Abstract
Purpose
Brain metastases are common in patients with limited-stage small cell lung cancer (LS-SCLC) due to the inability of most chemotherapeutics to penetrate the blood–brain barrier. Prophylactic cranial irradiation (PCI) is therefore recommended for use in patients with a good response to concurrent chemoradiotherapy. However, PCI is not always delivered; therefore, we investigated the reasons for PCI omission in patients who underwent therapy with curative intent.
Methods and materials
We retrospectively reviewed all patients with LS-SCLC who were treated with curative intent at our institution. Overall survival and cumulative incidence of brain metastasis were estimated by the Kaplan-Meier method. The Pearson χ2 test and Mann-Whitney U test were used to examine factors associated with PCI use, and prognostic factors were analyzed with Cox proportional hazards modeling.
Results
We examined 208 patients who were treated for LS-SCLC at our institution. A total of 115 patients (55%) received PCI. The most common documented reason for PCI omission was patient refusal due to neurotoxicity concerns (38%). Physician assessment of being medically unfit (33%) and of advanced age (8%) were the second and third most common reasons, respectively. Karnofsky performance status and clinical American Joint Committee on Cancer stage but not PCI were significantly associated with overall survival. Only clinical stage remained an independent factor on multivariate analysis.
Conclusions
Approximately half of patients with LS-SCLC ultimately receive PCI, generally for guideline-recommended reasons. The most common reason for PCI omission was patient concerns regarding neurotoxicity. Efforts to decrease PCI neurotoxicity, including hippocampal-sparing radiation and memantine use, may increase the use of this survival-improving intervention in eligible patients with LS-SCLC.
Summary.
We sought to define the reasons for omission of prophylactic cranial irradiation (PCI) in a cohort of 208 patients with limited-stage small cell lung cancer. Of the 93 patients who did not receive PCI, the most common reason for PCI omission was patient refusal due to neurotoxicity concerns (38%). Being medically unfit (33%) and of advanced age (8%) were the next most common reasons. Efforts to decrease PCI neurotoxicity may increase the use of this survival-improving intervention.
Alt-text: Unlabelled box
Introduction
Limited-stage small cell lung cancer (LS-SCLC) is typically treated with chemotherapy and radiation therapy (RT) to the primary tumor. However, the brain is a frequent site of metastasis due to the inability of most chemotherapeutics to penetrate the blood–brain barrier and may be the sole site of recurrence in patients with otherwise good responses to initial therapy. Brain metastasis is associated with significant neurocognitive symptoms and poor survival and has been observed in more than 50% of patients with SCLC.1 Although the use of prophylactic cranial irradiation (PCI) is debated in patients with very early stage SCLC, such as T1N0M0 disease,2 PCI is currently considered a standard-of-care intervention in patients with LS-SCLC who achieve a complete or partial response to initial chemotherapy.3
PCI has been studied extensively in clinical trials and was shown to improve overall survival (OS) in patients with SCLC in 2 meta-analyses of phase 2 and 3 clinical trials.4, 5 Current National Comprehensive Cancer Network guidelines recommend the use of PCI for a limited number of patients with SCLC who respond well to initial therapy.
PCI is associated with chronic neurocognitive deficits in attention, memory, and problem-solving ability6, 7; however, these toxicity concerns must be weighed against the potential for neurologic deficits caused by disease progression in the brain. Currently, data on the utilization rates of PCI and the precise reasons for omission are limited. Therefore, in this study, we examined the rate of PCI use and factors associated with a lack of use in patients with LS-SCLC at a large academic institution.
Methods and materials
Patient cohort
After receiving institutional review board approval, we retrospectively reviewed all patients with LS-SCLC who were treated with curative intent at our institution from 1999 to 2013. Demographic information, stage, treatment, and disease-related outcomes were extracted from the medical record. For patients who did not receive PCI, we reviewed the clinical notes from the treating oncologists (medical, radiation, and surgical) to ascertain the reason.
Patients were staged according to the 7th Edition of the American Joint Committee on Cancer (AJCC) classification system, and all patients with up to stage IIIB disease were considered to have LS-SCLC. All patients had pathologic confirmation of disease at our institution and underwent an extent-of-disease evaluation with body computed tomography (CT) or positron emission tomography scans and a brain CT scan or magnetic resonance imaging to rule out distant metastases. Follow-up after completion of all therapy typically consisted of a history review, physical examination, CT chest scan, and magnetic resonance imaging of the brain every 3 to 6 months or as clinically indicated.
Endpoints and statistical considerations
All endpoints were calculated from the date of pathologic diagnosis. OS was calculated from the start date of chemotherapy until the date of death or last follow-up. OS was estimated with the Kaplan-Meier method and analyzed using the Cox proportional hazards regression model. Cumulative incidence of brain metastasis was analyzed by the Fine-Gray competing risks regression model, and death without brain metastasis was considered a competing risk. Pearson's χ2 test and Mann-Whitney U test were used to examine factors associated with PCI use, and prognostic factors were analyzed by Cox proportional hazards modeling. Factors included in the analyses were age at diagnosis, Karnofsky performance status (KPS), sex, clinical AJCC stage, concurrent versus sequential delivery of RT, thoracic RT fractionation, and PCI use. PCI and factors with a P-value <.1 on univariate analysis were included in the multivariate analysis. All statistical tests were two-sided, and P < .05 was considered statistically significant.
Results
We identified 283 patients who were treated with curative intent with surgery or radiation for LS-SCLC at our institution. Surgical patients lost to follow-up with unknown chemotherapy receipt7 or patients who refused chemotherapy6 were excluded. All remaining 264 patients received systemic therapy as part of their initial therapy. Thoracic RT (TRT) was administered in 236 patients (89%), and surgery was performed in 28 patients (11%). All patients were restaged after CT scanning, and 48 patients were excluded from the study due to disease progression, 5 patients died during or shortly after initial therapy, and 7 patients were lost to follow-up. Of the 208 patients in the study cohort, the median follow-up for all patients was 29.7 months. The median age was 67 years (Table 1).
Table 1.
Patient demographics and baseline characteristics
| Total Cohort | PCI Administered | PCI Not Administered | P-valuea | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Patients | 208 | 115 | 55 | 93 | 45 | ||
| Median age (range), years | 67 (43-94) | 63 (44-85) | 71 (46-94) | <.001 | |||
| Median KPS (range) | 90 (40-100) | 90 (60-100) | 80 (40-100) | .004 | |||
| KPS ≤70 | 29 | 14 | 7 | 24 | 22 | 76 | |
| Sex | .121 | ||||||
| Female | 119 | 57 | 71 | 60 | 48 | 40 | |
| Male | 90 | 43 | 44 | 49 | 45 | 50 | |
| Clinical AJCC stage | .067 | ||||||
| IA | 22 | 11 | 6 | 27 | 16 | 73 | |
| IB | 9 | 4 | 3 | 33 | 6 | 67 | |
| IIA | 30 | 14 | 18 | 60 | 12 | 40 | |
| IIB | 12 | 6 | 7 | 58 | 5 | 42 | |
| IIIA | 94 | 45 | 55 | 59 | 39 | 41 | |
| IIIB | 41 | 20 | 26 | 63 | 15 | 37 | |
| Thoracic RT | <.001 | ||||||
| Yes | 181 | 87 | 109 | 60 | 72 | 40 | |
| No (surgical resection only) | 27 | 13 | 6 | 22 | 21 | 78 | |
| Thoracic RT fractionation | <.001 | ||||||
| BID | 104 | 50 | 74 | 71 | 30 | 29 | |
| QD | 77 | 37 | 35 | 45 | 42 | 55 | |
| Chemotherapy timing with thoracic RT | <.001 | ||||||
| Concurrent | 143 | 69 | 100 | 70 | 43 | 30 | |
| Sequential (pre-RT) | 38 | 18 | 15 | 39 | 50 | 32 | |
| PCIb | |||||||
| Median dose (IQR), Gy | 25.0 (25.0-31.0) | ||||||
| 10 fractions | 63 | 30% | |||||
| 12-13 fractions | 3 | 1% | |||||
| 15 fractions | 46 | 22% | |||||
AJCC, American Joint Committee on Cancer; BID, twice per day; IQR, interquartile range; KPS, Karnofsky performance status; PCI, prophylactic cranial irradiation; QD, once per day; RT, radiation therapy.
Independent-samples Mann-Whitney U test was performed for age, KPS, and thoracic RT dose. χ2 test was performed for all other variables.
A total of 112 of 115 patients with available fraction number and dose.
Treatment details
All patients received a platinum-based doublet chemotherapy regimen, most commonly with etoposide (97%), after or during local therapy. Twenty patients did not receive TRT but underwent surgical resection (n = 27; 13%), 19 of whom underwent lobectomy (70%). All but one had a T classification of T1 or T2 (96%). All patients who received TRT (n = 181; 87%) underwent a CT-based simulation in the supine position with custom immobilization and were treated to the involved field. Most patients were treated with 3-dimensional conformal or intensity modulated RT (n = 123; 68%). The majority of TRT patients received twice-daily (BID) fractionation (n = 104; 57%); all BID-treated patients received 45 Gy, with the exception of one patient who declined to complete TRT because of toxicity and ultimately received 30 Gy. For patients who received once-daily RT, the intended prescription dose was ≥54 to 60 Gy and the median dose was 54 Gy (interquartile range, 54-59.4 Gy; range, 23.4-70 Gy). The 6 patients who received ≤45 Gy in daily fractions had difficulty tolerating completion to the prescribed RT dose but 3 patients achieved a complete response and 3 patients a partial response to initial treatment. After recovery from acute toxicity, 3 patients later opted to receive PCI. The vast majority of patients (>90%) achieved a complete or partial response to thorax-directed TR. Chemotherapy sequencing was concurrent with RT for most patients (n = 143; 69%), and RT was generally initiated with the first or second cycle of chemotherapy (Table 2).
Table 2.
PCI utilization rates and reasons for declining treatment
| All patients | Date of diagnosis | P-valuea | |||||
|---|---|---|---|---|---|---|---|
| 2/2/2008 and prior | After 2/2/2008 | ||||||
| n | % | n | % | n | % | ||
| Number of patients | 208 | 100 | 105 | 50 | 103 | 50 | |
| Age, median [range] | 67 (43-94) | 67 (43-94) | 67 (46-86) | .685 | |||
| PCI utilization | .184 | ||||||
| PCI received | 115 | 55 | 53 | 50 | 62 | 60 | |
| Lack of PCI | 93 | 45 | 52 | 50 | 41 | 40 | |
| Brain imaging pre-RT | 195 | 94 | 101 | 96 | 94 | 91 | .147 |
| Reason for lack of PCI | .171 | ||||||
| Patient refused | 35 | 38 | 18 | 35 | 17 | 41 | |
| Medically unfit | 31 | 33 | 18 | 35 | 13 | 32 | |
| Medical Oncology | 21 | 68 | |||||
| Radiation Oncology | 10 | 32 | |||||
| Age | 7 | 8 | 4 | 8 | 3 | 7 | |
| Other | 4 | 4 | 3 | 6 | 1 | 2 | |
| Prior RT | 2 | 2 | 2 | 4 | 0 | 0 | |
| Unknown reasons | 14 | 15 | 7 | 13 | 7 | 17 | |
PCI, prophylactic cranial irradiation; RT, radiation therapy.
Independent-samples Mann-Whitney U test was performed for age and reason for lack of PCI. χ2 test was performed for all other variables.
Prophylactic cranial irradiation details and reasons for omission
A total of 115 patients (55%) received PCI. PCI was generally delivered in 25 Gy over 10 fractions (n = 62; 54%), with the next most frequent regimen being 30 Gy over 15 fractions (n = 47; 41%; Table 1).
Patients who did not receive PCI were more likely to be older (P < .001), have lower KPS scores (P = .004), receive sequential chemotherapy rather than concurrent (P < .001), undergo surgical resection for local therapy (P < .001), and receive daily fractionation for TRT instead of BID TRT (P < .001).
There was no difference in PCI utilization on the basis of the era of diagnosis as determined by the date of diagnosis (Table 2). Of those patients who did not subsequently receive PCI, the most common documented reason for omission of PCI was patient refusal due to concerns about potential toxicity (35 patients; 38%). The second most common reason was being deemed medically unfit after primary therapy by oncologist assessment (31 patients; 33%). The determination of medical fitness was made by radiation oncologists in 21 cases (68%) and by medical oncologists in 10 cases (32%). Patients being considered too advanced in age (7 patients; 8%) was another common reason for non-recommendation of PCI, where all but one patient was ≥77 years of age (range, 70-94 years). Two patients (2%) had received prior head and neck RT; therefore, PCI was not recommended. Four patients (4%) had other reasons for not receiving PCI. For 14 patients (15%), the reason for PCI omission was not clearly documented (Table 2).
Outcomes
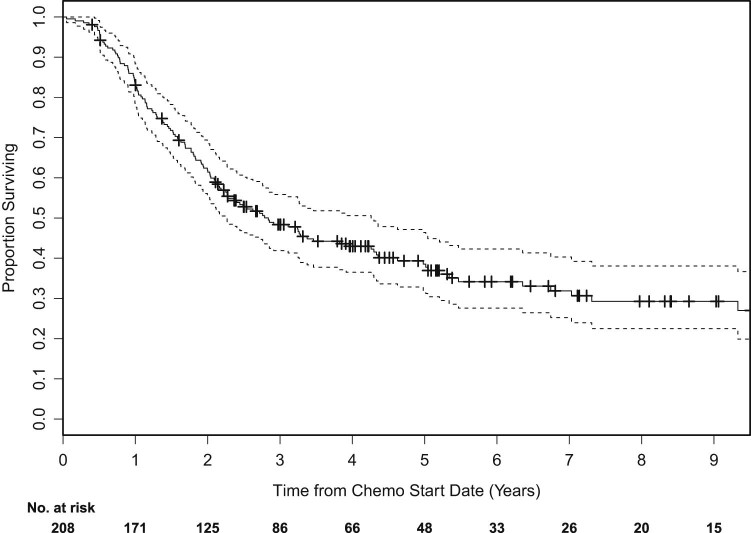
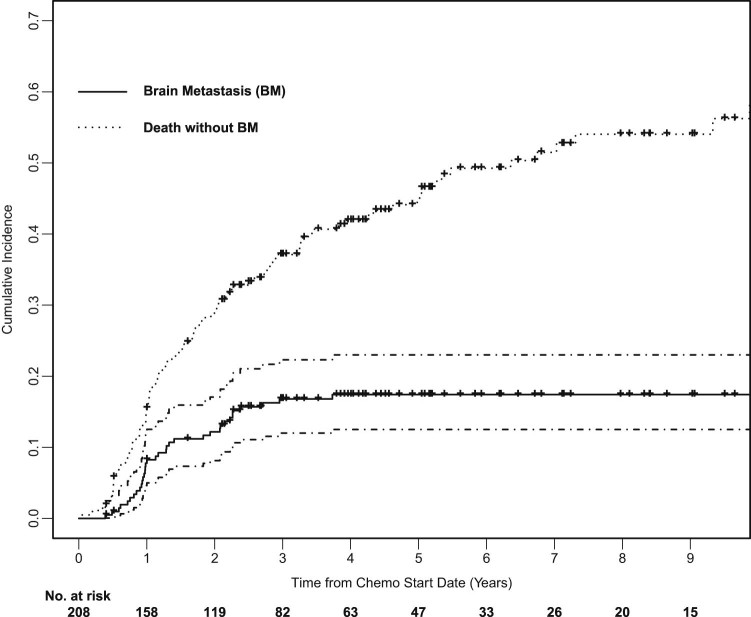
The median OS was 35.1 months (Fig 1). At 2 years, the OS rate was 64%; OS was 49% at 3 years. KPS (0.813; 95% confidence interval [CI], 0.983-1.023; P = .034) and clinical AJCC stage (1.209; 95% CI, 1.070-1.366; P = .002) but not PCI (0.917; 95% CI, 0.651-1.292; P = .62) were significantly associated with OS; only clinical stage remained an independent factor on multivariate analysis (1.208; 95% CI, 1.021-1.429; P = 0.027). Cumulative incidence of brain metastasis was 12% at 2 years and 17% at 3 years, and no factors were identified as significantly associated with cumulative incidence of brain metastasis (Fig 2).
Figure 1.
Kaplan-Meier survival curves of overall survival for all patients.
Figure 2.
Cumulative incidence of brain metastasis with death as a competing risk.
Discussion
Two large-scale Surveillance, Epidemiology, and End Results analyses and 2 meta-analyses of multiple phase 2 and 3 studies with large patient populations have demonstrated a significant association between PCI use and improved survival in patients with SCLC.4, 5, 8, 9 Therefore, PCI is commonly accepted to prevent brain metastasis and improve survival in patients with LS-SCLC, yet its use among patients is limited even in a large tertiary cancer center. This deficit substantially results from concerns about neurotoxicity.
Despite this strong association among PCI, intracranial control, and OS, roughly half of the patients at our large academic center do not receive PCI, mainly because of neurotoxicity concerns on the part of the patient, the oncologist, or both. Although late effects of dementia, ataxia, and urinary incontinence have been observed in a small percentage of long-term survivors after cranial irradiation, these findings are largely from early retrospective studies with small sample sizes or higher doses of radiation.10 No significant decline in neurocognitive function was observed between PCI and no-PCI groups in 2 large, randomized trials, and PCI continues to be recommended in the National Comprehensive Cancer Network guidelines for the treatment of LS-SCLC in patients who achieved a complete or partial response to initial chemoradiation therapy.1, 11 Given the benefits of PCI on intracranial disease control and survival, it is important to better understand the reasons for patient refusal and to appropriately address these concerns.
A recent study of patient choice in PCI use for NSCLC demonstrated that improved survival is the most important consideration, even at the expense of significant neurotoxicity.12 Interestingly, participants appeared to be more concerned about brain metastasis posttreatment and were more willing to accept some neurotoxicity for a decreased risk of brain metastasis, which is perhaps due to increased preoccupation with preventing disease recurrence as patients approached the completion of definitive therapy. The findings of our study confirm the primacy of patient concerns about neurotoxicity. We also found that physician concern about toxicity is a significant contributor to omission of PCI, with appropriate considerations that include age and medical fitness. Overall, concerns about PCI-associated neurotoxicity appear to be the major impediment preventing patients from receiving PCI. Therefore, investigating methods to minimize PCI-associated neurotoxicity could increase utilization rates by increasing the appeal of this intervention for both patients and practitioners. Two current methods of minimizing PCI-associated neurotoxicity are the use of memantine and/or hippocampal-sparing radiation techniques.
Understanding the mechanisms of radiation-related neurotoxicity could advance our ability to mitigate this adverse outcome. Brain irradiation has been associated with gradual damage to white matter, as observed on imaging.7, 13 Preclinical studies suggest an association among late neurotoxicity, inflammation, and damage to neural progenitor cells in the hippocampus and subventricular zone.14, 15, 16 A dose-volume histogram analysis of 2 prospective clinical trials demonstrated that radiation-induced neurotoxicity is predicted by radiation dose to specific regions of the brain, including the hippocampus, rather than dose to the whole brain.17 Because brain metastasis to the hippocampus is rare, hippocampal-sparing radiation may limit radiation-induced neurotoxicity while still providing adequate disease control.18
Hippocampal-sparing radiation has been evaluated in Radiation Therapy Oncology Group (RTOG) 0933, a phase 2 study of hippocampal avoidance during whole-brain radiation therapy (WBRT), in which neurocognitive outcomes were compared with the historical controls of WBRT patients treated without hippocampal sparing. Hippocampal sparing was found to be associated with memory preservation and improved quality of life.19 A randomized phase 2/3 trial, NRG-CC003, is currently ongoing to evaluate the efficacy of hippocampal-sparing PCI compared with PCI in SCLC.20 In RTOG 0614, a randomized, double-blind, placebo-controlled trial of WBRT with or without memantine, patients receiving memantine with WBRT demonstrated better cognitive function, with a reduced rate of decline in memory, executive function, and processing speed.21 In light of the results of these trials, a clinical trial of memantine and WBRT with or without hippocampal sparing was opened and is currently accruing.22 Validation of the benefit of these maneuvers may lead to increased use of PCI as neurotoxicity concerns are alleviated.
With respect to outcomes, our study did not demonstrate a significant difference in OS with the use of PCI, in contrast to the recent Surveillance, Epidemiology, and End Results analyses and meta-analyses.8, 9 Interestingly, in a retrospective review of a similar-sized cohort (n = 207), Giuliani et al demonstrated an association between PCI use and improved OS in their patient cohort.23 The lack of observed benefit with PCI in our study may be related to the differing rate of brain imaging used in our 2 studies (94% in the present study compared with “the majority” of the Giuliani cohort), which may have screened out many patients who already had brain metastasis in our cohort, among other factors.23, 24 Another important consideration is that our cohort was likely underpowered to detect an OS difference. Notably, of the 7 randomized clinical trials that were evaluated in the practice-defining meta-analysis, the relative risk of brain metastasis was shown to be decreased in some of the individual trials. However, no single trial demonstrated improved OS. It was only after a pooled analysis of 987 patients was conducted that a statistically significant improvement in OS was shown.5 Another study by Farooqi et al examined 658 patients with LS-SCLC who received chemotherapy and RT.25 The authors reported that 55% of these patients received PCI, which improved OS and brain metastasis–free survival. However, unlike our study and the one by Giuliani et al, the non-PCI group in the Farooq et al study included patients with no response or with early disease progression after local therapy, thereby including unfavorable patients in the analysis. With respect to our research question regarding reasons for PCI omission, Farooqi et al did not report the individual rates for the reasons for PCI omission, but they did postulate that neurotoxicity is a likely contributing factor.
Our study is inherently limited because it is a single-institution, retrospective study, and the reasons for omission of PCI were extracted from medical records; therefore, they may not be as comprehensively assessed or as accurate when compared with prospective studies. However, this study posits several hypotheses to investigate further. In our study, patients who were deemed medically unfit for PCI and patients of advanced age comprised the second and third largest groups who did not receive PCI, respectively. This suggests that both patients and physicians are concerned about the balance between disease control and neurotoxicity in patients with LS-SCLC, a subset of whom can become long-term survivors. In addition, patient perception of potential neurotoxicity is likely influenced by how medical and radiation oncologists present and frame the pros and cons of PCI. The treating oncologists may have some inherent biases about PCI. Continued investigation of the mechanism of radiation-induced neurotoxicity will be important in the effort to develop mitigators of toxicity and ultimately optimize PCI use among patients who would benefit from this therapy. In light of our data, the ongoing prospective trials of hippocampal-sparing radiation and memantine use with PCI are warranted to address both physician and patient concerns about PCI-related neurotoxicity.
Conclusion
Even at a large academic center, only approximately half of patients with LS-SCLC who responded to initial chemoradiotherapy ultimately received PCI, most commonly due to patient concerns about neurocognitive toxicity. PCI is withheld from a similar number of patients due to physician concerns with regard to tolerability. Overall, these results suggest that efforts to decrease neurotoxicity of PCI, including hippocampal-sparing radiation and memantine use, may broaden the application of this intervention in patients with LS-SCLC.
Footnotes
Sources of support: This work was supported in part by the National Cancer Institute at the National Institutes of Health (grant number P30 CA008748).
Conflicts of interest: The authors have declared no conflicts of interest.
References
- 1.Arriagada R., Chevalier T.L., Borie F. Prophylactic cranial irradiation for patients with small cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183–190. doi: 10.1093/jnci/87.3.183. [DOI] [PubMed] [Google Scholar]
- 2.Knisely J., Sharma R., Goenka A., Halthore A. PCI in resected small-cell lung cancer. Lancet Oncol. 2016;17:e415. doi: 10.1016/S1470-2045(16)30453-3. [DOI] [PubMed] [Google Scholar]
- 3.Rudin C., Ismaila N., Hann C. Treatment of small-cell lung cancer: American Society of Clinical Oncology endorsement of the American College of Chest Physicians guideline. J Clin Oncol. 2015;33:4106–4111. doi: 10.1200/JCO.2015.63.7918. [DOI] [PubMed] [Google Scholar]
- 4.Schild S., Foster N., Meyers J. Prophylactic cranial irradiation in small-cell lung cancer: Findings from a north central cancer treatment group pooled analysis. Ann Oncol. 2012;23:2919–2924. doi: 10.1093/annonc/mds123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auperin A., Arriagada R., Pignon J.P. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 6.Le Pechoux C., Laplanche A., Faivre-Finn C. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01) Ann Oncol. 2011;22:1154–1163. doi: 10.1093/annonc/mdq576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson B.E., Patronas N., Hayes W. Neurologic, computed cranial tomographic, and magnetic resonance imaging abnormalities in patients with small-cell lung cancer: Further follow-up of 6- to 13-year survivors. J Clin Oncol. 1990;8:48–56. doi: 10.1200/JCO.1990.8.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Eaton B., Kim S., Marcus D. SEER analysis in elderly patients. Cancer. 2013;119:3753–3760. doi: 10.1002/cncr.28267. [DOI] [PubMed] [Google Scholar]
- 9.Patel S., Macdonald O.K., Suntharalingam M. Evaluation of the use of prophylactic cranial irradiation in small cell lung cancer. Cancer. 2009;115:842–850. doi: 10.1002/cncr.24105. [DOI] [PubMed] [Google Scholar]
- 10.Vigliani M.C., Duyckaerts C., Hauw J.J., Poisson M., Magdelenat H., Delattre J.Y. Dementia following treatment of brain tumors with radiation therapy administered alone or in combination with nitrosourea-based chemotherapy: A clinical and pathological study. J Neurooncol. 1999;41:137–149. doi: 10.1023/a:1006183730847. [DOI] [PubMed] [Google Scholar]
- 11.Gregor A., Cull A., Stephens R.J. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: Results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC) Eur J Cancer. 1997;33:1752–1758. doi: 10.1016/s0959-8049(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 12.Lehman M., Gorayski P., Watson S., Edeling D., Jackson J., Whitty J. Patient preferences regarding prophylactic cranial irradiation: A discrete choice experiment. Radiother Oncol. 2016;121:225–231. doi: 10.1016/j.radonc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Constine L.S., Konski A., Ekholm S., McDonald S., Rubin P. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys. 1988;15:319–330. doi: 10.1016/s0360-3016(98)90011-6. [DOI] [PubMed] [Google Scholar]
- 14.Greene-Schloesser D., Moore E., Robbins M.E. Molecular pathways: Radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tofilon P.J., Fike J.R. The radioresponse of the central nervous system: A dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Monje M.L., Mizumatsu S., Fike J.R., Palmer T.D. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 17.Peiffer A.M., Leyrer C.M., Greene-Schloesser D.M. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology. 2013;80:747–753. doi: 10.1212/WNL.0b013e318283bb0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundapur V., Ellchuk T., Ahmed S., Gondi V. Risk of hippocampal metastases in small cell lung cancer patients at presentation and after cranial irradiation: A safety profile study for hippocampal sparing during prophylactic or therapeutic cranial irradiation. Int J Radiat Oncol Biol Phys. 2015;91:781–786. doi: 10.1016/j.ijrobp.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Gondi V., Pugh S.L., Tome W.A. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ClinicalTrials.gov Whole-brain radiation therapy with or without hippocampal avoidance in treating patients with limited stage or extensive stage small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02635009 Available at. Accessed March 1, 2017.
- 21.Brown P.D., Pugh S., Laack N.N. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov Memantine hydrochloride and whole-brain radiotherapy with or without hippocampal avoidance in reducing neurocognitive decline in patients with brain metastases. https://clinicaltrials.gov/ct2/show/NCT02360215 Available at. Accessed March 1, 2017.
- 23.Giuliani M., Sun A., Bezjak A. Utilization of prophylactic cranial irradiation in patients with limited stage small cell lung carcinoma. Cancer. 2010;116:5694–5699. doi: 10.1002/cncr.25341. [DOI] [PubMed] [Google Scholar]
- 24.Tai P., Assouline A., Joseph K., Stitt L., Yu E. Prophylactic cranial irradiation for patients with limited-stage small-cell lung cancer with response to chemoradiation. Clin Lung Cancer. 2013;14:40–44. doi: 10.1016/j.cllc.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Farooqi A.S., Holliday E.B., Allen P.K., Wei X., Cox J.D., Komaki R. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol. 2017;122:307–312. doi: 10.1016/j.radonc.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]