Abstract
Introduction
Due to the neurocognitive side effects of whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS) is being used with increasing frequency. The use of SRS is expanding for patients with multiple (>4) brain metastases (BM). This study summarizes our institutional experience with single-fraction, linear-accelerator-based SRS for multiple BM.
Methods and materials
All patients who were treated between January 1, 2013, and September 30, 2015, with single-fraction SRS for ≥4 BM were included in this institutional review board–approved, retrospective, single-institution study. Patients were treated with linear accelerator–based image guided SRS.
Results
A total of 59 patients with ≥4 BM were treated with single-fraction SRS. The median follow-up was 15.2 months, and the median overall survival for the entire cohort was 5.8 months. The median number of treated lesions per patient was 5 (range, 4-23). Per patient, the median planning target volume (PTV) was 4.8 cc (range, 0.7-28.8 cc). The prescribed dose across all 380 BM for the 59 patients ranged from 7 to 20 Gy. The median of the mean dose to the total PTV was 19.5 Gy. Although the number of treated lesions (4-5 vs ≥6) did not influence survival, better survival was noted for a total PTV <10 cc versus ≥10 cc (7.1 vs 4.2 months, respectively; P = .0001). A mean dose of ≥19 Gy to the entire PTV was also associated with increased survival (6.6 vs 5.0 months, respectively; P = .0172). Patients receiving a dose of >12 Gy to ≥10 cc of normal brain had worse survival (5.1 vs 8.6 months, respectively; P = .0028).
Conclusion
In single-fraction SRS for patients with multiple BM, smaller total tumor volume, higher total dose, and lower volume of normal brain receiving >12 Gy were associated with increased survival. These data suggest that using SRS for the treatment of multiple BM is efficacious and that outcomes may be affected more by total tumor volume than by the number of lesions.
Summary.
We describe the results of our retrospective analysis of single-fraction radiosurgery for multiple brain metastases. Our study is unique because most of our patients were treated with a single isocenter technique, for which there is little published clinical data. We delineate the patient and treatment characteristics as well as dosimetric data that correlate with better survival. Regardless of the number of lesions, we found an association between lower tumor volume and improved survival.
Alt-text: Unlabelled box
Introduction
Brain metastases (BM) can occur in up to 10% to 40% of patients with cancer.1, 2 Despite advances in diagnosis and treatment, BM are typically associated with a limited life expectancy.3, 4 Surgery and whole brain radiation therapy (WBRT) may improve local control and survival for patients with a limited number of BM.5, 6 However, WBRT causes late neurocognitive deficits without offering a survival advantage compared with a more focal radiation therapy approach such as stereotactic radiosurgery (SRS).7, 8, 9 The use of SRS alone provides very high rates of tumor response and local control.8, 10, 11
SRS is recommended for patients with a limited number (ie, 1-3) of BM.12, 13 Evolving radiation therapy and imaging technology and recognition of the long-term side effects associated with WBRT have increased interest in SRS for patients with larger numbers of BM.14 A prospective trial examining SRS alone for up to 10 BM demonstrated no survival or local recurrence differences in patients who were treated for 2 to 4 BM versus 5 to 10 BM.14 In fact, cumulative tumor volume and largest treated tumor diameter were more significant predictors of outcome than the number of treated lesions.
An obstacle to the use of SRS for larger numbers of BM is the treatment time required when each lesion is treated with a separate radiation therapy plan. Single-isocenter, multitarget (SIMT), volumetric modulated arc therapy (VMAT) for SRS planning and delivery enables the simultaneous treatment of several lesions. This technique was shown to substantially reduce treatment time, with possible improvements in conformity indices and normal brain dose, compared with multiple isocenter plans.15, 16 Data on clinical outcomes using this technique are sparse, with one study showing high local control and a 6-month overall survival (OS) rate of 60%.17
The choice between SIMT and WBRT for the treatment of patients with ≥4 BM remains an unresolved issue. This study was performed to explore our institutional experience with SIMT for the treatment of multiple BM to identify those patients who might benefit the most from this procedure.
Methods and materials
Study population
This study was a retrospective review performed at the Radiation Oncology Department of Duke University Medical Center in Durham, North Carolina. From a chart review, we identified patients who underwent SRS as a treatment for ≥4 BM between January 1, 2013, and September 30, 2015. The study was approved by our institutional review board. Inclusion criteria were age ≥18 years, ≥4 BM treated with single-fraction SRS, and histologically proven extracranial malignancy. Primary brain tumors were excluded, and BM biopsy was not required.
Collected data included patient demographics; disease characteristics; Karnofsky Performance Status; initial and salvage brain treatments; number and volume of treated BM; and dosimetric parameters such as technique, planning target volume (PTV) dose, and dose to organs at risk (OARs). Recursive partitioning analysis (RPA) and Graded Prognostic Assessment scores for BM were calculated from these clinical data.3, 18 Survival status and the date of death or last follow-up were documented.
Treatment
All patients underwent a computed tomography (CT) simulation with a frameless SRS thermoplastic mask (BrainLAB, Munich, Germany). A thin-cut (1 mm) CT scan of the brain was fused with a thin-cut, gadolinium contrast–enhanced, axial, 3-dimensional, T1-weighted magnetic resonance imaging (MRI) scan. Gross tumor volume included the enhancing lesions on the contrast-enhanced T1 sequence on the MRI scan. PTV was created by adding a 1-mm margin to the gross tumor volume. The dose was normalized so that the 100% isodose line encompassed all or nearly all of the target volume (typically >99%) such that the maximum dose ranged between 110% and 125%. This corresponded to selecting the 80% to 90% isodose line when the dose is normalized to be 100% at the maximum dose point.
Doses were prescribed on the basis of lesion size and volume. The Radiation Technology Oncology Group 90-05 dosing guidelines19 were typically followed, but doses were decreased at the treating physician's discretion according to tumor location (ie, brainstem), V12Gy for the brain, and doses to OAR from previous radiation treatments. All patients were contoured using the BrainLAB iPlan RT Image software (BrainLAB, Munich, Germany). VMAT treatment plans were then prepared with the Varian Eclipse (Varian Medical Systems, Palo Alto, CA) treatment planning system, using beam geometry and optimization criteria as previously described.20, 21 One patient was treated using dynamic conformal arcs with a single isocenter per target; the treatment plan was prepared using the BrainLAB iPlan RT Dose treatment planning software. Treatment was delivered on a Novalis TX linear accelerator (Varian Medical Systems) using orthogonal kV imaging and cone beam CT for 6-degree-of-freedom position adjustment prior to treatment.22
Statistical methods
The primary objective of this retrospective study was to describe OS in this patient population as a function of patient demographics, disease characteristics, and treatment parameters. OS-SRS was defined as the time from SRS until death or last follow-up, if the patient remained alive. OS-SRS was calculated using the Kaplan-Meier estimator. Univariate and multivariate Cox proportional hazards models were used to identify predictors of OS. Given the small sample size, these analyses are intended to be descriptive in nature because the study lacks the power to draw definitive conclusions. On the basis of the study size and number of events (deaths), the multivariate analyses focused on 4 potential predictors: age (≥65/ <65 years), total volume of all brain lesions (≥10/ <10 cc), mean dose to the entire PTV (≥19/ <19 Gy), and the volume of normal brain (total brain volume—PTV) exposed to ≥12 Gy (V12Gy; ≥10/<10 cc). The multivariate model using backward variable elimination employed a 0.10 significance level for variable retention. SAS (SAS Institute, Cary, NC) Version 9.3 was used for all analyses.
Results
Fifty-nine patients met the study inclusion criteria. As of February 6, 2016, the median follow-up was 15.2 months. Patient and treatment characteristics are summarized in Table 1. The average age was 61.8 years (range, 40.5-83.8 years). The most common primary histology was non-small cell lung cancer (35.6%). Most patients had a Karnofsky Performance Status of ≥70 (93.2%), an RPA ≥2 (93.2%), and a Graded Prognostic Assessment ≥1 (71.2%). More than half of patients (54.2%) had undergone previous brain radiation therapy, with 22 patients (37.3%) receiving WBRT alone and 8 (13.5%) previously treated with SRS alone. Four patients (6.8%) had previously undergone surgery for BM due to mass effect, symptoms, or size.
Table 1.
Patient and treatment characteristics
| Patient and Treatment characteristics | N (%)a | |
|---|---|---|
| Sex | Male | 27 (45.8) |
| Female | 32 (54.2) | |
| Age, mean (SD), y | 61.8 (11.1) | |
| Primary Tumor | Non-small cell lung cancer | 21 (35.6) |
| Breast | 15 (25.4) | |
| Melanoma | 14 (23.7) | |
| Renal Cell Carcinoma | 6 (10.2) | |
| Other | 3 (5.1) | |
| Previous brain radiation therapyb | WBRT | 22 (37.3) |
| PBRT | 1 (1.7) | |
| SRS alone | 8 (13.5) | |
| WBRT + SRS | 1 (1.7) | |
| Previous surgery | Yes | 4 (6.8) |
| Treated lesions | No. of treated lesions, median (range) | 5 (4-23) |
| Median volume of all lesions within a patient, median (range), cc | 0.40 (0.05-3.60) | |
| Total volume of all lesions within a patient, median (range), cc | 4.8 (0.7-28.8) | |
| Fractionation and dosing | Median dose to all lesions within a patient, median (range), Gy | 18 (10.5-20) |
| Mean dose to total PTV, median (range)c, cc | 19.5 (12.7-24.5) | |
| Treatment technique | Volumetric modulated arc therapy | 58 (98.3) |
| Dynamic conformal arcs | 1 (1.7) | |
| Isocenters | Single isocenter | 55 (93.2) |
| Further treatment | Repeat SRS | 12 (20.3) |
| WBRT | 6 (10.2) | |
PBRT, partial brain radiation therapy; PTV, planning target volume; SD, standard deviation; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
Except where noted otherwise.
The denominator for these percentages was the 32 patients who received any prior brain radiation therapy.
Mean dose to the PTV was calculated based on mean dose to the aggregate planning target volume, not the average of the mean doses to the individual lesions.
A median of 5 lesions were treated per patient (range, 4-23 lesions). The median of the total PTV of all treated lesions in a patient was 4.8 cc (range, 0.7-28.8 cc). Fifty-five patients (93.2%) were treated with a single isocenter. The most commonly used treatment technique was VMAT (98.3%). A single patient was treated using dynamic conformal arc planning with 4 isocenters. The PTV prescription dose across all doses ranged from 7 to 20 Gy. The median of the mean dose to the total treated PTV was 19.5 Gy (range, 12.7-24.5 Gy).
Dose constraints
Doses to OARs were well within normal limits. The median maximum point dose to the brainstem was 3.4 Gy (range, 0.4-12.8 Gy) for the entire cohort of 52 patients (88.1%) who did not have brainstem metastases. For the 3 patients who were treated with 2 isocenters, the median maximum brainstem dose was 2.3 Gy (range, 1.9-5.7 Gy).
The median maximum dose to the optic chiasm was 1.7 Gy (range, 0.3-9.1 Gy). For the 55 patients who were treated with SIMT, the median dose was 1.7 Gy (range, 0.3-9.1 Gy) versus 1.0 Gy (range, 0.5-3.2) for the 4 patients who were treated with more than 1 isocenter.
The median V12Gy was 13.7 cc (range, 3.8-59.5 Gy) for all patients. In the 55 SIMT patients, the median V12Gy was 13.7 cc (range, 3.8-59.5 Gy) versus 6.6 cc (range, 3.9-18.2 Gy) for the 4 patients who were treated with a single fraction but more than 1 isocenter.
Survival analysis
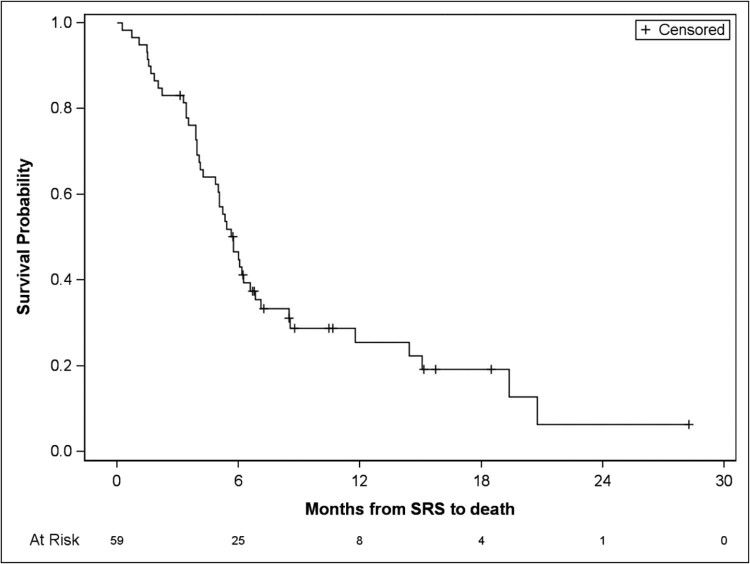
For the entire patient cohort, the median OS was 5.8 months (95% confidence interval [CI], 4.9-6.6; Fig 1). One-year survival was 25.5% (95% CI, 14.2%-38.4%), and 2-year survival was 6.4% (95% CI, 0.6%-22.9%). Univariate analyses provided no evidence of survival differences according to tumor histology, sex, age, prior treatments for BM (WBRT, SRS, or surgery), RPA classification, number of fractions used, or number of isocenters. In addition, the number of treated lesions did not appear to influence survival; no significant difference was found between patients who were treated for 4 to 5 lesions (n = 42) versus patients who were treated for 6 or more metastases (n = 17; median OS: 5.6 months [95% CI, 4.2-8.5] vs 5.8 months [95% CI, 3.4-6.8], respectively; P = .66).
Figure 1.
Overall survival for the entire patient cohort (n = 59).
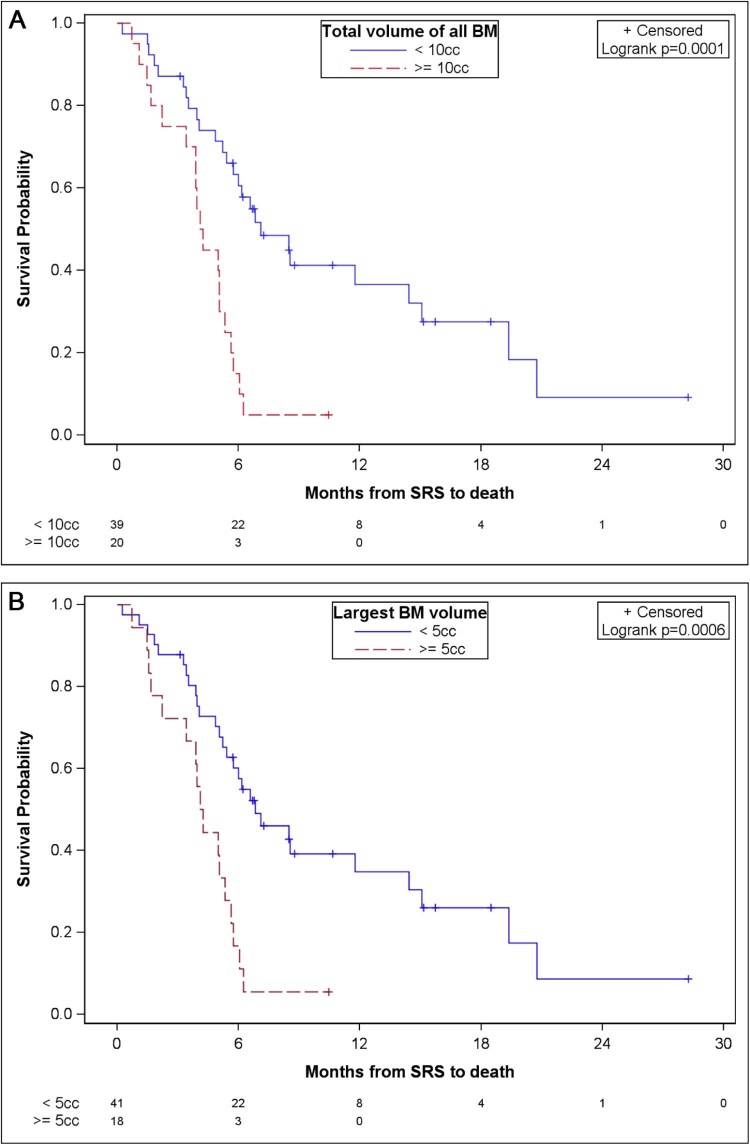
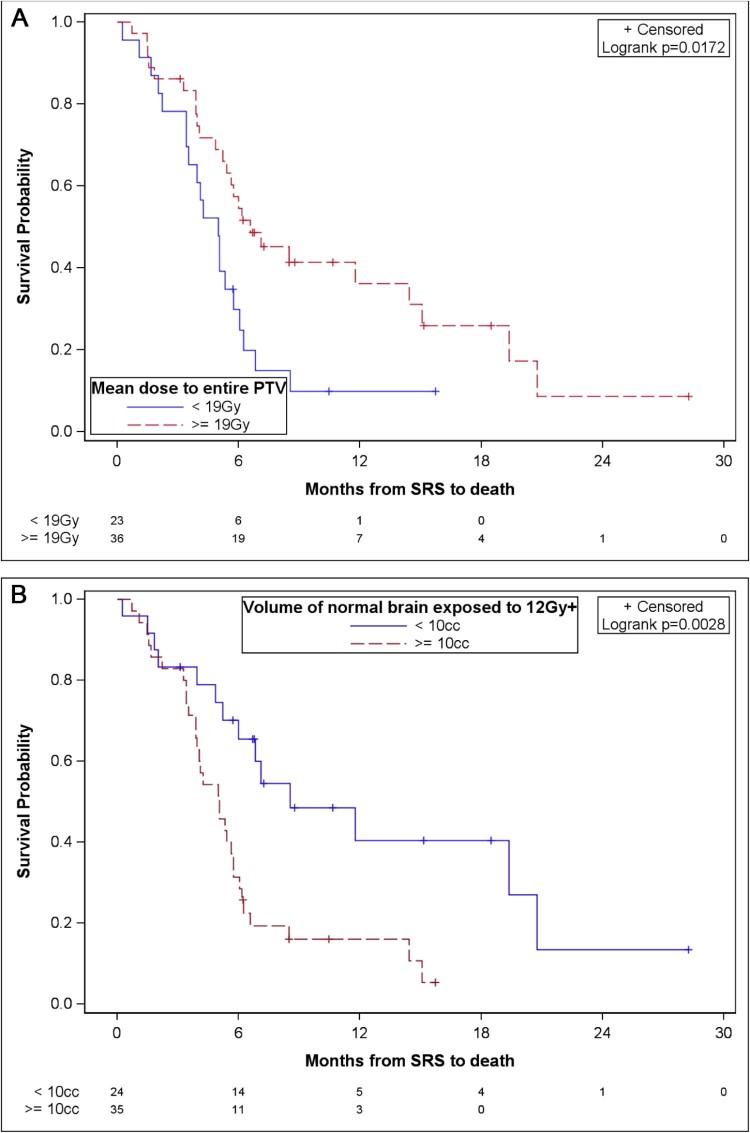
An analysis of parameters related to tumor volume (Fig 2) and dose (Fig 3) revealed potential impacts on survival. While these results need confirmation in a larger study with adequate power to detect true differences, our study provided evidence of a potential increase in survival in patients with a total PTV <10 cc compared with patients with a ≥ 10 cc total PTV (median OS: 7.1 months [95% CI,5.4-14.4] vs 4.2 months [95% CI, 2.2-5.3], respectively; P = .0001). Patients whose largest lesion was <5 cc versus ≥5 cc also had improved survival (median OS: 6.8 months [95% CI, 5.2-14.4] vs 4.2 months [95% CI, 2.2-5.3], respectively; P = .0006). A combined PTV mean dose of ≥19 Gy versus <19 Gy demonstrated a survival advantage (median OS: 6.6 months [95% CI, 5.2-14.4] vs 5 months [95% CI, 3.4-5.8], respectively; P = .0172). V12Gy >10 cc was associated with a poorer survival (median OS: 8.6 months vs 5.1 months; P = .0028).
Figure 2.
Overall survival according to volume parameters. (A) Overall survival according to total PTV of brain metastases. (B) Overall survival according to largest lesion volume.
Figure 3.
Overall survival according to dose parameters. (A) Overall survival according to mean dose to total PTV. (B) Overall survival according to volume of normal brain exposed to >12 Gy.
From the multivariate analysis including age, total volume of all BM, mean dose to the entire PTV, and V12Gy as potential predictors, the only significant predictor of survival was the total volume of BM, which showed worse survival in patients with a total PTV ≥10 cc (hazard ratio [HR]: 3.34; 95% CI, 1.74-6.43; P = .0003).
Salvage therapy
Of the 43 patients with post-SIMT SRS imaging, 5 patients had local failure; in all 5, local failure was accompanied by distant failure in the brain. Sixteen of the 59 patients in the study did not undergo post-SRS imaging due to death and/or progression of extracranial disease. The crude intracranial local failure rate was 11.6% (5 of 43 patients). All patients who had local failure also presented with distant failure. There were 21 cases of distant failure alone (crude rate, 48.8%). Two cases (4.7%) of radionecrosis were observed.
Sixteen patients (27.1%) underwent further radiation therapy, including 4 patients who received additional treatment with WBRT only, 2 who received WBRT and SRS, and 10 who received additional treatment only with SRS. Of the 12 patients (20.3%) who received additional SRS, 11 had 1 additional course and 1 had 3 additional courses. The median time between the first and second SRS treatments was 4.0 months. For the 6 patients (10.2%) who received post-SRS WBRT, the median time from SRS to WBRT was 4.1 months.
Discussion
As systemic therapy continues to improve with the growing role of immunotherapy, patient survival is improving. Treating BM while minimizing radiation's impact on quality of life and neurocognition becomes ever more crucial. The use of SRS has been traditionally limited to 1 to 3 BM, as illustrated by the inclusion criteria of pivotal SRS prospective trials.7, 23, 24 However, advances in treatment delivery and MRI have overcome the technical difficulties of simultaneously treating ≥4 BM. Little has been published on the efficacy of single-fraction, single-isocenter SRS for multiple BM.
This study provided no evidence that patient survival was significantly affected by the number of treated metastases. Although these analyses should be interpreted cautiously given the study's lack of power and the number of comparisons, the results suggest that volumetric and dose parameters were associated with survival, including total PTV, volume of the largest treated lesion, and normal brain V12Gy. These variables are interrelated and reflect, in essence, the significance of total intracranial tumor volume on survival in the setting of SRS treatment. Mean dose was also associated with improved survival. However, mean dose is inversely related to tumor volume, and patients with a higher PTV received lower delivered doses and exhibited a lower V12. Ultimately, the only factor that was significant in the multivariate analysis was total tumor volume.
Previously published results underscored that the number of intracranial metastases is not a prognostic factor for survival.25, 26, 27 The significant effect of cranial tumor volume on survival has been demonstrated in both retrospective26 and prospective trials.25, 27 Bhatnagar et al treated patients with 4 to 18 BM (median = 5) with SRS, and multivariate analyses indicated that smaller cranial tumor volume was associated with improved survival regardless of the number of tumors.26 Yamamoto et al demonstrated that the diameter of the largest tumor (≥1.6 cm) and cumulative tumor volume (≥1.9 mL) were each significant in influencing survival, whereas a number of tumors greater than 4 was not significant.25
Lower total lesion volume is associated with improved SRS response and may also permit higher treatment doses to each lesion, which is another parameter that is associated with better survival. As reported in other SRS trials for 1 to 3 metastases, local control as well as survival are influenced by the dose to each lesion.28 A similar impact of dose is also seen in this study, where a mean dose of ≥19 Gy to the entire PTV had a beneficial effect on survival.
Survival was adversely affected by the volume of normal brain receiving doses higher than 12 Gy. Normal brain exposure in SRS treatments as measured by V12Gy has been associated with increased toxicity, including radionecrosis and radiographic changes.29, 30 To our knowledge, a relationship between V12Gy and other potential side effects, such as neurocognitive toxicity and neurologic death, has not been reported. Similarly, the significance of tumor volume in survival warrants an analysis of whether patients with larger-volume BM are more likely to experience neurologic death or whether these patients have a larger burden of systemic disease that leads to higher mortality rates.
In other trials of patients with multiple BM, median survival after SRS ranged from 6.2 to 8.6 months.25, 26, 27, 31 Our median survival was 5.8 months and was not comparable to these published studies because approximately 60% of our patients were treated previously with brain radiation therapy, including SRS and WBRT. With improved chemotherapy and immunotherapy, patients face the dilemma of repeat brain radiation with increasing frequency. In patients who previously received WBRT, salvage SRS is often optimal for local control of recurrent BM while maximizing quality of life.
Much has been published about the negative effects of WBRT.7, 32 Most recently, data from the prospective, randomized QUARTZ trial indicate that in patients with NSCLC and BM, WBRT does not improve survival or quality of life when compared with supportive care alone.33 Rather than using WBRT, patients may receive multiple courses of SRS safely.34, 35 Our results also illustrate that appropriately chosen patients with multiple metastases may be treated with repeated courses of SIMT SRS. Factors that determine the decision to use repeat SRS versus WBRT for salvage include the number of distant BM, radioresistant histology, time to failure, or previous whole brain administration. Retreatment for each patient is considered on a case-by-case basis, but in general, fewer BM, melanoma or renal cell histology, previous whole brain administration, or longer time to failure would support the use of salvage SRS.
Conclusions
These findings are hypothesis-generating and are limited by the study sample size, lack of power, number of comparisons, and the study's retrospective and single-institution nature. To our knowledge, this is the largest reported experience to examine single-fraction SIMT for multiple BM. In our experience, this technique is feasible, readily implemented, and well tolerated by patients, although robust quality assurance and careful correction of translational/rotational deviations in position are essential. SIMT is associated with favorable survival in patients with 4 or more BM, particularly when the total metastatic lesion volume, rather than the number of lesions, is low. A prospective trial examining SIMT in patients with 4 to 10 BM has been opened to better define its efficacy and effect on neurocognition (NCT02886572 at clinicaltrials.gov).
Footnotes
Meeting information: This work was presented in poster format at the 2016 Annual Meeting of the American Society for Radiation Oncology, September 25-28, 2016, Boston, MA.
Sources of support: This research was supported by Varian Medical Systems, Grant Number 283-0092.
Conflicts of interest: John Kirkpatrick and Justus Adamson own Clearsight Radiotherapy Products LLP. Justus Adamson reports a consulting arrangement with Immunolight LLC. Both of these relationships are unrelated to the study. Fang-Fang Yin, John Kirkpatrick, and Grace Kim have received research funding from Varian Medical Systems.
References
- 1.Barnholtz-Sloan J.S., Sloan A.E., Davis F.G., Vigneau F.D., Lai P., Sawaya R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Schouten L.J., Rutten J., Huveneers H.A., Twinstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 3.Sperduto P.W., Chao S.T., Sneed P.K. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Barnholtz-Sloan J.S., Yu C., Sloan A.E. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012;14:910–918. doi: 10.1093/neuonc/nos087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patchell R.A., Tibbs P.A., Walsh J.W. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 6.Vecht C.J., Haaxma-Reiche H., Noordijk E.M. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama H., Shirato H., Tago M. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 8.Tsao M., Xu W., Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 9.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Neill B.P., Iturria N.J., Link M.J., Pollock B.E., Ballman K.V., O'Fallon J.R. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169–1176. doi: 10.1016/s0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 11.Minniti G., Clarke E., Lanzetta G. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Central Nervous System Cancers. 2016. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf Version 1; Available at.
- 13.Tsao M.N., Rades D., Wirth A. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jairam V., Chiang V.L., Yu J.B. Role of stereotactic radiosurgery in patients with more than four brain metastases. CNS Oncol. 2013;2:181–193. doi: 10.2217/cns.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark G.M., Popple R.A., Young P.E., Fiveash J.B. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys. 2010;76:296–302. doi: 10.1016/j.ijrobp.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Kim H., Choi J., Lee S., Sung K., Lee S., Lee K. Volumetric modulated arc therapy for multiple brain metastases: A more efficient modality than dynamic arc stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;84:S787. [Google Scholar]
- 17.Lau S.K., Zakeri K., Zhao X. Single-isocenter frameless volumetric modulated arc radiosurgery for multiple intracranial metastases. Neurosurgery. 2015;77:233–240. doi: 10.1227/NEU.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaspar L.E., Scott C., Murray K., Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 19.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 20.Morrison J., Hood R., Yin F.F., Salama J.K., Kirkpatrick J., Asamson J. Is a single isocenter sufficient for volumetric modulated arc therapy radiosurgery when multiple itracranial metastases are spatially dispersed? Med Dosim. 2016;41:285–289. doi: 10.1016/j.meddos.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Stanhope C., Chang Z., Wang Z. Physics considerations for single-isocenter, volumetric modulated arc radiosurgery for treatment of multiple intracranial targets. Pract Radiat Oncol. 2016;6:207–213. doi: 10.1016/j.prro.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Chang Z., Wang Z., Ma J. Six degree-of-freedom image guidance for frameless intracranial stereotactic radiosurgery with kilo-voltage cone-beam CT. J Nucl Med Radiat Ther. 2010;1:101. [Google Scholar]
- 23.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 24.Kondziolka D., Patel A., Lunsford L.D., Kassam A., Flickinger J.C. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M., Serizawa T., Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar A.K., Flickinger J.C., Kondziolka D., Lunsford L.D. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Hunter G.K., Suh J.H., Reuther A.M. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:1394–1398. doi: 10.1016/j.ijrobp.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Park S.H., Hwang S.K., Kang D.H. Gamma knife radiosurgery for multiple brain metastases from lung cancer. J Clin Neurosci. 2009;16:626–629. doi: 10.1016/j.jocn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Varlotto J.M., Flickinger J.C., Niranjan A., Bhatnagar A.K., Kondziolka D., Lunsford L.D. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003;57:452–464. doi: 10.1016/s0360-3016(03)00568-6. [DOI] [PubMed] [Google Scholar]
- 30.Korytko T., Radivoyevitch T., Colussi V. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419–424. doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 31.Serizawa T., Higuchi Y., Nagano O. Testing different brain metastasis grading systems in stereotactic radiosurgery: Radiation Therapy Oncology Group's RPA, SIR, BSBM, GPA, and modified RPA. J Neurosurg. 2012;117:31–37. doi: 10.3171/2012.8.GKS12710. [DOI] [PubMed] [Google Scholar]
- 32.Sneed P.K., Mendez J., Vemer-van den Hoek J.G. Adverse radiation effect after stereotactic radiosurgery for brain metastases:Incidence, time course, and risk factors. J Neurosurg. 2015;123:373–386. doi: 10.3171/2014.10.JNS141610. [DOI] [PubMed] [Google Scholar]
- 33.Brown P.D., Ballman K.V., Cerhan J.H. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for eesected metastatic brain disease. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvenna P., Nankivell M., Barton R. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minniti G., Scaringi C., Paolini S. Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neurooncol. 2016;126:91–97. doi: 10.1007/s11060-015-1937-4. [DOI] [PubMed] [Google Scholar]