Abstract
Purpose
Radiation-induced cognitive decline is relatively common after treatment for primary and metastatic brain tumors; however, identifying dosimetric parameters that are predictive of radiation-induced cognitive decline is difficult due to the heterogeneity of patient characteristics. The memory function is especially susceptible to radiation effects after treatment. The objective of this study is to correlate volumetric radiation doses received by critical neuroanatomic structures to post–radiation therapy (RT) memory impairment.
Methods and materials
Between 2008 and 2011, 53 patients with primary brain malignancies were treated with conventionally fractionated RT in prospectively accrued clinical trials performed at our institution. Dose-volume histogram analysis was performed for the hippocampus, parahippocampus, amygdala, and fusiform gyrus. Hopkins Verbal Learning Test-Revised scores were obtained at least 6 months after RT. Impairment was defined as an immediate recall score ≤15. For each anatomic region, serial regression was performed to correlate volume receiving a given dose (VD(Gy)) with memory impairment.
Results
Hippocampal V53.4Gy to V60.9Gy significantly predicted post-RT memory impairment (P < .05). Within this range, the hippocampal V55Gy was the most significant predictor (P = .004). Hippocampal V55Gy of 0%, 25%, and 50% was associated with tumor-induced impairment rates of 14.9% (95% confidence interval [CI], 7.2%-28.7%), 45.9% (95% CI, 24.7%-68.6%), and 80.6% (95% CI, 39.2%-96.4%), respectively.
Conclusions
The hippocampal V55Gy is a significant predictor for impairment, and a limiting dose below 55 Gy may minimize radiation-induced cognitive impairment.
Summary.
The present study defines specific volumetric radiation therapy dose thresholds for critical neural structures on the basis of Hopkins Verbal Learning Test performance in patients treated with cranial radiation treatment (CRT). Although a radiation-induced cognitive decline has been reported in patients undergoing cranial radiation treatment, dosimetric thresholds for damage to specific brain structures remain undefined. In this study, hippocampal V55Gy significantly predicted impairment and provides additional evidence for the correlation between dose to the hippocampus and cognitive impairment.
Alt-text: Unlabelled box
Introduction
Cranial radiation therapy (CRT) is commonly used to treat brain tumors, particularly for high-grade or malignant lesions. Unfortunately, radiation-induced neurocognitive toxicity after CRT is a relatively common complication of CRT, occurring in 50% to 90% of treated patients.1, 2, 3, 4, 5, 6 The treatment options for neurocognitive toxicity are quite limited once symptoms are present.5 Survival rates for patients who experience radiation-induced cognitive decline (RICD) have been increasing due to the improved survival of patients with malignant brain tumors.7 Furthermore, indications for therapy in patients with aggressively behaving benign tumors such as atypical meningiomas8 and low-grade gliomas continue to increase.9
The time course and underlying mechanisms of CRT-related neurocognitive changes are complex.10 Early-onset symptoms are usually associated with transient demyelination, and delayed symptoms are associated with vascular abnormalities, demyelination, and/or white matter necrosis.2 Processes leading to chronic RICD are believed to occur at doses below those that result in radiographically detectable anatomic injuries such as radionecrosis.
RICD has been reported in patients receiving doses <60 Gy to brain regions that are involved in adult neurogenesis.11 However, dosimetric thresholds for damage to such brain structures have not been convincingly defined. Identifying dosimetric factors that are predictive of RICD is difficult due to the heterogeneity of patients who receive CRT as well as the contribution of confounding factors such as baseline neurocognitive status, disease progression, medical comorbidities, and use of chemotherapy. Furthermore, cognitive outcomes can be affected by tumor progression and regression. Patients with greater tumor regression after whole brain radiation therapy (WBRT) tend to have better recovery of executive and fine motor functions after treatment.12 However, even in the setting of achieving local tumor control, it appears that immediate recall and delayed recall, as assessed with the Hopkins Verbal Learning Test (HVLT), declines after WBRT.13
The susceptibility of memory function to radiation effects makes it a useful outcome for which to establish dosimetric thresholds for neurocognitive decline after treatment. The objective of this study is to correlate volumetric dose received by critical neural structures to cognitive decline through the use of cognitive function test scores as a measure of overall cognition.
Methods and materials
Patient population
Patients in this study were treated for a primary brain tumor between February 2008 and October 2011 in one of two National Cancer Institute–approved prospective clinical trials (WFU97100/91105)14, 15 assessing the use of donepezil (Aricept) in patients who receive CRT. Patients treated at our institution with available dosimetric data were included in the analysis. All patients were ≥18 years of age with a clinically predicted life expectancy of ≥30 weeks and Karnofsky Performance Status ≥70. Electronic medical records were reviewed to determine patient characteristics (ie, age, sex, Eastern Cooperative Oncology Group performance status, prior WBRT, prior surgery, and education level) and disease characteristics (ie, tumor histology, tumor size, and location).
Treatment
Patients were treated with partial- or whole-brain CRT. All treatments were planned with the Pinnacle Treatment Planning System (Philips, Andover, MA). Doses and treatment fields were determined by the treating physician but were generally based on the guidelines used in major Radiation Therapy Oncology Group trials. Each patient underwent pre-CRT computed tomography (CT) and magnetic resonance imaging (MRI) of the brain. Patients also had follow-up MRI brain scans to assess tumor response ≥6 months after treatment.
Cognitive testing
Per clinical trial criteria, all patients were enrolled at least 6 months after completing brain RT.14 Postrandomization, baseline cognitive batteries were administered. We analyzed baseline HVLT-Revised (HLVT-R) scores as a measure of cognitive functioning and Functional Assessment of Cancer Therapy-Brain (FACT-Br) as a measure of health-related quality of life. Because no pre-RT baseline data could be collected, impairment was defined as an HVLT-R immediate recall score of ≤15 based on studies reporting optimal sensitivity and specificity for detecting impairment with HVLT-R cutoff scores of 14.5 to 15.5.16, 17, 18, 19 HVLT total score and subsection scores (total recall, delayed recall,retention [percent retained], and recognition discrimination index) were correlated to patient and treatment characteristics.
Region of interest volume determination
Selected anatomical regions of interest (ROIs) represent functional targets of cognitive decline after CRT.1, 11 Briefly, the hippocampus was chosen because of its role in spatial and working memory. The fusiform gyrus has a role in verbal memory. Damage to the amygdala has been implicated previously in affecting HVLT performance. Other disease processes that affect the parahippocampal gyrus have also been found to affect HVLT performance.
Recoverable treatment plans were included in dosimetric analyses. Pre-CRT CT and MRI image sets were obtained and exported to MIM Maestro Version 6.4 (MIM Software, Cleveland, OH) for ROI delineation. Pre-CRT MRI and CT image sets were fused using rigid registration. Brain ROIs were defined bilaterally using standard anatomical landmarks on the pretreatment and posttreatment T1-weighted gadolinium-enhanced MRI axial sequences (Fig 1).
Figure 1.
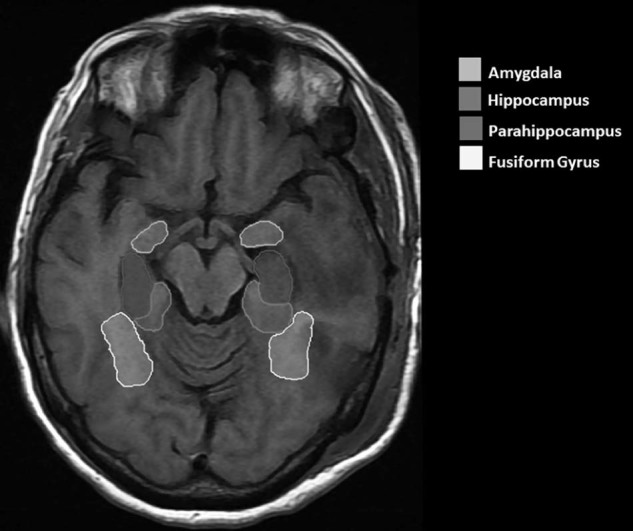
Delineation of regions of interest on T1-weighted magnetic resonance imaging of the brain. The hippocampus, amygdala, parahippocampus, and fusiform gyrus are delineated. All structure volumes included both the left and right sides.
The hippocampus was contoured from its head to the floor of the temporal horn of the lateral ventricle.20 The lateral edge of the quadrigeminal cistern was used to define the medial boundary of the hippocampus bilaterally. The amygdala, which lies anterior and superior to the hippocampus, was segmented from the hippocampus using the alveus of the hippocampus as the inferior boundary. Variations in signal intensity between the white matter of the parahippocampal gyrus and the gray matter of the hippocampus were used to distinguish the hippocampus from the surrounding parahippocampal gyrus. Similarly, the fusiform gyrus was identified below the parahippocampal gyrus. ROIs were manually delineated by a single observer after passing intraobserver reliability tests.
Dosimetric analysis
CRT plans were imported and reconstructed in MIM for total absolute dose. MRI contours of ROIs were used to construct dose-volume histograms (DVHs) for structures that are critical to memory and recognition, namely the hippocampus, parahippocampal gyrus, amygdala, and fusiform gyrus. Volumes were normalized to determine the percentage of volume receiving a given dose, VD(Gy). The VD(Gy) was determined for the entire dose range in 0.1 Gy intervals.
Statistical analysis
The relationship between binary HVLT immediate outcome and patient variables (ie, age, sex, performance status, and education), tumor characteristics (ie, histology, grade, and size), treatment characteristics (ie, total dose, fractionation scheme, and treatment location), and health-related quality of life outcomes (FACT-Br score) were assessed using Mann-Whitney U test and Fisher's exact test.
Serial logistic regressions were performed for the predictor variable VD(Gy) for each discrete dose between 0 Gy and the maximum dose received by any patient at 0.1 Gy intervals. The binary outcome variable evaluated by these logistic regressions was post-CRT HVLT-R ≤ 15 or HVLT-R > 15. The Akaike information criterion (AIC) was calculated for each VD(Gy) model to compare the quality of the models. Multiple logistic regression for HVLT (binary) with the patient variable age and VD(Gy) was conducted to account for expected age-related cognitive decline.
Results
Between February 2008 and October 2011, 81 patients were treated with CRT at our institution in the (WFU97100/91105) trials. Of the 81 patients, 53 had archived RT plans that could be recovered from the treatment planning software and were included in dosimetric analysis. The following tumor types were included in the analysis: glioblastoma (13%), primitive neuroectodermal (21%), and low-grade/benign tumors (66%). The median age was 49 years (range, 19-84 years). All patients had an Eastern Cooperative Oncology Group performance status score of ≤2.
The majority (81%) of these patients were treated with conventionally fractionated partial brain radiation therapy, and 10 patients received WBRT with a regional boost. (Table 1) The hippocampus was included in the planning target volume for 10 patients. The median prescribed radiation dose was 54 Gy (range, 40.0-60.6 Gy) delivered in 1.8 Gy per fraction (range, 1.5-2.5 Gy per fraction).
Table 1.
Participant and treatment characteristics
| Characteristic | n (%) |
|---|---|
| Total analyzed (% Total) | 53 (65.4) |
| Participant Characteristics | |
| Median age, years (range) | 49 (20-84) |
| Sex (Male) | 26 (49.1) |
| Race (White) | 48 (90.5) |
| Handedness (Right) | 44 (83.0) |
| ECOG PS | |
| 0 | 26 (49.1) |
| 1 | 26 (49.1) |
| 2 | 1 (1.9) |
| 3 | 0 (0) |
| 4 | 0 (0) |
| Education | |
| High school or less | 16 (30.8) |
| College/Vocational | 23 (44.2) |
| Graduate | 13 (25.0) |
| Tumor Characteristics | |
| Hemisphere Involved | |
| Bilateral | 8 (15.4) |
| Left | 23 (44.2) |
| Right | 21 (40.4) |
| Brain Regions included in PTV | |
| Frontal lobe | 15 (28.3) |
| Temporal lobe | 7 (13.2) |
| Parietal lobe | 4 (7.5) |
| Occipital lobe | 18 (34.0) |
| Whole brain | 10 (18.9) |
| Hippocampus | 10 (18.9) |
| Tumor Type | |
| Glioblastoma | 4 (8) |
| Anaplastic glioma | 3 (6) |
| Primitive neuroectiodermal | 11 (21) |
| Benign/Low-grade | 35 (66) |
| Treatment Characteristics | |
| Median Maximum RT Dose, cGy (range) | 5400 (4000-6060) |
| Median Fractional Dose, cGy (range) | 180.00 (150-200) |
ECOG PS, Eastern Cooperative Oncology Group performance status; PTV, planning target volume; RT, radiation therapy.
Cognitive testing was performed at a median of 10 months (range, 6-26 months) after the completion of CRT. Impairment based on HVLT score ≤15 was identified in 24.5% of patients (n = 13). The median score for HVLT-R immediate recall score was 21 (range, 2-32). Median FACT-Br score was 130 (range, 68-178). Patients with HVLT scores of ≤15 were significantly older (P = .004) and tended to receive higher total doses (P = .08) than patients with higher post-CRT HVLT scores (Table 2). No significant correlation was observed between tumor type and impairment.
Table 2.
Characteristics stratified by Hopkins Verbal Learning Test-Revised immediate recall score
| Impairment | HVLT Score ≤15 | HVLT Score >15 | |
|---|---|---|---|
| n | 13 | 40 | P-value |
| Age, median (range) | 58.37 (29.06-84.73) | 44.20 (19.78-78.95) | .004 |
| Irradiation dose, median (range) | 5507.00 (4500.00-6016.00) | 5360.00 (4000.00-6060.00) | .08 |
| Dose per fraction, median (range) | 180.00 (150.00-200.00) | 180.00 (150.00-200.00) | .166 |
| HVLT total, median (range) | 10.00 (2.00-15.00) | 23.00 (17.00-32.00) | < .001 |
| HVLT-DR, median (range) | 0.00 (0.00-7.00) | 8.00 (1.00-12.00) | < .001 |
| HVLT-discrim, median (range) | 7.00 (0.00-11.00) | 10.50 (5.00-12.00) | .001 |
| HVLT-sav, median (range) | 0.00 (0.00-133.33) | 81.82 (14.29-120.00) | .008 |
| HVLT-recog, median (range) | 8.00 (5.00-12.00) | 11.00 (5.00-12.00) | .009 |
discrim, discrimination; HVLT, Hopkins Verbal Learning Test; DR, Hopkins Verbal Learning Test-Revised-Delayed Recall; recog, recognition; sav, stress and verbal.
Dosimetric results
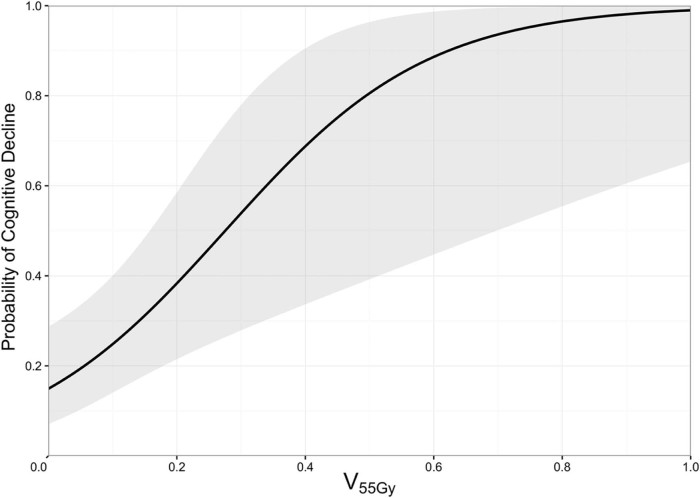
Hippocampal V53.4Gy to V60.9Gy was a statistically significant predictor of post-CRT HVLT scores ≤15 (P < .05; Fig 2; Suppl. Table S1) Hippocampal V55Gy was the most significant predictor (P = .004; AIC = 53.4). V55Gy of 0%, 25%, and 50% was associated with postradiation impairment rates of 14.9% (95% confidence interval [CI], 7.2%-28.7%), 45.9% (95% CI, 24.7%-68.6%), and 80.6% (95% CI, 39.2%-96.4%), respectively. The multivariate model including V55Gy and age was strongly predictive for lower posttreatment HVLT scores (P = .0006; AIC = 42.92). Fusiform gyrus V18.5Gy to V59.9Gy were also significant predictors of total HVLT scores, with the most significant relationship at V46.5Gy (P = .003). No significant dosimetric relationship was observed for the parahippocampus V0.1Gy to V60Gy.
Figure 2.
The probability of cognitive impairment for hippocampal V55 Gy, the most significant predictor of post–cranial radiation therapy cognitive impairment, as defined by Hopkins Verbal Learning Test-Revised immediate recall scores ≤15, (P = .004). The 95% confidence interval is shown.
Discussion
RICD is a significant long-term toxicity that is associated with prior CRT. RICD can affect both quality of life and performance status after CRT.21 Prevention and risk stratification are imperative because deficits are often permanent with no therapies that have been proven to reverse symptoms. Injury to the hippocampus is thought to play an important role in the development of RICD; however, dosimetric constraints have thus far not been defined.22 In this study, hippocampal V55Gy was found to better predict tumor-induced impairment than parahippocampal, fusiform gyrus, amygdala, or lesser hippocampal volumetric dose. The rate of cognitive impairment in patients whose treatment plans allowed for a V55 = 0% was 14.9% compared with a rate of 45.9% in patients where V55 ≥25%, which suggests that limiting the amount of the hippocampus that receives 55 Gy may be meaningful when considering late neurocognitive function effects of treatment.
Earlier studies examining RICD suggested that volumetric dose to the hippocampal formation is important.13, 22, 23 Studies investigating hippocampal sparing indicate that the degree of RICD risk, particularly for deficits in learning, memory, and spatial processing, is largely dependent on the volume that is spared.23 In a prior dosimetric study, investigators performed a DVH analysis of multiple regions in the brain that were suspected of contributing as target structures for radiation damage. In this preliminary analysis, the volume of the hippocampus receiving 60 Gy was found to be predictive of global cognitive functioning.11 This study was important because it confirmed that although other structures within the brain may contribute to RICD, dose to the temporal lobes and hippocampus had a significant effect on cognitive function after CRT. Gondi et al prospectively evaluated 18 patients with benign or low-grade brain tumors and correlated DVHs to cognitive performance. The results of this analysis showed that patients with >40% of the bilateral hipocampi receiving dose >7.3 Gy had worsened impairment on the Wechsler Memory Scale delayed recall test.23 The results from the present study are consistent with these prior studies and support the focus on the hippocampus over other limbic structures, which suggests that clinically encountered doses (ie, >55 Gy) may be sufficient to result in RICD.
Although the frequency of tumor recurrence in the regions that were spared high-dose radiation is relatively low, avoidance of target structures that are involved in cognition may increase this risk.24, 25 Treating the limbic system as a series circuit and sparing selected structures may be a possible and potentially feasible solution to minimize the risk of treatment-related cognitive impairment. Identifying dose constraints for the hippocampus is important; however, equally important is identifying regions that are less sensitive to radiation and not associated with an increased risk for RICD. Data from the present study suggest that clinically relevant doses to the parahippocampus and amygdala are not associated with RICD and do not support involvement of these regions in radiation sparing.
Although this study provides additional evidence for the correlation between dose to the hippocampus and treatment-related cognitive impairment, it has several limitations. The correlation between V55 and cognitive decline could be confounded by the fact that the volume receiving 55 Gy often contained tumor and the presence of tumor or prior surgery probably contributes to the degree of cognitive decline. Furthermore, a single time point of patients' cognitive decline was used. Pre-CRT cognitive testing would allow for a more complete understanding of the evolution of cognitive decline in these patients. In addition, although HVLT is a proper assessment of hippocampal-dependent memory and cognition, non–hippocampal-dependent memory may also contribute to RICD and may not be addressed with the instrument that was used to define RICD in this study.
Given the increasing recognition of cortical networks in cognitive function, an evaluation of the role of irradiation of networks as well as specific structures in RICD is important. Although hippocampal avoidance may improve cognitive function over conventional 3-dimensional techniques, cognition may still be adversely affected by CRT. Future prospective studies are needed to validate the findings of the current study.
Footnotes
Funding Source: Wake Forest NCORP grant (UG CA189824-01)
Conflicts of interest: None.
Supplementary material related to this article can be found at https://doi.org/10.1016/j.adro.2017.08.013.
Supplementary data
The following is the supplementary data to this article:
Hippocampal dosimetric parameters significantly predictive for post-treatment HVLT scores.
References
- 1.Gondi V., Tomé W.A., Mehta M.P. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene-Schloesser D., Robbins M.E., Peiffer A.M., Shaw E.G., Wheeler K.T., Chan M.D. Radiation-induced brain injury: A review. Front Oncol. 2012;2:1–18. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown B.P., Buckner J.C., Fallon J.R.O. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination. J Clin Oncol. 2003;21:2519–2524. doi: 10.1200/JCO.2003.04.172. [DOI] [PubMed] [Google Scholar]
- 4.Crossen B.J., Garwood D., Glatstein E., Neuwelt E.A. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J Clin Oncol. 2016;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 5.Attia A., Page B.R., Lesser G.J., Chan M. Treatment of radiation-induced cognitive decline. Curr Treat Options Oncol. 2014;15:539–550. doi: 10.1007/s11864-014-0307-3. [DOI] [PubMed] [Google Scholar]
- 6.Greene-Schloesser D., Robbins M.E. Radiation-induced cognitive impairment-from bench to bedside. J Neurooncol. 2012;14:37–44. doi: 10.1093/neuonc/nos196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Chan M.D., Tatter S.B., Lesser G., Shaw E.G. Radiation oncology in brain tumors: Current approaches and clinical trials in progress. Neuroimaging Clin N Am. 2010;20:401–408. doi: 10.1016/j.nic.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Shaw E.G., Wang M., Coons S.W. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: Initial results of RTOG 9802. J Clin Oncol. 2012;30:3065–3070. doi: 10.1200/JCO.2011.35.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink J., Born D., Chamberlain M.C. Radiation necrosis: Relevance with respect to treatment of primary and secondary brain tumors. Curr Neurol Neurosci Rep. 2012;12:276–285. doi: 10.1007/s11910-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 11.Peiffer A.M., Leyrer C.M., Greene-Schloesser D.M. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology. 2013;80:747–753. doi: 10.1212/WNL.0b013e318283bb0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Bentzen S.M., Renschler M., Mehta M.P. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 13.Chang E.L., Wefel J.S., Hess K.R. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 14.Shaw E.G., Rosdhal R., D'Agostino R.B. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 15.Rapp S.R., Case L.D., Peiffer A. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33:1653–1659. doi: 10.1200/JCO.2014.58.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank R.M., Byrne G.J. The clinical utility of the Hopkins Verbal Learning Test as a screening test for mild dementia. Int J Geriatr Psychiatry. 2000;15:317–324. doi: 10.1002/(sici)1099-1166(200004)15:4<317::aid-gps116>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Hogervorst E., Combrinck M., Lapuerta P., Rue J., Swales K., Budge M. The Hopkins Verbal Learning Test and screening for dementia. Dement Geriatr Cogn Disord. 2002;13:13–20. doi: 10.1159/000048628. [DOI] [PubMed] [Google Scholar]
- 18.Kuslansky G., Katz M., Verghese J. Detecting dementia with the Hopkins Verbal Learning Test and the mini-mental state examination. Arch Clin Neuropsychol. 2004;19:89–104. [PubMed] [Google Scholar]
- 19.Shi J., Tian J., Wei M., Miao Y., Wang Y. The utility of the Hopkins Verbal Learning Test (Chinese version) for screening dementia and mild cognitive impairment in a Chinese population. BMC Neurol. 2012;12:136. doi: 10.1186/1471-2377-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chera B.S., Amdur R.J., Patel P., Mendenhall W.M. A radiation oncologist's guide to contouring the hippocampus. Am J Clin Oncol. 2009;32:20–22. doi: 10.1097/COC.0b013e318178e4e8. [DOI] [PubMed] [Google Scholar]
- 21.Soffietti R., Kocher M., Abacioglu U.M. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 22.Gondi V., Pugh S.L., Tomé W.A. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gondi V., Hermann B.P., Mehta M.P., Tomé W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85:345–354. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Ghia A., Tomé W.A., Thomas S. Distribution of brain metastases in relation to the hippocampus: Implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68:971–977. doi: 10.1016/j.ijrobp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Gondi V., Tomé W.A., Marsh J. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: Safety profile for RTOG 0933. Radiother Oncol. 2010;95:327–331. doi: 10.1016/j.radonc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hippocampal dosimetric parameters significantly predictive for post-treatment HVLT scores.