Abstract
Standard methods for disease response assessment in patients with lymphoma, including positron emission tomography and computed tomography scans, are imperfect. In other hematologic malignancies, particularly leukemias, the ability to detect minimal residual disease (MRD) is increasingly influencing treatment paradigms. However, in many subtypes of lymphoma, the application of MRD assessment techniques, like flow cytometry or polymerase chain reaction–based methods, has been challenging because of the absence of readily detected circulating disease or canonic chromosomal translocations. Newer MRD detection methods that use next-generation sequencing have yielded promising results in a number of lymphoma subtypes, fueling the hope that MRD detection may soon be applicable in clinical practice for most patients with lymphoma. MRD assessment can provide real-time information about tumor burden and response to therapy, noninvasive genomic profiling, and monitoring of clonal dynamics, allowing for many possible applications that could significantly affect the care of patients with lymphoma. Further validation of MRD assessment methods, including the incorporation of MRD assessment into clinical trials in patients with lymphoma, will be critical to determine how best to deploy MRD testing in routine practice and whether MRD assessment can ultimately bring us closer to the goal of personalized lymphoma care. In this review article, we describe the methods available for detecting MRD in patients with lymphoma and their relative advantages and disadvantages. We discuss preliminary results supporting the potential applications for MRD testing in the care of patients with lymphoma and strategies for including MRD assessment in lymphoma clinical trials.
INTRODUCTION
Our understanding of the biology and heterogeneity of lymphoma is rapidly growing and fueling advances in tailored therapy. Despite this, we still have only a limited ability to determine the optimal intensity and duration of treatment for individual patients. At the present time, we rely on relatively insensitive methods to evaluate lymphoma tumor burden and disease response. The most useful such methods for most lymphoma subtypes remain positron emission tomography (PET) and computed tomography (CT) imaging. However, those methods are costly and associated with radiation exposure and possible downstream negative health consequences.1-3 Although they do provide valuable information, these techniques can produce false-positive results that may incorrectly inform decision making4-7 or false-negative results regarding clinical remissions that ultimately end in relapse, presumably because they fail to identify residual lymphoma cells that are below the limit of imaging detection. In addition, imaging-based surveillance of patients with lymphoma in remission is not associated with benefit.8-10
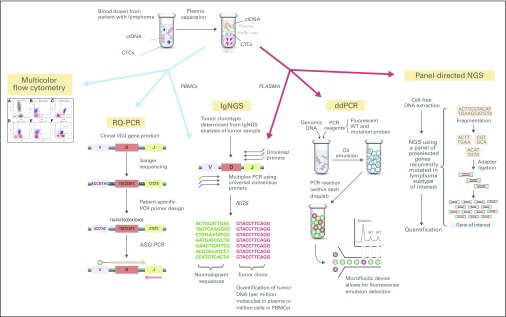
Given these limitations, we need more sensitive methods to detect the presence of minimal residual disease (MRD) in patients treated for lymphoma. Several methods are currently being developed and studied, which may significantly affect the management of lymphoma in the coming years. MRD can be identified by detecting circulating tumor cells (CTCs), including the genomic tumor DNA within CTCs, or by detecting cell-free circulating tumor DNA (ctDNA) that is either secreted directly into the bloodstream by tumor cells or released during necrosis or apoptosis. MRD can be detected in the blood or bone marrow (BM) using a variety of techniques, including flow cytometry (FC), polymerase chain reaction (PCR) –based methods, and next-generation sequencing (NGS) –based techniques (Fig 1).11-15 Beyond the detection of CTCs or ctDNA, the amount of CTCs or ctDNA detected in a blood or BM sample can provide important information about tumor burden.14-25 Therefore, MRD assessment can potentially serve many purposes, including providing prognostic information before treatment initiation, measuring depth of treatment response, monitoring for disease recurrence, and, through NGS, tracking the clonal evolution of tumors. There is an ever-expanding arsenal of cytotoxic, immunologic, and targeted antilymphoma therapies associated with varying intensity and risk, which would ideally be rationally targeted toward specific subgroups of patients. MRD assessment has the potential to drive a more personalized approach to the deployment of these antilymphoma therapies. To realize that potential, MRD assessment methods and MRD-guided treatment approaches require additional validation in prospective lymphoma clinical trials.
Fig 1.
Minimal residual disease assessment methods in patients with lymphoma. ASO, allele-specific oligonucleotide; CTC, circulating tumor cell; ctDNA, circulating tumor DNA; ddPCR, digital droplet polymerase chain reaction; IgNGS, immunoglobulin gene next-generation sequencing; NGS, next-generation sequencing; PBMC, peripheral blood mononuclear cell; PCR, polymerase chain reaction; RQ-PCR, real-time quantitative polymerase chain reaction; WT, wild type.
In this review, we describe currently available MRD detection methods, their limitations and advantages, their potential applications, and possible strategies for incorporating MRD assessment into clinical trials. This review focuses on two lymphoma subtypes as prototypes for the application of MRD: mantle cell lymphoma (MCL) and diffuse large B-cell lymphoma (DLBCL); however, the methods and strategies described have the potential for broader application to other lymphoma subtypes.
STATE OF THE SCIENCE: MRD ASSESSMENT METHODS
FC and PCR-Based Methods
Until recently, the primary methods available for assessing MRD in lymphoma were FC and PCR-based techniques. Through the use of multicolor FC (MCFC), in which cells are exposed to fluorescently labeled antibodies and specific cell populations of interest are identified on the basis of light scatter (size) and fluorescence emitted (immunophenotype), malignant circulating or BM tumor cells can be identified, with a detection limit of 10−4 to 10−5.23-26 MCFC is well established as an MRD method in leukemias and has also been studied in MCL, where MRD levels at diagnosis correlate with stage, lactate dehydrogenase level, and MCL International Prognostic Index score.24-26
PCR enables the detection of tumor-specific DNA sequences, including chromosomal rearrangements, through the design of primers specific for genetic regions of interest (eg, IGH-CCND1 in MCL or allele-specific oligonucleotides [ASOs]) and subsequent amplification of the tumor-specific sequences.23 In B-cell lymphomas, each tumor has a clonal immunoglobulin gene product, which can serve as a tumor-specific sequence for detection and measurement using quantitative PCR, with a detection limit of 10−5.23 Real-time quantitative PCR (RQ-PCR) is validated in MCL as a dynamic biomarker throughout a patient’s treatment course. Quantitative MRD levels detected by RQ-PCR track with treatment response to therapy.23,24,27-32 The presence of MRD detected by RQ-PCR at the end of induction (EOI) therapy, after autologous stem-cell transplantation (ASCT), and during surveillance after therapy completion (induction or ASCT) is associated with poorer outcomes23,24,27-35 (Table 1). Digital droplet PCR (ddPCR) is a high-throughput PCR technology that allows for the highly sensitive detection and quantification of tumor-specific somatic mutations. A study of this technique in DLBCL demonstrated that potentially therapeutically relevant somatic mutations could be detected in the blood of patients with DLBCL at a sensitivity of 0.05%.42
Table 1.
MRD Assessment in Patients With DLBCL and MCL Who Received Treatment As Part of a Prospective Clinical Trial
NGS-Based Methods
With the advent of NGS, novel and highly sensitive MRD assessment methods have been developed and studied in lymphoma. NGS-based MRD assessment can identify CTCs present in peripheral blood mononuclear cells (PBMCs) or BM or ctDNA shed by tumors into the bloodstream and detectable in the plasma.13,15 Two NGS-based MRD assessment methods have been studied for lymphoma: NGS of immunoglobulin or T-cell receptor genes (hereafter IgNGS; a discussion of T-cell lymphoma is beyond the scope of this review) via ClonoSEQ (Adaptive Biotechnologies, Seattle, WA) and detection of somatic genetic alterations using ultrasensitive, panel-directed NGS (eg, CAPP-SEQ [cancer personalized profiling by deep sequencing]). These methods have been studied in a range of lymphoma subtypes, including MCL and DLBCL.
IgNGS relies on the determination of the tumor clonotype by PCR using universal primers and then NGS of the immunoglobulin genes present in tumor tissue (or blood if there is a sufficient circulating tumor component). Multiple tumor clones can be detected and tracked, and immunoglobulin clones are considered malignant if they have a frequency of > 5% among all immunoglobulin sequences measured in the tumor specimen. PCR and NGS of the immunoglobulin VDJ region is then performed on a blood sample spiked with a known quantity of normal reference DNA to quantify the fractional presence of this clonotype (number of malignant relative to nonmalignant sequencing reads) in PBMCs (CTCs) or plasma (ctDNA).
IgNGS has been used to evaluate untreated patients with MCL21,40,41 and can provide information about depth of response to treatment (Table 1).40,41 IgNGS can also detect MRD in the plasma and PBMCs of untreated patients with DLBCL, including ≥ 80% of patients at diagnosis.17,19 Moreover, MRD levels detected by IgNGS track with treatment response in patients with DLBCL, and the interim presence of ctDNA during initial treatment and presence of ctDNA at EOI are associated with poorer progression-free survival (PFS).16,17,19 In addition, preliminary studies in DLBCL suggest that IgNGS is sensitive and highly specific for the detection of relapse after the completion of standard therapy16,17 and possibly also after allogeneic stem-cell transplantation.43 In one study of IgNGS monitoring in DLBCL, 91% of patients who experienced relapse after first-line therapy were MRD positive before or at the time of relapse, with a median lead time of 3.5 months between MRD positivity and imaging-confirmed relapse.
Like IgNGS, panel-directed NGS MRD detection exploits the genetic features of a lymphoma to identify tumor-specific ctDNA in a patient’s blood. Using library preparation and bioinformatic pipelines optimized for low-input DNA, panel-directed NGS interrogates patient tissue (eg, plasma) for a panel of single-nucleotide variants, insertions or deletions, and chromosomal translocations known to frequently occur in specific lymphoma subtypes (to date, primarily DLBCL). Panels can be tailored to the disease under study, and the panel breadth and sequencing depth can be adjusted to optimally balance sensitivity, throughput, and cost. Comparison of normal and variant reads generated by deep sequencing the loci of interest, enriched using amplification- or hybrid capture–based methods, permits quantitative detection of ctDNA. Thus far, studies have demonstrated that genetic alterations detected using panel-directed NGS MRD assessment of blood are highly concordant with NGS of DLBCL tumor samples.18,22,44 Similar to IgNGS, panel-directed methods can identify ctDNA in patients with DLBCL before treatment, quantify ctDNA levels that track with treatment response, and detect molecular relapse before and at the time of clinical relapse.18,20,22,45 In one study, DLBCL-associated mutations became undetectable in patients who responded to standard therapy, whereas patients with refractory disease had persistent mutations, including new mutations that emerged during therapy, consistent with noninvasive detection of clonal evolution.22 In another study, panel-directed NGS of plasma detected ctDNA in all patients with DLBCL at the time of radiographic disease progression and before relapse in 73%. Patients with detectable ctDNA at any point after remission had significantly worse PFS compared with ctDNA-negative patients.18 The assay was highly specific, as confirmed by a lack of ctDNA detected in healthy controls and patients with DLBCL in long-term remission.
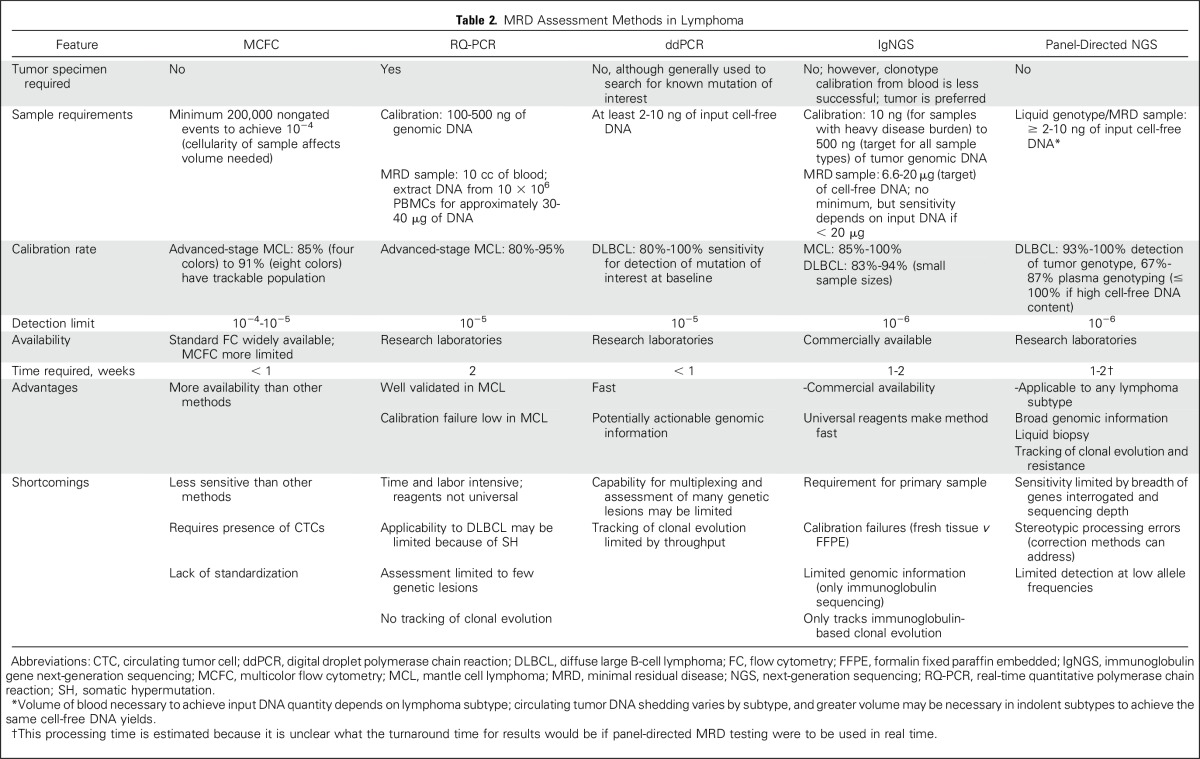
Limitations and Advantages of MRD Assessment Methods
Each method for assessing MRD in patients with lymphoma has its particular shortcomings and advantages (Table 2). Some lymphomas (eg, MCL) are characterized by the frequent presence of a significant circulating disease component (CTCs), which theoretically makes them good candidates for FC-based MRD assessment. However, in other lymphoma subtypes, especially DLBCL, circulating disease is not present or the levels are too low for FC-based detection.46 Even in MCL, a disease in which CTCs are common, MCFC is not as sensitive for MRD detection as other methods, such as RQ-PCR.25,26
Table 2.
MRD Assessment Methods in Lymphoma
RQ-PCR is applicable in the great majority (80% to 95%) of patients with MCL; however, aside from the minority of patients in whom consensus primers are able to detect t(11;14), RQ-PCR requires a time- and labor-intensive process of patient-specific ASO primer development. As a result, outside of centers where RQ-PCR has been established and validated, access to this method is limited. In addition, RQ-PCR may not be applicable in some patients in whom sequencing of the IgH PCR product is unsuccessful or in whom an unstable junctional region precludes the design of an ASO primer.23 In DLBCL, most tumors do not have a chromosomal rearrangement that can be tracked by PCR-based methods.47 RQ-PCR of the immunoglobulin genes or methylation of p57KIP2 gene promoter has been explored in DLBCL and was applicable in 70% to 84% of the small number of patients with DLBCL studied, but frequent somatic hypermutation limited its applicability.
In contrast to RQ-PCR, NGS-based MRD assessment methods do not require patient-specific reagents. IgNGS uses universal PCR primers to amplify the clonal immunoglobulin gene products present in a lymphoma for subsequent sequencing, MRD detection, and quantification. Current panel-directed NGS methods use locked-in panels of genetic alterations, which also avoid the patient-specific issues of ASO RQ-PCR.
Like RQ-PCR, NGS-based methods are applicable in the great majority of patients with MCL,21,40 but they are also applicable in DLBCL, where other MRD methods have been largely unsuccessful.16-20,43 Studies have demonstrated that IgNGS can identify a suitable immunoglobulin clone for subsequent MRD assessment in 83% to 94% of patients with DLBCL16,17,19 and 85% to 100% of patients with MCL.21,43 However, IgNGS does require the availability of a sufficient amount and quality of tumor tissue to establish the tumor clonotype. Indeed, the immunoglobulin clone calibration rate is higher when fresh tumor tissue is used as compared with formalin-fixed paraffin-embedded tissue.17 The amount of input DNA used in the IgNGS reaction also affects the clone calibration success rate.17 In the case of DLBCL, although it is possible to identify an immunoglobulin clone for subsequent MRD assessment from blood, the calibration rate from blood using IgNGS seems to be lower than that in panel-directed methods.16,18,22 Depending on the panel breadth and sequencing depth used, panel-directed methods are also applicable in most patients with DLBCL. Thus far, panel-directed methods have detected somatic alterations from tumor tissue in 93% to 100% of patients with DLBCL and 67% to 87% of pretreatment plasma samples (up to 100% when tumor genotypes are already established), with sensitivity affected by input DNA quantity, variant allele frequency of mutations, and possibly the sequencing breadth and depth of the assay.18,20,22 The use of molecular barcoding and digital error suppression techniques can minimize stereotypic background errors observed at low allele fractions that are introduced through library preparation and sequencing, which can improve the sensitivity and specificity of panel-directed MRD assessment and enhance liquid genotyping capacity.48 Panel-directed MRD assessment has the highest sensitivity (97% to 100%) for detecting ctDNA in plasma samples when there is a higher total cell-free DNA content (> five haploid genome equivalents per milliliter) or when evaluating abundant mutations with a higher variant allele frequency (≥ 20%) in the tumor tissue.14,15
A primary advantage of NGS-based MRD assessment is its improved sensitivity for the detection of MRD in blood (10−6) compared with RQ-PCR (10−5) and MCFC (10−4 to 10−5). Panel-directed MRD assessment may be more sensitive than IgNGS, with ctDNA detected at time points at which no MRD was detected by IgNGS in one study.18 In addition, panel-directed MRD assessment can provide genomic information that may be valuable for precision-medicine approaches. Panel-directed ctDNA assessment can serve as a so-called liquid biopsy, providing a tumor genotype and perhaps a more comprehensive snapshot of tumor genomic heterogeneity, because ctDNA can arise from any tumor site rather than mutations identified through biopsy of one tumor location.13,15,18,22,44 The genomic information produced through panel-directed NGS is highly reproducible across tissue and blood samples from the same time point, and allele frequencies are highly correlated among paired tumor and plasma samples.18 In addition to somatic mutations, clinically relevant chromosomal rearrangements (eg, MYC, BCL2, and BCL6 in DLBCL) can be identified through ctDNA analysis.18 Panel-directed MRD assessment can also provide information about clonal evolution and detect new mutations that arise during therapy that may have therapeutic implications.18,20,22 In one example, ctDNA was used to accurately and noninvasively distinguish nontransformed follicular lymphoma from transformed DLBCL arising from follicular lymphoma, and histologic transformation could be predicted ahead of time through analysis of ctDNA in plasma. ddPCR can also provide information about prespecified mutations; however, ddPCR has less capacity to evaluate multiple mutations simultaneously compared with panel-directed methods, thereby limiting the application of ddPCR as an MRD assessment method in the setting of clonal evolution.
THE WAY FORWARD: MRD ASSESSMENT IN CLINICAL TRIALS
With rapid technical improvements in MRD assessment methods, the ability to assess MRD in all patients with lymphoma may be attainable in the near future. However, the critical question regarding MRD assessment in lymphoma remains to be answered: can these tools improve patient outcome? To determine the usefulness of MRD assessment in the care of patients with lymphoma, incorporation of these assays into prospective lymphoma clinical trials will be essential to provide further validation and refinement of the performance characteristics of the assays. More importantly, MRD testing can be integrated into clinical trial design to provide information that can dynamically guide therapy. In this section, we will discuss strategies for the inclusion of MRD assessment in lymphoma clinical trials, with a focus on DLBCL and MCL.
Liquid Biopsy
One practical application of panel-directed NGS MRD assessment is as a liquid biopsy in clinical trials that use precision-medicine approaches. An increasing proportion of clinical trials include acquisition of pretreatment tumor tissue for genomic assessment, which may be technically difficult or impossible if prior biopsy tissue is unavailable. ctDNA assessment of blood can potentially provide similar and possibly superior (given the broader sampling v single-site biopsy) genomic information while avoiding the cost, risk, and discomfort of a biopsy. For clinical trials that stratify patients on the basis of genomic factors (eg, cell of origin in DLBCL, which can be determined through ctDNA assessment18), ctDNA can potentially provide the necessary genetic information. When trials evaluate targeted therapies that have mutations associated with response or resistance, ddPCR or panel-directed assessment of ctDNA could be used to aid in participant selection or during the study to identify the emergence of mutations associated with treatment failure to justify study of a regimen modification (eg, addition of a second agent). For example, Camus et al42 described the detection of potentially targetable mutations by ddPCR in the blood of patients with DLBCL, and Scherer et al18 demonstrated the ability of panel-directed ctDNA assessment to detect BTK mutations in the blood of patients with DLBCL treated with ibrutinib.
Prognostic Value of MRD
Another potential application of MRD assessment is to leverage the prognostic value of pretreatment MRD abundance to risk stratify patients for specific management approaches. Currently, clinical prognostic factors are commonly used to delineate high- versus low-risk patients and test the application of more or less intensive treatment strategies. Quantitative MRD levels as determined by NGS-based MRD assessment in patients with DLBCL correlate with classic measures of tumor burden, including stage,16,18 lactate dehydrogenase,16-18 and metabolic tumor volume by PET-CT.17,18 Furthermore, in one DLBCL study, pretreatment ctDNA level as assessed by panel-directed NGS was associated with inferior PFS.18 Given its potential role as a prognostic biomarker, if further validated, pretreatment MRD level could be used to identify a high-risk target population for study of treatment intensification (eg, addition of an agent to R-CHOP [rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone] in patients with MRD-high, advanced-stage DLBCL). However, dynamic changes in MRD will likely prove more useful in guiding therapy escalation or de-escalation than single pretreatment measurements.
Depth of Response and MRD-Guided Therapy
Perhaps the greatest potential of MRD assessment in lymphoma care is the dynamic, real-time information gained about depth of response to treatment and the resulting opportunities to study MRD-based changes in management. A key question will be whether real-time MRD data can be used to tailor therapy in patients with lymphoma patients—to escalate, consolidate, prolong, de-escalate, or discontinue therapy on the basis of MRD status or change in MRD level. MRD status at key therapeutic time points, as determined in multiple analyses of RQ-PCR in patients with MCL and in preliminary studies of NGS-based MRD assessment in patients with DLBCL, are highly correlated with response to chemotherapy-based treatment and subsequent disease progression.16-18,22-24,27-35 Validation of these findings in MCL using NGS-based MRD assessment methods and additional studies validating the association between MRD levels and response rates and PFS after specific therapies in MCL and DLBCL will be critical.
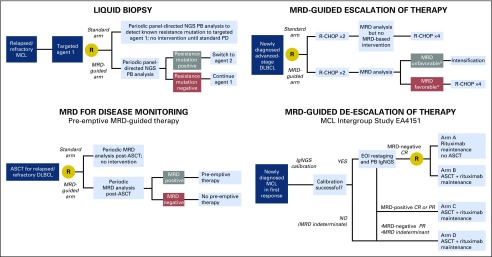
The information that MRD assessment provides about response depth could be used to design MRD-driven clinical trials that personalize management decisions at various time points throughout a patient’s course. If an early interim MRD assessment is associated with subsequent treatment outcome, as suggested in some studies,16,20 one could envision clinical trials in which therapy is changed during the trial on the basis of MRD results (Fig 2). With such an approach, the patients most likely to have favorable outcomes with the baseline regimen would not be exposed to intensification. Instead, the study could focus more intensive interventions on patients who, in real time, exhibit an inadequate response.
Fig 2.
Examples of clinical trial designs incorporating minimal residual disease (MRD) assessment and MRD-based intervention. ASCT, autologous stem-cell transplantation; CR, complete response; DLBCL, diffuse large B-cell lymphoma; EOI, end of induction; IgNGS, immunoglobulin gene next-generation sequencing; MCL, mantle cell lymphoma; NGS, next-generation sequencing; PB, peripheral blood; PD, progressive disease; PR, partial response; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. (*) MRD unfavorable versus favorable terminology is used because with early interim assessment the threshold may be more complicated than just MRD positive versus MRD negative (eg, a quantitative decrease in MRD level by a certain threshold).
Caution must be exercised in using interim response assessment data to guide therapeutic changes, because studies of interim PET-based treatment modification have not shown improved outcomes in DLBCL.49-51 The challenges of interim PET-based therapy in DLBCL offer important lessons for the development of MRD-driven clinical trials. Success requires both an accurate test (interim PET may be insufficiently sensitive or specific to merit treatment modification) as well as an effective intervention (chemotherapy intensification may be inadequate therapy for tumors exhibiting early signs of chemotherapy resistance). Clearly, the performance characteristics of the MRD test and the implications of the MRD results on which treatment decisions will be based must be well understood before embarking on such an approach. The type of intervention that can be justified on the basis of MRD testing will depend on test characteristics, tolerability of the planned treatment, and expected outcomes for the disease under study. For example, interim intensification of first-line therapy (eg, salvage chemotherapy and ASCT or chimeric antigen receptor–modified T cells) in a curable disease like DLBCL will require high specificity in an MRD test to ensure that potentially curative therapy is not discontinued early and patients are not exposed to toxicity on the basis of a false-positive test. Whereas, in an incurable disease like MCL, where consolidation treatment with ASCT is commonly used across the entire population of eligible patients in an effort to prolong PFS but without curative intent, MRD status after induction therapy could be used to study rational de-escalation of therapy (eg, ASCT v maintenance strategy). This type of approach is being evaluated in the upcoming Intergroup MCL consolidation trial (EA4151).
In addition to real-time decision making, MRD-based depth of response may be used as a surrogate end point to guide the evaluation of novel treatments. The association between MRD at EOI and PFS has already been demonstrated in MCL and may therefore be used to evaluate treatment strategies more rapidly than the traditional end point of PFS, because modern regimens are associated with median PFS in excess of 5 years.27,34 Recent trials have reported IgNGS-based MRD data in MCL.40,41 However, additional validation of its potential to predict PFS and overall survival will be necessary before MRD becomes a principal end point in clinical trials.
It is also important to remember that the value of MRD assessments may vary with the type of therapy under consideration. The patterns of MRD clearance and their implications for outcome should be separately established for different antilymphoma modalities, as was done, for example, in a recent study of panobinostat plus rituximab therapy in patients with relapsed or refractory DLBCL.20 Targeted therapies are now sometimes administered continuously until progression. However, dynamic MRD information may allow the safe discontinuation of treatment of certain patients, with significant potential gains in safety and cost. Another possible application of MRD is disease monitoring during immunotherapy (eg, checkpoint inhibitors) for lymphomas. Indeterminate PET results have been well described with those agents52; therefore, it would be useful to examine whether MRD assessment could provide a more tumor-specific method of assessing the burden of active lymphoma in patients with an indeterminate response, which could help to inform decisions about subsequent treatment.
MRD for Disease Monitoring
Another important application of MRD is in post-treatment surveillance. PET and CT scans may occasionally detect clinically occult relapse, but the benefit of imaging surveillance has not been demonstrated in lymphoma, particularly DLBCL or MCL.8-10,53,54 MRD studies in MCL and DLBCL provide preliminary evidence that MRD assessment may provide a sensitive and specific method for disease monitoring after therapy completion.16-18,23,24,28-36 The ability to detect relapse ahead of clinical symptoms does not necessarily imply a clinical benefit, and it remains to be proven whether early MRD-based intervention can affect outcome. The availability of sensitive and specific tools at least allows that question to be addressed in clinical trials. Here again, more data are needed on the conditions under which MRD most accurately predicts relapse, which will require inclusion of MRD assessment in clinical trials, even if MRD-based intervention is not considered.
The Nordic group has already evaluated MRD-based surveillance and pre-emptive therapy in MCL.55 In one study, patients with MCL who were identified as MRD positive by RQ-PCR after ASCT received pre-emptive rituximab therapy in the absence of clinical relapse. The study demonstrated that rituximab could reinduce molecular remission (87% of the time), and 48% of patients remained in clinical remission. In patients who did have subsequent clinical relapse, the median interval between rituximab and relapse was 55 months.55 These findings are provocative, but MRD-based pre-emptive rituximab has not become standard, because no definitive benefit could be demonstrated in the absence of a control group. With the increasing availability of well-tolerated, effective treatments and the association between MRD status and outcome in MCL, there is a strong rationale for the development of MRD-driven pre-emptive therapy. However, it is critical that clinical trials of MRD-guided interventions in lymphoma include control groups to determine whether pre-emptive therapy can actually improve outcomes.
FUTURE DIRECTIONS AND CONCLUSION
Although this review has focused on the evidence accumulated on the use of MRD assessment in MCL and DLBCL, these tools, particularly NGS-based methods, have broader applicability. Even in the case of classic Hodgkin lymphoma (cHL), a disease in which the tumor cells comprise only a small proportion of the tumor mass, NGS-based MRD assessment methods have demonstrated initial promise. In early studies, IgNGS identified a tumor clone for tracking in 56% to 71% of patients, and ctDNA was detected in 73% of patients.43,56 Using a separate NGS method, Vandenberghe et al57 identified cHL-specific genomic imbalances in ctDNA at diagnosis that became undetectable after treatment. Finally, XPO1 mutations were detected at diagnosis in patients with cHL using digital PCR, and the persistence of this mutation in patients with a negative PET scan at EOI showed a possible association with relapse.58 Further development and validation of MRD techniques in cHL is required, but these results and others suggest that MRD detection may ultimately be feasible across all lymphoma subtypes.
In conclusion, MRD assessment has the potential to be a highly sensitive and specific tool for assessing the presence of subclinical disease in patients with lymphoma. Although several techniques are currently being studied, NGS-based methods will likely have the broadest applicability, because they exploit universal features of lymphoid malignancies. To realize the promise of MRD assessment for improving the care of patients with lymphoma, it is essential that adequate sample collection at appropriate time points be incorporated into clinical trials. Technical requirements for tissue and blood collection, processing, and storage should be considered to ensure the quality of the downstream MRD data that are obtained. In certain lymphoma subtypes, there may be discordant MRD findings between PBMCs and plasma41,43; therefore, collection of both should be considered. Ultimately, clinical trials testing MRD-guided management of patients with lymphoma could transform the treatment paradigm in all lymphoma subtypes. With the power to provide real-time, quantitative data about tumor burden, noninvasive genomic profiling, and even tracking of clonal dynamics, MRD assessment can help bring us closer to the goal of personalized lymphoma care.
ACKNOWLEDGMENT
We thank Mark Murakami, MD, for his thoughtful review of the manuscript and Jared Millar and the City of Hope Creative Solutions Department for their assistance with digital artwork.
Footnotes
Supported by a Lymphoma Research Foundation Clinical Investigator Larry and Denise Mason Career Development Award and National Cancer Institute, National Institutes of Health, Awards No. NIH 2K12CA001727-21 and P50CA107399 (A.F.H.) and by the Harold and Virginia Lash Foundation (P.A.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Alex F. Herrera
Data analysis and interpretation: Alex F. Herrera
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Minimal Residual Disease Assessment in Lymphoma: Methods and Applications
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Alex F. Herrera
Consulting or Advisory Role: Bristol-Myers Squibb, Pharmacyclics, Merck, Genentech
Research Funding: Pharmacyclics (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Seattle Genetics (Inst), Genentech (Inst), Immune Design (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Philippe Armand
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Pfizer
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst), Affimed Therapeutics (Inst), Otsuka (Inst), Sigma-Tau (Inst), Roche (Inst), Tensha Therapeutics (Inst), Sequenta (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
REFERENCES
- 1.Huntington SF, Svoboda J, Doshi JA: Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. J Clin Oncol 33:1467-1474, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Berrington de González A, Mahesh M, Kim KP, et al. : Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 169:2071-2077, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DJ, Hall EJ: Computed tomography: An increasing source of radiation exposure. N Engl J Med 357:2277-2284, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz CH, Schöder H, Teruya-Feldstein J, et al. : Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol 28:1896-1903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulaner GA, Lilienstein J, Gönen M, et al. : False-positive [18F]fluorodeoxyglucose-avid lymph nodes on positron emission tomography-computed tomography after allogeneic but not autologous stem-cell transplantation in patients with lymphoma. J Clin Oncol 32:51-56, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Avivi I, Zilberlicht A, Dann EJ, et al. : Strikingly high false positivity of surveillance FDG-PET/CT scanning among patients with diffuse large cell lymphoma in the rituximab era. Am J Hematol 88:400-405, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Zinzani PL, Stefoni V, Tani M, et al. : Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol 27:1781-1787, 2009 [DOI] [PubMed] [Google Scholar]
- 8.El-Galaly TC, Jakobsen LH, Hutchings M, et al. : Routine imaging for diffuse large B-cell lymphoma in first complete remission does not improve post-treatment survival: A Danish-Swedish population-based study. J Clin Oncol 33:3993-3998, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Thompson CA, Ghesquieres H, Maurer MJ, et al. : Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol 32:3506-3512, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheah CY, Dickinson M, Hofman MS, et al. : Limited clinical benefit for surveillance PET-CT scanning in patients with histologically transformed lymphoma in complete metabolic remission following primary therapy. Ann Hematol 93:1193-1200, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Bardelli A, Pantel K: Liquid biopsies, what we do not know (yet). Cancer Cell 31:172-179, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Schwarzenbach H, Hoon DS, Pantel K: Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 11:426-437, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Diaz LA, Jr, Bardelli A: Liquid biopsies: Genotyping circulating tumor DNA. J Clin Oncol 32:579-586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roschewski M, Staudt LM, Wilson WH: Dynamic monitoring of circulating tumor DNA in non-Hodgkin lymphoma. Blood 127:3127-3132, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alix-Panabières C, Pantel K: Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 6:479-491, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Roschewski M, Dunleavy K, Pittaluga S, et al. : Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: A correlative biomarker study. Lancet Oncol 16:541-549, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtz DM, Green MR, Bratman SV, et al. : Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 125:3679-3687, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherer F, Kurtz DM, Newman AM, et al. : Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med 8:364ra155, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armand P, Oki Y, Neuberg DS, et al. : Detection of circulating tumour DNA in patients with aggressive B-cell non-Hodgkin lymphoma. Br J Haematol 163:123-126, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Assouline SE, Nielsen TH, Yu S, et al. : Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood 128:185-194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladetto M, Brüggemann M, Monitillo L, et al. : Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 28:1299-1307, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Rossi D, Diop F, Spaccarotella E, et al. : Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood 129:1947-1957, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Hoster E, Pott C: Minimal residual disease in mantle cell lymphoma: Insights into biology and impact on treatment. Hematology (Am Soc Hematol Educ Program) 2016:437-445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pott C, Hoster E, Delfau-Larue MH, et al. : Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: A European MCL intergroup study. Blood 115:3215-3223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Böttcher S, Ritgen M, Buske S, et al. : Minimal residual disease detection in mantle cell lymphoma: Methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica 93:551-559, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Cheminant M, Derrieux C, Touzart A, et al. : Minimal residual disease monitoring by 8-color flow cytometry in mantle cell lymphoma: An EU-MCL and LYSA study. Haematologica 101:336-345, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermine O, Hoster E, Walewski J, et al. : Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): A randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 388:565-575, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Johnson JL, Koval G, et al. : Detection of minimal residual disease following induction immunochemotherapy predicts progression free survival in mantle cell lymphoma: Final results of CALGB 59909. Haematologica 97:579-585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen NS, Pedersen L, Elonen E, et al. : Primary treatment with autologous stem cell transplantation in mantle cell lymphoma: Outcome related to remission pretransplant. Eur J Haematol 71:73-80, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Pott C, Schrader C, Gesk S, et al. : Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood 107:2271-2278, 2006 [DOI] [PubMed] [Google Scholar]
- 31. doi: 10.1182/blood-2016-03-704023. Albertsson-Lindblad A, Kolstad A, Laurell A, et al: Lenalidomide-bendamustine-rituximab in untreated mantle cell lymphoma > 65 years, the Nordic Lymphoma Group phase I+II trial NLG-MCL4. Blood [epub ahead of print on June 27, 2016] [DOI] [PubMed] [Google Scholar]
- 32.Kolstad A, Laurell A, Jerkeman M, et al. : Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood 123:2953-2959, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowan AJ, Stevenson PA, Cassaday RD, et al. : Pretransplantation minimal residual disease predicts survival in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in complete remission. Biol Blood Marrow Transplant 22:380-385, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisler CH, Kolstad A, Laurell A, et al. : Nordic MCL2 trial update: Six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support—Still very long survival but late relapses do occur. Br J Haematol 158:355-362, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Pott C, Macintyre E, Delfau-Larue MH, et al: MRD eradication should be the therapeutic goal in mantle cell lymphoma and may enable tailored treatment approaches: Results of the Intergroup trials of the European MCL Network. Blood 124, 2014 (abstr 147) [Google Scholar]
- 36.Geisler CH, Kolstad A, Laurell A, et al. : Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 112:2687-2693, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pott C, Delfau-Larue M, Beldjord K, et al: R-CHOP vs R-FC followed by maintenance with rituximab of IFN: First results of MRD assessment within the randomized trial for elderly patients with MCL. Ann Oncol 22, 2011 (suppl 4; abstr 233)
- 38.Callanan MB, Delfau MH, Macintyre E, et al. : Predictive power of early, sequential MRD monitoring in peripheral blood and bone marrow in patients with mantle cell lymphoma following autologous stem cell transplantation with or without rituximab maintenance: Interim results from the LyMa-MRD project, conducted on behalf of the Lysa Group. Blood 126, 2015. (abstr 338) [Google Scholar]
- 39.Kaplan LD, Jung SH, Stock W, et al. : Bortezomib maintenance (BM) versus consolidation (BC) following aggresive immunochemotherapy and autologous stem cell transplant (ASCT) for untreated mantle cell lymphoma (MCL): CALGB (Alliance) 50403. Blood 126, 2015. (abstr 337) [Google Scholar]
- 40.Armand P, Redd R, Bsat J, et al. : A phase 2 study of rituximab-bendamustine and rituximab-cytarabine for transplant-eligible patients with mantle cell lymphoma. Br J Haematol 173:89-95, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Chen RW, Li H, Bernstein SH, et al. : RB but not R-HCVAD is a feasible induction regimen prior to auto-HCT in frontline MCL: Results of SWOG study S1106. Br J Haematol 176:759-769, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camus V, Sarafan-Vasseur N, Bohers E, et al. : Digital PCR for quantification of recurrent and potentially actionable somatic mutations in circulating free DNA from patients with diffuse large B-cell lymphoma. Leuk Lymphoma 57:2171-2179, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Herrera AF, Kim HT, Kong KA, et al. : Next-generation sequencing-based detection of circulating tumour DNA After allogeneic stem cell transplantation for lymphoma. Br J Haematol 175:841-850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohers E, Viailly PJ, Dubois S, et al. : Somatic mutations of cell-free circulating DNA detected by next-generation sequencing reflect the genetic changes in both germinal center B-cell-like and activated B-cell-like diffuse large B-cell lymphomas at the time of diagnosis. Haematologica 100:e280-e284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurtz DM, Scherer F, Newman AM, et al: Dynamic noninvasive genome monitoring for outcome prediction in diffuse large B-cell lymphoma. Blood 126, 2015 (abstr 130)
- 46.Mancuso P, Calleri A, Antoniotti P, et al. : If it is in the marrow, is it also in the blood? An analysis of 1,000 paired samples from patients with B-cell non-Hodgkin lymphoma. BMC Cancer 10:644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tibiletti MG, Martin V, Bernasconi B, et al. : BCL2, BCL6, MYC, MALT 1, and BCL10 rearrangements in nodal diffuse large B-cell lymphomas: A multicenter evaluation of a new set of fluorescent in situ hybridization probes and correlation with clinical outcome. Hum Pathol 40:645-652, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Newman AM, Lovejoy AF, Klass DM, et al. : Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol 34:547-555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sehn LH, Hardy ELG, Gill KK, et al: Phase 2 trial of interim PET scan-tailored therapy in patients with advanced stage diffuse large B-cell lymphoma (DLBCL) in British Columbia (BC). Blood 124, 2014 (abstr 392) [Google Scholar]
- 50.Mamot C, Klingbiel D, Hitz F, et al. : Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol 33:2523-2529, 2015 [DOI] [PubMed] [Google Scholar]
- 51. Duehrsen U, Huttmann A, Muller S, et al: Positron emission tomography (PET) guided therapy of aggressive lymphomas: A randomized controlled trial comparing different treatment approaches based on interim PET results (PETAL trial). Blood 124, 2014 (abstr 391) [Google Scholar]
- 52. doi: 10.1182/blood-2016-05-718528. Cheson BD, Ansell S, Schwartz L, et al: Refinement of the Lugano classification response criteria for lymphoma in the era of immunomodulatory therapy. Blood 128:2489-2496, 2016. [DOI] [PubMed] [Google Scholar]
- 53.Hosein PJ, Pastorini VH, Paes FM, et al. : Utility of positron emission tomography scans in mantle cell lymphoma. Am J Hematol 86:841-845, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Gill S, Wolf M, Prince HM, et al. : [18F]fluorodeoxyglucose positron emission tomography scanning for staging, response assessment, and disease surveillance in patients with mantle cell lymphoma. Clin Lymphoma Myeloma 8:159-165, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Kolstad A, Pedersen LB, Eskelund CW, et al. : Molecular monitoring after autologous stem cell transplantation and preemptive rituximab treatment of molecular relapse: Results from the Nordic mantle cell lymphoma studies (MCL2 and MCL3) with median follow-up of 8.5 years. Biol Blood Marrow Transplant 23:428-435, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Oki Y, Neelapu SS, Fanale M, et al. : Detection of classical Hodgkin lymphoma specific sequence in peripheral blood using a next-generation sequencing approach. Br J Haematol 169:689-693, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandenberghe P, Wlodarska I, Tousseyn T, et al. : Non-invasive detection of genomic imbalances in Hodgkin/Reed-Sternberg cells in early and advanced stage Hodgkin’s lymphoma by sequencing of circulating cell-free DNA: A technical proof-of-principle study. Lancet Haematol 2:e55-e65, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Camus V, Stamatoullas A, Mareschal S, et al. : Detection and prognostic value of recurrent exportin 1 mutations in tumor and cell-free circulating DNA of patients with classical Hodgkin lymphoma. Haematologica 101:1094-1101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]