Abstract
The ordering of molecular genetic tests by health providers not well trained in genetics may have a variety of untoward effects. These include the selection of inappropriate tests, the ordering of panels when the assessment of individual or fewer genes would be more appropriate, inaccurate result interpretation and inappropriate patient guidance, and significant unwarranted cost expenditure. We sought to improve the utilization of molecular genetic tests by requiring providers without specialty training in genetics to use genetic counselors and molecular genetic pathologists to assist in test selection. We used a genetic and genomic test review process wherein the laboratory-based genetic counselor performed the preanalytic assessment of test orders and test triage. Test indication and clinical findings were evaluated against the test panel composition, methods, and test limitations under the supervision of the molecular genetic pathologist. These test utilization management efforts resulted in a decrease in genetic test ordering and a gross cost savings of $1,531,913 since the inception of these programs in September 2011 through December 2013. The combination of limiting the availability of complex genetic tests and providing guidance regarding appropriate test strategies is an effective way to improve genetic tests, contributing to judicious use of limited health care resources.
CME Accreditation Statement: This activity (“JMD 2015 CME Program in Molecular Diagnostics”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“JMD 2015 CME Program in Molecular Diagnostics”) for a maximum of 36 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Genetic and genomic testing is clinically available for >4000 genetic conditions, a number that has tripled in the past decade (GeneTests, https://www.genetests.org/disorders, last accessed September 12, 2013). This category of tests, although fairly low volume relative to other laboratory tests, contributes substantial cost to laboratory medicine in our institution, in part because of the increasing availability and complexity of molecular test options. A study of United Healthcare members found that spending on molecular genetic tests increased 14% per year between 2008 and 2010.1 Given the rarity of most genetic disorders and the growing array of testing options, it is perhaps not surprising that 8% to 30% of genetic tests are ordered incorrectly.2, 3 Indeed, many physicians report feeling unprepared to order genetic testing or perform clinical tasks related to genetics because of lack of knowledge, confidence, and experience with genetic disorders.4, 5, 6 The impact of these factors on patient care is difficult to quantify but almost certainly contributes to delayed time to diagnosis and an increase in the risk of erroneous result interpretation. Given the desire to provide appropriate testing coupled with the need to address the rapidly increasing cost of molecular genetic testing, our institution recognized an opportunity to optimize genetic test utilization among our clinicians.
Materials and Methods
Two initiatives were undertaken to improve molecular genetic test utilization at our institution.
Initiative I: Clinical Decision Support Tools
We limited the electronic ordering of molecular genetic tests. This process also required a clinical genetics consultation for any inpatient testing. This initiative, launched in November 2011, was piloted with select genetic tests that represented the highest annual cost to our institution.
Two types of electronic clinical decision support tools (CDSTs) were generated to function in the computerized provider order entry system within our electronic medical record system (Epic Systems, Verona, WI). The first CDST restricted users from filing all inpatient orders for the selected tests and required a consultation with a clinical geneticist for tests that could not reasonably be deferred to an ambulatory setting. This was designed to decrease unnecessary inpatient testing, while still preserving an avenue for testing if it was considered to be absolutely necessary. The second CDST limited outpatient genetic test orders to a self-selected group of clinicians who reported routine use of genetic testing in their clinical practice; this group was designated deemed users. All other users were prevented from filing orders for these tests and encouraged to obtain consultation with clinical genetics. After a successful pilot phase, this initiative was expanded in February 2012 to include approximately 40 complex genetic and genomic tests; additional tests were added as they became available in our system.
We reviewed the number of test orders that were prevented by the CDST and cost savings achieved by not performing these tests. There was potential revenue lost in the outpatient setting on the basis of this initiative, but the amount is not known because the reimbursement for these tests is variable based on decisions made by the individual providers (ie, some claims may have been denied, some fully reimbursed, and some partially reimbursed).
Initiative II: Genetic and Genomic Test Review and Guidance
We used a genetic counselor (J.D.R.) in daily order review and guidance for genetic and genomic testing. Although the CDST initiative targeted high-cost, high-complexity genetic tests, the engagement of a genetic counselor implemented a daily review of all genetic and genomic test orders, including those originating with the deemed users. Working with our Center for Pathology Informatics, daily pending logs were generated to capture all defined genetic and genomic test orders, as well as all miscellaneous test orders, a significant percentage of which were esoteric genetic and genomic tests.
The daily genetic and genomic test review (GGTR) began as a manual process in September 2011, with a more comprehensive and consistent review process implemented in August 2012 using electronic pending logs. The GGTR process involved the identification of molecular test orders from the daily pending list generated from the laboratory information system by the laboratory genetic counselor. The test indication and clinical findings were evaluated against the test requested, to assess whether the test composition (eg, genes or mutations included), method (eg, technology or platform used in genotyping and deletion/duplication analysis), and limitations (eg, targeted genotyping, depth of coverage of next-generation sequencing, or resolution of chromosomal microarray coverage) would appropriately address the clinical diagnostic question. Customized communication with ordering clinicians commenced on completion of the case review. Criteria for further inquiry included the following: requests for testing of multiple genes or gene panels, discordance between physician specialty and type of test, discordance between the clinical diagnosis and test ordered, and cost >$1000. The patient electronic medical record was accessed to review the ordering physician's notes and any previous genetic testing. Cases requiring in-depth medical review were elevated to the molecular genetic pathologist (F.L.L.), who is also certified by the American Board of Medical Genetics in clinical genetics. On the basis of these reviews, orders either proceeded unchanged or a member of the GGTR team contacted the ordering clinician to elicit additional information and suggest alternative testing strategies as appropriate for patient care or cost efficiency. Tests were approved, canceled, or modified (eg, from multigene panel to reflexed testing strategy), depending on the collaborative decision with the clinician. The numbers of interactions, GGTR outcomes, and cost savings associated with these interventions were recorded and monitored monthly.
Results
Initiative I
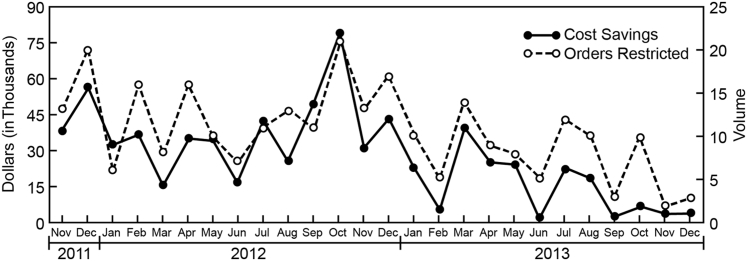
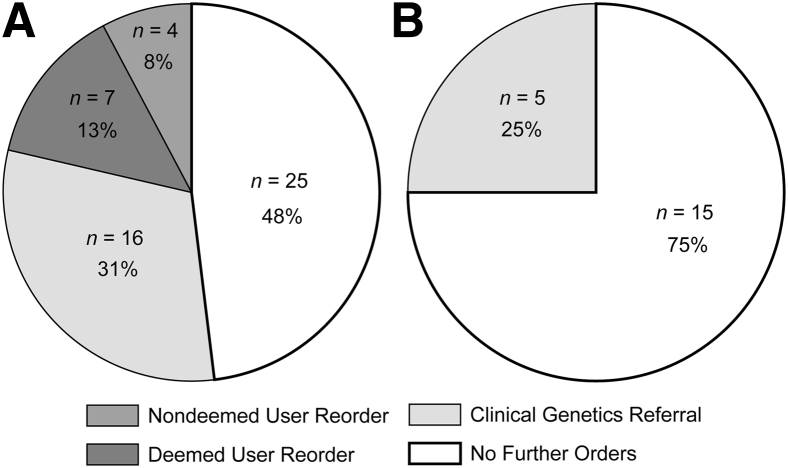
The CDST initiative restricted 273 molecular genetic test orders from its launch in November 2011 through December 2013 (ie, 26 months) . On the basis of the institutional cost of these tests, the gross cost avoidance achieved was $711,026 (Figure 1). There was an average cost avoidance of $27,347 per month because of this intervention. The hospital information system enabled tracking of attempted orders for the selected tests in both inpatient and ambulatory settings. There were no significant changes in the patient or physician census during the time period included in this study. Representative cross-sectional data from July to December 2012 demonstrate the outcomes (Figure 2). In both settings, the most likely response to encountering a CDST was to abandon the pursuit of genetic testing altogether (in 48% of ambulatory cases and 75% of inpatient cases). For those cases, we could not totally exclude the possibility that limited access to genetic testing prompted pursuit of additional evaluations that contributed to the overall cost of patient care. In >25% of cases, clinicians placed an order for a clinical genetics consultation after encountering a CDST. In both settings, the least likely response to the CDST was to avert the system and find a way to place the order without a genetics consultation or reorder by a deemed user in the ambulatory setting.
Figure 1.
Volume reductions and cost savings associated with clinical decision support tools, calculated from a monthly report of restricted test order attempts and their associated institutional costs.
Figure 2.
Impact of clinical decision support tools (CDST) initiative (July to December 2012). A: Ambulatory test orders (n = 52). B: Inpatient test orders (n = 20). Nondeemed user reorder indicates that the user ordered the test (usually as a miscellaneous order, for which a CDST cannot be used) without using the recommended strategies of either referring the patient to clinical genetics or consulting with another deemed user who could place the order. No further orders represent cases where no additional attempts to order genetic testing and no referrals to clinical genetics were identified during that episode of care.
Initiative II
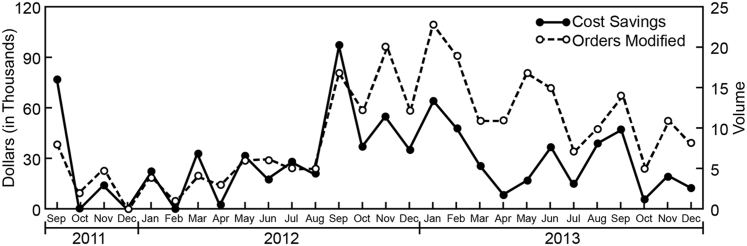
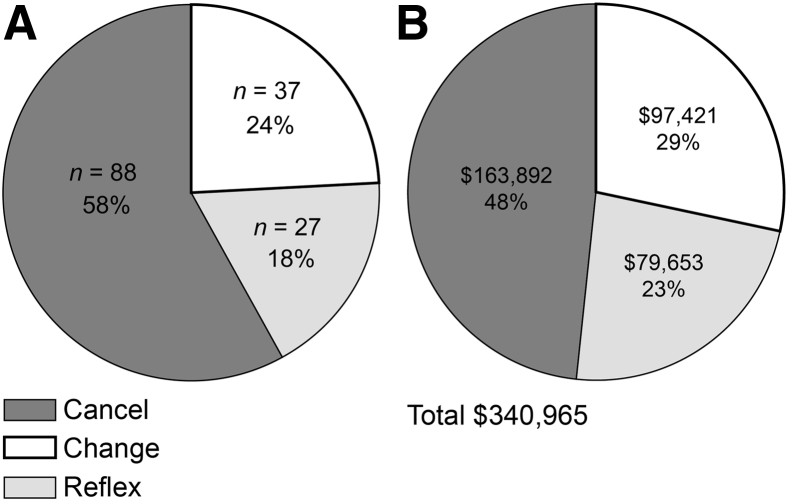
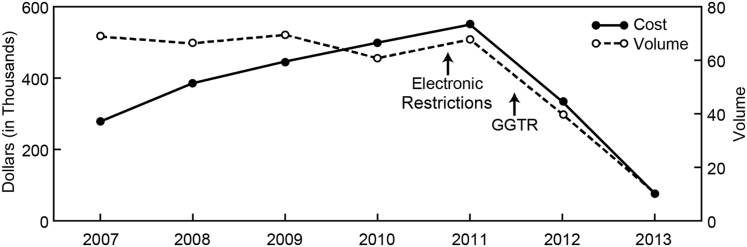
Through the GGTR initiative, interactions between the genetic counselor and molecular genetic pathologist with the ordering clinicians resulted in the modification of 261 orders from September 2011 through December 2013 (ie, 28 months). This resulted in a gross cost avoidance of $820,887 (Figure 3). There was an average cost avoidance of $29,317 per month because of this intervention. A notable increase in the number of GGTR interventions was seen in September 2012, corresponding to the implementation of an electronic pending log, allowing for a more comprehensive review. With use of the electronic log for the full year, 152 GGTR cases were handled in 2013 (Figure 4A). Of these cases, 58% were canceled, 18% were specifically changed to a reflexed test strategy, and 24% were changed in some other way (eg, test method or reference laboratory choice). In reflexed cases, after discussion with the ordering provider, testing proceeded stepwise through the genes of interest or an agreed-on test algorithm until a genetic etiology was identified or all testing was completed. The total cost savings in 2013 was $340,965 (Figure 4B), with a mean cost savings of $2243 per case. A review of the ordering patterns from 2007 through 2011, before implementation of test utilization management (UM) initiatives, demonstrated that the volume of the five most costly gene sequencing panels remained fairly steady, averaging 67 orders per year (Figure 5). The cost of testing, however, increased at an average rate of 19.25% per year. In 2012, after a full year of CDST and five months of daily GGTR with electronic pending logs, the volume and costs of these tests decreased by approximately 40% each. In 2013, additional volume and cost reductions of >60% were realized. The aggregate gross cost savings from all institutional test UM initiatives from their inception to December 2013 was >$1.96 million. The cost savings for the CDST and GGTR initiatives, respectively, represent 36.2% and 41.8% of the total savings. Most important, these UM initiatives provided evidence of improved utilization of genetic and genomic testing, in support of good patient care. Case vignettes that support this model as an improvement over the previously unregulated system are provided in Table 1.
Figure 3.
Orders modified and cost savings associated with genetic and genomic test review, September 2011 to December 2013, on the basis of institutional cost of canceled tests or the difference in cost between the original and the modified test order. The dramatic increase in both orders modified and cost savings from August 2012 coincides with the implementation of a more comprehensive and consistent review process using daily pending logs.
Figure 4.
Outcome of 2013 genetic and genomic test review cases (n = 152). A: Type of order alteration. B: Associated cost savings. Cancel represents cases where no genetic testing was ordered. Change includes modifications in gene tested, method, or testing laboratory. Reflex specifically represents a change from multiple concurrent tests or a multigene panel to a strategy of reflexed testing.
Figure 5.
Impact of genetic test utilization management initiatives on multigene test panel orders from 2007 to 2013. GGTR, genetic and genomic test review.
Table 1.
Case Vignettes from GGTR
| GGTR-identified test issue | Test order | Indication | Physician specialty | Action |
|---|---|---|---|---|
| Duplicate order | GAA sequencing, laboratory A | Low GAA enzyme activity; suspected Pompe disease | Genetics | Discuss laboratory choice with both physicians and cancel one order |
| GAA sequencing, laboratory B | Genetics | |||
| Unrelated diagnosis | Hereditary neuropathy with liability to pressure palsies evaluation | Family history of hereditary nonpolyposis colon cancer | Internal medicine | Cancel order after discussion with clinician and recommend referral to genetic counselling |
| Single-gene vs multigene panel testing | Complete ataxia evaluation | Tremor and ataxia | Neurology | Inpatient order restricted by CDST, inpatient genetics consultation, and approve single-gene testing |
| Previous diagnostic result | Comprehensive mtDNA analysis and NGS | Stroke, hemiplegia, tremor, and ptosis | Pediatric neurology | Review reveals previous mtDNA mutation identified and inpatient order canceled after discussion with clinician |
| Appropriate test method | DMD sequencing | Muscle weakness in 2-year-old boy | Pediatric neurology | Discuss method with ordering physician and change order to DMD deletion/duplication analysis |
CDST, clinical decision support tool; GGTR, genetic and genomic test review; mtDNA, mitochondrial DNA; NGS, next-generation sequencing.
In one example, GGTR revealed an order by an internal medicine physician for hereditary neuropathy with liability to pressure palsies (HNPP) evaluation. A review of the physician's medical record note indicated that the patient was interested in genetic testing on the basis of a family history of hereditary nonpolyposis colorectal cancer (HNPCC). Contact with the ordering clinician confirmed that the incorrect order had been entered. Testing was canceled, and the patient was referred for genetic counseling to review the family history because several genes are known to be associated with inherited colon cancer risk. This intervention prevented completely inappropriate testing from being performed and facilitated a referral for a genetics consultation to ensure that the appropriate testing is ordered in the future.
In another case, DMD gene sequencing was ordered for a young boy suspected to have Duchenne muscular dystrophy. A review of the patient's medical record gave no indication that previous testing for gene deletions and duplications, the most common cause of Duchenne muscular dystrophy, had been done. The medical resident who entered the order was educated, and the correct order was subsequently placed. The patient was found to have a large duplication in the gene, causative of his phenotype. Review of the order provided an opportunity to educate and avoided a delayed, or even missed, diagnosis for the patient. Incidentally, this resulted in a decreased cost for testing, saving $800.
Discussion
Traditional approaches to improving test utilization are being challenged in the current health care climate. There is an obligation to improve practice within the context of a paradigm shift in genetic and genomic test utilization and a value-based payment scheme. In our institution, there are more nongenetic clinicians ordering multigene test panels that require complex analysis and interpretation by specialized reference laboratories. In addition to test validity and utility, test cost and reimbursement are of concern in our health care institution. We investigated two UM methods to optimize the use of genetic and genomic tests for our patients.
We suggested that because certain drugs are restricted to select physician groups on the basis of their subspecialty expertise (eg, only oncologists give chemotherapy, and certain antimicrobial agents are restricted to infectious disease specialists), it would be reasonable to limit highly complex genetic and genomic tests to those physicians who routinely use them in their practices (ie, deemed users). A clinical genetics consultation was required for inpatient testing, and only deemed users could order the test in the outpatient setting. As an initial step, this CDST proved to be fruitful because there was institutional leadership support. Developing an effective CDST involves a significant initial time investment by both the test UM and information technology teams. The cost of this investment factors into the net cost savings of test UM initiatives, but such calculations were beyond the scope of this report. As a result of close partnership with information technology colleagues, the CDST was relatively easy to implement and requires little ongoing management. It is undisputed that there is substantial waste in the health care system in the United States.7 In the experience of our institution, molecular genetic tests are the most expensive laboratory tests, and significantly contribute to the overall cost of laboratory testing. The CDST initiatives have been largely accepted by clinicians in our institution, who are aware of the increasing pressure to provide high-quality evidence-based health care while reducing cost.
In addition to cost and reimbursement of tests, lack of familiarity with testing options, including validity and utility, was identified as a barrier in incorporating genetic tests in clinical practice.1 However, this barrier did not seem to prevent various medical specialties from ordering genetic and genomic tests for their patients. We introduced the GGTR process in our institution, and we realized positive outcomes in test utilization in addition to significant cost savings. Unlike the CDST, this initiative requires an ongoing daily time investment by a laboratory genetic counselor and supervising molecular genetic pathologist, the cost of which was not calculated in the current report. The GGTR intervention not only averted test duplication and inappropriate orders but assisted in the use of appropriate test methods that increased yield for mutation detection. There was an opportunity for review of molecular test results for the patient that dictated subsequent genetic testing considerations. This process facilitated preanalytic guidance and postanalytic assessment of genetic and genomic tests ordered by both genetic and nongenetic experts, generating the opportunity for learning and collaboration between the laboratory-based GGTR team and clinicians.
When coupled with access to and support from clinical and laboratory genetics and genomics teams, such test UM initiatives are welcomed by most clinicians who have neither the time nor the resources to develop a genetic testing strategy for each patient. Working together to determine a plan that is targeted to the most likely genetic etiologies can result in shorter time to diagnosis, more personalized care and management, increased patient satisfaction, and improved cost-effectiveness. Experiences at this institution demonstrate the successful integration of genetic test UM initiatives in a large academic health care system. As a result of these efforts, this institution realized an increase in appropriate genetics referrals, improved utilization of genetic testing, and significant cost savings.
Footnotes
Supported in part through a cooperative agreement with the Cleveland Clinic with funds provided in part by the Division of Laboratory Science and Standards, Office of Surveillance, Epidemiology, and Laboratory Services, CDC, under Cooperative Agreement U47CI000831 (G.W.P.).
The findings herein are those of the authors and do not necessarily represent the official views of the CDC.
Disclosures: None declared.
References
- 1.UnitedHealth Center for Health Reform and Modernization. Working Paper 7: Personalized Medicine: Trends and Prospects for the New Science of Genetic Testing and Molecular Diagnostics. Minnetonka, 2012. Available at http://www.unitedhealthgroup.com/∼/media/UHG/PDF/2012/UNH-Working-Paper-7.ashx. Last updated March 2012, accessed September 11, 2013
- 2.Miller C.E., Krautscheid P., Baldwin E.E., Tvrdik T., Openshaw A.S., Hart K., LaGrave D. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. Am J Med Genet A. 2014;9999:1–8. doi: 10.1002/ajmg.a.36453. [DOI] [PubMed] [Google Scholar]
- 3.Kotzer K.E., Riley J.D., Conta J.H., Anderson C.M., Schahl K.A., Goodenberger M.L. Genetic testing utilization and the role of the laboratory genetic counselor. Clin Chim Acta. 2014;427:193–195. doi: 10.1016/j.cca.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Nippert I., Harris H., Jualian-Reynier C., Kristoffersson U., ten Kate L.P., Anionwu E., Benjamin C., Challen K., Schmidtke J., Nippert R.P., Harris R. Confidence of primary care physicians in their ability to carry out basic medical genetic tasks: a European survey in five countries: part 1. J Community Genet. 2011;2:1–11. doi: 10.1007/s12687-010-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mainous A.G., III, Johnson S.P., Chirina S., Baker R. Academic family physicians' perception of genetic testing and integration into practice: a CERA study. Fam Med. 2013;45:257–262. [PubMed] [Google Scholar]
- 6.Salm M., Abbate K., Appelbaum P., Ottman R., Chung W., Marder K., Leu C.S., Alcalay R., Goldman J., Curtis A.M., Leech C., Taber K.J., Klitzman R. Use of genetic tests among neurologists and psychiatrists: knowledge, attitudes, behaviors, and needs for training. J Genet Couns. 2014;23:156–163. doi: 10.1007/s10897-013-9624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IOM (Institute of Medicine): The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary, Learning Health System Series. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]