mutations in mucosal and uveal melanomas is low. We studied only one leptomeningeal case, however, so we cannot address the frequency of BRAF mutations at this site.
In the present study, histopathological classification of nonacral primary cutaneous melanoma did not reliably distinguish BRAF-mutated from unmutated cases; approximately 66% of superficial spreading type, 50% of nodular, and 20% of cases of lentigo maligna melanomas showed BRAF mutation. These findings are in keeping with other reports,15 and further corroborate the notion that histological subtype is not a surrogate for BRAF mutation status and that it is less informative than other parameters, such as patient age, anatomical site of the primary neoplasm, and degree of solar elastosis at the primary site.19, 20
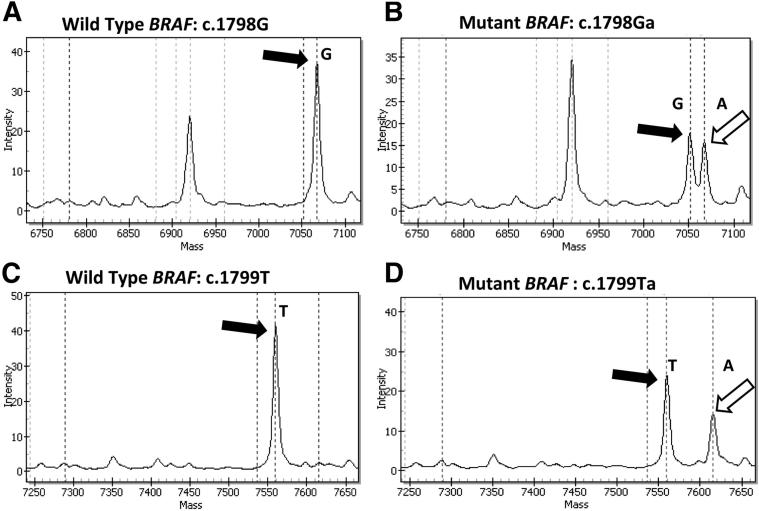
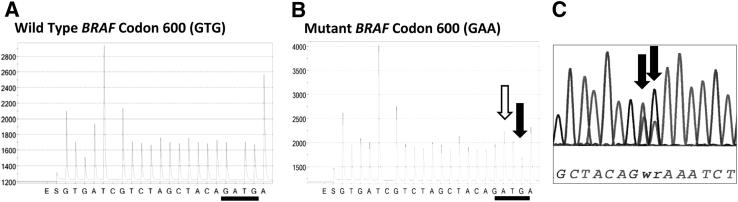
A number of molecular approaches are widely used in clinical practice to detect BRAF mutations in melanoma. These methods include (but are not restricted to) melting-curve analysis of PCR products, PCR-based DNA sequencing such as pyrosequencing and Sanger sequencing,2, 15, 16, 21, 22, 23, as well as high-throughput methods based on DNA primer extension, such as MALDI-TOF MS (a SNP array) and next-generation sequencing platforms. In addition to detecting known BRAF mutations, DNA sequencing approaches may provide the added advantage of discovery of novel sequence variants. However, MALDI-TOF MS is limited to the detection of only a single predetermined nucleotide position per primer, and requires meticulous assay design using multiple primer sets to detect the relevant mutations (Table 1 and Figure 1). Additionally, homopolymeric variations may be difficult to interpret by pyrosequencing; we therefore used Sanger sequencing to confirm all such abnormalities (eg, GTG>AAG and GTG>GAA) detected by pyrosequencing in our patient samples (Figure 2).
Figure 1.
Detection of BRAF c.1798_1799delinsAA (p.V600K) by single-base primer extension MALDI-TOF MS. A two-primer strategy was used to detect the variant nucleotides in the first and second base positions of codon 600. A and C: Single peaks representing the WT nucleotides at the first and second positions of codon 600, G and T, respectively (black arrows). B and D: Heterozygous variant peaks (white arrows) representing an indel (GT>AA) involving positions 1 and 2 of BRAF codon 600, resulting in a p.V600K change.
Figure 2.
Detection of BRAF c.1799_1800delinsAA (the so-called p.V600E2) in melanoma by pyrosequencing and Sanger sequencing. A: Pyrosequencing tracing shows the WT BRAF codon 600 as GTG. B: In mutant BRAF codon 600, a prominent peak represents A after the first G (white arrow), and a reduced peak represents the third base G (black arrow). C: The precise variant sequence (GAA) is confirmed by Sanger sequencing, which demonstrates a heterozygous pattern of T/A G/A at the second and third base positions of codon 600 (arrows).
The FDA recently approved vemurafenib to treat patients with late-stage (metastatic) or unresectable melanoma harboring the BRAF p.V600E mutation, and also approved a companion diagnostic assay, the cobas 4800 BRAF V600 mutation test, designed to detect BRAF p.V600E mutations (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm268241.htm, accessed January 2, 2013). The relatively high frequency of non-p.V600E mutations found in the present study is likely to have implications for the detection rate of BRAF mutations using the FDA-approved companion diagnostic test, and may also have implications regarding the efficacy of vemurafenib therapy. In one large clinical trial, the cobas 4800 BRAF mutation test detected a subset of non-p.V600E BRAF mutations, including 10 patients with BRAF p.V600K, four of whom showed a partial clinical response to vemurafenib.5 Others also have reported that the cobas 4800 BRAF V600 mutation test detects a subset of non-p.V600E BRAF mutations, most often p.V600K, and less often p.V600D and p.V600E2.7, 8 Although the proportion of non-p.V600E mutations that are detected using the FDA approved assay remains somewhat unclear, a recent study showed that the cobas 4800 mutation test detected 70% of 40 p.V600K mutations.8 It therefore seems reasonable to conclude that the cobas 4800 BRAF V600 mutation test may miss a subset of patients who have non-p.V600E BRAF mutations but who may nonetheless respond to vemurafenib therapy. However, to address the frequency of these potential misses would require a side-by-side comparison of the cobas 4800 BRAF V600 mutation test and other BRAF mutation detection methods on samples from patients with known response to vemurafenib.
In conclusion, we have shown that non-p.V600E BRAF mutations occur in almost 25% of patients with melanoma. The p.V600K mutation is the most common non-p.V600E variant, with an overall frequency approaching 20%. Although some patients with p.V600K mutations have reportedly shown response to anti-BRAF targeted therapy,2, 5, 7 the precise performance characteristics of these patients as a distinct subgroup when treated with these agents is not clear from the literature and warrants further study. Furthermore, the inadvertent inclusion of patients with the p.V600K mutation in clinical trials using FDA approved cobas 4800 mutation test could lead to its misclassification as p.V600E. This could be a potential confounder in design of such studies. Additionally, the response profile to anti-BRAF targeted therapies is unclear for patients with rare non-p.V600E BRAF mutations. Although rare, these variants should not be ignored, especially in this era of personalized medicine, when the ultimate goal is therapy based on the individual molecular genetic profile of each patient. Our data also indicate that exon 11 BRAF mutations are very rare (<1%) in melanoma; we suggest, therefore, that routine screening for exon 11 BRAF mutations in melanoma is not warranted.
References
- 1.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein J.C., Sznol M., Pavlick A.C., Ariyan S., Cheng E., Bacchiocchi A., Kluger H.M., Narayan D., Halaban R. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty K.T., Puzanov I., Kim K.B., Ribas A., McArthur G.A., Sosman J.A., O’Dwyer P.J., Lee R.J., Grippo J.F., Nolop K., Chapman P.B. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A., Kim K.B., Schuchter L.M., Gonzalez R., Pavlick A.C., Weber J.S., McArthur G.A., Hutson T.E., Flaherty K.T., Moschos S.J., Lawrence D.P., Hersey P., Kefford R.F., Chmielowski B., Puzanov I., Li J., Nolop K.B., Lee R.J., Joe A.K., Sosman J.A. BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma (abstract) J Clin Oncol. 2011;29(15 Suppl):8509. [Google Scholar]
- 5.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., Hogg D., Lorigan P., Lebbe C., Jouary T., Schadendorf D., Ribas A., O’Day S.J., Sosman J.A., Kirkwood J.M., Eggermont A.M., Dreno B., Nolop K., Li J., Nelson B., Hou J., Lee R.J., Flaherty K.T., McArthur G.A., collaborators Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph E.W., Pratilas C.A., Poulikakos P.I., Tadi M., Wang W., Taylor B.S., Halilovic E., Persaud Y., Xing F., Viale A., Tsai J., Chapman P.B., Bollag G., Solit D.B., Rosen N. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci USA. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halait H., Demartin K., Shah S., Soviero S., Langland R., Cheng S., Hillman G., Wu L., Lawrence H.J. Analytical performance of a real-time PCR-based assay for V600 mutations in the BRAF gene, used as the companion diagnostic test for the novel BRAF inhibitor vemurafenib in metastatic melanoma. Diagn Mol Pathol. 2012;21:1–8. doi: 10.1097/PDM.0b013e31823b216f. [DOI] [PubMed] [Google Scholar]
- 8.Anderson S., Bloom K.J., Vallera D.U., Rueschoff J., Meldrum C., Schilling R., Kovach B., Lee J.R., Ochoa P., Langland R., Halait H., Lawrence H.J., Dugan M.C. Multisite analytic performance studies of a real-time polymerase chain reaction assay for the detection of BRAF V600E mutations in formalin-fixed paraffin-embedded tissue specimens of malignant melanoma. Arch Pathol Lab Med. 2012;136:1385–1391. doi: 10.5858/arpa.2011-0505-OA. [DOI] [PubMed] [Google Scholar]
- 9.Amanuel B., Grieu F., Kular J., Millward M., Iacopetta B. Incidence of BRAF p.Val600Glu and p.Val600Lys mutations in a consecutive series of 183 metastatic melanoma patients from a high incidence region. Pathology. 2012;44:357–359. doi: 10.1097/PAT.0b013e3283532565. [DOI] [PubMed] [Google Scholar]
- 10.Jakob J.A., Bassett R.L., Jr., Ng C.S., Curry J.L., Joseph R.W., Alvarado G.C., Rohlfs M.L., Richard J., Gershenwald J.E., Kim K.B., Lazar A.J., Hwu P., Davies M.A. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118:4014–4023. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma S., Greaves W.O., Ravandi F., Reddy N., Bueso-Ramos C.E., O’Brien S., Thomas D.A., Kantarjian H., Medeiros L.J., Luthra R., Patel K.P. Rapid detection and quantitation of BRAF mutations in hairy cell leukemia using a sensitive pyrosequencing assay. Am J Clin Pathol. 2012;138:153–156. doi: 10.1309/AJCPL0OPXI9LZITV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim N., Haluska F.G. Molecular pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol. 2009;4:551–579. doi: 10.1146/annurev.pathol.3.121806.151541. [DOI] [PubMed] [Google Scholar]
- 13.Demunter A., Stas M., Degreef H., De Wolf-Peeters C., van den Oord J.J. Analysis of N- and K-ras mutations in the distinctive tumor progression phases of melanoma. J Invest Dermatol. 2001;117:1483–1489. doi: 10.1046/j.0022-202x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- 14.Tsao H., Goel V., Wu H., Yang G., Haluska F.G. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platz A., Egyhazi S., Ringborg U., Hansson J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008;1:395–405. doi: 10.1016/j.molonc.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J.H., Choi J.W., Kim Y.S. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol. 2011;164:776–784. doi: 10.1111/j.1365-2133.2010.10185.x. [DOI] [PubMed] [Google Scholar]
- 17.Ribas A., Flaherty K.T. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat Rev Clin Oncol. 2011;8:426–433. doi: 10.1038/nrclinonc.2011.69. [DOI] [PubMed] [Google Scholar]
- 18.Curtin J.A., Busam K., Pinkel D., Bastian B.C. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado J.L., Fridlyand J., Patel H., Jain A.N., Busam K., Kageshita T., Ono T., Albertson D.G., Pinkel D., Bastian B.C. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 20.Shinozaki M., Fujimoto A., Morton D.L., Hoon D.S. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753–1757. doi: 10.1158/1078-0432.ccr-1169-3. [DOI] [PubMed] [Google Scholar]
- 21.Spittle C., Ward M.R., Nathanson K.L., Gimotty P.A., Rappaport E., Brose M.S., Medina A., Letrero R., Herlyn M., Edwards R.H. Application of a BRAF pyrosequencing assay for mutation detection and copy number analysis in malignant melanoma. J Mol Diagn. 2007;9:464–471. doi: 10.2353/jmoldx.2007.060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay R., MacRae E., Barber D., Khalil M., Demetrick D.J. BRAF mutations in melanocytic lesions and papillary thyroid carcinoma samples identified using melting curve analysis of polymerase chain reaction products. Arch Pathol Lab Med. 2007;131:1361–1367. doi: 10.5858/2007-131-1361-BMIMLA. [DOI] [PubMed] [Google Scholar]
- 23.Willmore-Payne C., Holden J.A., Tripp S., Layfield L.J. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005;36:486–493. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]