Abstract
Purpose
The use of anthracycline chemotherapy is associated with heart failure (HF) among survivors of non-Hodgkin lymphoma (NHL). We aimed to understand the contribution of preexisting cardiovascular risk factors to HF risk among NHL survivors.
Methods
Using Danish registries, we identified adults diagnosed with aggressive NHL from 2000 to 2010 and sex- and age-matched general-population controls. We assessed HF from 9 months after diagnosis through 2012. We used Cox regression analysis to assess differences in risk for HF between survivors and general population controls. Among survivors only, preexisting cardiovascular factors (hypertension, dyslipidemia, and diabetes) and preexisting cardiovascular disease were ascertained. We used multivariable Cox regression to model the association of preexisting cardiovascular conditions on subsequent HF.
Results
Among 2,508 survivors of NHL and 7,399 controls, there was a 42% increased risk of HF among survivors compared with general population controls (hazard ratio [HR], 1.42; 95% CI, 1.07 to 1.88). Among survivors (median age at diagnosis, 62 years; 56% male), 115 were diagnosed with HF during follow-up (median years of follow-up, 2.5). Before NHL diagnosis, 39% had ≥ 1 cardiovascular risk factor; 92% of survivors were treated with anthracycline-containing regimens. In multivariable analysis, intrinsic heart disease diagnosed before lymphoma was associated with increased risk of HF (HR, 2.71; 95% CI, 1.15 to 6.36), whereas preexisting vascular disease had no association with HF (P > .05). Survivors with cardiovascular risk factors had an increased risk of HF compared with those with none (for 1 v 0 cardiovascular risk factors: HR, 1.63; 95% CI, 1.07 to 2.47; for ≥ 2 v 0 cardiovascular risk factors: HR, 2.86; 95% CI, 1.56 to 5.23; joint P < .01).
Conclusion
In a large, population-based cohort of NHL survivors, preexisting cardiovascular conditions were associated with increased risk of HF. Preventive approaches should take baseline cardiovascular health into account.
INTRODUCTION
Survivors of non-Hodgkin lymphoma (NHL) are at increased risk for heart failure, with an incidence approximately five times greater than that of the general population.1,2 Although anthracycline-based chemotherapy is a major contributor to increased risk, patients with NHL, whose mean age at diagnosis is 66 years, may have preexisting conditions that further increase their risk of heart failure and other cardiovascular diseases.3,4 Lifestyle factors, hypertension, diabetes, dyslipidemia, and older age contribute to heart failure in the general population.5 Older age and preexisting cardiovascular risk factors are associated with heart failure and other cardiovascular diseases in cancer survivors, particularly among those who received anthracyclines.6,7
Much of our understanding of heart failure risk in NHL survivors arises from clinical trials that exclude patients with comorbidities, clinical populations that are not necessarily representative of larger populations, or cohorts of childhood cancer survivors who do not have cardiovascular risk factors before their cancer diagnosis.1,8 We used data from the Danish population-based registries, which include information on a full range of important cardiovascular risk factors for an entire national population of lymphoma survivors and general population controls. We aimed to determine whether preexisting cardiovascular risk factors are associated with heart failure among survivors of aggressive NHL, which is generally treated with curative intent and can have a substantial disease-free survivorship period.
METHODS
Data Sources
Denmark has a national health insurance system, and there is a comprehensive health record of each resident filed under a unique identification number, enabling the merging of demographic and clinical data from different databases. Denmark’s Central Population Register, established in 1968, contains emigration and vital status data.9 Cause of death is recorded in the Register of Causes of Death.10 The Danish National Lymphoma Registry, called LYFO, includes diagnosis and treatment data for patients diagnosed with lymphoma starting in 2000.11 The National Patient Register contains data regarding hospital discharges, diagnoses, and procedures from 1978 (with outpatient records complete after 1993).12 These are recorded with the Danish modified version of the International Classification of Diseases, eighth revision (ICD-8) until 1993 and ICD, 10th revision (ICD-10) from 1994.13 The Danish Cancer Registry contains tumor characteristics for all cancers diagnosed after 1943.9 The National Prescription Register records all prescription drugs distributed in community pharmacies since 1994.14 All registries were complete through 2012, with two exceptions: the Register of Causes of Death was complete through 2010, and the Cancer Registry was complete through 2011.
Sample
Survivors of aggressive NHL had the following histologies: diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, primary effusion lymphoma, Burkitt lymphoma, follicular lymphoma grade 3, peripheral T-cell lymphoma not otherwise specified, angioimmunoblastic T-cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, anaplastic large-cell lymphoma, hepatosplenic T-cell lymphoma, enteropathy-associated T-cell lymphoma, and NK/T-cell lymphoma nasal type. We included survivors diagnosed at age ≥ 15 years with aggressive NHL between 2000 and 2010, followed from 9 months after diagnosis (landmark analysis) until December 2012 or a censoring event. By 9 months after diagnosis, most patients have completed first-line therapy or, in the setting of primary refractory disease and in patients eligible for stem cell transplantation, second-line therapy and subsequent consolidation. We excluded survivors who had a diagnosis of heart failure or a cancer other than NHL before the landmark.
Outcome Ascertainment
The primary outcome was heart failure, diagnosed during inpatient or outpatient hospital visits or as cause of death after the landmark. ICD-10 codes for heart failure are I11.0, I13.0, I13.2, I50, I50.0, I50.1, and I50.9. By restricting our outcome to heart failure diagnosed at a hospital visit or death, we conservatively aimed to capture the symptomatic and most severe cases.15
Treatment
Treatment data included chemotherapy regimens, use of radiation, and receipt of autologous hematopoietic cell transplantation. Prescribed doses of anthracyclines were determined algorithmically on the basis of standard regimens and cycles noted in LYFO. Radiation field was determined algorithmically on the basis of site of treatment. Receipt of anthracyclines and chest radiation was coded dichotomously. Missing radiation and chemotherapy data were abstracted from the medical record.
Preexisting Cardiovascular Disease
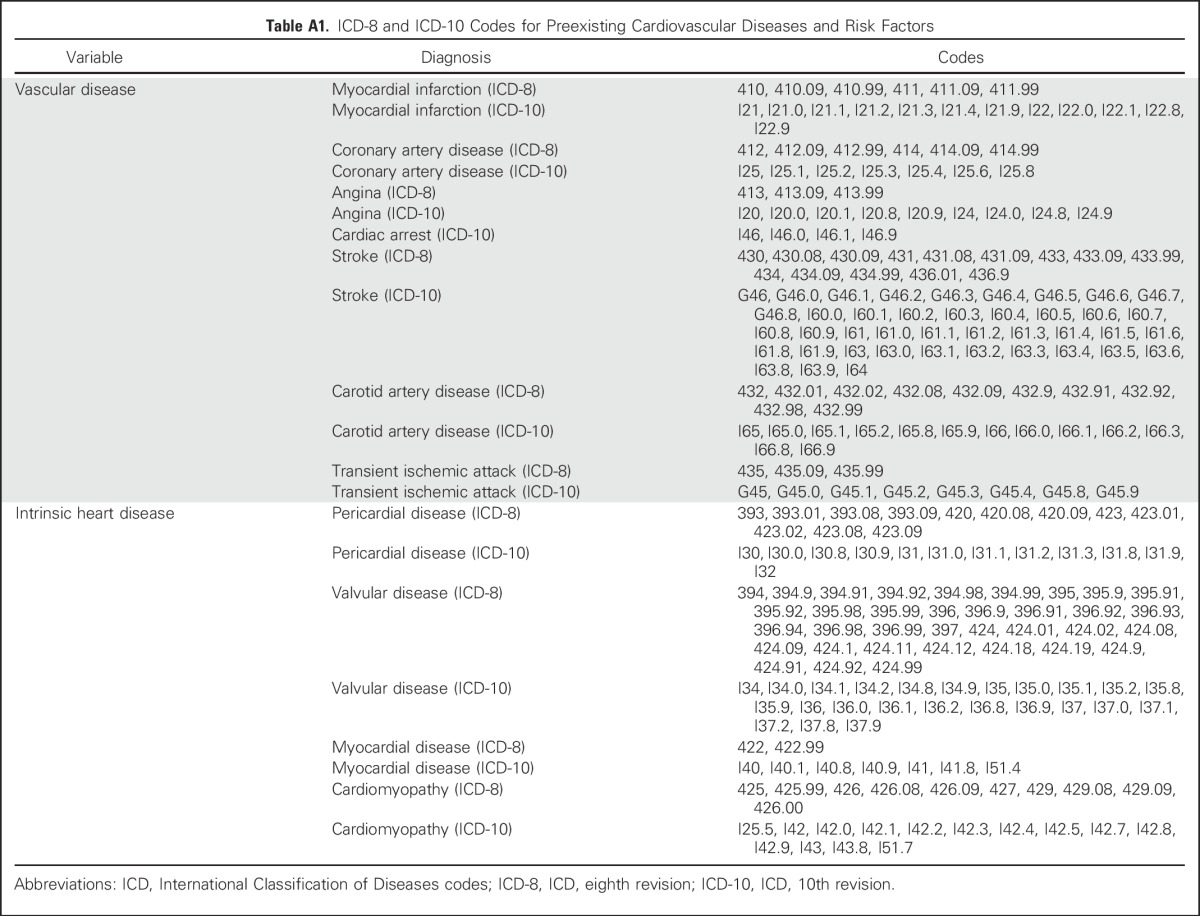
We assessed the presence of cardiovascular disease that was diagnosed before lymphoma diagnosis using the National Patient Register (see Appendix Table A1, online only, for ICD-8 and ICD-10 codes). Vascular disease included myocardial infarction, coronary artery disease, stable and unstable angina, cardiac arrest, stroke, carotid artery disease, and transient ischemic attacks. Intrinsic heart disease (not including heart failure, for which survivors were excluded from analysis) included pericardial disease, valvular disease, myocardial disease, and cardiomyopathy without clinical heart failure.
Preexisting Cardiovascular Risk Factors
Cardiovascular risk factors included hypertension, dyslipidemia, and diabetes. Hypertension and dyslipidemia were ascertained using prescriptions for these conditions noted in the National Prescription Register. Hypertension was defined by the prescription of an antihypertensive, including calcium channel blockers, diuretics, angiotensin inhibitors, beta blockers, or alpha blockers. Dyslipidemia was defined by the prescription of statins. Diabetes was ascertained in the National Patient Register. We coded each survivor as having 0, 1, or > 1 risk factor present before diagnosis of lymphoma.
Additional Covariables
We included sex from the Central Population Register and age at diagnosis and histology from LYFO.
General Population Controls
Controls were matched three to one on age at diagnosis and sex, drawn randomly from the Central Population Register. Controls were alive, had not emigrated, and had no diagnosis of cancer or heart failure before the landmark.
Analysis
To determine whether NHL diagnosis was associated with increased risk of heart failure, we fit a Cox regression model stratified by matched sets; the main effect of interest was survivor or control status. The remaining analyses used data only from survivors. To investigate potential confounding by indication, we cross-tabulated preexisting cardiovascular risk factors, including the number of cardiovascular risk factors, vascular disease, and intrinsic heart disease, with receipt of anthracyclines and tested for associations using Fisher’s exact test.
Follow-up time for heart failure continued until the first diagnosis of a heart failure event. Censoring events were emigration, second primary malignancy (SPM), relapse, or death from any cause. Because data in the Cancer Registry and the Register of Causes of Death were not complete through 2012, this approach did not count heart failure diagnosed as a cause of death (without a prior hospitalization) after 2010, and a diagnosis of a SPM in 2012 would be missed as a censoring event. We assumed that SPMs and heart failure diagnosed at death are rare occurrences and that the benefits of including available data after 2010 would outweigh the impact of these missing data.
A multivariable Cox regression model included age, sex, receipt of anthracyclines, cumulative dose of anthracyclines, receipt of mediastinal radiation, receipt of autologous transplantation, presence of preexisting cardiovascular diseases (intrinsic heart disease and vascular disease), and number of preexisting cardiovascular risk factors (hypertension, dyslipidemia, and diabetes mellitus). Interaction effects between cardiovascular risk factors and cumulative anthracycline dose were assessed using the likelihood ratio test. Statistical analyses were conducted using R software, version 3.2.5 (R Core Development Team, Vienna, Austria), including the survival package.
RESULTS
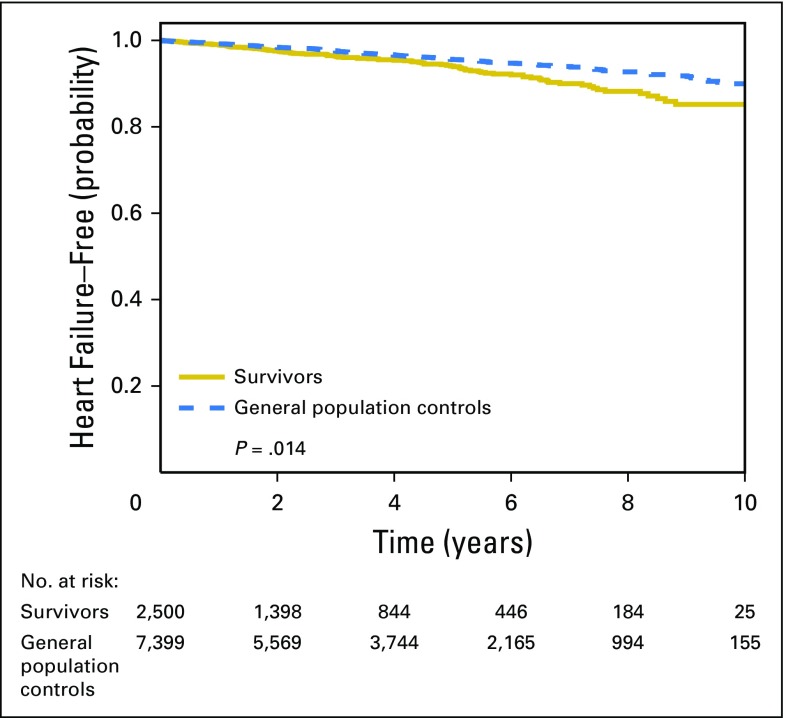
Our cohort included 2,508 people who were diagnosed with incident aggressive NHL between 2000 and 2010, and 7,399 cancer-free general population controls (Table 1). Compared with age- and sex-matched controls, NHL survivors had a 42% increased risk of heart failure (HR, 1.42; 95% CI, 1.07 to 1.88; Fig 1).
Table 1.
Characteristics of 2,508 Survivors and 7,399 Matched General Population Controls
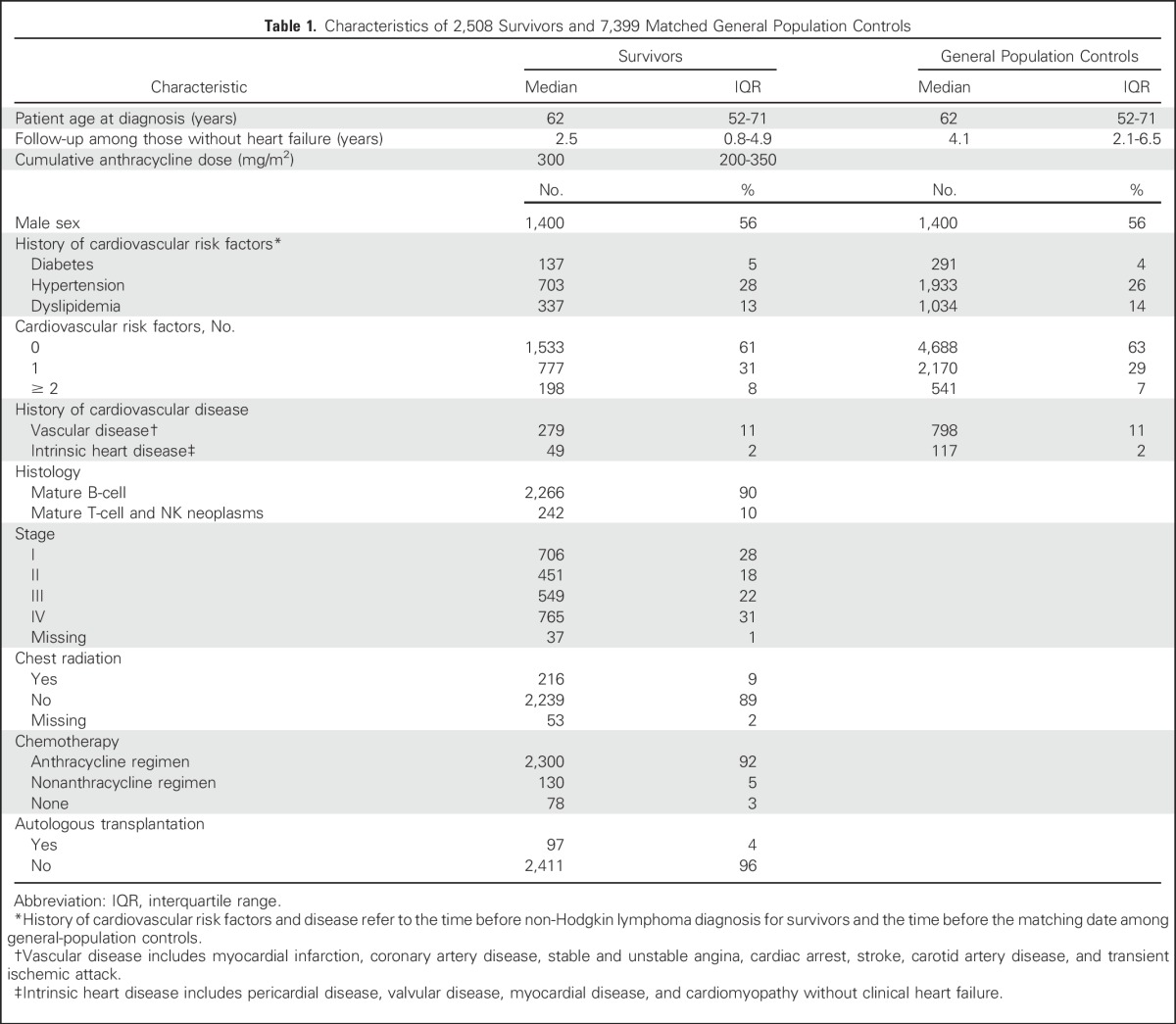
Fig 1.
Kaplan-Meier estimates of remaining free of heart failure among lymphoma survivors and general population controls.
Among survivors, 575 were censored because of relapse, 285 were censored because of death, 153 were censored because of SPM, and 10 were censored because of emigration. Before NHL diagnosis, 703 (28%) were diagnosed with hypertension, 337 (13%) with dyslipidemia, and 137 (6%) with diabetes. Thirty-nine percent had ≥ 1 cardiovascular risk factor.
Ninety-two percent of survivors received an anthracycline, with a median prescribed cumulative dose of 300 mg/m2 (interquartile range, 200-350 mg/m2). Almost half of survivors (45%) were prescribed a cumulative dose of 300 mg/m2. Nine percent received chest radiation, and 4% received an autologous hematopoietic cell transplant.
Median follow-up for survivors without an event was 2.5 years (range, 0.0 to 10.8 years); at 5 years, 604 survivors remained at risk. During follow-up, 115 patients with heart failure were identified.
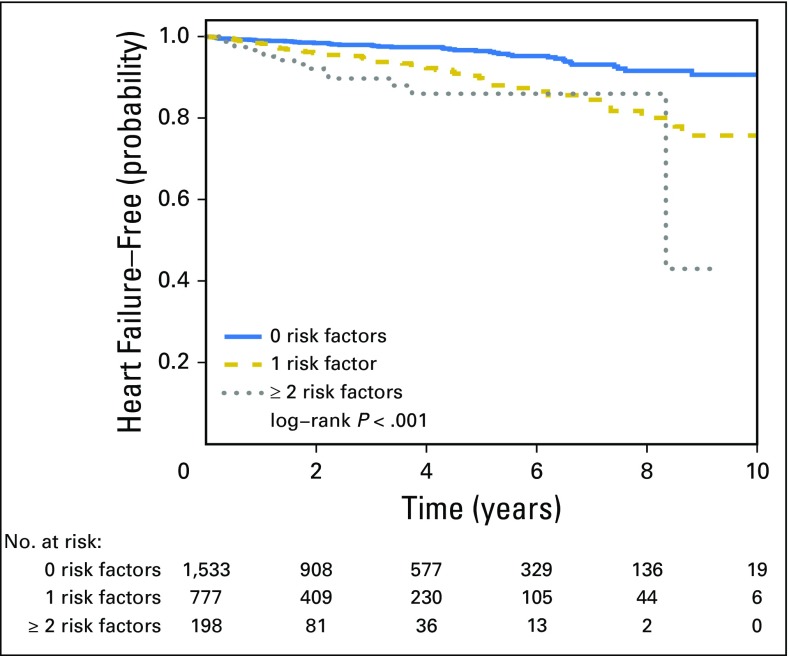
In unadjusted analyses, having more cardiovascular risk factors was associated with increased risk of later heart failure, compared with having none (for 1 v 0 cardiovascular risk factors: HR, 2.61; 95% CI, 1.75 to 3.89; for ≥ 2 v 0 cardiovascular risk factors: HR, 4.39; 95% CI, 2.44 to 7.92; Fig 2). Older age at diagnosis, having a history of intrinsic heart disease, and having a history of vascular disease were all associated with increased risk of later heart failure. (Table 2).
Fig 2.
Kaplan-Meier estimates of remaining free of heart failure among lymphoma survivors with 0, 1, or ≥ 2 risk factors.
Table 2.
Predictors of Heart Failure Among Survivors in the Follow-Up Period (N = 2,508)
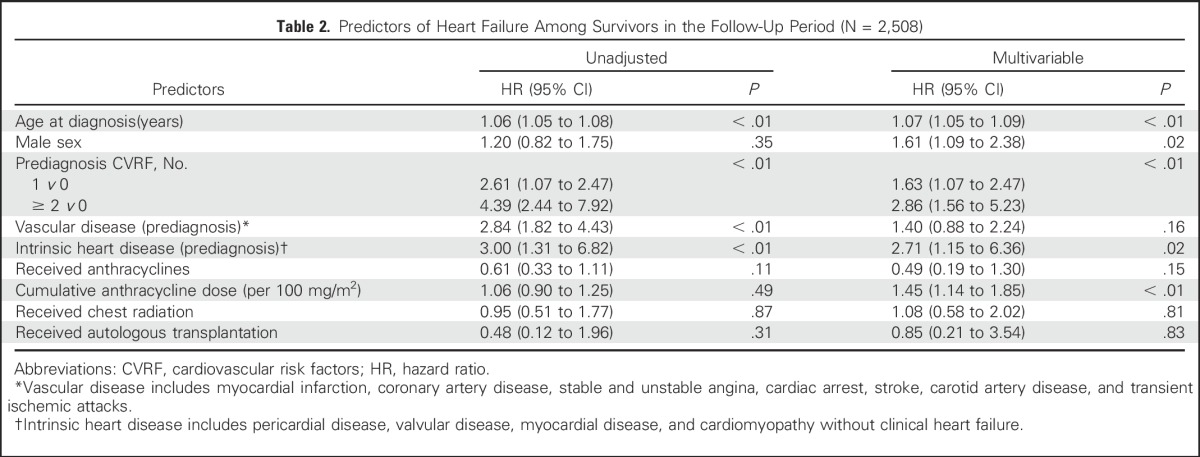
In the multivariable model, the number of cardiovascular risk factors was associated with an increased risk of heart failure (1 v 0 cardiovascular risk factors: HR, 1.63; 95% CI, 1.07 to 2.47; ≥ 2 v 0 cardiovascular risk factors: HR, 2.86; 95% CI, 1.56 to 5.23; joint P < .01; Table 2). Preexisting intrinsic heart disease was also significantly associated with an increased risk of heart failure (HR, 2.71; 95% CI, 1.15 to 6.36), although preexisting vascular disease was not (P > .05). In addition, age and sex were strongly associated with risk of subsequent heart failure, as was cumulative anthracycline dose.
A model including an interaction term between cumulative anthracycline dose and number of cardiovascular risk factors had a P value of .19 for the interaction term. The main model, therefore, did not contain an interaction term.
We also found evidence of confounding by indication, because the prescription of anthracyclines was associated with the number of preexisting cardiovascular risk factors (P < .01), preexisting vascular disease (P < .01), and preexisting intrinsic heart disease (P < .01), such that survivors with these conditions less frequently received anthracyclines.
We conducted a sensitivity analysis in which follow-up time began 12 months after diagnosis, allowing for the possibility that NHL treatment may extend beyond 9 months. The model results did not differ substantially from the primary model with regard to either magnitude or significance of effects (results not shown).
Because heart failure may sometimes be erroneously coded as cardiomyopathy, we conducted an additional sensitivity analysis. Survivors with any intrinsic heart disease diagnosed before lymphoma diagnosis were excluded, because those individuals may have had heart failure and thus would no longer be at risk. This model showed no meaningful difference compared with the main model (results not shown).
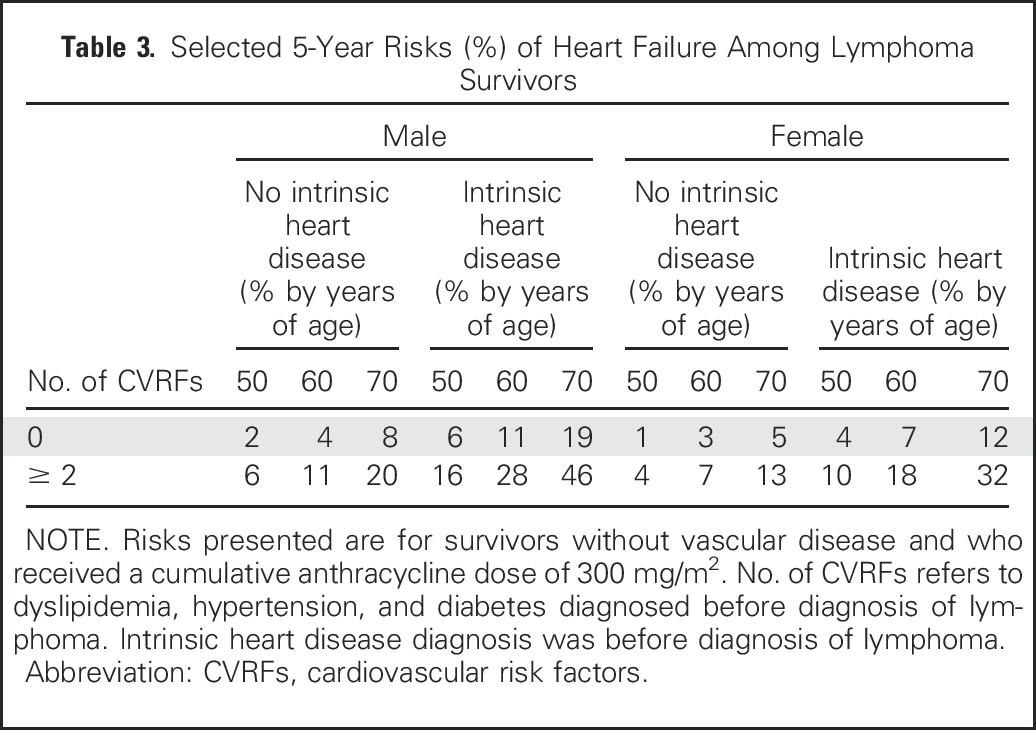
To illustrate the differences in absolute risk of heart failure across survivors with various levels of important risk factors for heart failure, we calculated selected 5-year predicted risks of heart failure for survivors who did not receive radiation, did not undergo transplantation, did not have preexisting vascular disease, and received a cumulative dose of 300 mg/m2 of anthracyclines (Table 3). For example, for a 50-year-old man without preexisting intrinsic heart disease and without preexisting cardiovascular risk factors, the 5-year risk of heart failure is 2%. This risk rises to 16% in the presence of intrinsic heart disease and 2 cardiovascular risk factors.
Table 3.
Selected 5-Year Risks (%) of Heart Failure Among Lymphoma Survivors
DISCUSSION
In a large, national, contemporarily treated cohort of survivors of aggressive NHL and matched general population controls, survivors had an elevated risk of heart failure, even within the first 5 years of follow-up. This is congruent with a prior pooled analysis of four European trials that included 476 patients with aggressive NHL treated with anthracyclines, in which 12% of patients with aggressive NHL were diagnosed with heart failure within 5 years of diagnosis.1 Although cardiovascular risk is likely to increase over time, the significant short-term risk of a serious post-treatment cardiac event demonstrates the necessity of early intervention in this high-risk population.
Comorbid conditions were prevalent in our cohort of NHL survivors, enabling us to demonstrate the importance of preexisting cardiovascular risk factors and comorbidities in contributing to the development of heart failure. Although treatment of NHL confers substantial risk of heart failure, the presence of cardiovascular risk factors and intrinsic heart disease before lymphoma diagnosis dramatically heightens the risk of heart failure months to years after treatment completion.
There has been limited investigation of the contribution of cardiovascular risk factors on later heart failure in NHL survivors. In the pooled analysis of aggressive NHL, preexisting hypertension was identified as a risk factor for cardiovascular disease (HR, 4.1; 95% CI, 2.1 to 8.9).1 A retrospective registry-based analysis of older patients diagnosed with diffuse large B-cell lymphoma between 1991 and 2002 similarly found elevated risks of heart failure among patients with preexisting hypertension and diabetes.16 A retrospective analysis among a large managed care organization found that NHL survivors with multiple cardiovascular risk factors (including hypertension, dyslipidemia, and diabetes) were at higher risk for developing ischemic heart disease, stroke, and heart failure compared with those without cancer with the same number of risk factors; however, this analysis did not adjust for treatment.7 Our population-based analysis among a contemporarily treated cohort demonstrates that cardiovascular risk factors present at diagnosis are independently associated with the risk of heart failure conferred by cardiotoxic treatment. Investigations of the etiology of cardiovascular late effects need to account for these common contributors to heart disease.
The presence of preexisting cardiovascular risk factors can inform heart failure prevention among NHL survivors. New guidelines from the American Society for Clinical Oncology recommend prevention and monitoring of cardiac dysfunction after treatment completion among adult cancer survivors.17 In these guidelines, high-risk survivors are defined by a combination of cumulative dose of anthracyclines, dose of radiation therapy to the heart, age, history of cardiovascular disease, and the number of traditional cardiovascular risk factors (including smoking, hypertension, diabetes, dyslipidemia, and obesity) present during or after treatment. Our findings confirm the roles of anthracyclines and age, and extend this definition by highlighting the additional risk posed by risk factors present even before cancer treatment. Most survivors in our cohort received a cumulative dose of anthracyclines of at least 300 mg/m2, which in itself is considered high risk. For asymptomatic high-risk survivors, providers can implement routine screening with techniques such as echocardiography, cardiac magnetic resonance imaging, or multiple-gated acquisition scanning to detect subclinical decline in cardiac function. In addition, the guidelines suggest referral to a cardiologist and aggressive targeting of cardiovascular risk factors.
In recent years, there has been heightened recognition of preclinical disease states for key cardiovascular risk factors, including hypertension, diabetes, and dyslipidemia. Guidelines in the general population recommend aggressive risk-reducing strategies for individuals at high risk for cardiovascular disease, with treatment goals for cardiovascular risk factors below levels that define preclinical disease.18-20 High-risk cancer survivors may similarly benefit from aggressive management of hypertension, diabetes, and hyperlipidemia.
Chest radiation, an acknowledged risk factor for heart failure among cancer survivors, was not associated with heart failure in our cohort. Chest radiation has become increasingly focused, minimizing exposure to the myocardium.21 Effects of chest radiation are more likely to be identified years after diagnosis and may not be relevant in the first few years after treatment completion.
When interpreting our findings, it is important to understand the study limitations. We opted to analyze follow-up data through 2012 (among those not previously censored) despite some missing data. Specifically, we were unable to ascertain SPMs (a censoring end point) in 2012. However, SPMs were rare in our cohort before 2012, with one to 31 patients per calendar year. Therefore, this is unlikely to have affected our findings meaningfully. Similarly, we were unable to ascertain heart failure as a cause of death beyond 2010. From 2000 through 2010, only four of the 77 heart failure events (5%) were diagnosed as a cause of death without a prior hospital discharge for heart failure. We extrapolated that with 38 heart failure events ascertained as a hospital discharge in 2011 and 2012, no more than two additional heart failure events would have been ascertained as a cause of death (not preceded by a hospital discharge). We do not believe this presents a significant bias in our findings. Our approach to include additional years of follow-up provided significant analytic power and enabled us to include all of our important predictors in the multivariable model.
The Danish population registries lacked granular data that may have improved our model. Tobacco use data were unavailable, although the effects of tobacco on heart failure are generally indirect, mediated through preexisting cardiovascular conditions included in our model. Clinical metrics, such as left ventricular function and blood pressure measured at the start of follow-up, were also unavailable. Similarly, we cannot ascertain the indication for the medications used to identify hypertension. However, even within the limitations of a registry cohort, important associations of cardiovascular conditions and heart failure were still detected, and they were robust to multiple sensitivity analyses. A consequence of using an observational cohort is confounding by indication, with healthier patients more likely to receive anthracyclines. By modeling anthracyclines as both an indicator of whether they were prescribed and the prescribed cumulative dose, we were able to account for some bias. The Danish population is predominantly white, which limits the generalizability of our findings. However, the well-known Framingham risk factors were originally developed in a white population and were later found to predict cardiovascular mortality equally well in non-Hispanic black and Mexican American participants.22 Similarly, consistent magnitudes of associations between risk factors and myocardial infarction across international populations suggest that differences in cardiovascular risk result from variations in exposure.23 Thus, our model may generalize to more diverse populations. Finally, the follow-up time precludes analysis of long-term heart failure risk. However, with a median age of 62 years, even short-term risk is relevant to this older population.
Our study demonstrated the excess risk of heart failure, even in the short term, among survivors of aggressive NHL. It further quantified the contribution of preexisting cardiovascular risk factors to the later development of heart failure among survivors of aggressive NHL. This confirms common practice among oncologists for limiting the use and dosage of anthracyclines in older patients with multiple cardiovascular risk factors. The balance of potential benefits and harms in the use of anthracyclines as part of curative therapy for aggressive NHL should be considered with up-front therapy. For survivors of NHL, the presence of cardiovascular risk factors before the diagnosis of lymphoma in combination with anthracycline treatment should alert providers to heightened risk of heart failure even shortly after treatment. Clinicians should focus on preventive strategies among newly diagnosed patients with NHL with existing cardiovascular risk factors to avoid heart disease that can manifest in the early years of survivorship.
Appendix
Table A1.
ICD-8 and ICD-10 Codes for Preexisting Cardiovascular Diseases and Risk Factors
Footnotes
Supported by a Clinical Research Scholar Award from the Leukemia & Lymphoma Society (T.S.), National Institutes of Health grant (R03 CA186223; T.S.), National Institutes of Health grant to Memorial Sloan-Kettering, Cancer Center Support Grant (P30 CA008748), and The Danish Cancer Society.
Presented at the Best Abstracts session of the Biennial Cancer Survivorship Research Symposium, Washington, DC, June 16, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: Talya Salz, Emily C. Zabor, Peter de Nully Brown, Susanne Oksbjerg Dalton, Nirupa J. Raghunathan, Matthew J. Matasar, Richard Steingart, Andrew J. Vickers, Kevin C. Oeffinger, Christoffer Johansen
Financial support: Talya Salz, Christoffer Johansen
Administrative support: Talya Salz
Provision of study materials or patients: Peter de Nully Brown, Susanne Oksbjerg Dalton, Peter Svenssen Munksgaard, Christoffer Johansen
Collection and assembly of data: Emily C. Zabor, Peter de Nully Brown, Christoffer Johansen
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Preexisting Cardiovascular Risk and Subsequent Heart Failure Among Non-Hodgkin Lymphoma Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Talya Salz
No relationship to disclose
Emily C. Zabor
No relationship to disclose
Peter de Nully Brown
No relationship to disclose
Susanne Oksbjerg Dalton
No relationship to disclose
Nirupa J. Raghunathan
No relationship to disclose
Matthew J. Matasar
Honoraria: Genentech
Consulting or Advisory Role: Genentech, Kite Pharma, Rocket Pharmaceuticals
Research Funding: Genentech, GlaxoSmithKline, Spectrum Pharmaceuticals
Richard Steingart
Consulting or Advisory Role: Pfizer
Andrew J. Vickers
Stock or Other Ownership: OPKO Diagnostics
Patents, Royalties, Other Intellectual Property: Royalties for 4Kscore test
Peter Svenssen Munksgaard
No relationship to disclose
Kevin C. Oeffinger
No relationship to disclose
Christoffer Johansen
No relationship to disclose
REFERENCES
- 1.Moser EC, Noordijk EM, van Leeuwen FE, et al. : Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood 107:2912-2919, 2006 [DOI] [PubMed] [Google Scholar]
- 2.André M, Mounier N, Leleu X, et al. : Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: A GELA cohort study on 2837 patients. Blood 103:1222-1228, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Carver JR, Shapiro CL, Ng A, et al. : American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol 25:3991-4008, 2007 [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Lin CC, Mariotto AB, et al. : Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252-271, 2014 [DOI] [PubMed] [Google Scholar]
- 5.He J, Ogden LG, Bazzano LA, et al. : Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 161:996-1002, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Yancik R, Ganz PA, Varricchio CG, et al. : Perspectives on comorbidity and cancer in older patients: Approaches to expand the knowledge base. J Clin Oncol 19:1147-1151, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Armenian SH, Xu L, Ky B, et al. : Cardiovascular disease among survivors of adult-onset cancer: A community-based retrospective cohort study. J Clin Oncol 34:1122-1130, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hequet O, Le QH, Moullet I, et al. : Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol 22:1864-1871, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Pedersen CB: The Danish Civil Registration System. Scand J Public Health 39:22-25, 2011. (suppl 7)https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21775345&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Helweg-Larsen K: The Danish Register of Causes of Death. Scand J Public Health 39:26-29, 2011. (suppl 7) [DOI] [PubMed] [Google Scholar]
- 11.Arboe B, El-Galaly TC, Clausen MR, et al. : The Danish National Lymphoma Registry: Coverage and data quality. PLoS One 11:e0157999, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynge E, Sandegaard JL, Rebolj M: The Danish National Patient Register. Scand J Public Health 39:30-33, 2011. (7, suppl) [DOI] [PubMed] [Google Scholar]
- 13.Dalton SO, Steding-Jessen M, Gislum M, et al. : Social inequality and incidence of and survival from cancer in a population-based study in Denmark, 1994-2003: Background, aims, material and methods. Eur J Cancer 44:1938-1949, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kildemoes HW, Sørensen HT, Hallas J: The Danish National Prescription Registry. Scand J Public Health 39(7, Suppl)38-41, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Kümler T, Gislason GH, Kirk V, et al. : Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail 10:658-660, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, McBride RB, Eisenberger A, et al. : Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J Clin Oncol 26:3159-3165, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Armenian SH, Lacchetti C, Barac A, et al. : Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 35:893-911, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. : The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 289:2560-2572, 2003 [DOI] [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) : Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circ 106:3143-3421, 2002 [PubMed] [Google Scholar]
- 20.American Diabetes Association : Standards of medical care in diabetes--2012. Diabetes Care 35:S11-S63, 2012. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Nimwegen FA, Ntentas G, Darby SC, et al. : Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood 129:2257-2265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley LP, Dickinson LM, Estacio RO, et al. : Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ Cardiovasc Qual Outcomes 3:181-187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezzati M: How can cross-country research on health risks strengthen interventions? Lessons from INTERHEART. Lancet 364:912-914, 2004 [DOI] [PubMed] [Google Scholar]