Abstract
Greenhouse gases (GHGs) emissions from streams are important to regional biogeochemical budgets. This study is one of the first to incorporate stream GHGs (CO2, CH4 and N2O) concentrations and emissions in rivers of the Tibetan Plateau. With one-time sampling from 32 sites in rivers of the plateau, we found that most of the rivers were supersaturated with CO2, CH4 and N2O during the study period. Medians of partial pressures of CO2 (pCO2), pCH4 and pN2O were presented 864 μatm, 6.3 μatm, and 0.25 μatm respectively. Based on a scaling model of the flux of gas, the calculated fluxes of CO2, CH4 and N2O (3,452 mg-C m2 d−1, 26.7 mg-C m2 d−1 and 0.18 mg-N m2 d−1, respectively) in rivers of the Tibetan Plateau were found comparable with most other rivers in the world; and it was revealed that the evasion rates of CO2 and CH4 in tributaries of the rivers of the plateau were higher than those in the mainstream despite its high altitude. Furthermore, concentrations of GHGs in the studied rivers were related to dissolved carbon and nitrogen, indicating that riverine dissolved components could be used to scale GHGs envision in rivers of the Tibetan Plateau.
Introduction
The input of carbon (C) and nitrogen (N) from land to water leads most rivers in the world to be supersaturated with greenhouse gases (GHGs, i.e. CO2, CH4 and N2O) and therefore net sources of GHGs to the atmosphere1–5. Rivers are reactors for degradation and metabolic processes among aqueous C and N, making them active areas of GHGs with the atmosphere2,6–10. For example, after entering into the aquatic system from the land and atmosphere, part of the organic carbon will undergo degradation and result in GHGs emissions2,9–12. At the same time, denitrification and nitrification in aquatic system will also alter the nitrogen pools and emit N2O gas, which has a global warming potential approximately 265 times that of CO2, to the atmosphere13–15. It was estimated that CO2 emissions from global streams are at 1.8 × 106 Gg C d−1 3, while the size of inland water CH4 and N2O evasion were estimated at 0.2 Gg C d−1 and 32.2 Gg N d−1, respectively4,16,17.
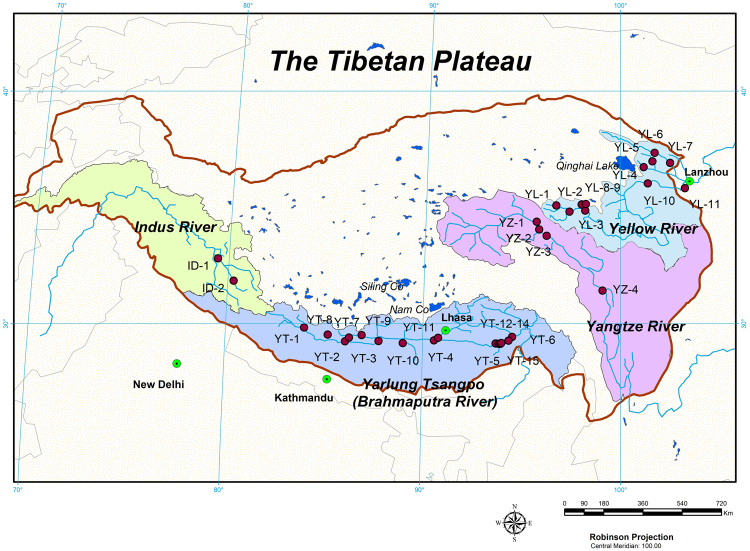
There are more than ten large rivers in Asia originating from the Tibetan Plateau that provide water resources for billions of people18. With climate change and increasing anthropogenic activities, changes in global riverine C and N have been suggested during the last decades19–21, including rivers on the Tibetan Plateau22,23. It was reported that concentrations of dissolved inorganic carbon (DIC) are elevated in river basins of the plateau, due to the extensive topographic relief and soil erosion24. In addition, though concentrations of dissolved organic carbon (DOC) were low, average concentrations of riverine nitrogen on the Tibetan Plateau were close to the world’s mean level, which leads to low DOC/DON ratios (C/N)24. Low C/N in rivers usually means that the dissolved organic matters are more bioavailable and more easily decomposed into GHGs emissions25. Therefore, along with climate change and increasing anthropogenic activities on the Tibetan Plateau, a growing export of bioavailable riverine carbon and nitrogen can be expected24, which will possibly influence GHGs emissions in rivers of the plateau. Furthermore, compared with rivers in other regions of the world, rivers on the Tibetan Plateau have large slopes, due to their huge drop in topography26. Large slopes of the river catchments usually lead to large stream velocity, which will result in a high gas transfer velocity (k)27. Nevertheless, few literatures have been documented on the Tibetan Plateau, despite their potential interaction with the GHGs budgets in this critical region13,14,28. Here we present data from four major watersheds (the Indus, the Yarlung Tsangpo, the Yangtze River and the Yellow River, Fig. 1) on the Tibetan Plateau to 1) assess the spatial distributions of GHGs (CO2, CH4 and N2O) concentrations across the rivers, and 2) investigate the potential factors (e.g., dissolved riverine carbon and nitrogen matter, water temperature, precipitation and land cover types, etc.) that affect GHGs emissions from rivers in this ecological-fragile region.
Figure 1.
Sampling sites for greenhouse gases (GHGs) in rivers of the Tibetan Plateau. Note: the Indus River is abbreviated to “ID”, the Yarlung Tsangpo is to “YT”, the Yangtze River is to “YZ” and the Yellow River is to “YL”; information on selected rivers and detailed data are shown in Table S1. Hydrographical data of the drainage area are from26 and the map was generated by ArcGIS 10.2 (ESRI Inc.).
Results
Spatial distribution of pGHGs in the rivers of the Tibetan Plateau
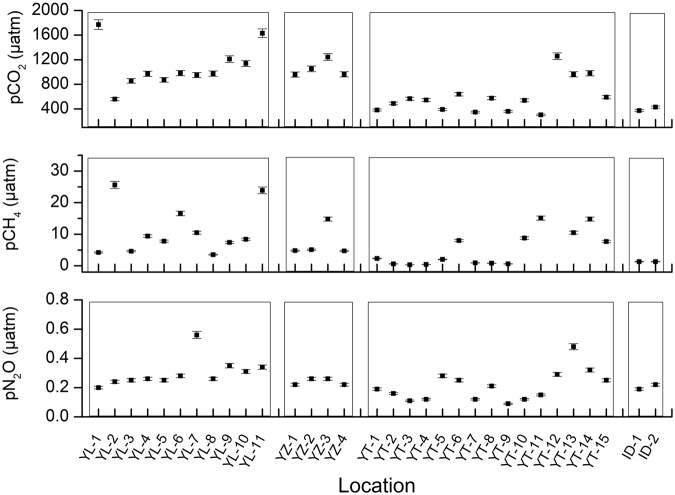
Partial pressures of GHGs (pGHGs, including pCO2, pCH4 and pN2O) in the waters and atmosphere of the studied rivers showed that most of the sampling sites in the watersheds were supersaturated with CO2, CH4 and N2O during the sampling period (Table S3). The pGHGs on the Tibetan Plateau varied largely within the different river basins. For instance, the maximum and minimum pCO2 of the Yellow River was 1771 μatm and 560 μatm, respectively (Fig. 2). Similarly, two end values of pCH4 in Yarlung Tsangpo were 0.3 μatm and 200.5 μatm. To avoid the defect of such abnormal extreme values, medians for the pGHGs instead of averages were employed in this study in order to discuss the GHGs in rivers of the Tibetan Plateau.
Figure 2.
Partial pressures of GHGs distribution in rivers of the Tibetan Plateau. “YL”, “YZ”, “YT” and “ID” are presented for sampling sites in the Yellow River, the Yangtze River, the Yarlung Tsangpo and Indus, respectively. The figure was plotted by Origin 8.5 (Originlab Corp.).
The pCO2 on the Tibetan Plateau ranged from 304 μatm to 1771 μatm with a median of 864 μatm, pCH4 ranged from 0.3 to 200.5 μatm with a median of 6.3 μatm, and pN2O ranged from 0.09 to 0.56 with a median of 0.25 μatm. The Yellow River and the Yangtze River had higher pGHGs than those in the Yarlung Tsangpo and the Indus (Fig. 2). pCO2 in rivers of the Tibetan Plateau were lower than the other streams in the world. For instance, concentrations of CO2 in rivers of Alaska, Canada and conterminous U.S. ranged from 570–3100 μatm2,29,30, while pCO2 in Amazon was as high as ~4000 μatm, four times higher than that in rivers of the Tibetan Plateau31. CH4 concentrations in rivers of the Tibetan Plateau were similar with streams in Alaska30, but much lower than those waters in fens or marsh (>40) in the boreal area32. Low pGHGs in rivers of the Tibetan Plateau may be mainly due to its high altitude (low atmospheric pressure) and low concentrations of nutrients. It was observed that pGHG in tributaries was higher than those in mainstreams. This means that streams in the lower order have a higher GHGs emission rate to the atmosphere, which is consistent with previous studies3. For instance, partial pressures of CO2 in the small tributaries of Yarlung Tsangpo (YT-7, 8 and YT-12~15) ranged from 347 to 1257 μatm with a median of 777 μatm, while pCO2 in the mainstream ranged from 304–639 with a lower median of 489 μatm (Fig. 2). Similarly, pCH4 in tributaries of the Yarlung Tsangpo were also higher than that of the mainstream (~6 times). We infer that high pCH4 in tributaries corresponded with the complex wetland area with reductive conditions in the southeast part of the river basin33.
Identifying N2O emissions is important for evaluating climate change scenarios and assessing mitigation options34. Hu et al. (2016) calculated that N2O emissions from the global rivers is 32.2 Gg N d−1 17, and it was proposed that N2O emissions from rivers were still increasing as a result of human activities on land, thereby enhancing nitrogen export to aquatic systems5. However, during the sampling period in this study, in-stream pN2O was lower than that in the atmosphere in sites YT-2, 3, 4 and YT- 7,8,9, indicating that this area of Yanglung Tsangpo on the Tibetan Plateau might act as a sink of N2O rather than the source. Despite the sites mentioned above with regard to acting as a N2O sink, it was also found that rivers in the western and southern Tibetan Plateau (the Indus and the Yarlung Tsangpo) presented lower N2O concentrations than rivers of the eastern and northern plateau. Partial pressures of N2O in small tributaries on the Tibetan Plateau were from 0.09 to 0.48 μatm with a median of 0.25 μatm, also higher than those in the mainstream (0.22 μatm). Different with that of CO2 and CH4, concentrations of N2O on the Tibetan Plateau were similar to most other pristine rivers in the world and much lower than those under intensive anthropogenic activities35,36.
Flux of GHGs in the rivers of the Tibetan Plateau
All streams in the studied river basins on the Tibetan Plateau showed sources of CO2 and CH4 to the atmosphere during the sampling season. Among them, fluxes of CO2 and CH4 in the Yellow River (median ~6,333 mg CO2-C m−2 d−1, 48.2 mg CH4-C m−2 d−1) were significantly higher than those in the Yangtze River (median ~3,276 mg CO2-C m−2 d−1, 13.6 mg CH4-C m−2 d−1), the Yarlung Tsangpo (~2,442 mg CO2-C m−2 d−1, 11.9 mg CH4-C m−2 d−1) and the Indus (~2,085 g CO2-C m−2 d−1, 2.4 mg CH4-C m−2 d−1), while the flux of N2O in the Yarlung Tsangpo was only 0.07 mg N2O-N m−2 d−1 – much lower than that in the other three rivers (Table S3). Emission rates of both CO2 and CH4 generally illustrated a decreased trend with an increasing stream order, whereas N2O presented an opposite trend. We found first-order streams had high CO2 and CH4 fluxes relative to the second and third-order stream sites on the Tibetan Plateau. For example, in YL-1 and YL-11 – two small branches of the Yellow River – the CO2 fluxes were more than 7,000 mg CO2-C m−2 d−1, which were almost three times higher than those in the Amazon basin (Table 1). Nevertheless, due to steep slopes and high stream velocities, GHGs transfer velocities at some sites of the rivers (e.g., YL-1, 4, 9, YT-8, and so on) are fairly high (Table S2), despite its high altitude and low atmospheric pressure. The flux of CO2 and CH4 from stream surfaces across the four catchments during the study period had a large range (619–14,260 mg CO2-C m−2 d−1 and −6.0–817.9 mg CH4-C m−2 d−1, respectively). However, the median values were a bit lower than those of global streams4. Although N2O fluxes in the Tibetan rivers (−2.46–4.8 mg N2O-N m−2 d−1) were small relative to those of CO2 and CH4 (Tables 1 and 2), notably, N2O emission rates of these rivers are similar to those of many other rivers in the world (Table 3), despite the pristine aquatic environment.
Table 1.
CO2 flux estimates from rivers on the Tibetan Plateau and other river basins in the world (mg-C m−2 d−1).
| Water Type (Location) | Estimated flux |
|---|---|
| Stream (Yellow River, Tibetan Plateau, China) | 6,333 |
| Stream (Yangtze River, Tibetan Plateau, China) | 3,276 |
| Stream (Yarlung Tsangpo, Tibetan Plateau, China) | 2,442 |
| Stream (Indus, Tibetan Plateau, China) | 2,085 |
| Stream (interior Alaska)30 | 5,400 |
| Stream (Northern, Sweden)52 | 7,679 |
| Stream (Finland)53 | 975 |
| Small stream (Ontario, Canada)54 | 1,079 |
| Small stream (Quebec, Canada)29 | 3,121 |
| Small stream (Sweden)55 | 8,279 |
| Headwater Stream (conterminous U.S.)2 | 2,844 |
| Stream (Amazon basin)31 | 2,268 |
| Stream (Mississippi)56 | 3,241 |
| Stream (Mid and downstream of Yangtze River, China)57 | 3,551 |
| Stream (Xinjiang river, China)58 | 3,277 |
| Stream (Temperate zone)2 | 6,493 |
Table 2.
CH4 flux estimates from rivers on the Tibetan Plateau and other water bodies in the world (mg-C m−2 d−1).
| Water Type (Location) | Estimated flux |
|---|---|
| Streams (Yellow River, Tibetan Plateau, China) | 48.2 |
| Streams (Yangtze River, Tibetan Plateau, China) | 13.6 |
| Streams (Yarlung Tsangpo, Tibetan Plateau, China) | 11.9 |
| Stream (Indus, Tibetan Plateau, China) | 2.4 |
| Stream (interior Alaska)30 | 7.7 |
| Beaver pond (Manitoba, Canada)59 | 80.8 |
| Alpine Fen (Alaska)32 | 217.8 |
| Marsh (Alaska)32 | 79.7 |
| Poor fens (interior Alaska)60 | 134.8 |
| Reservoir (Finland)61 | 26.0 |
| Rich fen (control treatment, interior Alaska)62 | 73.7 |
| Stream in peatland (Scotland)63 | 176.2 |
| Stream (Ontario, Canada)64 | 134.8 |
| Stream (Tennessee, U.S.A.)65 | 9.9 |
Table 3.
N2O flux estimates from rivers on the Tibetan Plateau and other water bodies and river basins in the world (mg-N m−2 d−1).
| Water Type (Location) | Estimated flux |
|---|---|
| Streams (Yellow River, Tibetan Plateau, China) | 0.34 |
| Streams (Yangtze River, Tibetan Plateau, China) | 0.18 |
| Streams (Yarlung Tsangpo, Tibetan Plateau, China) | 0.07 |
| Stream (Indus, Tibetan Plateau, China) | 0.13 |
| Stream (Neuse River, North Carolina, U.S.A.)66 | 0.36 |
| Stream (Hudson River, U.S.A)67 | 0.16 |
| Stream (Southeast China)35 | 0.76–9.51 |
| Stream (Amazon basin)68 | 0.27 |
| River estuary (Tamar, England)69 | 0.27 |
| River estuary (Yangtze River, China)36 | 1.64 |
| Lakes (Finland)61 | −0.005–0.008 |
Discussion
Potential factors affect the delivery of GHGs in rivers of the Tibetan Plateau
Relationships between the GHGs and other dissolved components in waters, such as DIC/N and DOC/N, added to environmental variables such as pH and water temperature, were explored in order to investigate the potential factors that affect GHGs in the rivers of the Tibetan Plateau (Table 4). These relationships likely result from a joint influence from the chosen index and changes with the equilibrium distribution of the air-water system.
Table 4.
Pearson correlation (r2) between pGHGs (μatm) and dissolved carbon and nitrogen (mg L−1) and meteorological (water temperature (°C) and average annual precipitation (mm)).
| DIC (n = 10) | DOC (n = 10) | Temp. (n = 30) | Prep. (n = 10) | pH (n = 22) | |
|---|---|---|---|---|---|
| pCO2 | 0.76** | 0.02 | 0.01 | 0.21 | 0.30 (−)** |
| DIC (n = 10) | DOC (n = 10) | Temp. (n = 30) | Prep. (n = 10) | ||
| pCH4 | 0.79** | 0.27 | 0.01 | 0.05 | |
| DIN (n = 10) | DON (n = 10) | Temp. (n = 10) | Prep. (n = 10) | ||
| pN2O | 0.23 | 0.05 | <0.01 | 0.01 (−) |
It was estimated that ~60% DIC exported in York River was lost as CO2 evasion to the atmosphere10. Partial pressure of CO2 in streams of the Tibetan Plateau appears to be significantly correlated with DIC (r2 > 0.76, 0.05) within the catchment (Table 4). In addition to being correlated with dissolved carbon matter, pCO2 is also correlated with precipitation (Table 4). Precipitation events mainly control in-stream gas concentrations in two ways: (1) increasing gas concentrations by flushing inorganic and organic carbon from the landscape into streams, and (2) decreasing gas concentrations by diluting stream water and facilitating gas exchange37. Interestingly, the correlation between the precipitation and pCO2 is stronger than that with DOC concentrations and water temperature. We infer that there are two factors which possibly are responsible for the strong correlation between precipitation and CO2 concentrations. First, rates of precipitation usually correlate with terrestrial ecosystem fluxes such as annual net primary production38 and secondly, the higher annual precipitation usually leads to higher flushing and delivery of soil and riparian/wetland CO2 to streams and river2. Therefore, we propose that precipitation impacts stream CO2 evasion not only on long seasonal timescales but also on short time scales associated with CO2 production and flushing processes. A negative relationship between pCO2 and pH is expected, as dissolved CO2 acts as an acid in water and poorly buffered systems30. Moreover, pH can be a strong indicator of the dissolved CO2 in the stream39.
Similar to pCO2, partial pressures of CH4 in rivers of the Tibetan Plateau were also elevated with dissolved carbon. Concentrations of dissolved carbon, including DIC and DOC, explained most of the variability in pCH4 (Table 4). As a product of anaerobic decomposition of organic matter, there was a positive trend (Table 4) in CH4 concentrations with the increased DOC concentrations in the rivers of the Tibetan Plateau, indicating that water temperature placed a certain influence on driving pCH4 increased in anaerobic decomposition in these Tibetan rivers. Partial pressures of N2O were correlated with dissolved nitrogen (DIN and DON, Table 4). Anthropogenic activities have been important sources of dissolved nitrogen to rivers for several centuries40, and it was estimated that more than 90% of the current N2O emissions from rivers and estuaries in the world can be considered anthropogenic sources13. Most of the large cities on the Tibetan Plateau were located along the river catchment. Therefore, with growing anthropogenic activities such as urbanization, industrial and agricultural activities, more nitrogen substances will enter the mainstream of the rivers than enter the tributaries. This may also explain why the N2O concentrations in mainstreams of the Tibetan rivers are higher than those in tributaries.
This is one of the first studies to incorporate stream GHGs (CO2, CH4 and N2O) concentrations and emissions in four large river basins on the Tibetan Plateau, where the most important permafrost area in the mid-latitude region is distributed. Despite the defect that the results are based on single sampling at each observation site in the summer half year of the plateau, we found that most waters in the studied rivers of the Tibetan Plateau were consistently supersaturated with GHGs (CO2, CH4 and N2O) during the sampling season. In-stream pGHGs on the Tibetan Plateau ranged from 300 to 1,800 μatm with pCO2 median of 864 μatm, pCH4 of 6.3 μatm and pN2O of 0.25 μatm, respectively. Concentrations of GHGs in the rivers showed a different spatial pattern across the plateau. In-stream pCO2 and pCH4 in the Yellow River and the Yangtze River were over two times higher than those in Yarlung Tsangpo and Indus, while the pN2O in the Yellow River was the lowest. It was observed that CH4 and N2O concentrations were one and two orders of magnitude lower than that of CO2, respectively. Evasion rates of CO2 and CH4 in the tributaries were at least two times higher than those in the mainstream of the rivers. The concentrations of GHGs in waters of the Tibetan Plateau rivers were related to dissolved carbon and nitrogen matter, indicating that compared with climatic conditions (i.e., water temperature, precipitation) that could influence the aqueous pGHGs, riverine dissolved components are the key drivers that control the GHGs envision in rivers of this region. Due to high GHGs transfer velocity (k) in rivers of the Tibetan Plateau, the flux of GHGs (~660–14,300 mg-C and N m2 d−1) presented in a manner similar to many other streams in the world, despite its high altitude and pristine river water environment on the plateau. Nevertheless, with growing agricultural and industrial activities in this ecological-fragile region, a large amount of nutrients will be transported to the aquatic system and increasing GHGs emissions from the rivers can be anticipated. Further studies based on intensive observations (e.g., monthly, seasonally and annually) are needed to identify the full roles of the rivers act in GHGs emissions of the Tibetan Plateau.
Materials and Methods
Sampling information and studied river basins
The sampling work was conducted one-time at each observation site with triple parallel samples during the year of 2014 and 2015. The detailed sampling information are listed in Table S1. The studied river basins are 1) The Indus, one of the largest river systems (3,180 km) draining the Himalaya and running across West China, Pakistan and North India41; 2) the Yarlung Tsangpo – the upper reach of the Brahmaputra River, running across the South Tibetan Plateau, India and Bangladesh42; 3) the Yangtze River, with a drainage area of 1.80 × 106 km2 in Euro-Asian Continent, the third longest (6,300 km) and fourth for freshwater flow (900 × 109 m3 d−1) in the world43; 4) the Yellow River, the second largest river (5,464 km) which runs across China44. On the plateau region, the drainage basins of the Yarlung Tsangpo and the Yangtze River are larger than those of the Indus River and the Yellow River (Fig. 1). These two rivers are prominently influenced by the India/South Asia monsoon and receive more precipitation in the summer45. The four river catchments cover almost half of the Tibetan Plateau (Fig. 1) and have variable meteorological and landscape characteristics26. Besides gas samples collected from the mainstreams of the headwater of the four rivers, gases from the mainstem and small tributaries of the Yarlung Tsangpo and the Yellow River were also collected. Three stream orders were defined, based on the joint level of the tributaries with the main river stream. Order 3 stands for the main stem of the river, while order 2 stands for the main tributaries that join to the main stem of order 3. Order 1 stands for the small headwater tributaries that join to the main tributaries in order 2 (Table S1). The hydrological conditions (e.g., discharge, stream velocity, etc.) of the studied rivers were listed in Table S2.
Method of GHGs & water samples collection and measurement
We used the headspace equilibration method1 to collect dissolved greenhouse gas (GHGs) in the studied rivers. Using this method, 40 ml of stream water was equilibrated with 20 ml of ambient air by shaking for 2 minutes underwater to maintain constant temperature. 15 mL of the equilibrated headspace was then sub-sampled and stored in air-tight Exetainer vials. Samples were taken at all study sites in duplicate or triplicate for quality control. Gas concentrations were analyzed using a Shimadzu gas chromatograph (GC-2014) with a flame ionization detector and electron capture detector at the Yale Analytical and Stable Isotope Center.
Water samples for dissolved riverine carbon and nitrogen were collected at approximately 10 cm depth below the surface and filtered with 0.7 μm glass fiber filters in the field. Samples for DIC measurement were stored at room temperature in 500-ml gas-tight brown glass bottles and preserved with 100 μl HgCl2 to avoid photic or biological degradation, while DOC and dissolved nitrogen samples were stored in 500-ml acid-cleaned polypropylene bottles and stored in containers at −18 °C until laboratory measurement46,47. Concentrations of dissolved inorganic nitrogen (DIN, including NO3 −, NH4 + and NO2 −) in the water were detected by (HPLC) Dionex ICS 2000 and Dionex ICS 2500. DIC (comprising HCO3 −, CO3 2− and CO2), DOC, and total dissolved nitrogen (TDN) were measured with a TOC analyzer (SHIMADZU-TOC-VCPH).
GHGs concentration and flux calculation
The initial calculation of the equilibrated headspace concentration of trace gases measured by GC was in units of ppmv. We calculated the trace GHG concentrations in the stream water prior to equilibration by using the law of conservation of mass, Henry’s Law, and the Ideal Gas Law. Henry’s Law can be written as:
| 1 |
where kH is a temperature dependence constant48, Caq is the gas concentration in the aqueous solution in mol/L, and Pgas is the gas concentration in the air in units of atm. KH therefore has units of mol/L*atm−1.
The ideal gas law is written as:
| 2 |
where P is the pressure of the gas, V is the volume of the gas, n is the amount of the gas measured in moles, T is the temperature in Kelvin, and R is the universal gas constant-equal to 0.08206 L*atm*mol−1*K−1.
Stream water gas concentrations prior to equilibration were calculated as follows:
| 3 |
I: initial, F: final, solving for CaqF is:
| 4 |
The initial concentration of gas in the steam (CaqI) was found by substituting in Henry’s Law and inputting measured values from the gas chromatograph.
Equations (3) and (4) can be reorganized and combined to yield:
| 5 |
We used the ideal gas law to convert CgasI and CgasF from units of ppmv to mol/L, using the temperature of the water, estimated atmospheric pressure, and R. Substituting in constants and calculated values into equation (3) allowed us to solve for the concentration of dissolved GHGs in the water (CaqI).
Flux rates of trace GHGs were calculated using the equation:
| 6 |
where k is the gas transfer velocity and ([CO2]aq − [CO2]sat) is the concentration gradient between the actual CO2 concentration that would be present when CO2 is in equilibrium with the atmosphere49. [CO2]sat is found by multiplying the current partial pressure of (pCO2)50 in μatm by kH.
Gas transfer velocity was estimated with the models from Raymond et al. (2012), which are not regional estimates and have been used against other methods51. With the caution against the use of these models when attempting to perform process-based studies, we employed the one below (equation 7) to conduct the k calculation in rivers of the Tibetan Plateau.
| 7 |
where V is stream velocity (m s−1) and S is slope (unitless)27.
Electronic supplementary material
Acknowledgements
This work was funded by the National Nature Science Foundation of China (4171101266, 41675130, 41630754), State Key Laboratory of Cryospheric Science (SKLCS-ZZ-2017), the Academy of Finland (decision number 268170) and the Startup Foundation for Introducing Talent of NUIST (No. 2018r001). Dr. Shaopeng Gao and Dr. Xiaoqing Cui are thanked for their assistance with the measurement of C and N with TOC analyzer, and Dr. Wei Yan is thanked for his assistance with the meteorological data extraction from the China Meteorological Forcing Dataset. Dr. Zhiyong Wang is thanked for the hydrographical data collection from the Hydrological Yearbook of China.
Author Contributions
P.R., C.L., M.S., S.K. and B.Q. designed the research. B.Q., K.A., F.Y. and C.L. organized the fieldwork. B.Q. and C.L. led the writing of the manuscript, which was improved by P.R. and M.S. All authors commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16552-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bin Qu, Email: bin.b.qu@outlook.com.
Chaoliu Li, Email: lichaoliu@itpcas.ac.cn.
References
- 1.Cole JJ, Caraco NF, Kling GW, Kratz TK. Carbon dioxide supersaturation in the surface waters of lakes. Science-AAAS-Weekly Paper Edition. 1994;265:1568–1569. doi: 10.1126/science.265.5178.1568. [DOI] [PubMed] [Google Scholar]
- 2.Butman D, Raymond PA. Significant efflux of carbon dioxide from streams and rivers in the United States. Nature Geosci. 2011;4:839–842. doi: 10.1038/ngeo1294. [DOI] [Google Scholar]
- 3.Raymond PA, et al. Global carbon dioxide emissions from inland waters. Nature. 2013;503:355–359. doi: 10.1038/nature12760. [DOI] [PubMed] [Google Scholar]
- 4.Crawford JT, et al. CO2 and CH4 emissions from streams in a lake‐rich landscape: Patterns, controls, and regional significance. Global Biogeochem. Cycles. 2014;28:197–210. doi: 10.1002/2013GB004661. [DOI] [Google Scholar]
- 5.Kroeze C, Dumont E, Seitzinger SP. New estimates of global emissions of N2O from rivers and estuaries. Environ. Sci. 2005;2:159–165. doi: 10.1080/15693430500384671. [DOI] [Google Scholar]
- 6.Striegl, R. G., Dornblaser, M. M., McDonald, C. P., Rover, J. R. & Stets, E. G. Carbon dioxide and methane emissions from the Yukon River system. Global Biogeochem. Cycles26 (2012).
- 7.Meybeck M. Carbon, nitrogen, and phosphorus transport by world rivers. Am. J. Sci. 1982;282:401–450. doi: 10.2475/ajs.282.4.401. [DOI] [Google Scholar]
- 8.Seitzinger SP, Kroeze C, Styles RV. Global distribution of N2O emissions from aquatic systems: natural emissions and anthropogenic effects. Chemosphere-Global Change Science. 2000;2:267–279. doi: 10.1016/S1465-9972(00)00015-5. [DOI] [Google Scholar]
- 9.Lapierre, J.-F. L., Guillemette, F., Berggren, M. & Del Giorgio, P. A. Increases in terrestrially derived carbon stimulate organic carbon processing and CO2 emissions in boreal aquatic ecosystems. Nat. Commun. 4 (2013). [DOI] [PubMed]
- 10.Raymond PA, Bauer JE, Cole JJ. Atmospheric CO2 evasion, dissolved inorganic carbon production, and net heterotrophy in the York River estuary. Limnol. Oceanogr. 2000;45:1707–1717. doi: 10.4319/lo.2000.45.8.1707. [DOI] [Google Scholar]
- 11.Reeburgh, W. et al. A CH4 emission estimate for the Kuparuk River basin, Alaska. J. Geophys. Res. Atmos. 103 (1998).
- 12.Holgerson, M. A. & Raymond, P. A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nature Geosci. 9 (2016).
- 13.Seitzinger SP, Kroeze C. Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Global Biogeochem. Cycles. 1998;12:93–113. doi: 10.1029/97GB03657. [DOI] [Google Scholar]
- 14.Ramanathan V, Cicerone RJ, Singh HB, Kiehl JT. Trace gas trends and their potential role in climate change. J. Geophys. Res. Atmos. 1985;90:5547–5566. doi: 10.1029/JD090iD03p05547. [DOI] [Google Scholar]
- 15.IPCC. Intergovernmental Panel on Climate Change, the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Rep. 1535 (Cambridge Univeristy. Press, Cambridge, U. K., and New York, 2013).
- 16.Quéré CL, et al. Global carbon budget 2014. Earth System Science Data Discussions. 2015;7:521–610. doi: 10.5194/essdd-7-521-2014. [DOI] [Google Scholar]
- 17.Hu M, Chen D, Dahlgren RA. Modeling nitrous oxide emission from rivers: a global assessment. Glob. Chang. Biol. 2016;22:3566–3582. doi: 10.1111/gcb.13351. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J, Huang Q. Distribution and variation of lakes in Tibetan Plateau and their comparison with lakes in other part of China. Water Resources Protection. 2004;4:24–27. [Google Scholar]
- 19.Sillanpää, M. Natural Organic Matter inWater, 1st Edition, Characterization and Treatment Methods. ISBN: 9780128017197 (2014).
- 20.Evans C, Monteith D, Cooper D. Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ. Pollut. 2005;137:55–71. doi: 10.1016/j.envpol.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Howarth R, Schneider R, Swaney D. Metabolism and organic carbon fluxes in the tidal freshwater Hudson River. Estuaries. 1996;19:848–865. doi: 10.2307/1352302. [DOI] [Google Scholar]
- 22.Tong Y, et al. Riverine nitrogen loss in the Tibetan Plateau and potential impacts of climate change. Sci. Total Environ. 2016;553:276–284. doi: 10.1016/j.scitotenv.2016.02.099. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Xu S, Yang J, Yin H. Silicate weathering and CO2 consumption deduced from the seven Chinese rivers originating in the Qinghai-Tibet Plateau. Chem. Geol. 2008;249:307–320. doi: 10.1016/j.chemgeo.2008.01.025. [DOI] [Google Scholar]
- 24.Qu, B. et al. Export of dissolved carbonaceous and nitrogenous substances in rivers of the “Water Tower of Asia”. Journal of Environmental Sciences (2017). [DOI] [PubMed]
- 25.Thurman, E. In Organic geochemistry of natural waters 7–65 (Springer, 1985).
- 26.MWR. The Ministry of Water Resources of the People’s Republic of Chinahttp://www.mwr.gov.cn/ (2015).
- 27.Raymond PA, et al. Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers. Limnology & Oceanography: Fluids & Environments. 2012;2:41–53. [Google Scholar]
- 28.Andreae, M. O. & Schimel, D. S. In Exchange of trace gases between terrestrial ecosystems and the atmosphere Vol. 238, 926–932 (Wiley 1989).
- 29.Teodoru, C. R., Del Giorgio, P. A., Prairie, Y. T. & Camire, M. Patterns in pCO2 in boreal streams and rivers of northern Quebec, Canada. Global Biogeochem. Cycles23 (2009).
- 30.Crawford JT, Striegl RG, Wickland KP, Dornblaser MM, Stanley EH. Emissions of carbon dioxide and methane from a headwater stream network of interior Alaska. J. Geophys. Res. Biogeosci. 2013;118:482–494. doi: 10.1002/jgrg.20034. [DOI] [Google Scholar]
- 31.Richey JE, Melack JM, Aufdenkampe AK, Ballester VM, Hess LL. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2. Nature. 2002;416:617–620. doi: 10.1038/416617a. [DOI] [PubMed] [Google Scholar]
- 32.Sebacher DI, Harriss RC, Bartlett KB, Sebacher SM, Grice SS. Atmospheric methane sources: Alaskan tundra bogs, an alpine fen, and a subarctic boreal marsh. Tellus B. 1986;38:1–10. doi: 10.3402/tellusb.v38i1.15059. [DOI] [Google Scholar]
- 33.Hou, X. Vegetation atlas of China. Chinese Academy of Science, the editorial board of vegetation map of China (2001).
- 34.Howarth, R. W. et al. In Nitrogen cycling in the North Atlantic Ocean and its watersheds 75–139 (Springer, 1996).
- 35.Yang L, Yan W, Ma P, Wang J. Seasonal and diurnal variations in N2O concentrations and fluxes from three eutrophic rivers in Southeast China. Journal of Geographical Sciences. 2011;21:820–832. doi: 10.1007/s11442-011-0882-1. [DOI] [Google Scholar]
- 36.Wang D, Chen Z, Xu S. The fluxes of CH4, CO2 and N2O from Yangtze estuary intertidal flat in summer season. Geochim. Cosmochim. Acta Suppl. 2008;72:A995. [Google Scholar]
- 37.Zeng FW, Masiello CA, Hockaday WC. Controls on the origin and cycling of riverine dissolved inorganic carbon in the Brazos River, Texas. Biogeochemistry. 2011;104:275–291. doi: 10.1007/s10533-010-9501-y. [DOI] [Google Scholar]
- 38.Knapp AK, Smith MD. Variation among biomes in temporal dynamics of aboveground primary production. Science. 2001;291:481–484. doi: 10.1126/science.291.5503.481. [DOI] [PubMed] [Google Scholar]
- 39.Waldron S, Scott EM, Soulsby C. Stable isotope analysis reveals lower-order river dissolved inorganic carbon pools are highly dynamic. Environ. Sci. Technol. 2007;41:6156–6162. doi: 10.1021/es0706089. [DOI] [PubMed] [Google Scholar]
- 40.Vitousek PM, et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecological applications. 1997;7:737–750. [Google Scholar]
- 41.Karim A, Veizer J. Weathering processes in the Indus River Basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem. Geol. 2000;170:153–177. doi: 10.1016/S0009-2541(99)00246-6. [DOI] [Google Scholar]
- 42.Coleman JM. Brahmaputra River: channel processes and sedimentation. Sediment. Geol. 1969;3:129–239. doi: 10.1016/0037-0738(69)90010-4. [DOI] [Google Scholar]
- 43.Yan, W., Zhang, S., Sun, P. & Seitzinger, S. P. How do nitrogen inputs to the Changjiang basin impact the Changjiang River nitrate: a temporal analysis for 1968–1997. Global Biogeochem. Cycles17 (2003).
- 44.Wang, X., Ma, H., Li, R., Song, Z. & Wu, J. Seasonal fluxes and source variation of organic carbon transported by two major Chinese Rivers: The Yellow River and Changjiang (Yangtze) River. Global Biogeochem. Cycles26 (2012).
- 45.Yang K, et al. Recent climate changes over the Tibetan Plateau and their impacts on energy and water cycle: A review. Global Planet. Change. 2014;112:79–91. doi: 10.1016/j.gloplacha.2013.12.001. [DOI] [Google Scholar]
- 46.Raymond PA, et al. Controls on the variability of organic matter and dissolved inorganic carbon ages in northeast US rivers. Mar. Chem. 2004;92:353–366. doi: 10.1016/j.marchem.2004.06.036. [DOI] [Google Scholar]
- 47.Seitzinger S, Sanders R. Contribution of dissolved organic nitrogen from rivers to estuarine eutrophication. Mar. Ecol. Prog. Ser. 1997;159:12. doi: 10.3354/meps159001. [DOI] [Google Scholar]
- 48.Sander R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015;15:4399–4981. doi: 10.5194/acp-15-4399-2015. [DOI] [Google Scholar]
- 49.Raymond PA, Caraco NF, Cole JJ. Carbon dioxide concentration and atmospheric flux in the Hudson River. Estuaries and Coasts. 1997;20:381–390. doi: 10.2307/1352351. [DOI] [Google Scholar]
- 50.Blasing, T. Recent greenhouse gas concentrations. Updated April 2016, 10.3334/CDIAC/atg.032 (2016).
- 51.Hall RO, Jr, Tank JL, Baker MA, Rosi-Marshall EJ, Hotchkiss ER. Metabolism, gas exchange, and carbon spiraling in rivers. Ecosystems. 2016;19:73–86. doi: 10.1007/s10021-015-9918-1. [DOI] [Google Scholar]
- 52.Jonsson A, et al. Integrating aquatic carbon fluxes in a boreal catchment carbon budget. J. Hydrol. 2007;334:141–150. doi: 10.1016/j.jhydrol.2006.10.003. [DOI] [Google Scholar]
- 53.Huotari J, Haapanala S, Pumpanen J, Vesala T, Ojala A. Efficient gas exchange between a boreal river and the atmosphere. Geophys. Res. Lett. 2013;40:5683–5686. doi: 10.1002/2013GL057705. [DOI] [Google Scholar]
- 54.Koprivnjak, J. F., Dillon, P. & Molot, L. Importance of CO2 evasion from small boreal streams. Global Biogeochem. Cycles24 (2010).
- 55.Humborg C, et al. CO2 supersaturation along the aquatic conduit in Swedish watersheds as constrained by terrestrial respiration, aquatic respiration and weathering. Glob. Chang. Biol. 2010;16:1966–1978. doi: 10.1111/j.1365-2486.2009.02092.x. [DOI] [Google Scholar]
- 56.Dubois, K. D., Lee, D. & Veizer, J. Isotopic constraints on alkalinity, dissolved organic carbon, and atmospheric carbon dioxide fluxes in the Mississippi River. J. Geophys. Res. Biogeosci. 115 (2010).
- 57.Liu, S. et al. Dynamic biogeochemical controls on river pCO2 and recent changes under aggravating river impoundment: an example of the subtropical Yangtze River. Global Biogeochem. Cycles (2016).
- 58.Yao G, et al. Dynamics of CO2 partial pressure and CO2 outgassing in the lower reaches of the Xijiang River, a subtropical monsoon river in China. Sci. Total Environ. 2007;376:255–266. doi: 10.1016/j.scitotenv.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 59.Louis VLS, Kelly CA, Duchemin É, Rudd JW, Rosenberg DM. Reservoir Surfaces as Sources of Greenhouse Gases to the Atmosphere: A Global Estimate Reservoirs are sources of greenhouse gases to the atmosphere, and their surface areas have increased to the point where they should be included in global inventories of anthropogenic emissions of greenhouse gases. Bioscience. 2000;50:766–775. doi: 10.1641/0006-3568(2000)050[0766:RSASOG]2.0.CO;2. [DOI] [Google Scholar]
- 60.Wickland, K. P., Striegl, R. G., Neff, J. C. & Sachs, T. Effects of permafrost melting on CO2 and CH4 exchange of a poorly drained black spruce lowland. J. Geophys. Res. Biogeosci. 111 (2006).
- 61.Huttunen JT, et al. Fluxes of methane, carbon dioxide and nitrous oxide in boreal lakes and potential anthropogenic effects on the aquatic greenhouse gas emissions. Chemosphere. 2003;52:609–621. doi: 10.1016/S0045-6535(03)00243-1. [DOI] [PubMed] [Google Scholar]
- 62.Turetsky, M. et al. Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland. J. Geophys. Res. Biogeosci. 113 (2008).
- 63.Hope D, Palmer SM, Billett MF, Dawson JJ. Carbon dioxide and methane evasion from a temperate peatland stream. Limnol. Oceanogr. 2001;46:847–857. doi: 10.4319/lo.2001.46.4.0847. [DOI] [Google Scholar]
- 64.Baulch, H. M., Dillon, P. J., Maranger, R. & Schiff, S. L. Diffusive and ebullitive transport of methane and nitrous oxide from streams: Are bubble‐mediated fluxes important? J. Geophys. Res. Biogeosci. 116 (2011).
- 65.Jones JB, Mulholland PJ. Methane input and evasion in a hardwood forest stream: effects of subsurface flow from shallow and deep pathways. Limnol. Oceanogr. 1998;43:1243–1250. doi: 10.4319/lo.1998.43.6.1243. [DOI] [Google Scholar]
- 66.Stow CA, Walker JT, Cardoch L, Spence P, Geron C. N2O emissions from streams in the Neuse River watershed, North Carolina. Environ. Sci. Technol. 2005;39:6999–7004. doi: 10.1021/es0500355. [DOI] [PubMed] [Google Scholar]
- 67.Cole JJ, Caraco NF. Emissions of nitrous oxide (N2O) from a tidal, freshwater river, the Hudson River, New York. Environ. Sci. Technol. 2001;35:991–996. doi: 10.1021/es0015848. [DOI] [PubMed] [Google Scholar]
- 68.Richey JE, Devol AH, Wofsy SC, Victoria R, Riberio MN. Biogenic gases and the oxidation and reduction of carbon in Amazon River and floodplain waters. Limnology and Oceanography. 1988;33:551–561. doi: 10.4319/lo.1988.33.4.0551. [DOI] [Google Scholar]
- 69.Law C, Rees A, Owens N. Temporal variability of denitrification in estuarine sediments. Estuarine, Coastal and Shelf Science. 1991;33:37–56. doi: 10.1016/0272-7714(91)90069-N. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.