Abstract
Control of biospecimen quality that is linked to processing is one of the goals of biospecimen science. Consensus is lacking, however, regarding optimal sample quality-control (QC) tools (ie, markers and assays). The aim of this review was to identify QC tools, both for fluid and solid-tissue samples, based on a comprehensive and critical literature review. The most readily applicable tools are those with a known threshold for the preanalytical variation and a known reference range for the QC analyte. Only a few meaningful markers were identified that meet these criteria, such as CD40L for assessing serum exposure at high temperatures and VEGF for assessing serum freeze-thawing. To fully assess biospecimen quality, multiple QC markers are needed. Here we present the most promising biospecimen QC tools that were identified.
One of the main goals in biospecimen science is to identify and control the potential bias due to biospecimen processing or quality on the molecular analyses. Several recent studies have recognized the influence of preanalytical variables (eg, warm or cold ischemia times, or delays in processing1) on the integrity of biomolecules.
For example, gene profiling of peripheral blood cells of patients has the potential to advance our understanding of a variety of human diseases. Appropriate biomarkers can improve diagnosis and clinical management of patients. However, to optimize cell gene expression data for translational studies, it is essential to control the preanalytical variables that produce changes in gene expression, variables that are unrelated to the disease condition being studied.
Controlling preanalytical variables is a particularly challenging and complex issue, because the influence of the quality of a sample on the molecular data obtained from its analysis depends not only on the class of biomolecule analyzed (DNA, RNA, protein, metabolite) but also on the type of analytical method (multiplex versus singleplex, qualitative versus quantitative) and the specificity, sensitivity, and robustness of the method against specific preanalytical variations. Biobanking method validation requires both knowledge of the preanalytical variables that need to be controlled and identification of those factors that do not affect the quality of the biospecimen for a given type of research.
To address this major goal of controlling for preanalytical variables, two main approaches are used in biospecimen science-driven biobanking. The first is to optimize the quality of biospecimens and thus to directly minimize and/or control the preanalytical bias. Unfortunately, in most clinical settings there is only limited ability to control preanalytical variables influencing biomolecule integrity, such as surgery or warm ischemia time. In a clinical context, therefore, a second approach must be to retrospectively apply appropriate tests to accurately assess the global biomolecular integrity status of each biospecimen. This process becomes critical for high-throughput, quantitative downstream assays implemented as clinical molecular diagnostics.
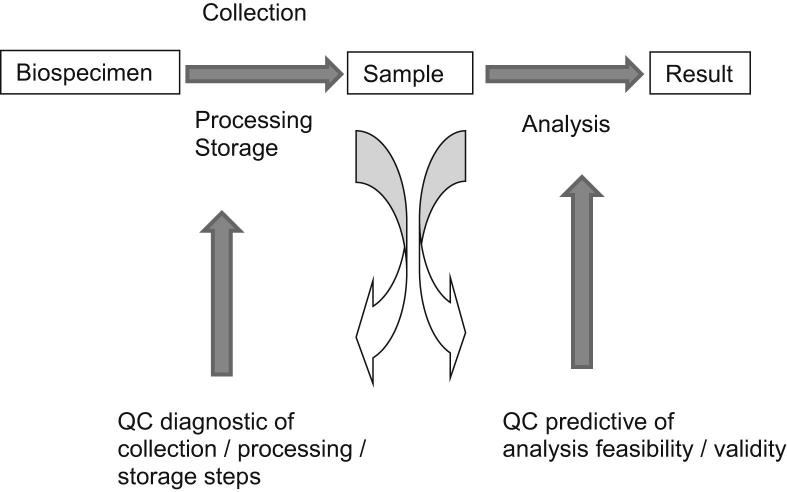
Once the most critical points in a biospecimen processing method have been identified, specific tests or markers to assess the quality of the biospecimen are needed. These may be called surrogate quality biomarkers or surrogate quality indicators. At present, there are few appropriate quality-control tools that either are predictive of downstream method feasibility [eg, DNA methylation analysis on DNA extracted from formalin-fixed, paraffin-embedded (FFPE) tissue] and reliability (eg, feasibility of methylation analysis does not guarantee its accuracy) or are diagnostic of upstream biospecimen processing steps (eg, tissue fixation time) (Figure 1). Quality control (QC) in the form of diagnostic tests of upstream biospecimen processing steps is called biospecimen molecular diagnostics or preanalytical characterization. Ultimately, preanalytical characterization should allow researchers to assess the reliability of a specific type of downstream analysis.
Figure 1.
Quality control assays, applied on biological samples, can be either diagnostic of upstream collection, processing, and/or storage conditions or predictive of the feasibility and/or validity of downstream analysis performed with the samples.
Several steps are needed to solve these issues. First, the scientific community must agree on the elements from the life cycle of the biospecimen that should be documented in scientific publications, as has been proposed in the Biospecimen Reporting for Improved Study Quality (BRISQ) recommendations.2 Second, these elements must be codified, so that their recording and communication is standardized. For example, the ISBER Biospecimen Science Working Group has developed a standard preanalytical code to identify the main preanalytical variables for both fluid and solid-tissue biospecimens and their simple derivatives.3 Finally, it is critical to perform biospecimen research to identify the key biomarkers that will predict sample integrity and quality.4
Different QC assays are used to characterize viable and nonviable biospecimens. In the first case, viability and functionality (eg, pluripotency, response to antigens, motility) are assessed through microscopy, flow cytometry, or immunoenzymatic assays. In the case of nonviable specimens, molecular integrity (eg, protein phosphorylation status, epitope conformation, rRNA degradation, DNA cross-linking degree) is generally assessed through immunoenzymatic, electrophoretic, and molecular biologic assays.5
It is critical to define and standardize the assays used to assess the molecular integrity of biospecimens procured in a clinical setting, because there is no consensus in the literature on which biospecimen quality markers or tools provide the best biospecimen molecular diagnostic performance and information. For example, standard approaches to assessing RNA integrity, such as ribosomal RNA measurements and RNA integrity number (RIN), are neither sensitive nor specific enough to assess potential bias in downstream gene expression analysis.6
The goal of this literature review was to identify tools (markers and assays) that can be used to assess preanalytical variations for both fluid and solid tissue-derived samples. Defining the most appropriate assays used to assess biospecimen quality, as well as the methods for standardization between laboratories through external quality assessment, remains an open question. Furthermore, it is imperative to define the analytical data that can be reliably obtained within different sample quality categories, and to develop methods to overcome pitfalls linked to issues of sample quality. The scope of the present review is limited to upstream QC of the biospecimen. We did not address downstream QC of the end-use results, such as gene expression microarray parameters. The scope of the review encompasses both clinical biology and research laboratory settings: on the one hand, information that has previously been published in a clinical setting can be applied to research; on the other hand, high-throughput assays initially performed only in research settings may later be implemented in clinical practice.
Evidence-Based Biospecimen QC Tools
Biospecimen Science-Specific Literature Compilation
Members of the ISBER Biospecimen Science Working Group searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed) using keywords such as analyte, stability, preanalytical, and specimen, and then compiled all identified publications that dealt with biomolecules and biospecimen analytical behavior. Relevant literature from other sources on the same subjects was also included, including proceedings from meetings, as well as guidelines and recommendations. Additional potential sources of information were identified by members of the working group; these included selected publications on basic and clinical research and DNA, RNA, and protein analysis from studies that used samples from tissue resources (the Cooperative Human Tissue Network, Cooperative Group Banks) and other programs (Innovative Molecular Analysis Technologies) of the U.S. National Cancer Institute.
Biospecimen Science-Specific Literature Review
Members of the ISBER Biospecimen Science Working Group performed a critical review of these literature sources to find published data demonstrating that specific biomarkers are particularly unstable and sensitive to preanalytical variations, or to find assays that can be used to assess such variations. The objective of the working group to identify unstable analytes was thus directly opposite to the typical focus of studies conducted to identify the analytical stability of specific analytes or high-throughput signatures.
References were classified and reviewed in five thematic categories: i) single analytes; ii) hormones, cytokines, and nutritional indicators; iii) high-throughput methods; iv) functional assays; and v) pathogens.
Publications on single analytes (primarily in blood or urine) were expected to contain information on the most immediately useful diagnostic markers. We attempted to identify markers that had an on/off response to specific preanalytical variations, such as those in which enzymatic or immunological activity is completely lost. This condition makes it clear that the change is significant, and that absolute reference values are not needed. A considerable degree of degradation (eg, a 60% drop in 30 minutes at room temperature) was also considered to be very important, because we can assume that the presence of the corresponding marker or activity will completely disappear under more stressful conditions (eg, 2 hours at room temperature).
In publications on hormones, cytokines, and nutritional indicators in blood, reference values were expected to be found, at least for clinically important analytes.
High-throughput methods (-omics) include arrays (DNA, RNA) and mass spectrometry. No absolute reference values were expected to be found, but publications addressing these methods were expected to provide information on the most immediately useful predictive markers.
For publications on functional assays (eg, in blood cells), no absolute reference values were expected to be found.
Data from publications on pathogens could support development of spiking-based QC tools.
A set of data items was extracted and recorded from each relevant publication in a common matrix, as follows: reference; type or types of samples studied; preanalytical variable or variables studied; range of preanalytical variable studied (range of variation applied); preanalytical threshold identified; potential QC tool or marker (sample characteristic assessed); QC method [eg, enzyme-linked immunosorbent assay (ELISA), RT-PCR]; type of method (qualitative or quantitative, simple or multiplex); range of the potential QC tool/marker (range of measures observed as a result of the preanalytical variation applied); control samples used as baseline; and, in the case of quantitative methods, the reference ranges (expected values of the potential QC marker in a general population). Based on these elements, the most appropriate QC tools, in terms of molecular diagnostic performance and feasibility of application, were selected by consensus.
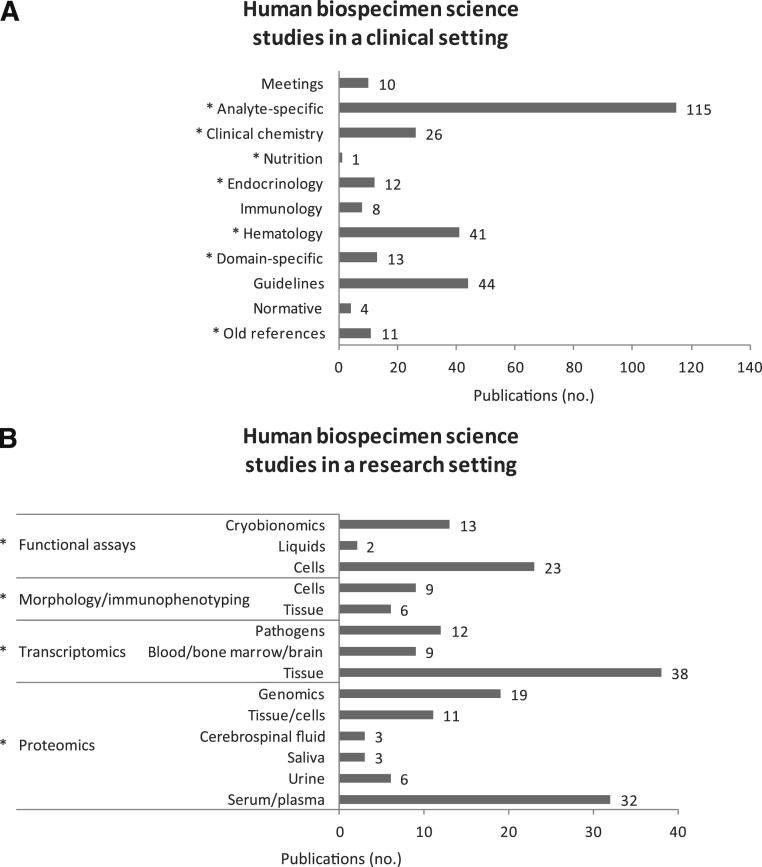
In all, 494 references on human biospecimen science were collected between 2008 and 2010 and reviewed in 2011; of these, 287 were studies performed in a clinical setting, 185 were studies performed in a research setting (Figure 2), and 22 were publications from the Cooperative Human Tissue Network. The full compilation is available at http://www.isber.org/wg/bs/documents/isberbswgliteraturecompilation.pdf (3rd edition, September 2011; last accessed May 2012). The 494 publications came from 225 different journals. The most frequently cited journals were Clinical Chemistry (67 publications), Clinical Chemistry and Laboratory Medicine (28 publications), and Cancer Epidemiology, Biomarkers & Prevention (17 publications). All of the remaining journals had fewer than 10 publications.
Figure 2.
Biospecimen science literature compilation of human biospecimen science studies in a clinical (A) or a research (B) setting. Asterisks mark groups of publications reviewed for this study.
Classification of Potential QC Tools
Based on the data from the literature review, we evaluated some of the marker tools using four criteria: type of QC tool, evidence base, applicability grade, and accessibility grade.
Type of QC Tool
The QC tools can be categorized into two main types: diagnostic and predictive.
-
1.
Diagnostic tools assess the processing steps of the biospecimen, such as delay of processing, time or type of fixation, or storage duration.
-
2.
Predictive tools assess the feasibility and/or reliability of the downstream analysis. This is particularly important for high-throughput methods, as predictive of successful method performance.
Evidence Based
QC tools from recommendations that were not evidence-based were not retained for further review.
Applicability Grade
Applicability was assessed in three grades: immediate, potential, and no applicability.
-
1.
For a grade of immediate applicability, a threshold is identified for the preanalytical variation (eg, a maximum delay of processing) and the absolute value of a reference (control population) range is known. Only a few articles met these criteria.
-
2.
For a grade of potential applicability, a threshold is identified but the absolute value of a reference range is not known. In other cases of potential applicability, accelerated aging studies are performed but real-time stability testing is not performed, or native concentrations of analytes are too low for immediate application but a spiking approach with a recombinant antigen or a synthetic molecule may be possible.
-
3.
For a grade of not immediately applicable, more stressful conditions would need to be applied to the biospecimen to assess a suitable threshold.
QC tools graded as of immediate applicability require further validation studies. QC tools in the other two grades require feasibility or proof-of-principle studies.
Accessibility Grade
Another key element for assessing the usefulness of a QC tool is how user-friendly and accessible the corresponding method may be: readily, potentially, or not immediately accessible.
-
1.
The readily accessible grade includes classical laboratory methods (eg, ELISA, PCR, flow cytometry).
-
2.
The potentially accessible grade includes methods requiring high-throughput platforms (eg, microarray platform, mass spectrometry platform) that are typically available as a centralized service.
-
3.
The not immediately accessible grade includes laboratory-developed (in-house) methods.
Identification of Potential QC Tools
Potential QC tools were identified in the above-mentioned five thematic categories.
The single analyte group of publications consisted primarily of targeted stability studies. The specific analytes were measured by methods usually applied in clinical biology.
The hormones, cytokines, and nutritional indicators group of publications consisted of stability studies of these specific molecules.
The high-throughput group of publications, gene expression microarray or mass spectrometry data were compared for samples that had undergone stressful conditions versus their baseline conditions.
In the functional assays group of publications, a variety of cytology specimen types were studied (including FFPE, cervical cytology, bone marrow, saliva, buccal specimens, blood, and urine), with checking for changes in DNA under different collection or storage conditions as the overall goal. Most of these publications used DNA yield as the endpoint for QC; in some of the studies, researchers looked for DNA amplification by PCR, and in others at the level of whole-genome analysis, single-nucleotide polymorphism analysis, or single-strand breaks. Also, blood cells (peripheral blood mononuclear cells, natural killer cells, and T cells) and sperm were checked for cell viability and cell biological functions using standard QC tests for IFN-γ, CD4+, natural killer cell activity, CD8+, HIV infectivity, and cytokine production (IL-2, IL-6, IFN-γ, TNF-α, GM-CSF). Different types of cells (eg, cell lines, tissue, sperm, cornea, and stem cells) were tested for cell viability and DNA integrity that had undergone various processing and storage conditions. Mass spectrometry studies were performed on semen and urine, and QC was determined by the number of chromatographic peaks generated.
The pathogens group of publications consisted primarily of stability studies of viral pathogen nucleic acids in serum or plasma.
Diagnostic QC Tools
The most readily applicable and accessible evidence-based QC tools are summarized in Table 1.
Table 1.
Biospecimen Molecular Diagnostic Tools Identified, with QC Scope and Evaluation
| QC tool | References | Analyte type | Sample type | QC scope | Applicability grade | Accessibility grade | Future research required |
|---|---|---|---|---|---|---|---|
| Transferrin receptor | 7 | Protein | Serum | Precentrifugation delay | 1 | 1 | |
| Ascorbic acid | 8 | Vitamin | Serum, EDTA plasma | Precentrifugation delay, storage conditions | 1 | 2 | Longer precentrifugation delay to get 100% degradation |
| K+ | 9 | Ion | Serum | Precentrifugation delay at 4°C | 1 | 1 | Plasma |
| GM-CSF, IL-1α, G-CSF | 10 | Protein | EDTA plasma ± PI | Precentrifugation delay | 1 | 1 | |
| C3f peptides, fibrinopeptide A | 11 | Peptide | Serum, plasma | Postcentrifugation delay | 3 | 2 | |
| ACTH | 12 | Hormone | EDTA plasma, serum | Postcentrifugation delay | 3 | 1 | Longer delays to assess the threshold of ACTH complete degradation in both serum and plasma |
| sCD40L | 13 | Protein | Serum | Exposure to room temperature | 1 | 1 | |
| Vitamin E | 14 | Vitamin | EDTA plasma | Storage conditions | 1 | 1 | |
| MMP-9 | 15 | Protein | Citrated plasma | Storage conditions | 2 | 1 | healthy donors, Degradation in EDTA plasma and serum |
| VEGF | 16 | Protein | Serum | Freeze thawing, storage conditions | 2 | 1 | Real-time stability testing |
| IL-1β, IL-10, IL-15 | 17 | Protein | Heparin plasma | Storage conditions | 2 | 1 | Stability in other anticoagulants and in serum |
| MMP-7 | 18 | Protein | Serum | Freeze thawing | 1 | 1 | Stability in plasma and saliva |
| IL-15, IL-17, IFN-γ | 17 | Protein | Heparin plasma | Freeze thawing | 2 | 1 | Stability in other anticoagulants and in serum |
| ICAM1, SLC7A5 | 24 | RNA | EDTA PBMCs | Precentrifugation delay at room temperature | 2 | 1 | More subjects, other anticoagulants, define reference intervals |
| Adenosine A2a receptor, T cell receptor α locus, T54 protein, tumor necrosis factor superfamily member 14 putative lymphocyte G0/G1 switch gene, inhibitor of DNA binding 1 dominant negative helix-loop-helix protein, diphtheria toxin receptor | 19 | RNA | Citrate PBMCs | Precentrifugation delay at room temperature | 2 | 1 | More subjects, other anticoagulants, define reference intervals |
| NR4A2, AREG///LOC653193, MAFF | 20 | RNA | ACD PBMCs | Precentrifugation delay at room temperature | 2 | 1 | More subjects, other anticoagulants, define reference intervals |
| TNF-α | 21 | Protein | Urine | Preprocessing delay | 2 | 1 | Spiking approach |
| Epinephrine, dopamine | 22 | Compound | Urine | Storage conditions | 1 | 2 | Effect of freeze thawing |
| α-1-Antitrypsin | 23 | Protein | Urine | Freeze thawing | 3 | 1 | Longer preprocessing delays in order to define a threshold for 100% degradation and more freeze thaw cycles. |
| Truncated cystatin-C | 24 | Protein | CSF | Storage conditions | 3 | 3 | Produce mAbs once confirmation in other sample types |
| T-cell IFN-γ response | 25 | Protein | Viable PBMCs | Storage conditions | 3 | 1 | More prolonged cryopreservation |
| DUSP1 expression | 26 | RNA | Fresh prostatic tissue | Warm ischemia time | 1 | 1 | Other tissue types |
| Dusp1, 1a, Egr1, bHLHe40 (alias bHLHb2), Ppp1r15a (alias Gadd34; alias Myd116), Slc25a25, Btg2, Cxcl1, Zfp36, Jun | 27 | RNA | Frozen tissue | Cold ischemia time | 2 | 1 | Different types of human tissues and reference ranges |
| p-Tyrosine, ERBB2 (alias HER2; alias Neu)-Tyr1248, FAK | 28 | Protein | Breast tissue | Cold ischemia time | 1 | 1 | Other tissue types |
| Myosin heavy chain, smooth muscle isoform | 38 | Protein | Prostatic tissue | Cold ischemia | 3 | 2 | Other tissue types, reference values |
ACD, acid-citrate-dextrose; ACTH, adrenocorticotropic hormone; CSF, cerebrospinal fluid; mAbs, monoclonal antibodies; PBMC, peripheral blood mononuclear cell; PI, protease inhibitors.
For Serum and Plasma Specimens
Transferrin receptor
De Jongh et al7 showed a 90% increase of soluble transferrin receptor, measured by ELISA in serum, after an 8-hour blood precentrifugation delay; they reported a reference range of 171 to 212 U/mL.
Ascorbic acid
Karlsen et al8 suggested ascorbic acid, measured by chromatography, as a potential QC tool in serum and plasma. Ascorbic acid showed a 70% decrease in serum or EDTA plasma, after a 6-hour blood precentrifugation delay at room temperature, as well as a 100% decrease in serum and EDTA plasma after 3 months of storage at −20°C. The authors did not report a reference range, but this is known from the clinical chemistry to be 26 to 85 μmol/L.30
Potassium
Heins et al9 showed that blood precentrifugation delay at 4°C induced a dramatic increase in potassium concentration about 200% after 1 day and up to 500% after 7 days of processing delay. The increase was less pronounced after delay at room temperature, because of the temperature-dependent activity of the Na+-K+-ATPase. The mean baseline value has been reported as 3.92 mmol/L, measured by indirect potentiometry,9 and the standard reference range is 3.29 to 4.50 mmol/L.31
GM-CSF, IL-1α, and G-CSF
Ayache et al10 performed ELISA to examine the global chemokine and cytokine profile in EDTA plasma collected with or without protease inhibitors. A 2-hour precentrifugation delay at room temperature induced an 11- to 20-fold increase of GM-CSF, IL-1α, and G-CSF in blood collected without protease inhibitors and a 7- to 10-fold increase of the same proteins in blood collected with protease inhibitors. Baseline reference levels were reported as 214 ± 163 pg/mL for GM-CSF, 9.4 ± 7.7 pg/mL for IL-1α, and 119 ± 60 pg/mL for G-CSF.
C3α chain and fibrinogen peptides
Using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry, Marshall et al11 showed for the first time a characteristic family of complement C3α chain and fibrinogen peptides generated in citrated plasma and exposed to room temperature for 4 hours. These findings have been confirmed by Yi et al32 in serum and in EDTA, citrated, and heparinated plasma and by West-Nørager et al33 for a 2-hour postcentrifugation delay of serum at either room temperature or 4°C using MALDI-TOF mass spectrometry. The following peptides were identified as involved in postsampling modifications in serum: complement C3f, aa 2-16 (SKITHRIHWESASLL); complement C3f, aa 1-16 (SSKITHRIHWESASLL); complement C3f, aa 1-17 (SSKITHRIHWESASLLR); fibrin α C term fragment, aa 81-105 (SSSYSKQFTSSTSYNRGDSTFESKS); fibrin α C term fragment, aa 81-106 (SSSYSKQFTSSTSYNRGDSTFESKSY); fibrinopeptide A, aa 1-12 (EGDFLAEGGGVR); fibrinopeptide A, aa 3-16 (SGEGDFLAEGGGVR); fibrinopeptide A, aa 2-16 (DSGEGDFLAEGGGVR); fibrinopeptide A (modifications: Ser-3 phosphorylated), aa 1-16 (ADSGEGDFLAEGGGVR); kininogen, aa 439-456 (HNLGHGHKHERDQGHGHQ).
ACTH and BNP
Evans et al12 studied the stability of plasma and serum hormones and confirmed the instability of adrenocorticotrophic hormone (ACTH) and brain natriuretic peptide (BNP). Reference ranges are 10 to 64 pmol/L (measured by immunoradiometric assay) for ACTH and 34 to 153 pmol/L (measured by radioimmunoassay) for BNP. However, BNP levels in healthy donors are at or near the detection limit of the assay; this quality marker can be useful only in heart failure subjects and is therefore not of general use. A clinically significant decrease in ACTH levels was observed after storage at room temperature for 8 hours in EDTA plasma and after only 1 hour in serum.
CD40L
Lengelle et al13 reported soluble CD40L (sCD40L) as a QC marker that allows assessment of serum exposure to elevated temperatures. They found that that sCD40L, measured by ELISA, undergoes complete degradation in 12 hours at 37°C or in 48 hours at room temperature. Also, freeze-thaw cycles had no effect on sCD40L levels in serum. They reported a reference range of 7 to 17 ng/mL, and the precise threshold below which significant exposure to elevated temperatures can be ascertained as 4.3 ng/mL.
Vitamin E
Ocke et al14 reported that vitamin E in EDTA plasma decreased by more than 90% when plasma was stored for more than 24 months at −20°C. The analytical method used was high performance liquid chromatography. They reported the reference range as 19 to 31 μmol/L.
MMP-9
Rouy et al15 showed complete degradation of MMP-9 measured by ELISA in citrated plasma after 25 to 36 months of storage at −80°C. Their reported reference range for MMP-9, established in a population of patients with acute myocardial infarction, was 80 to 800 ng/mL.
VEGF
Kisand et al16 recently showed that VEGF in serum is labile to freeze-thawing and to storage duration either at −20°C or at −80°C. When VEGF was measured by ELISA, it became undetectable after one to six freeze-thaw cycles. Accelerated aging testing and Arrhenius plots allow extrapolation to predict that VEGF becomes undetectable after 11 months of storage at −20°C or after 4.5 years of storage at −80°C. The VEGF reference range reported by enzyme immunoassay kit manufacturers (eg, R&D Systems, Inc., package insert: Quantikine Human Total MMP7 Immunoassay. Minneapolis, MN) is 62 to 707 pg/mL in serum. Although the results reported by Kisand et al16 seem interesting, the lability of VEGF exposed to freeze-thaw cycles has not been confirmed,34 and application in plasma is not feasible, given that reference levels in plasma may start from 0 pg/mL.
Interleukin cytokines
De Jager et al17 examined recovery and stability of spiked cytokines in heparin plasma and serum and showed that IL-1β, IL-10, and IL-15 were completely degraded after 4 years of storage at −80°C, and IL-15, IL-17, and IFN-γ were completely degraded after four freeze-thaw cycles. However, for all of these cytokines the reference ranges in healthy donors start from 0 pg/mL and are reported by ELISA kit manufacturers to be lower than the lowest calibration point (eg, R&D Systems, Inc., package insert: Quantikine Human IL-10 Immunoassay. Minneapolis, MN). Therefore, unless ultra-sensitive detection methods are developed and used, only a spiking strategy is possible for development of corresponding QC tools.
MMP-7
Chaigneau et al18 showed complete loss of MMP-7 as measured by ELISA in serum after 30 freeze-thaw cycles. The reference ranges reported by the kit manufacturer are 1.07 to 4.40 ng/mL in serum and 1.10 to 4.59 ng/mL and 3.80 to 28.3 ng/mL in plasma and saliva, respectively (R&D Systems, Inc., package insert: Quantikine Human Total MMP7 Immunoassay. Minneapolis, MN).
Adenosine A2a receptor and other genes
Using Affymetrix microarrays, Baechler et al,19 found that a variety of signaling pathways are activated in peripheral blood cells collected on CPT cell preparation tubes and stabilized in RNAlater reagent after ex vivo overnight incubation. Many of the genes sensitive to ex vivo incubation are involved in transcriptional regulation, cell cycle progression, and apoptosis. Several of these genes also encode proteins that perform functions essential to the immune response. Most down-regulated (>40-fold) by the precentrifugation delay were the adenosine A2a receptor, the T-cell receptor α locus, the T54 protein, and the tumor necrosis factor superfamily member 14 coding genes. Most up-regulated (>70-fold) by the precentrifugation delay were the putative lymphocyte G0/G1 switch gene, the inhibitor of DNA binding 1 dominant negative helix-loop-helix protein, and the diphtheria toxin receptor coding genes.
NR4A2, AREG///LOC653193, and MAFF genes
A similar study performed by Barnes et al20 on peripheral blood mononuclear cells collected in acid-citrate-dextrose tubes and stabilized in TRIzol reagent, with a 4-hour delay, demonstrated that the most sensitive genes (those with a greater than sevenfold change after a 4-hour precentrifugation delay) were NR4A2, AREG///LOC653193, and MAFF.
Epinephrine and dopamine
Boomsma et al22 showed complete loss of epinephrine and dopamine measured by high performance liquid chromatography and fluorometry in heparinized plasma after suboptimal storage conditions. The suboptimal storage condition threshold identified was 2 to 3 days of storage at room temperature. Their reported reference values were 0.24 ± 0.10 nmol/L for epinephrine and 0.11 ± 0.03 nmol/L for dopamine.
For Urine Specimens
TNF-α
Banks et al21 described complete loss of TNF-α measured by ELISA in urine after a preprocessing delay of 30 minutes at 37°C. However, given the low reference values in urine (0.1 ± 0.2 pg/μmol),35 only a spiking approach would be feasible. [TNF-α concentration in serum or plasma from healthy donors is <16 pg/mL, which is the lowest calibration point of most ELISA kits (R&D Systems, Inc., package insert: Quantikine Human TNF-α Immunoassay. Minneapolis, MN).]
Epinephrine and dopamine
Boomsma et al22 showed complete loss of epinephrine and dopamine measured by high performance liquid chromatography and fluorometry in unpreserved urine after suboptimal storage conditions. The suboptimal storage condition threshold identified was 10 days of storage at room temperature. Only the mean baseline reference values were reported: 0.25 mmol/L for epinephrine and 1.86 mmol/L for dopamine.
α1 Anti-trypsin
Tencer et al23 studied the stability of α1 anti-trypsin and found a significant 35% decrease when the urine prefreezing delay was either 7 days at room temperature or 30 days at 4°C, independent of the presence of additives in the urine specimens. The α1 anti-trypsin levels decreased by 35% after 1 day of storage at −20°C and by 62% after 180 days of storage at that temperature. The authors concluded that the degradation was due to thawing, rather than to cryostorage. An important interindividual variability was observed, with reference ranges of 3 to 66 mg/L (median, 16 mg/L) obtained by single radial immunodiffusion or automated immunoturbidimetry.
For Cerebrospinal Fluid Specimens
Cystatin-C
Carrette et al24 reported that 3 months of storage at −20°C induced an N-terminal truncation of cystatin C, as detected by MALDI-TOF mass spectrometry. Implementation of such a QC assay would necessitate development of monoclonal antibodies specific to the truncated N-terminal end and to intact epitopes, thus allowing the performance of ELISA and calculation of a ratio.
For Viable Blood Cell Specimens
IFN-γ production
Owen et al25 studied the effect of cryopreservation on apoptosis and T-cell responses to different protein and peptide antigens. They showed that long-term (1 year) cryopreservation increased apoptosis, as measured by activated caspase 3 antibody staining, and diminished CD4+ and CD8+ T-cell responses to both cytomegalovirus lysate and to staphylococcal enterotoxin B in HIV patients, with acute and chronic infection, respectively, measured by IFN-γ production. The mean absolute decrease in the percentage of responding T cells was only about 1%. Further research must be done to determine whether more prolonged cryopreservation has a more dramatic effect on these functional assays.
For Solid-Tissue Specimens
HPRT
Foss et al36 proposed RT-PCR of the hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA (168 bp) to assess the quality of paraffin-embedded tissue. Two technical advantages of this method are that the primers function with both human and mouse RNA (and so such a QC tool can be applied to both types of specimens) and that they preclude amplification of genomic DNA. However, although amplification was effective on mouse spleen tissues fixed with Omnifix and Carnoy’s fixative, it was not effective on FFPE tissues.
DUSP1
Lin et al26 explored the effects of surgery-linked warm ischemia on prostate tissue and showed that the DUSP1 gene was significantly up-regulated (14-fold change) after surgery and ischemia. DUSP1 expression was measured by both microarray and quantitative RT-PCR. The CT value was shown to be <8. The reported reference range in presurgical tissue was 7.5 < CT < 11.
Hspa1, Jun, and other genes
Thompson et al27 explored the effect of cold ischemia at either room temperature or 37°C and the effect of thawing on RIN and global gene expression profiles of rat liver tissue. They showed that cold ischemia induced significant up-regulation of the rat genes Dusp1, Hspa1a and Hspa1b, Egr1, bHLHe40 (alias bHLHb2), Ppp1r15a (aliases Gadd34, Myd116), Slc25a25, Btg2, Cxcl1, Zfp36, and Jun. The threshold in terms of number of hours of incubation at 37°C was not clearly indicated, and reference ranges remain to be determined.
Fos
Almeida et al37 showed significant up-regulation of the Fos gene expression with cold ischemia in mouse liver. Fos gene expression was increased by a factor of 12 after 3 hours at room temperature, as measured by quantitative RT-PCR.
p-Tyr, ERBB2-Tyr1248, and PTK2
De Cecco28 studied the effect of cold ischemia time on global gene expression and on the phosphorylation status of molecular targets in breast tissue. The phosphorylated epitopes p-Tyr, ERBB2-Tyr1248, and PTK2 (alias FAK) were completely denatured after 24 hours of cold ischemia and were undetectable on tissue protein extract Western blot. Gene expression signatures were also studied; but the identities of the most sensitive genes were not reported.
Myosin heavy chain
Using two-dimensional differential gel electrophoresis, Jackson et al38 compared the proteomic profiles between prostatic tissue of different cold ischemia times. Myosin heavy chain smooth muscle isoform increased by six- to sevenfold after 5 hours of cold ischemia.
Predictive QC Tools
One of the main obstacles in performing high-throughput DNA analysis on clinical samples is the limited starting amounts of genomic DNA that can be obtained from these samples. Whole-genome amplification has allowed applying high-throughput analysis in samples from which only minute amounts of genomic DNA can be extracted. Such samples include single cells,38 buccal swabs and blood spots,39 fine-needle aspirates, and tissue microdissection.40 The difficulty is even greater for FFPE tissues, in which intra- and intermolecular cross-linking further limits the access to and the quality of the biomolecules (Sigma-Aldrich, Whole gene amplification from archived formalin-fixed, paraffin-embedded tissues. St. Louis, MO). Whole-genome amplification has been key in generating larger quantities of DNA, thereby permitting the application in these samples of high-throughput genomic techniques, including single-nucleotide polymorphism arrays39, 40, 41 and methylation analysis (Illumina, Inc., Comprehensive DNA methylation analysis on the Illumina Infinium assay platform. San Diego, CA).
Several amplification approaches are available, including multiple-displacement amplification,39, 41 OmniPlex technology,39, 41, 42 and restriction and circularization-aided rolling circle amplification (RCA-RCA).29 Of note, little information is available on sample quality parameters that would allow predicting the success of downstream whole-genome amplification in FFPE tissues.
Multiplex GAPDH PCR
Wang et al29 developed a simple approach to assessing DNA fragmentation in very small clinical specimens of widely different origins, including archival specimens. Their approach predicts the likelihood of success of whole-genome amplification using RCA-RCA and subsequent PCR. The method is based on a multiplex PCR using four GAPDH amplicons of varying sizes. Even if minimal quantities of longer PCR fragments (approximately 300 to 400 bp) are visible, the subsequent RCA-RCA and PCR-based assays are still successful. Only colon and glioblastoma were studied (after 5 to 7 years or 10 to 12 years of fixation, respectively); other tissues and formalin fixation times remain to be explored. More importantly, this tool was used to evaluate the effect of formalin fixation time on the quality of DNA.
RT-PCR Efficiency
Player et al43 analyzed a panel of RNA quality parameters to predict successful hybridization on a microarray chip. Parameters considered included general ranges of absorbance, rRNA ratios, and RIN (before the array). More importantly, according to the authors’ protocol, 1000-fold amplification should happen in the first round of RT-PCR. Less than expected amplification is indicative of poor RNA quality and should preclude proceeding with the microarray experiment. It would be important to consistently apply these criteria to RNA from tissues (frozen or even FFPE tissues), and not only to RNA from cultured cells.
28S RNA Quantitative RT-PCR
Roberts et al44 identified simple QC quantitative RT-PCR assays as tools to predict success in subsequent microarray analysis of formalin fixed specimens. A ΔΔCT value of <15 for the 28S RNA between archival tissue and the universal RNA predicts array results of good quality (40% calls in 21/24 samples). In addition, if the absolute value of the 28S RNA CT is <15, the quality of the array data is similar to that of data from frozen specimens. The authors reported other data regarding conventional RNA quality parameters, including the finding that RIN > 4 correlates with >35% calls. It would be useful to expand the tissue types analyzed (lung and colon were the main tissues studied), as well as to analyze minute dissected specimens (only whole tissue sections were studied).
Phosphoproteins
Espina et al45 studied phosphoprotein profiles in tissues subjected to different lengths of time at room temperature to assess phosphoprotein stability. They measured 53 signal pathway phosphoproteins over time. Phosphoprotein stability is significantly affected by time at room temperature in tissues. Both up- and down-regulation of several phosphoproteins were observed as early as within 30 minutes after excision. This information is important for assessment of the overall phosphoprotein stability of solid-tissue specimens.
RNA Integrity Number
Thompson et al27 showed the predictive value of RIN on the gene expression microarray performance. RIN ≤ 7 was associated with significant decreases of the microarray sensitivity and specificity. It will be important to assess this data on both frozen and fixed human specimens.
Discussion
Several studies have addressed the effects of preanalytical variables on the molecular quality of biospecimens. Such concerns have been reflected in continued discussions at scientific meetings (eg, the annual ISBER and BRN symposia), as well as further work by ISBER and by the National Cancer Institute in developing evidence-based best practices for biospecimen collection, storage, and processing. However, as the present literature review indicates, additional research is still required to clearly elucidate the effects of preanalytical variables on biospecimens, along with the effects on downstream research activity.
There is still a lack of consensus regarding which markers or tools are the most useful for assessing sample quality. As the effects of biospecimen processing on the quality of research data become better recognized and better understood, we can hope that a more general effort in identifying useful quality-assurance and quality-control (QA/QC) markers and tools will be appreciated by the biospecimen science community.
This review does not address current widely used QC assays, such as nucleic acid spectrophotometry, RIN, or PCR, nor historical QC tools (eg, brain tissue pH, assumed to result from hypoxia46), such as have recently been reviewed in the third edition of the ISBER Best Practices5 or suggested in the literature in the form of recommendations.47 Instead, here we review indices for novel and evidence-based QC assays with reasonable biospecimen molecular diagnostic potential.
Most of the publications reviewed were observational studies, in that they observed biospecimen behavior relative to differences in processing or characterization methods. We believe that fundamental biospecimen research is also needed to explain the underlying mechanisms (eg, protease enzymatic activities underlying protein concentration changes, activation of cellular pathways underlying gene expression changes, oxidation and single-strand breaks underlying DNA degradation, or methylation-chromatin conformation underlying stem cell pluripotency status). This kind of research could lead to better solutions for stabilizing biospecimens.
The main goal of the present literature review was to identify markers and assays for the evaluation of quality of specimens. However, another approach in biospecimen science is to increase the range of molecular techniques that can be applied to specimens, circumventing their inherent challenges, particularly those presented by archival FFPE tissues. For example, antigen retrieval for FFPE material has not only improved immunohistochemical and proteomics analysis of tissues48, 49, 50, 51), but has also led to further development of DNA and RNA extraction from FFPE tissues, shedding light on this difficult topic of biospecimen QC.48, 52
Although some findings have been confirmed by multiple research teams, it appears that the most reliable QC tools will have to be defined and validated for each type of biospecimen. For example, multiple teams have confirmed the instability of Fos and Junb gene expression with cold ischemia in tissues,38, 53, 54 as well as the instability of ascorbic acid with blood precentrifugation delay and serum storage conditions8, 55 and of the serum complement C3 and fibrinopeptides with postcentrifugation delays.11, 32, 33
It is unlikely that a single marker can provide all of the information required regarding sample quality. For example, the most frequently used characterization tool, nucleic acid quantification, is not enough. The question arises as to what might be the lower threshold of total RNA input needed to obtain consistent high-throughput data, such as RNA arrays. Ten nanograms might be enough if pristine RNA is used from cell lines, but not necessarily if from tissue RNA, in which case the biomolecule integrity might be compromised.56 Furthermore, a single universal QC assay cannot cover all aspects of biospecimen characterization. The use of the suggested QC tools is not to certify a biospecimen as being of high or low quality, but rather to diagnose specific preanalytical conditions (eg, a serum sample having undergone more than 24 hours at room temperature conditions). Obviously, the significance of such conditions differs, depending on the specific downstream analyte, with different analytes having different robustness to the same conditions. Therefore, a panel of carefully selected QC markers will be needed to comprehensively assess biospecimen quality, and specific knowledge will be required on the influence of specific collection and/or processing conditions on each downstream assay.
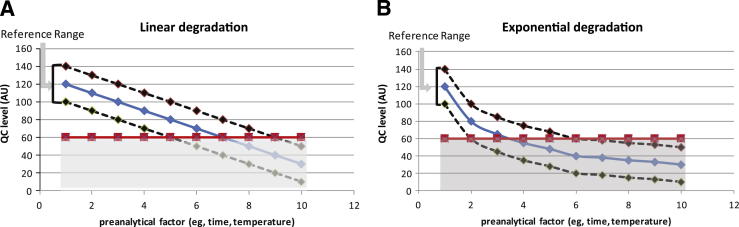
Another challenge is the lack of population reference ranges for the majority of research biomarkers. The usefulness of a surrogate quality biomarker can be assessed only if we know the corresponding population reference ranges. We present a model of surrogate QC markers with either linear or exponential degradation rate on specific preanalytical conditions (Figure 3). Knowledge of the baseline reference ranges in the population of interest is necessary to be able to define the lower threshold value of the analyte, below which we can be certain that the biospecimen underwent conditions more stressful than those of the corresponding intercept. With linear degradation (Figure 3A), biospecimens that show <60 arbitrary units (AU) of analyte (minus the analytical uncertainty of the method) have undergone >5 preanalytical factor stress units. Similarly, with exponential degradation (Figure 3B), biospecimens that show <60 AU of analyte (minus the analytical uncertainty of the method) have undergone >2 preanalytical factor stress units. Diagnostic performance depends on the degradation kinetics. It is obvious, with the degradation kinetics shown in Figure 3, that a biospecimen molecular diagnostic tool with linear degradation will be more specific, whereas one with exponential degradation will be more sensitive.
Figure 3.
Models of QC tools. Baseline reference ranges are indicated as mean values in the population (blue diamonds) and as upper (red diamonds) and lower (green diamonds) reference values. The (shaded area) red squares corresponds to an arbitrary threshold of the QC marker below which diagnostic conclusions as to the upstream processing of the sample can be drawn. A: Linear degradation of the QC marker. B: Exponential degradation of the QC marker. AU, arbitrary units.
To define QC thresholds, one must know the reference ranges in both healthy and diseased subjects4 and also take into account the analytical imprecision. Only then can a marker cutoff be defined, beyond which it is certain that a biospecimen has undergone a minimum amount of a specific preanalytical stress independent of the initial level of the marker used. For high-throughput studies in which thousands of data points are generated on a single sample, a traditional absolute value for the reference is not available.
Development of novel QC tools through spiking approaches can overcome difficulties linked to very low levels of the native analytes in the biospecimen. In this case, contents of the primary collection tubes can be spiked with a recombinant antigen or synthetic molecule, if it has been demonstrated that this molecule undergoes degradation once in the native biospecimen environment. The spiking approach could be applicable to QC tool development based on observations of pathogen nucleic acid instability in biospecimens, such as instability of hepatitis C viral RNA in serum.57 The spiking approach would require that a critical amount of the spiking molecule is included up front in the collection tubes by the manufacturers. However, such an approach would prevent downstream applications targeting these pathogen spiking molecules.
For high-throughput studies, few reports identified markers with predictive value for downstream analysis. Most studies assessed the quality of the results after the downstream assay had been performed; for example, quality parameters such as noise, background, 3′:5′ ratio, or percentage present calls might be assessed for a 54,000-gene array. However, a method to predict the success of the downstream analysis is important, not only because high-throughput approaches are usually expensive, but also because precious clinical specimens might be the limiting factor, especially for research.
For predictive markers, our literature review revealed that, although authors may recommend some particular number or threshold, such as using RNA with certain parameters, this recommendation is usually not based on proven evidence.
In some cases, animal models can allow identification of QC tools applicable to human biospecimens. For example, postmortem salmon RNA stability has been shown to be tissue-type dependent,58 and mouse phosphorylated protein status has been shown to be greatly influenced by the postmortem interval.59 A QC marker identified in an animal model may be applied to human biospecimens if the corresponding molecule or pathway is expressed in both.
We derived potential QC tools from publications that were not intended to identify quality-assurance and quality-control (QA/QC) markers. Most studies had a different aim, such as the development of a technique. In most of these cases, the data need to be validated on a larger scale. Only through large-scale international validation exercises will we be able to reach a consensus on the most reliable QC markers, introduce them into proficiency testing programs60 and into biobank accreditation schemes, and establish reference ranges, and so ultimately incorporate them into future reporting recommendations.61
To standardize the assays to be used to assess the molecular integrity of biospecimens, candidate QC tools such as those identified in the present review should be validated by large biospecimen research project consortia. They should also be cross-checked against previously established upstream QC tools, such as the RIN (method comparison), and against downstream QC metrics, such as microarray metrics (method performance). Critical windows of preanalytical variations and biospecimen quality ranges, such as would be acceptable for specific applications, could ultimately be identified by large biospecimen research consortia studying different applications, including high-throughput sequencing, multiplex PCR, epigenetics, gene expression, microRNA, and protein arrays.
Footnotes
Disclosure: F.B. was previously employed by the Picardie Biobank, which holds a French patent on the use of sCD40L as a quality-control marker in serum, based on her previous work.
The 2011 Biospecimen Science Working Group consisted of Fotini (Fay) Betsou (Chair), Garry Ashton, Michael Barnes, Erica E. Benson, Rodrigo Chuaqui, Judith Clements, Domenico Coppola, Yvonee De Souza, Annemieke De Wilde, James F. Eliason, Barbara Glazaer, Katrina Goddard, Fiorella Guadagni, Elaine Gunter, Keith Harding, Jae-Pil Jeon, Olga Kofanova, Conny Mathay, Rolf Muller, Francesca Poloni, Katheryn E. Shea, Amy P.N. Skubitz, Mark E. Sobel, Stella Somiari, and Gunnel Tybring.
References
- 1.Moore H.M., Compton C.C., Lim M.D., Vaught J., Christiansen K.N., Alper J. 2009 Biospecimen Research Network Symposium: advancing cancer research through biospecimen science. Cancer Res. 2009;69:6770–6772. doi: 10.1158/0008-5472.CAN-09-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore H.M., Kelly A., Jewell S.D., McShane L.M., Clark D.P., Greenspan R., Hainaut P., Hayes D.F., Kim P., Mansfield E., Potapova O., Riegman P., Rubinstein Y., Seijo E., Somiari S., Watson P., Weier H.U., Zhu C., Vaught J. Biospecimen reporting for improved study quality. Biopreserv Biobank. 2011;9:57–70. doi: 10.1089/bio.2010.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betsou F., Lehmann S., Ashton G., Barnes M., Benson E.E., Coppola D., DeSouza Y., Eliason J., Glazer B., Guadagni F., Harding K., Horsfall D.J., Kleeberger C., Nanni U., Prasad A., Shea K., Skubitz A., Somiari S., Gunter E., International Society for Biological and Environmental Repositories (ISBER) Working Group on Biospecimen Science Standard preanalytical coding for biospecimens: defining the sample PREanalytical code. Cancer Epidemiol Biomarkers Prev. 2010;19:1004–1011. doi: 10.1158/1055-9965.EPI-09-1268. [DOI] [PubMed] [Google Scholar]
- 4.Betsou F., Barnes R., Burke T., Coppola D., Desouza Y., Eliason J., Glazer B., Horsfall D., Kleeberger C., Lehmann S., Prasad A., Skubitz A., Somiari S., Gunter E., International Society for Biological and Environmental Repositories (ISBER) Working Group on Biospecimen Science Human biospecimen research: experimental protocol and quality control tools. Cancer Epidemiol Biomarkers Prev. 2009;18:1017–1025. doi: 10.1158/1055-9965.EPI-08-1231. [DOI] [PubMed] [Google Scholar]
- 5.ISBER: 2012 best practices for repositories: collection, storage, retrieval, and distribution of biological materials for research. 3rd edition. Biopreserv Biobank. 2012;10:79–161. doi: 10.1089/bio.2012.1022. [DOI] [PubMed] [Google Scholar]
- 6.Opitz L., Salinas-Riester G., Grade M., Jung K., Jo P., Emons G., Ghadimi B.M., Beissbarth T., Gaedcke J. Impact of RNA degradation on gene expression profiling. BMC Med Genomics. 2010;3:36. doi: 10.1186/1755-8794-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Jongh R., Vranken J., Vundelinckx G., Bosmans E., Maes M., Heylen R. The effects of anticoagulation and processing on assays of IL-6, sIL-6R, sIL-2R and soluble transferrin receptor. Cytokine. 1997;9:696–701. doi: 10.1006/cyto.1997.0217. [DOI] [PubMed] [Google Scholar]
- 8.Karlsen A., Blomhoff R., Gundersen T.E. Stability of whole blood and plasma ascorbic acid. Eur J Clin Nutr. 2007;61:1233–1236. doi: 10.1038/sj.ejcn.1602655. [DOI] [PubMed] [Google Scholar]
- 9.Heins M., Heil W., Withold W. Storage of serum or whole blood samples? Effects of time and temperature on 22 serum analytes. Eur J Clin Chem Clin Biochem. 1995;33:231–238. doi: 10.1515/cclm.1995.33.4.231. [DOI] [PubMed] [Google Scholar]
- 10.Ayache S., Panelli M., Marincola F.M., Stroncek D.F. Effects of storage time and exogenous protease inhibitors on plasma protein levels. Am J Clin Pathol. 2006;126:174–184. doi: 10.1309/3WM7-XJ7R-D8BC-LNKX. [DOI] [PubMed] [Google Scholar]
- 11.Marshall J., Kupchak P., Zhu W., Yantha J., Vrees T., Furesz S., Jacks K., Smith C., Kireeva I., Zhang R., Takahashi M., Stanton E., Jackowski G. Processing of serum proteins underlies the mass spectral fingerprinting of myocardial infarction. J Proteome Res. 2003;2:361–372. doi: 10.1021/pr030003l. [DOI] [PubMed] [Google Scholar]
- 12.Evans M.J., Livesey J.H., Ellis M.J., Yandle T.G. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem. 2001;34:107–112. doi: 10.1016/s0009-9120(01)00196-5. [DOI] [PubMed] [Google Scholar]
- 13.Lengellé J., Panopoulos E., Betsou F. Soluble CD40 ligand as a biomarker for storage-related preanalytic variations of human serum. Cytokine. 2008;44:275–282. doi: 10.1016/j.cyto.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Ockè M.C., Schrijver J., Obermann-de Boer G.L., Bloemberg B.P., Haenen G.R., Kromhout D. Stability of blood (pro)vitamins during four years of storage at −20° C: consequences for epidemiologic research. J Clin Epidemiol. 1995;48:1077–1085. doi: 10.1016/0895-4356(94)00232-f. [DOI] [PubMed] [Google Scholar]
- 15.Rouy D., Ernens I., Jeanty C., Wagner D.R. Plasma storage at −80° C does not protect matrix metalloproteinase-9 from degradation. Anal Biochem. 2005;338:294–298. doi: 10.1016/j.ab.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Kisand K., Kerna I., Kumm J., Jonsson H., Tamm A. Impact of cryopreservation on serum concentration of matrix metalloproteinases (MMP)-7, TIMP-1, vascular growth factors (VEGF) and VEFG-R2 in Biobank samples. Clin Chem Lab Med. 2011;49:229–235. doi: 10.1515/CCLM.2011.049. [DOI] [PubMed] [Google Scholar]
- 17.De Jager W., Bourcier K., Rijkers G.T., Prakken B.J., Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaigneau C., Cabioch T., Beaumont K., Betsou F. Serum biobank certification and the establishment of quality controls for biological fluids: examples of serum biomarker stability after temperature variation. Clin Chem Lab Med. 2007;45:1390–1395. doi: 10.1515/CCLM.2007.160. [DOI] [PubMed] [Google Scholar]
- 19.Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Moser K., Ortmann W.A., Espe K.J., Balasubramanian S., Hughes K.M., Chan J.P., Begovich A., Chang S.Y., Gregersen P.K., Behrens T.W. Expression levels for many genes in human peripheral blood cells are highly sensitive to ex vivo incubation. Genes Immun. 2004;5:347–353. doi: 10.1038/sj.gene.6364098. [DOI] [PubMed] [Google Scholar]
- 20.Barnes M.G., Grom A.A., Griffin T.A., Colbert R.A., Thompson S.D. Gene expression profiles from peripheral blood mononuclear cells are sensitive to short processing delays. Biopreserv Biobank. 2010;8:153–162. doi: 10.1089/bio.2010.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banks R.E. Measurement of cytokines in clinical samples using immunoassays: problems and pitfalls. Crit Rev Clin Lab Sci. 2000;37:131–182. doi: 10.1080/10408360091174187. [DOI] [PubMed] [Google Scholar]
- 22.Boomsma F., Alberts G., van Eijk L., Man in ’t Veld A.J., Schalekamp M.A. Optimal collection and storage conditions for catecholamine measurements in human plasma and urine. Clin Chem. 1993;39:2503–2508. [PubMed] [Google Scholar]
- 23.Tencer J., Thysell H., Andersson K., Grubb A. Stability of albumin, protein HC, immunoglobulin G, kappa- and lambda-chain immunoreactivity, orosomucoid and alpha 1-antitrypsin in urine stored at various conditions. Scand J Clin Lab Invest. 1994;54:199–206. doi: 10.1080/00365519409088425. [DOI] [PubMed] [Google Scholar]
- 24.Carrette O., Burkhard P.R., Hughes S., Hochstrasser D.F., Sanchez J.C. Truncated cystatin C in cerebrospinal fluid: technical artefact or biological process? Proteomics. 2005;5:3060–3065. doi: 10.1002/pmic.200402039. [DOI] [PubMed] [Google Scholar]
- 25.Owen R.E., Sinclair E., Emu B., Heitman J.W., Hirschkorn D.F., Epling C.L., Tan Q.X., Custer B., Harris J.M., Jacobson M.A., McCune J.M., Martin J.N., Hecht F.M., Deeks S.G., Norris P.J. Loss of T cell responses following long-term cryopreservation. J Immunol Methods. 2007;326:93–115. doi: 10.1016/j.jim.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin D.W., Coleman I.M., Hawley S., Huang C.Y., Dumpit R., Gifford D., Kezele P., Hung H., Knudsen B.S., Kristal A.R., Nelson P.S. Influence of surgical manipulation on prostate gene expression: implications for molecular correlates of treatment effects and disease prognosis. J Clin Oncol. 2006;24:3763–3770. doi: 10.1200/JCO.2005.05.1458. [DOI] [PubMed] [Google Scholar]
- 27.Thompson K.L., Pine P.S., Rosenzweig B.A., Turpaz Y., Retief J. Characterization of the effect of sample quality on high density oligonucleotide microarray data using progressively degraded rat liver RNA. BMC Biotechnol. 2007;7:57. doi: 10.1186/1472-6750-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Cecco L., Musella V., Veneroni S., Cappelletti V., Bongarzone I., Callari M., Valeri B., Pierotti M.A., Daidone M.G. Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer. 2009;9:409. doi: 10.1186/1471-2407-9-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F., Wang L., Briggs C., Sicinska E., Gaston S.M., Mamon H., Kulke M.H., Zamponi R., Loda M., Maher E., Ogino S., Fuchs C.S., Li J., Hader C., Makrigiorgos G.M. DNA degradation test predicts success in whole-genome amplification from diverse clinical samples. J Mol Diagn. 2007;9:441–451. doi: 10.2353/jmoldx.2007.070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee W., Roberts S.M., Labbe R.F. Ascorbic acid determination with an automated enzymatic procedure. Clin Chem. 1997;43:154–157. [PubMed] [Google Scholar]
- 31.Cembrowski G.S., Blakney G.B., Higgins T.N., Revers C.W., Podlosky K.L., Hofer T.L. Reference intervals for plasma potassium drawn into Becton Dickinson PST Plus plastic vacutainer tubes for the Vitros 950 and Advia 1650 (regular centrifugation) and the Beckman LX-20 (Beckman Power Processor Preanalytics centrifugation) (abstract). Presented at the American Association for Clinical Chemistry 2004 annual meeting, July 27-29, 2004. Clin Chem. 2004;50 A108 (abstract D-6) [Google Scholar]
- 32.Yi J., Kim C., Gelfand C.A. Inhibition of intrinsic proteolytic activities moderates preanalytical variability and instability of human plasma. J Proteome Res. 2007;6:1768–1781. doi: 10.1021/pr060550h. [DOI] [PubMed] [Google Scholar]
- 33.West-Nørager M., Kelstrup C.D., Schou C., Høgdall E.V., Høgdall C.K., Heegaard N.H. Unravelling in vitro variables of major importance for the outcome of mass spectrometry-based serum proteomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847:30–37. doi: 10.1016/j.jchromb.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 34.Azimi-Nezhad M., Lambert D., Ottone C., Perrin C., Chapel C., Gaillard G., Pfister M., Masson C., Tabone E., Betsou F., Meyronet D., Ungeheur M.N., Visvikis-Siest S. Influence of preanalytical variables on VEGFA gene expression and circulating protein concentrations. Biopreserv Biobank. 2012;10:454–461. doi: 10.1089/bio.2012.0016. [DOI] [PubMed] [Google Scholar]
- 35.De Reijke T.M., de Boer E.C., Kurth K.H., Schamhart D.H. Urinary cytokines during intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: processing, stability and prognostic value. J Urol. 1996;155:477–482. [PubMed] [Google Scholar]
- 36.Foss R.D., Guha-Thakurta N., Conran R.M., Gutman P. Effects of fixative and fixation time on the extraction and polymerase chain reaction amplification of RNA from paraffin-embedded tissue. Comparison of two housekeeping gene mRNA controls. Diagn Mol Pathol. 1994;3:148–155. doi: 10.1097/00019606-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Almeida A., Paul Thiery J., Magdelénat H., Radvanyi F. Gene expression analysis by real-time reverse transcription polymerase chain reaction: influence of tissue handling. Anal Biochem. 2004;328:101–108. doi: 10.1016/j.ab.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Jackson D., Rowlinson R.A., Eaton C.K., Nickson J.A., Wilson I.D., Mills J.D., Wilkinson R.W., Tonge R.P. Prostatic tissue protein alterations due to delayed time to freezing. Proteomics. 2006;6:3901–3908. doi: 10.1002/pmic.200500794. [DOI] [PubMed] [Google Scholar]
- 39.Barker D.L., Hansen M.S., Faruqi A.F., Giannola D., Irsula O.R., Lasken R.S., Latterich M., Makarov V., Oliphant A., Pinter J.H., Shen R., Sleptsova I., Ziehler W., Lai E. Two methods of whole-genome amplification enable accurate genotyping across a 2320-SNP linkage panel. Genome Res. 2004;14:901–907. doi: 10.1101/gr.1949704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aaltonen K.E., Ebbesson A., Wigerup C., Hedenfalk I. Laser capture microdissection (LCM) and whole genome amplification (WGA) of DNA from normal breast tissue–optimization for genome wide array analyses. BMC Res Notes. 2011;4:69. doi: 10.1186/1756-0500-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergen A.W., Haque K.A., Qi Y., Beerman M.B., Garcia-Closas M., Rothman N., Chanock S.J. Comparison of yield and genotyping performance of multiple displacement amplification and OmniPlex whole genome amplified DNA generated from multiple DNA sources. Hum Mutat. 2005;26:262–270. doi: 10.1002/humu.20213. [DOI] [PubMed] [Google Scholar]
- 42.Panelli S., Damiani G., Espen L., Micheli G., Sgaramella V. Towards the analysis of the genomes of single cells: further characterisation of the multiple displacement amplification. Gene. 2006;372:1–7. doi: 10.1016/j.gene.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Player A., Wang Y., Rao M., Kawasaki E. Gene expression analysis of RNA purified from embryonic stem cells and embryoid body-derived cells using a high-throughput microarray platform. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01b02s2. Chapter 1:Unit 1B.2. [DOI] [PubMed] [Google Scholar]
- 44.Roberts L., Bowers J., Sensinger K., Lisowski A., Getts R., Anderson M.G. Identification of methods for use of formalin-fixed, paraffin-embedded tissue samples in RNA expression profiling. Genomics. 2009;94:341–348. doi: 10.1016/j.ygeno.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Espina V., Edmiston K.H., Heiby M., Pierobon M., Sciro M., Merritt B., Banks S., Deng J., VanMeter A.J., Geho D.H., Pastore L., Sennesh J., Petricoin E.F., 3rd, Liotta L.A. A portrait of tissue phosphoprotein stability in the clinical tissue procurement process. Mol Cell Proteomics. 2008;7:1998–2018. doi: 10.1074/mcp.M700596-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kingsbury A.E., Foster O.J., Nisbet A.P., Cairns N., Bray L., Eve D.J., Lees A.J., Marsden C.D. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28:311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- 47.Jewell S.D., Srinivasan M., McCart L.M., Williams N., Grizzle W.H., LiVolsi V., MacLennan G., Sedmak D.D. Analysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue Network. Am J Clin Pathol. 2002;118:733–741. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 48.Shi S.R., Shi Y., Taylor C.R. Antigen retrieval immunohistochemistry: review and future prospects in research and diagnosis over two decades. J Histochem Cytochem. 2011;59:13–32. doi: 10.1369/jhc.2010.957191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fowler C.B., Cunningham R.E., O’Leary T.J., Mason J.T. ‘Tissue surrogates’ as a model for archival formalin-fixed paraffin-embedded tissues. Lab Invest. 2007;87:836–846. doi: 10.1038/labinvest.3700596. [DOI] [PubMed] [Google Scholar]
- 50.Fowler C.B., Cunningham R.E., Waybright T.J., Blonder J., Veenstra T.D., O’Leary T.J., Mason J.T. Elevated hydrostatic pressure promotes protein recovery from formalin-fixed, paraffin-embedded tissue surrogates. Lab Invest. 2008;88:185–195. doi: 10.1038/labinvest.3700708. [DOI] [PubMed] [Google Scholar]
- 51.Fowler C.B., Evers D.L., O’Leary T.J., Mason J.T. Antigen retrieval causes protein unfolding: evidence for a linear epitope model of recovered immunoreactivity. J Histochem Cytochem. 2011;59:366–381. doi: 10.1369/0022155411400866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evers D.L., Fowler C.B., Cunningham B.R., Mason J.T., O’Leary T.J. The effect of formaldehyde fixation on RNA: optimization of formaldehyde adduct removal. J Mol Diagn. 2011;13:282–288. doi: 10.1016/j.jmoldx.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perou C.M., Sørlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., Fluge O., Pergamenschikov A., Williams C., Zhu S.X., Lønning P.E., Børresen-Dale A.L., Brown P.O., Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 54.Soriano M.A., Tessier M., Certa U., Gill R. Parallel gene expression monitoring using oligonucleotide probe arrays of multiple transcripts with an animal model of focal ischemia. J Cereb Blood Flow Metab. 2000;20:1045–1055. doi: 10.1097/00004647-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Chung W.Y., Chung J.K., Szeto Y.T., Tomlinson B., Benzie I.F. Plasma ascorbic acid: measurement, stability and clinical utility revisited. Clin Biochem. 2001;34:623–627. doi: 10.1016/s0009-9120(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez A.S., Espina B.H., Espina V., Liotta L.A. Automated laser capture microdissection for tissue proteomics. Methods Mol Biol. 2008;441:71–90. doi: 10.1007/978-1-60327-047-2_5. [DOI] [PubMed] [Google Scholar]
- 57.Halfon P., Khiri H., Gerolami V., Bourliere M., Feryn J.M., Reynier P., Gauthier A., Cartouzou G. Impact of various handling and storage conditions on quantitative detection of hepatitis C virus RNA. J Hepatol. 1996;25:307–311. doi: 10.1016/s0168-8278(96)80116-4. [DOI] [PubMed] [Google Scholar]
- 58.Seear P.J., Sweeney G.E. Stability of RNA isolated from post-mortem tissues of Atlantic salmon (Salmo salar L.) Fish Physiol Biochem. 2008;34:19–24. doi: 10.1007/s10695-007-9141-x. [DOI] [PubMed] [Google Scholar]
- 59.Li J., Gould T.D., Yuan P., Manji H.K., Chen G. Post-mortem interval effects on the phosphorylation of signaling proteins [Erratum appeared in Neuropsychopharmacology 2003, 28:1219] Neuropsychopharmacology. 2003;28:1017–1025. doi: 10.1038/sj.npp.1300112. [DOI] [PubMed] [Google Scholar]
- 60.Poloni F., Ashton G., Coppola D., De Souza Y., De Wilde A., Douglas J., Eliason J., Guadagni F., Gunter E., Kofanova O., Lehmann S., Mathay C., Shea K., Sobel M., Tybring G., Zink M., Betsou F., ISBER Biospecimen Science Working Group Biorepository proficiency testing for the quality control of biospecimens for the global biobanking community. Biopreserv Biobank. 2011;9:415–417. doi: 10.1089/bio.2011.9402. [DOI] [PubMed] [Google Scholar]
- 61.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics: Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]