Abstract
Prostaglandin E2 (PGE2) is associated with proliferation and angiogenesis in colorectal tumours. The role of prostaglandin transporter OATP2A1/SLCO2A1 in colon cancer tumorogenesis is unknown. We evaluated mice of various Slco2a1 genotypes in a murine model of colon cancer, the adenomatous polyposis (APC) mutant (Apc ∆716/+) model. Median lifespan was significantly extended from 19 weeks in Slco2a1 +/+/Apc Δ716/+ mice to 25 weeks in Slco2a1 −/−/Apc Δ716/+ mice. Survival was directly related to a reduction in the number of large polyps in the Slco2a1 −/− /Apc ∆716/+ compared to the Slco2a1 +/+/Apc Δ716/+ or Slco2a1 +/−/Apc Δ716/+mice. The large polyps from the Slco2a1 −/− /Apc ∆716/+ mice had significant reductions in microvascular density, consistent with the high expression of Slco2a1 in the tumour-associated vascular endothelial cells. Chemical suppression of OATP2A1 function significantly reduced tube formation and wound-healing activity of PGE2 in human vascular endothelial cells (HUVECs) although the amount of extracellular PGE2 was not affected by an OATP2A1 inhibitor. Further an in vivo model of angiogenesis, showed a significant reduction of haemoglobin content (54.2%) in sponges implanted into Slco2a1 −/−, compared to wildtype mice. These studies indicate that OATP2A1 is likely to promote tumorogenesis by PGE2 uptake into the endothelial cells, suggesting that blockade of OATP2A1 is an additional pharmacologic strategy to improve colon cancer outcomes.
Introduction
Colon cancer is one of the most common malignancies in humans and epidemiological studies have shown that ingesting aspirin, a nonsteroidal anti-inflammatory drug (NSAID), produces significant reductions in colorectal cancer death rate among individuals1. NSAIDs inhibit prostaglandin E2 (PGE2) production by COX2,3 and in animal models and humans inhibition of COX by NSAIDs suppresses colorectal tumour growth4,5. Genetic ablation of COX-1 or 2 in mice increases colon cancer survival rates, further supporting a role for suppressing PGE2 formation as a strategy to block colon cancer6. Multiple approaches have been described for disrupting the formation of PGE2 through COX inhibition7,8. However, COX-2, a rate-limiting enzyme of PGE2 synthesis, is upregulated in human colorectal tumours and their metastases9,10. This suggests that the dose of NSAID may be insufficient to inhibit the increased amounts of COX-2 in tumours and alternative strategies to disrupt the PGE2 pathway might be successful. For instance, the cell surface receptors for PGE2 are viable candidates because animals lacking the receptors EP111 and EP212 have increased survival rates in colon cancer models12. While PGE2 formation and its receptors contribute to colorectal cancer progression, it is unknown if the transporters which transport PGE2 have a role in colon cancer.
Extracellular PGE2 after binding to its cognate receptors activates a downstream signalling pathway that contributes to colon cancer progression by promoting the expression of genes involved in cell survival, tissue invasion and metastasis8–10. Generally anionic PGE2 does not readily cross biological membranes. The efficient release of PGE2 from cells requires a high affinity exporter, such as ABCC413. PGE2 can be reimported and requires an uptake carrier14–19. The organic anion transporting polypeptide (OATP) 2A1 encoded by SLCO2A1 (also known as PGT) is a high affinity uptake transporter for PGE2 17,18. A previous study suggested that reduced OATP2A1 expression in colorectal carcinoma might enhance colorectal cancer20; however, there is no direct in vivo or in vitro evidence for OATP2A1 contributing to processes affecting colorectal tumour progression.
The present study evaluated if OATP2A1 expression impacted colorectal tumorigenesis in a murine model. To accomplish this, mice with or without Slco2a1 were interbred with mice harbouring the APC adenomatous polyposis mutant (Apc ∆716/+) allele likely to mice prone gastrointestinal tumours21. Here, we present the first evidence that reduction in OATP2A1 levels or function has a beneficial role in promoting colon cancer survival by altering tumorigenesis.
Results
Impact of Slco2a1 on colon cancer in a murine model (ApcΔ716/+)
Mice lacking Slco2a1were intercrossed with mice harbouring a truncated form of the adenomatous polyposis coli gene (APC/Apc) (Apc Δ716/+) which predisposes mice to lethal gastrointestinal tumours21. The absence of Slco2a1 promoted survival as the median lifespan of 19 weeks in Slco2a1 +/+/Apc Δ716/+ mice (n = 38) was extended to 22 and 25 weeks in Slco2a1 +/−/Apc Δ716/+ (n = 35) and Slco2a1 −/−/Apc Δ716/+ mice (n = 9), respectively (p = 0.008 and 0.005) (Fig. 1a). These findings suggest that lower Oatp2a1 levels are protective and provide a survival advantage, whereas higher levels are not.
Figure 1.
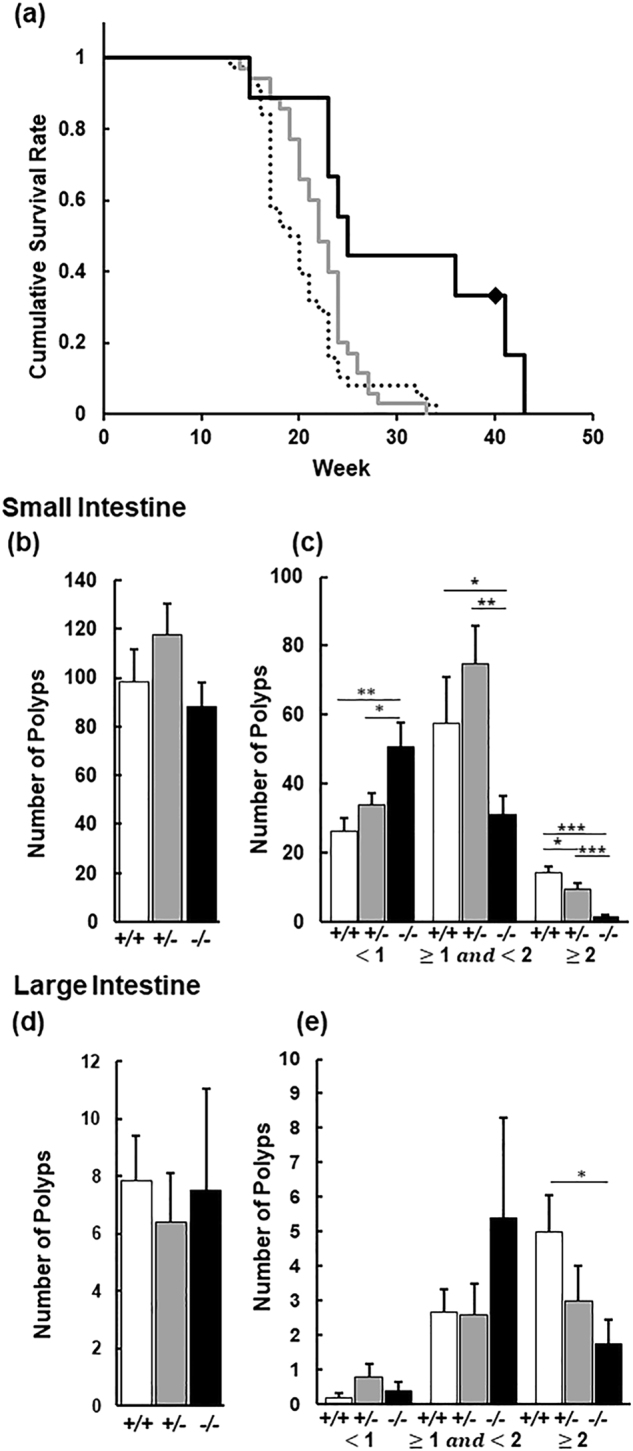
Impact of deletion of Slco2a1 on survival and growth of intestinal polyps in Apc Δ716/+ mice. (a) Survival curves of Slco2a1 +/+/Apc ∆716/+ (broken line, n = 38 including 23 males and 15 females), Slco2a1 +/−/Apc Δ716/+ (grey line, n = 35 including 17 males and 18 females), and Slco2a1 −/−/Apc Δ716/+ (solid line, n = 9 including 6 males and 3 females). Diamond shows censored data. Cumulative survival rate was analysed by Kaplan-Meier method for each cohort. (b,d) Number of overall total polyps in the small and large intestine in Slco2a1 +/+/Apc ∆716/+ (+/+, white), Slco2a1 +/−/Apc Δ716/+ (+/−, grey), and Slco2a1 −/−/Apc Δ716/+ (−/−, black). Number accounts for all polyps under the stereomicroscope. (c,e) Number of polyps are shown for three different size based on diameter in the small and large intestines. Each mouse was sacrificed at age of 13 weeks. Each bar represents the mean ± SEM. (n = 5–8). *, **, and *** indicate significant difference in polyp number of polyps by Student’s t-test with p < 0.05, <0.01, and <0.001, respectively.
We next assessed if polyp formation by Apc Δ716/+ was altered by the amount of Oatp2a1. Mice were examined at 13 weeks for the polyp size and number in the small and large intestines of the Apc Δ716/+ mutant mice that had been intercrossed with mice having either a single or no Slco2a1 allele. The total number of polyps in the small intestine of Slco2a1 +/+/Apc Δ716/+, Slco2a1 +/−/Apc Δ716/+, and Slco2a1 −/−/Apc Δ716/+ mice were 98.2 ± 13.4, 117.6 ± 12.8, and 88.0 ± 9.9, respectively. Although there was no significant difference in total polyp number observed between the Slco2a1 genotypes (Fig. 1b), the size of the polyps was affected by the Slco2a1 genotype. Notably, polyps less than 1 mm in diameter were more frequent in Slco2a1 −/−/Apc Δ716/+ mice and accounted for more than 50% of the total polyps. In contrast, larger polyps (between 1-to-2 mm as well as >2 mm) were significantly less frequent in the Slco2a1 −/−/Apc Δ716/+ mice than those in Slco2a1 +/+/Apc Δ716/+ and Slco2a1 +/−/Apc Δ716/+ mice. Large polyps (>2 mm) in Slco2a1 −/−/Apc Δ716/+ mice accounted for only 1.3 ± 0.59% of total polyps vs. over 10% of the polyps in the Slco2a1 +/+/Apc ∆716/+ mice (Fig. 1c). There was no significant difference in the total polyps in the large intestine between the three Slco2a1 genotypes (Fig. 1d). However, larger polyps were significantly less frequent in the Slco2a1 −/−/Apc Δ716/+ mice (Fig. 1e). The EP4 receptor appears to contribute to colon carcinogenesis22,23. Expression of the EP4 receptor in the polyps from the small intestine revealed comparable amounts in Slco2a1 +/+/Apc Δ716/+ and Slco2a1 −/−/Apc Δ716/+ mice (Supplementary Figure S1).
Slco2a1 deficiency affects microvascular density (MVD) in the small intestine
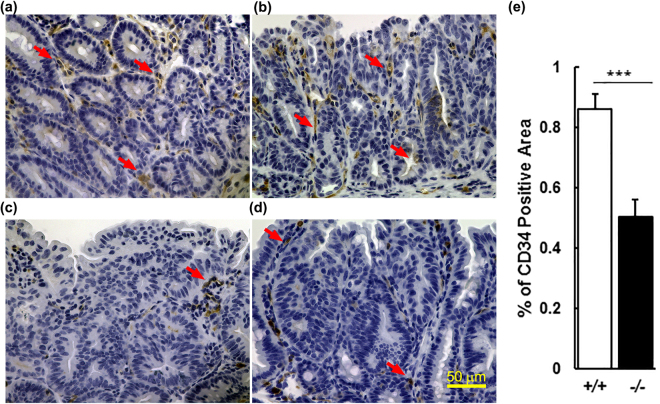
The size of colon cancer polyps has been related to vasculature and angiogenesis24. MVD is often an indicator of angiogenic capability of endothelial cells25. To quantify the MVD in polyps from the small intestine, an antibody against the endothelial marker CD34 was used. The extent of the CD34-positive area was compared in intestinal polyps between the Slco2a1 +/+/Apc ∆716/+ (Fig. 2a and b) and Slco2a1 −/−/Apc Δ716/+ (Fig. 2c and d) mice. The CD34 immunoreactivity was more frequently detected in the polyps taken from Slco2a1 +/+ /Apc Δ716/+ mice. The proportion of CD34-positive area within the polyps of Slco2a1 −/−/Apc Δ716/+ mice was reduced to 58.7%, and significantly lower (p = 10−5) than that in Slco2a1 +/+ /Apc Δ716/+ mice (Fig. 2e). This result suggests that angiogenesis is suppressed in the absence of Slco2a1.
Figure 2.
Effect of Slco2a1 on MVD in intestinal polyps. CD34 expression in paraffin section (4 μm) of polyps in small intestines prepared from two different Slco2a1 +/+/Apc ∆716/+ (+/+) (a,b) and Slco2a1 −/−/Apc Δ716/+ (−/−) (c,d). Pictures were taken under BZ-9000 with a magnification (× 40). The section was incubated with anti-CD34 antibody and stained brown by immunoenzymatic reaction with DAB. Typical DAB stain is indicated by red arrows. Nuclei were counter-stained blue with haematoxylin. Indicated arrows show immunoreactivity for anti-CD34 antibody. Experiments were repeated at least three times, and most representative pictures are shown. (e) CD34 positive area (%) in the region of determined polyps. The region of interest was quantitatively determined by Image J and bar graph represents the mean values of 48 individual section from 4 mice (12 sections from each). *** indicates significant difference by Student’s t-test with p < 0.001.
Immunolocalization of Oatp2a1 in intestinal polyps
Immunohistochemical analysis was performed to determine Oatp2a1 expression in normal small intestine and colon compared to polyp in the small intestine of Slco2a1 +/+ /Apc Δ716/+ mice (Fig. 3a–c). Oatp2a1 protein was prominent in the blood vessel endothelia (as indicated by a red arrowhead), but also in some cells with a round morphology (by red arrows) in the stromal tissues of the normal small intestine (Fig. 3a) and colon (Fig. 3b) of Slco2a1 +/+/Apc ∆716/+ mice. Notably, the Oatp2a1 immunoreactivity appears much more intense in the stromal tissues of intestinal polyps of Slco2a1 +/+ /Apc Δ716/+ mice (Fig. 3c), compared to the much weaker immunoreactivity in the normal small intestine and colon. As expected no Oatp2a1 immunoreactivity was detected in the corresponding region of Slco2a1 −/− /Apc Δ716/+ mice (Fig. 3d–f). To confirm Oatp2a1-expression in endothelial cells (versus macrophage), intestinal polyp samples were prepared from Slco2a1 +/+ /Apc Δ716/+ mice and co-labelled with anti-CD34 (Fig. 3g) or anti-F4/80 (a marker of activated macrophage) (Fig. 3h) and anti-Oatp2a1 antibody. Red fluorescence for Oatp2a1 mostly co-localized with the green fluorescence for anti-CD34 (Fig. 3g, merged). Red fluorescence only partly co-localized with green for anti-F4/80 (Fig. 3h, merged), indicating Oatp2a1 is primarily expressed in vasculature endothelial cells.
Figure 3.
Immunohistochemical localization of Oatp2a1 in normal gut and intestinal polyps. Paraffin section (4 μm) of mouse small intestine (a,d), colon (b,e), and intestinal polyps (c,f) of Slco2a1 +/+/Apc ∆716/+ (+/+, a–c) and Slco2a1 −/−/Apc Δ716/+ (−/−, d–f) was incubated with anti-Oatp2a1 antibody and stained brown by immunoenzymatic reaction with DAB. Nuclei were counter-stained blue with haematoxylin. Red arrowhead and arrow indicate endothelial and stromal cells, respectively. Co-localization of CD34 (g, green) or F4/80 (h, green) with Oatp2a1 (red) was detected in polyps of small intestines of Slco2a1 +/+/Apc ∆716/+. Nuclei were counterstained with Hoechst33342 (blue). Indicated white arrowheads show typical positive fluorescence for each reaction. Experiments were repeated at least three times and representative images are shown.
Role of OATP2A1 on angiogenic capability of HUVECs
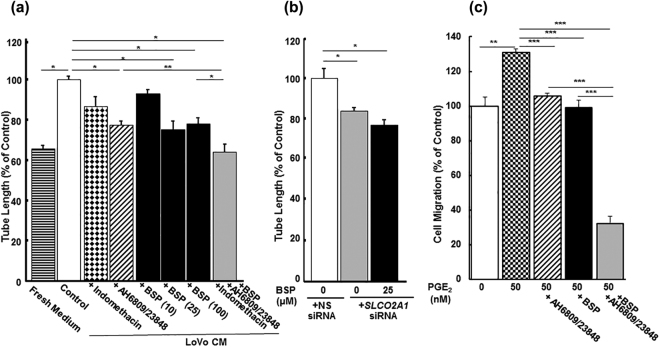
Both the formation of tube-like structures and endothelial cell migration are hallmark features of angiogenesis26. Transformed epithelial cells release many soluble factors, including PGE2 27. To assess the role of soluble factors and OATP2A1, the “tube formation” assay in HUVECs was used. Our initial experiments used conditioned medium (CM) from the colon cancer, LoVo cell line. The PGE2 concentration in the CM from LoVo cells was approximately 6-fold higher than fresh medium (23.2 ± 3.13 vs 4.0 ± 1.23 pM), and HUVECs treated with CM significantly increased the total tube length by 52.7% (Fig. 4a). Tube formation observed in CM was reduced by the presence of either the COX inhibitor, indomethacin, or the EP antagonists, AH6809 and AH23848. BSP, an OATP2A1 inhibitor, suppressed tube formation modestly at 10 μM and and maximally at 100 μM. The reduced tube formation displayed by indomethacin and EP antagonists was further decreased by the addition of BSP. Moreover, HUVEC tube formation was significantly reduced by silencing SLCO2A1 and as expected it was not further suppressed by the presence of BSP (Fig. 4b). The SLCO2A1 mRNA was decreased to a level that was 12.8% of control cultures transfected with the NS siRNA HUVECs (n = 6, p = 0.0001). Because solutes other than PGE2 might produce the effects observed by the CM, the impact of PGE2 on HUVECs was assessed by wound healing assay in FBS-free EBM™ supplemented with EGM™-2. HUVEC migration significantly increased by 31% in the presence of PGE2 (Fig. 4c). Migratory activity of HUVECs treated with PGE2 was reduced significantly in the presence of the AH6809 and AH23848, or BSP, and further declined in the presence of the both antagonists and BSP as observed in the tube formation assays undertaken in LoVo CM.
Figure 4.
Angiogenic capability of HUVECs is affected by OATP2A1. Tube formation by HUVECs was measured in the CM from human colorectal cancer LoVo cells, which were incubated in Ham’s F-12 for 24 h (a). HUVECs were incubated with fresh medium (stripe) or the CM in the absence (as control, open) or the presence of indomethacin (10 µM, diamond), mixture of EP antagonists (AH6809; 10 µM, AH23848; 25 µM, hatched), BSP (10, 25, or 100 µM, closed), or all agents (grey) on Matrigel® for 4 h. Tube formation assay was observed in HUVECs knocked-down for SLCO2A1 (b). Wound healing assay of HUVECs untreated (as control, open) or treated with PGE2 (50 nM, check) in the absence or presence of mixture of EP antagonists (AH6809; 10 µM and AH23848; 25 µM, hatched), BSP (25 µM, closed), or the both (grey) for 10 h (c). Experiments were repeated at least three times with triplicate, and each point represents results with the mean ± SEM. (n = 3). *, ** and *** indicate significant difference in tube length from by Student’s t-test with p < 0.05, <0.01 and <0.001, respectively.
To determine the impact of OATP2A1 on angiogenesis in vivo, we used the sponge subcutaneous implantation model28 and measured haemoglobin content because it correlates with angiogenesis in this model29. We compared haemoglobin content of the sponge granular tissue in Slco2a1 +/+ and Slco2a1 −/− mice that were implanted with a sponge that had been injected with either vehicle or BSP. Haemoglobin content was reduced by 54.2% in Slco2a1-deficient mice, compared to Slco2a1 +/+ mice. Further, sponge treatment with BSP produced a comparable reduction in haemoglobin content. Therefore, these results strongly indicate that OATP2A1 function is required to promote angiogenesis (Fig. 5).
Figure 5.

Impact of Oatp2a1 on in vivo angiogenic capability of endothelial cells. Haemoglobin was measured in implanted sponges in Slco2a1 wildtype (+/+, white bar) or Slco2a1 −/− (−/−, grey) mice (with mixed genetic background of BL/6 and SV129). BSP in physiological saline (5 nmol/100 μL/day/mouse) was consecutively injected into the sponge for 9 days (black), otherwise mice were injected with sterilized physiological saline. On Day 10, haemoglobin content was measured and normalized with wet weight of sponge granular tissues. Each bar represent of the mean value of 3–8 mice with SEM. * indicates significant difference in tube length from by Student’s t-test with p < 0.05, and <0.01, respectively.
Expression of functional OATP2A1 in HUVECs
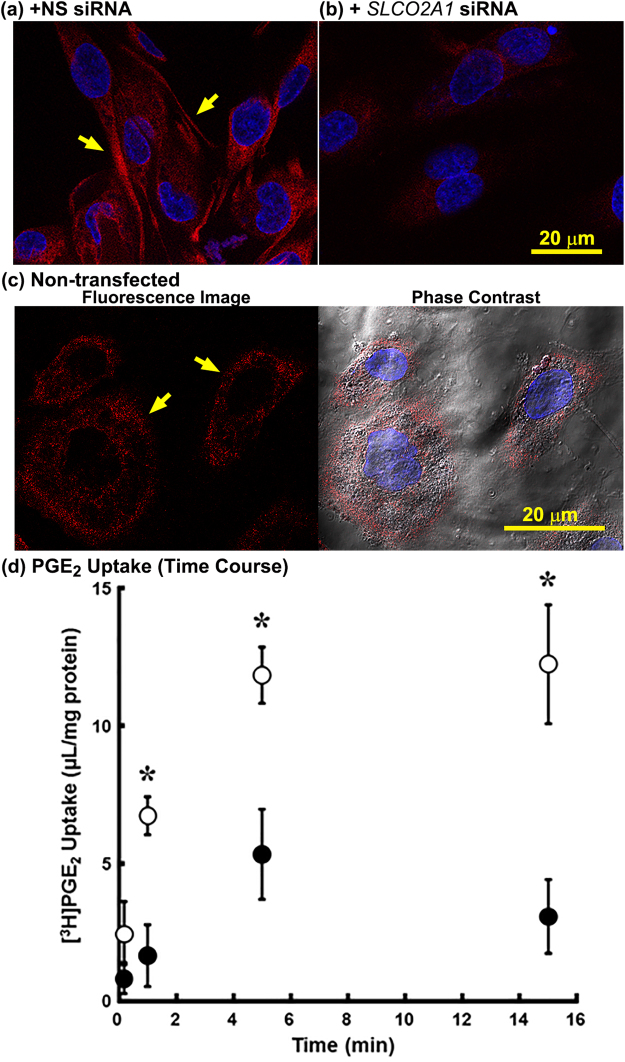
Expression of OATP2A1 was investigated in HUVECs by means of immunocytochemistry. The red fluorescence revealed by the anti-OATP2A1 antibody indicated OATP2A1 is mostly at the plasma membranes of HUVECs transfected with NS siRNA (Fig. 6a). The specificity of the anti-OATP2A1 is shown by the strong reduction in the immunodetectable OATP2A1 in the SLCO2A1 siRNA (Fig. 6b). The merged optical/fluorescence images of HUVECs show that OATP2A1 primarily localizes to the plasma membrane and/or submembranous structure (Fig. 6c). Accordingly, the [3H]PGE2 uptake by HUVECs and its intracellular accumulation at steady state were significantly reduced in the presence of BSP (Fig. 6d), consistent with the role of OATP2A1 in determining uptake and intracellular accumulation of PGE2.
Figure 6.
OATP2A1 function affects PGE2 accumulation in HUVECs. Fluorescence immunocytochemistry was performed with anti-OATP2A1 antibody in HUVECs transfected with NS siRNA (a) or SLCO2A1 siRNA (b), and none (c), followed by counterstaining with Hoechst 33342 for nuclei (blue). Fluorescence was merged with confocal phase contrast (c, phase contrast). Yellow arrow indicates fluorescence on the plasma membranes. Experiments were repeated at least three times, and representative image is shown. Uptake of [3H]PGE2 (1.5 nM) by HUVECs (d). Cellular uptake was observed in the absence (as control, open circle) or presence of BSP (25 µM, closed circle) over 15 min at 37 °C and pH 7.4. Each point represents the mean ± SEM. (n = 3). * indicates significant difference in uptake from control value by Student’s t-test with p < 0.05.
Effect of PGE2 taken up by HUVECs on their migratory activity
Finally, to determine whether PGE2 taken up by cells is involved in angiogenesis, HUVECs were treated with indomethacin for 16 hrs, and then the impact of BSP on their migratory activity was assessed for 10 hrs in the presence of PGE2. For control cells, the migration distance increased in a time-dependent manner. In contrast BSP-treated cells showed reduced migration at every time point (Fig. 7a). Unexpectedly, there was no significant difference in extracellular PGE2 between untreated and BSP-treated HUVECs (Fig. 7b). Consistent with the migration assay, under the same condition, intracellular accumulation of [3H]PGE2 in HUVECs was significantly reduced by BSP, and reached a plateau in 1 hr (Fig. 7c). Moreover, the effect of BSP on mRNA expression of adhesion molecules, which play critical role in migration, in HUVECs was determined by quantitative RT-PCR (Fig. 7d). mRNA expression of VE-cadherin, integrin αV and β3 was significantly decreased in HUVECs treated with BSP. These data suggest that PGE2 taken up by cells plays a role in migration of endothelial cells independently cell surface EP receptor.
Figure 7.
Effect of exogenous PGE2 on Migratory Capability of HUVECs. (a) Migratory activity of HUVECs pre-treated with indomethacin (100 μM) for 16 hrs. Migration distance of HUVECs was determined in EGM-2 containing PGE2 (3 nM) and indomethacin (100 μM) in the absence (Control, open circles) or the presence of BSP (25 μM, closed circles). Each point represents the mean ± SEM. (n = 4). (b) Extracellular PGE2 concentration was monitored for 10 hrs under the same condition. (c) Cellular uptake of [3H]PGE2 (10 nM) by HUVECs. HUVECs were pre-incubated with EGM-2 containing indomethacin (100 μM) for 16 hrs before experiment. Uptake was measured in the absence (Control, open circles) or presence of BSP (25 µM) (closed circles) at 37 °C and pH 7.4. Each point represents the mean ± SEM. (n = 3). (d) mRNA expression of cell adhesion-related genes (e.g. VE-cadherin, integrin αV and integrin β3) was evaluated by quantitative RT-PCR. HUVECs were pre-incubated with EGM-2 containing indomethacin (100 μM) for 16 hrs before experiment. Then, HUVECs were incubated with EGM-2 containing indomethacin in the absence (Control) or presence of BSP (25 μM, closed columns) for 10 hrs. Each bar represents the mean ± SEM. (n = 3). * indicates the significant different expression from Control by Students t-test (p < 0.05).
Discussion
Previous studies have established that PGE2, a product derived from arachidonic acid via COX, facilitates colorectal tumour progression and that either disruption of its synthesis or the blockade of prostanoid E receptors delays disease progression5,6,8–10, strongly suggesting that interfering with this pathway improves colon cancer survival. However, the spectrum of potential contributors to the PGE2 pathway is incomplete. One candidate is OATP2A1, a transporter that has been suggested20, but not demonstrated, to affect PGE2 uptake in colon cancer cells30,31. However, to date, there is no definitive studies that have shown that OATP2A1 affects the genesis of colon cancer. The present study demonstrated that genetic ablation of Slco2a1 strongly enhanced survival and this was associated with a marked suppression in the number of large colorectal cancer polyps.
The expression of the EP4 receptor is associated with colon cancer tumorogenesis22,23. We hypothesized that changes in the expression of the EP4 receptor accounts for the increased survival in the Slco2a1-null mice. Our immunohistochemical analysis indicated that EP4 receptor expression was similar among Slco2a1-null and wildtype mice (Supplementary Figure S1), suggesting that it does not account for the reduced tumorogenesis among Slco2a1-null mice. It is conceivable that PGE2 is elevated in the extracellular space of Slco2a1-null tumours, however, at this time we are not able to reliably measure the PGE2 in the extracellular space of tumours.
How does absence of Slco2a1 affect colon cancer tumorogenesis? Our studies support a mechanism whereby OATP2A1 expression in the endothelial vasculature of the tumour, is required for maximal tumorigenesis in the APC mutant (Apc ∆716/+) mouse model. While the number of small polyps was not impacted by Slco2a1 absence, the formation of large malignant polyps was markedly reduced by the loss of Oatp2a1 (Fig. 1). Notably, the endothelial marker CD34 (a marker of endothelial vasculature), was significantly lower in the stromal tissue of intestinal polyps from Slco2a1-deficient Apc Δ716/+ mice (Fig. 2). This is consistent with findings showing increased CD34 levels in colorectal tumours32,33 are a significant and independent poor prognostic factor in colon cancer34, and would be in accord with the finding that angiogenesis correlated with the size of malignant adenoma polyps8,9. Our in vivo studies showed Oatp2a1 was expressed in the tumour’s endothelial vasculature, which agrees with the previous study in human small intestines35. We speculate that OATP2A1 plays a role in neovascularization because vascular stresses are known factors to induce OATP2A1 expression36. However, it is currently unclear exactly how OATP2A1 expression in the vasculature of endothelial cells contributes to tumour angiogenesis. The present findings might be unique to the tumour micro-environment because Syeda et al. reported that pharmacological inhibition of Oatp2a1 in a diabetic mouse model promoted angiogenesis30,37. Nonetheless, the reduced microvascular density in Slco2a1-deficient polyps coupled with OATP2A1 affecting angiogenesis, as shown both in vitro tube formation and wound healing assays in HUVECs (Fig. 4) and in vivo sponge subcutaneous implantation model (Fig. 5), suggests that OATP2A1 contributes to tumour angiogenesis. Moreover, under PGE2-depeleted conditions, migration of HUVECs were associated with intracellular PGE2 levels rather than extracellular PGE2, and cell adhesion-related gene expressions were down-regulated (Fig. 7). Suppression of angiogenesis is related to decreased OATP2A1-mediated uptake both in vitro and in vivo and supports the idea that OATP2A1-mediated transport of PGE2 contributes to angiogenesis independent of EP receptors.
To elucidate how OATP2A1 affected angiogenesis we used the “tube formation” assay in human vascular endothelial cells. Tube formation in these cells was first evaluated with CM from the LoVo colon cancer cell line. The tube formation was reduced by varying combination of either COX inhibition (indomethacin) or EP antagonists, or BSP (an inhibitor of OATP2A1) suggesting each one of these factors contributes to angiogenesis (Fig. 4a). Our initial experiments did not determine if PGE2 alone affected tube formation because OATP2A1 can transport multiple prostaglandins and we did not want to exclude any one prostaglandin from consideration. Subsequently, we demonstrated that PGE2 alone affected angiogenesis using the wound healing assay in EBMTM (Fig. 4 and 7). Driving angiogenesis related to OATP2A1-mediated uptake might be attributed to PGE2 activating the peroxisome proliferator-activated receptor (PPAR) γ38,39, which is likely considering PPARγ is expressed in endothelial cells40. PPARγ activation might induce angiogenesis in endothelial cells, possibly by COX-2 upregulation which could increase PGE2 production. The reduction of tube formation by the COX-2 inhibitor, indomethacin, in HUVECs is consistent with this proposition. We also demonstrated that some of the HUVEC angiogenesis is mediated by EP receptor activation because the EP receptor antagonists suppressed tube formation (Fig. 4). At this point, we have not elucidated the relative importance of the EP receptor mediated pathway vs the “intracrine” pathway suggested by our findings with the Slco2a1 knockout mouse and our in vitro model systems. A hypothesized model is illustrated in Fig. 8. Indeed, the biological action of intracellular PGE2 has been shown in mammalian endothelial cells41, human prostate cancer PC3 cells42, and human endometrial stromal cells43. Hence, blocking both pathways might be synergistically effective to suppress angiogenesis. Future studies will dissect the relative importance of each of these components to angiogenesis and colon cancer tumorogenesis.
Figure 8.

Hypothesized role of OATP2A1 in intracrine PGE2 signalling. Secreted PGE2 from any cells, including normal and transformed epithelial cells, stromal cells (e.g. fibroblasts and macrophages) and endothelial cells may contribute to PGE2 signalling via cell surface EP receptors in endothelial cells, which affects angiogenesis. OATP2A1 expressed in the plasma membranes and/or subapical structures in endothelial cells actively imports PGE2. Based on the previous research PGE2 taken up by cells is assumed to be metabolized; however, our present study suggests that it may transmit signalling through intracellular intermediates to promote transcription of adhesion molecules.
The present findings suggest OATP2A1 modulates angiogenesis by promoting neovascularization through PGE2 uptake. While a previous study showed that OATP2A1 mRNA was reduced in colon tumours20 it did relate this to patient outcome. However, the role of OATP2A1 expression in colon cancer patient survival was to our knowledge unknown. Our preliminary studies, in humans, suggest higher expression of SLCO2A1 is a poor prognostic factor. However, a caveat to this interpretation is that this data is from a small cohort and does not quite achieve statistical significance (Supplementary Figure S2). Certainly future studies, are necessary to establish a clear relationship between SLCO2A1 expression in colorectal tumours and disease survival. Such studies may pave the way toward a therapeutic approach where OATP2A1 is used as a pharmacological target to suppress colorectal tumour progression. On the other hand, loss-of-function mutations of SLCO2A1 is a causative gene for primary hypertrophic osteoarthropathy and chronic non-specific ulcers in small intestine, which are related to aberrant catabolism of PGE2 35,44,45; therefore, adverse effects of OATP2A1 blocking may be concerned as well.
In conclusion, in Slco2a1 −/− /Apc Δ716/+ mice, the reduced number of large polyps associated with increased survival suggested that absence of Slco2a1 delays or blocks the formation of large polyps, possibly by suppressing angiogenesis, due to reduced PGE2 uptake. This would fit with the increase in small polyps in mice with Slco2a1 insufficiency. Our mouse model showed that the amount of OATP2A1 impacted colon cancer survival, thereby implying pharmacological blockade of OATP2A1 may increase colon cancer survival. Indeed, OATP2A1 expression in vascular endothelial cells coupled with our in vitro studies showing that angiogenesis is suppressed in HUVECs by OATP2A1 inhibition strongly supports this as a potential pharmacological target to improve colon cancer outcomes.
Materials and Methods
Materials
[5,6,8,11,12,14,15-3H]PGE2 ([3H]PGE2; 163.6 Ci/mmol) was purchased from PerkinElmer Life Science (Boston, MA). Bromosulfophthalein (BSP) was obtained from Sigma-Aldrich (St. Louis, MO) and Tokyo Chemical Industry (Tokyo, Japan), respectively. All other compounds were commercial products of reagent grade. Anti-mouse Oatp2a1 guinea pig and anti-human OATP2A1 rabbit polyclonal antibodies were kind gifts from Prof. Ken-ichi Hosoya (University of Toyama)46 and Michel A. Fortier (Université Laval, Canada)43, respectively.
Animals and quantification of intestinal polyps
All animal experimentation was carried out in accordance with the requirements of Kanazawa University Institutional Animal Care and Use Committee, and animal experiment protocols performed in this study were approved by the committee (Approved numbers; AP-153511 and AP-163750). Constructions of Apc Δ716/+ and Slco2a1 −/− mice were described previously21,47. Apc Δ716/+ mice were from a C57BL/6 strain48. The null allele of Slco2a1 was introduced into the Apc Δ716/+ mice by intercrossing the Apc Δ716/+ mice with Slco2a1 −/− mice to produce mice with a mixed genetic background of BL/6 and SV129. Compound heterozygotes for Slco2a1 and Apc (Slco2a1 +/− /Apc Δ716/+) on the mixed genetic background were interbreed to generate Apc mutant mice with three different Slco2a1 genotypes (Slco2a1 +/+ /Apc Δ716/+, Slco2a1 +/− /Apc Δ716/+ and Slco2a1 −/− /Apc Δ716/+−). Polyps in the small and large intestines of these mice were observed at age of 13 weeks (16.9–25.3 g, no significant difference between mice with different genotypes), and their number were counted under a stereo microscope at ×30–60 magnification described as previously21. Animal survival was analysed by Kaplan-Meier methods, and then compared by generalized Wilcoxon test.
Immunohistochemistry
Tissue samples were excised, and then fixed with 4% paraformaldehyde. Briefly, for light-microscopic analysis, paraffin-embedded sections were incubated with guinea pig anti-Slco2a1 IgG (1:100 dilution, overnight at 4 °C), rat anti-CD34 IgG (1:50 dilution, overnight at 4 °C, BD Biosciences, NJ) or anti-F4/80 IgG (1:100 dilution, 2 h at room temperature (rt), AbD Serotec, Raleigh, NC), followed by biotinylated or fluorescence-labelled secondary antibodies (1: 200–400 dilution). For DAB staining, the biotinylated IgG labelled-sections were reacted with horseradish peroxidase-conjugated streptavidin (ThermoFisher Scientific, MA), and developed with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA). The sections were observed with a conventional fluorescence microscope BZ-9000 (Keyence, Osaka, Japan) or a confocal laser microscope LSM710 (Carl Zeiss, Oberkochen, Germany). For evaluation of microvascular density (MVD), CD34-positive area was quantified by using Image J software49.
Tube formation assay
HUVECs (Lonza, Basel, Switzerland) were cultured in endothelial cell basal medium (EBM™, Cat. No. CC-3121, Lonza) supplemented with endothelial cell growth medium (EGM™-2, Cat. No. CC-4176, Lonza) and 2% FBS (Biosera, Kansas City, MO). Matrigel® (Corning, New York, NY) was solidified in a 96-well plate (45 µL/well), and then 9000 HUVECs were placed on each Matrigel® with Ham’s F-12 supplemented with 10% FBS, 100 U/mL Penicillin G and 100 μg/mL streptomycin (Wako Pure Chemical Industries, Osaka, Japan) or conditioned medium (CM) from human cancer LoVo cells, which were cultured at a density of 2 × 105 cells/cm2 in 2 mL growth medium for 24 h. After 4 h-incubation at 37 °C, tube formation was evaluated by measuring length of capillary-like structures in two-dimensional microscope images under BZ-9000 using Image J software49.
Wound healing assay
HUVECs were seeded into a multiple well plate at 1.8 × 104 cells/cm2 in EBM™ supplemented with EGM™-2 and the assay was conducted as described previously50. Next day each confluent monolayer was scraped by a 200-μL plastic pipette tip to make a cell-free area and then detached cells were washed off with EBM™. The cells were incubated at 37 °C in the absence or presence of EP antagonists or an OATP2A1 inhibitor BSP in FBS-free EBM™ supplemented with EGM™-2. To assess effect of exogenous PGE2, HUVECs were pre-treated with indomethacin (100 μM) for 16 hrs, and then the assay was performed in the presence or absence of BSP (25 μM) in the EGMTM-2 containing indomethacin. The cells were observed at 0 and 10 hrs using BZ-9000 and the distance between the edges of the cell-free areas was measured using Image J software49.
Knockdown of OATP2A1 in HUVECs
OATP2A1 was knocked down as described in previously48. Briefly, HUVECs were plated at a density of 0.25 × 106 cells/cm2, and then were transfected with 10 nM non-specific (NS) siRNA (Silencer® Select Negative Control #1 siRNA) or a mixture of two siRNAs to SLCO2A1 gene (Silencer® Select Validated siRNA s13097 and s13098), using Lipofectamine RNAi Max® (Life Technology) according to the manufacturer’s protocol. After the cells were cultured for 48 hrs, mRNA expression was measured by quantitative RT-PCR using oligonucleotide primers specific to SLCO2A1 (sense; 5′-ctgtggagacaat ggaatcgag-3′, antisense; 5′-cacgatcctgtctttgctgaag-3′), and then normalized with that of HPRT as previously described51. Protein expression of OATP2A1 was detected by immunocytochemistry as described below.
Immunocytochemistry for HUVECs
OATP2A1 was immunostained as described previously48. HUVECs were plated on glass slides (BD Falcon, Franklin Lakes, NJ) at a density of 5 × 104 cells/0.7 cm2. The cells were fixed in 4% paraformaldehyde, and permeabilized with 0.01% (w/v) Triton X100 in PBS. Immunoreaction was performed by incubating the cells with a 1:200 dilution of anti-human OATP2A1 rabbit polyclonal antibody for 3 hrs at rt, followed by staining with a 1:400 dilution of AlexaFluor® 594-conjugated anti-rabbit IgG (Life Technologies) for 1 hr at rt. The cells were counterstained with Hoechst 33342 (2 μg/mL) for nucleus (blue), and then mounted with Vectashield® (Vector Laboratories, Peterborough, UK). Fluorescence was examined by the use of a confocal laser microscope (LSM710, Carl Zeiss, Göttingen, Germany).
PGE2 uptake by HUVECs
Cells were cultured on collagen-coated plate at a density of 0.5 × 105 cells/cm2 for 2 days, and then used for [3H]PGE2 uptake was undertaken in the absence or presence of an OATP inhibitor as described before52. Intracellular accumulation of [3H]PGE2 was evaluated by measuring radioactivity in the cell lysates using a liquid scintillation counter (Hitachi Aloka Medical, Tokyo, Japan), and shown as cell-to-medium ratio normalized by protein content (μL/mg protein).
In vivo angiogenesis
In vivo angiogenesis was evaluated based on the previous studies28,53. Sponges (12 mm dia. × 3 mm height) were surgically implanted subcutaneously onto the dorsum of mice with Slco2a1 wildtype (Slco2a1 +/+) or null (Slco2a1 −/−) alleles, both of which have mixed genetic background of BL/6 and SV129 (ages 11–30 weeks) under general anaesthesia with pentobarbital sodium (50 mg/kg, i.p.). Slco2a1 +/+ mice were randomly divided into two groups, and sterilized physiological saline (100 μL, vehicle) or vehicle containing 5 nmol of an OATP inhibitor BSP was injected for 9 consecutive days into the implanted sponge, starting the day after the surgery, and then all animals were monitored daily and sacrificed at the 10th post-surgery day. The sponges were carefully removed and the contents were homogenized in sterilized water and centrifuged at 5000 × g for 10 min. Haemoglobin contained in the supernatant was quantified with Haemoglobin B-Test Wako according to manufacturer’s protocol (Wako Pure Chemical Industries).
Statistical analysis
Student’s t-test was used to assess significance of difference between in vitro assay results, with p < 0.05 as a criterion of significance.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
This research was carried out with the supports of a Grant-in-Aid for Scientific Research (KAKENHI) to T. N. (15H04755, and 15K15181) from the Japan Society for the Promotion of Science. This work was supported by NIH grants R01CA194057, P30CA21745, CA21865, CA194057, CA096832 and by ALSAC. We thank Mr. Kazuyuki Hayashi and Dr. Akio Nishiura at Ono Pharmaceutical Co. Ltd. for their helpful and constructive suggestions. We thank Dr. Douglas D. Ross at University of Maryland at Baltimore for his careful reading and suggestions. We also thank Mrs Junya Shimizu and Takatoshi Sakaguch at Kanazawa University for their technical assistances.
Author Contributions
The present study was designed by T.N., M.O. and I.T. Animal studies with A pc mouse models were developed by T.N., Y.O., R.A., H.S. and H.O. In vivo angiogenesis and in vitro studies with HUVECs were conducted with T.N., Y.O., S.K. and S.M. J.D.S. analysed Oncomine data. Manuscript was mainly produced by T.N. I.T. and J.D.S., and reviewed by all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16738-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thun MJ, Namboodiri MM, Heath CW., Jr. Aspirin use and reduced risk of fatal colon cancer. N. Engl. J. Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 2.Eisinger A, et al. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat. 2007;82:147–154. doi: 10.1016/j.prostaglandins.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshima M, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/S0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 5.Sheng H, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J. Clin. Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chulada PC, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- 7.Backlund MG, Mann JR, Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69(Suppl 1):28–32. doi: 10.1159/000086629. [DOI] [PubMed] [Google Scholar]
- 8.Castellone MD, Teramoto H, Gutkind JS. Cyclooxygenase-2 and colorectal cancer chemoprevention: the beta-catenin connection. Cancer Res. 2006;66:11085–11088. doi: 10.1158/0008-5472.CAN-06-2233. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart CE, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am. J. Gastroenterol. 2002;97:1037–1041. doi: 10.1111/j.1572-0241.2002.05625.x. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093–5096. [PubMed] [Google Scholar]
- 12.Sonoshita M, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat. Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 13.Reid G, et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamai I, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- 15.Sekine T, et al. Expression cloning and characterization of a novel multispecific organic anion transporter. J. Biol. Chem. 1997;272:18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- 16.Kimura H, et al. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J. Pharmacol. Exp. Ther. 2002;301:293–298. doi: 10.1124/jpet.301.1.293. [DOI] [PubMed] [Google Scholar]
- 17.Chan BS, et al. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter “PGT”. J. Biol. Chem. 1998;273:6689–6697. doi: 10.1074/jbc.273.12.6689. [DOI] [PubMed] [Google Scholar]
- 18.Chan BS, et al. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am. J. Physiol. Renal Physiol. 2002;282:F1097–F1102. doi: 10.1152/ajprenal.00151.2001. [DOI] [PubMed] [Google Scholar]
- 19.Gose T, et al. Prostaglandin transporter (OATP2A1/SLCO2A1) contributes to local disposition of eicosapentaenoic acid-derived PGE. Prostaglandins Other Lipid Mediat. 2016;122:10–17. doi: 10.1016/j.prostaglandins.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Holla VR, et al. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer Prev. Res. (Phila) 2008;1:93–99. doi: 10.1158/1940-6207.CAPR-07-0009. [DOI] [PubMed] [Google Scholar]
- 21.Oshima M, et al. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc. Natl. Acad. Sci. USA. 1995;92:4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutoh M, et al. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res. 2002;62:28–32. [PubMed] [Google Scholar]
- 23.Fujino H. The Roles of EP4 Prostanoid Receptors in Cancer Malignancy Signaling. Biol Pharm Bull. 2016;39:149–155. doi: 10.1248/bpb.b15-00840. [DOI] [PubMed] [Google Scholar]
- 24.Goodlad RA, et al. Inhibiting vascular endothelial growth factor receptor-2 signaling reduces tumor burden in the ApcMin/ + mouse model of early intestinal cancer. Carcinogenesis. 2006;27:2133–2139. doi: 10.1093/carcin/bgl113. [DOI] [PubMed] [Google Scholar]
- 25.Duff SE, et al. Lymphatic vessel density, microvessel density and lymphangiogenic growth factor expression in colorectal cancer. Colorectal. Dis. 2007;9:793–800. doi: 10.1111/j.1463-1318.2006.01199.x. [DOI] [PubMed] [Google Scholar]
- 26.Namkoong S, et al. Prostaglandin E2 stimulates angiogenesis by activating the nitric oxide/cGMP pathway in human umbilical vein endothelial cells. Exp. Mol. Med. 2005;37:588–600. doi: 10.1038/emm.2005.72. [DOI] [PubMed] [Google Scholar]
- 27.Tak FF. The PGE2-EP receptor axis in colorectal cancer and angiogenesis. J Tumor. 2014;2:208–218. [Google Scholar]
- 28.Andrade SP, Fan TP, Lewis GP. Quantitative in-vivo studies on angiogenesis in a rat sponge model. Br. J. Exp. Pathol. 1987;68:755–766. [PMC free article] [PubMed] [Google Scholar]
- 29.Muramatsu M, et al. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur. J. Pharmacol. 2000;402:181–191. doi: 10.1016/S0014-2999(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 30.Syeda MM, et al. Prostaglandin transporter modulates wound healing in diabetes by regulating prostaglandin-induced angiogenesis. Am. J. Pathol. 2012;181:334–346. doi: 10.1016/j.ajpath.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Chang HY, et al. Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice. Circulation. 2010;121:529–536. doi: 10.1161/CIRCULATIONAHA.109.862946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang JF, et al. Relationship and prognostic significance of SPARC and VEGF protein expression in colon cancer. J. Exp. Clin. Cancer Res. 2010;29:71. doi: 10.1186/1756-9966-29-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qasim BJ, Ali HH, Hussein AG. Immunohistochemical expression of PCNA and CD34 in colorectal adenomas and carcinomas using specified automated cellular image analysis system: a clinicopathologic study. Saudi. J. Gastroenterol. 2012;18:268–276. doi: 10.4103/1319-3767.98435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma YL, et al. Immunohistochemical analysis revealed CD34 and Ki67 protein expression as significant prognostic factors in colorectal cancer. Med. Oncol. 2010;27:304–309. doi: 10.1007/s12032-009-9210-3. [DOI] [PubMed] [Google Scholar]
- 35.Umeno J, et al. A Hereditary Enteropathy Caused by Mutations in the SLCO2A1 Gene, Encoding a Prostaglandin Transporter. PLoS Genet. 2015;11:e1005581. doi: 10.1371/journal.pgen.1005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topper JN, et al. Human prostaglandin transporter gene (hPGT) is regulated by fluid mechanical stimuli in cultured endothelial cells and expressed in vascular endothelium in vivo. Circulation. 1998;98:2396–2403. doi: 10.1161/01.CIR.98.22.2396. [DOI] [PubMed] [Google Scholar]
- 37.Liu, Z. et al. Inhibition of prostaglandin transporter (PGT) promotes perfusion and vascularization and accelerates wound healing in non-Diabetic and diabetic rats. PLoS One10, e0133615, 10.1371/journal.pone.0133615 (2015). [DOI] [PMC free article] [PubMed]
- 38.Shiraki, T. et al. Alpha,beta-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor gamma. J. Biol. Chem.280, 14145–14153, 10.1074/jbc.M500901200 (2005). [DOI] [PubMed]
- 39.Chou, W. L. et al. Identification of a novel prostaglandin reductase reveals the involvement of prostaglandin E2 catabolism in regulation of peroxisome proliferator-activated receptor gamma activation. J. Biol. Chem.282, 18162–18172, 10.1074/jbc.M702289200 (2007). [DOI] [PubMed]
- 40.Marx N, et al. PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARgamma as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.ATV.19.3.546. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharya M, et al. Nuclear localization of prostaglandin E2 receptors. Proc. Natl. Acad. Sci. USA. 1998;95:15792–15797. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Martinez AB, et al. Intracrine prostaglandin E(2) signalling regulates hypoxia-inducible factor-1alpha expression through retinoic acid receptor-beta. Int. J. Biochem. Cell Biol. 2012;44:2185–2193. doi: 10.1016/j.biocel.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Kang J, et al. Expression of human prostaglandin transporter in the human endometrium across the menstrual cycle. J. Clin. Endocrinol. Metab. 2005;90:2308–2313. doi: 10.1210/jc.2004-1482. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, et al. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am. J. Hum. Genet. 2012;90:125–132. doi: 10.1016/j.ajhg.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seifert W, et al. Mutations in the prostaglandin transporter encoding gene SLCO2A1 cause primary hypertrophic osteoarthropathy and isolated digital clubbing. Hum. Mutat. 2012;33:660–664. doi: 10.1002/humu.22042. [DOI] [PubMed] [Google Scholar]
- 46.Tachikawa M, et al. Role of the blood-cerebrospinal fluid barrier transporter as a cerebral clearance system for prostaglandin E(2) produced in the brain. J. Neurochem. 2012;123:750–760. doi: 10.1111/jnc.12018. [DOI] [PubMed] [Google Scholar]
- 47.Nakanishi T, Ross DD, Mitsuoka K. Methods to evaluate transporter activity in cancer. Methods Mol. Biol. 2010;637:105–120. doi: 10.1007/978-1-60761-700-6_5. [DOI] [PubMed] [Google Scholar]
- 48.Takaku, K. et al. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell92, 645–656 (1998). [DOI] [PubMed]
- 49.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Med. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Aarag BY, et al. In vitro anti-proliferative and anti-angiogenic activities of thalidomide dithiocarbamate analogs. Int Immunopharmacol. 2014;21:283–292. doi: 10.1016/j.intimp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Kasai, T. et al. Role of OATP2A1 in PGE2 secretion from human colorectal cancer cells via exocytosis in response to oxidative stress. Exp. Cell Res.341, 123–131, 10.1016/j.yexcr.2016.02.002 (2016). [DOI] [PubMed]
- 52.Nakanishi, T., Ross, D. D. & Mitsuoka, K. Methods to evaluate transporter activity in cancer. Methods Mol. Biol. 637, 105–120, 10.1007/978-1-60761-700-6_5 (2010). [DOI] [PubMed]
- 53.Majima, M. et al. Significant roles of inducible cyclooxygenase (COX)-2 in angiogenesis in rat sponge implants. Jpn. J. Pharmacol.75, 105–114 (1997). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).