Abstract
Stereotactic body radiation therapy (SBRT) can positively influence an antitumor immune response by inducing necrotic cell death. SBRT also been shown to eliminate tumors outside the radiation therapy field through an immune-mediated process known as the abscopal effect. Recent advances in immunotherapy may provide new therapeutic approaches for patients with liver cancer. Therefore, understanding the immune status of patients with cancer will likely guide how immunotherapy might be used in combination with SBRT. We hypothesized that we would observe changes in circulating blood immune cell populations of patients who received SBRT for liver tumors. Therefore, we assessed 110 immunophenotypes in the peripheral blood of 10 patients with liver cancer or metastases to the liver pretreatment and 2 posttreatment time points. Patients with liver cancer and metastatic patients both exhibited several immunophenotypic abnormalities at baseline compared with a group of healthy volunteer controls. In longitudinal studies, SBRT caused a specific reduction in CD3+ T cell counts and immature CD56brCD16− NK cell counts. The immune profiling and potential identification of circulating biomarkers shown here could lead to the design of combinatorial approaches with SBRT and immunotherapy to optimize the timing of treatment and direct the most effective immunotherapy with SBRT.
Summary.
Stereotactic body radiation therapy (SBRT) contributes to an antitumor immune response through multiple mechanisms, but a detailed understanding of SBRT's effects on the immune system is incomplete. We assessed systemic immunity in patients prior to and after SBRT to understand the changes in peripheral blood leukocytes. The characterization of the changes may identify biomarkers that could be used to optimize the combination and/or timing of future immunotherapeutic approaches with SBRT.
Alt-text: Unlabelled box
Introduction
Primary liver and metastatic liver tumors represent a major source of the cancer burden in the United States and worldwide. In the United States in 2016, approximately 28,000 new cases of primary liver tumors (hepatocellular carcinoma [HCC] and intrahepatic cholangiocarcinoma [CCA]) were diagnosed, and this number is expected to continue to rise with the increasing prevalence of hepatitis C infections and nonalcoholic fatty liver disease.1 The liver is also a common organ of spread for many malignancies, most notably colorectal cancer, in which resection of limited liver metastases may be curative. Surgical resection remains the optimal treatment for patients with resectable liver tumors. Recently, stereotactic body radiation therapy (SBRT) has emerged as an effective local treatment modality for primary and metastatic liver tumors. Although local control rates of 80% to 90% have been achieved, the majority of patients treated with SBRT ultimately develop intrahepatic or extrahepatic (systemic) recurrence outside of the irradiated volume.2, 3, 4 Systemic therapy only has a modest impact on survival for patients with primary or metastatic liver tumors.
SBRT induces necrotic tumor cell death, a prerequisite for eliciting an antitumor immune response. Although a detailed understanding of SBRT's effects on the immune system is incomplete, reports of partial or complete eradication of tumors outside of the radiation therapy (RT) field (defined as the abscopal effect) suggest that SBRT is capable of priming and expanding tumor-reactive T cells within the irradiated tumor and among the draining lymph tissues. These activated, tumor-specific T cells are thought to migrate to and eliminate nonirradiated tumors. Preclinical models have established that the abscopal effect is T cell dependent.5, 6 Recently, immune checkpoint blockade at the level of immune priming (CTLA-4 blockade) or effector function (B7-H1/PD-1) is being investigated in many clinical trials and is yielding promising results. Whether a distinct RT regimen, such as SBRT, synergizes with immune checkpoint blockade and elicits a potential systemic curative response is an important question. The combination of anti-CTLA-4 and RT is capable of inducing abscopal effects in patients with melanoma.7, 8 B7-H1 or PD-1 blockade in preclinical RT models increased the rate of tumor regression and reflected results characteristic of PD-1 blockade in conjunction with other therapies in patients with advanced cancer.6 However, the impact of PD-1 blockade on SBRT-mediated abscopal effects and synergism with SBRT is largely unexplored.
For patients with liver tumors undergoing SBRT, further knowledge of the effect of SBRT on immune cell populations may help define the potential role of the combination of SBRT with immunotherapy, such as adoptive immunotherapy and/or checkpoint inhibition. Therefore, we tested whether SBRT induces changes in peripheral blood leukocytes using multiparameter flow cytometry on fresh, unmanipulated whole blood samples from patients with liver cancer and patients with liver metastases. A total of 110 immune cell phenotypes encompassing all major peripheral blood cell populations were assessed in this cohort. Immunophenotypic differences after SBRT as well as differences between healthy volunteers (HVs) and patients with cancer are described.
Methods and materials
Patients
Ten patients receiving SBRT for liver metastases (n = 4), CCA (n = 1), and HCC (n = 5) consented and were enrolled with the approval of the institutional review board. Patients were enrolled in accordance with the following inclusion criteria: age ≥18 years and Eastern Cooperative Oncology Group performance status score of 0 or 1; life expectancy >6 months; diagnosis of liver metastases, CCA, or HCC; received SBRT; and able to undergo blood draws. Other patient characteristics are listed in Table 1. The SBRT treatment technique used at our institution has been previously described.9 All patients had a single tumor treated. The median maximal tumor dimension was 3.8 cm (range, 2.2-4.9 cm). The target volume consisted of the liver tumor with a margin of 5 to 7 mm for setup uncertainty. The prescription dose was 50 to 60 Gy in 5 fractions or 54 Gy in 3 fractions delivered on consecutive weekdays. Each patient received a single course of treatment. A total of 11 age-matched HV samples were collected, and an additional 29 samples from a previous study were included for analyses.10
Table 1.
Patient characteristics
| Patient No. | Age | Sex | Histology | Previous treatments | Interval between last previous treatment and SBRT | 3-month disease status |
|---|---|---|---|---|---|---|
| 1 | 50 | F | Endometrial adenocarcinoma | Surgery, pelvic external beam radiation therapy + brachytherapy, carboplatin/paclitaxel, bevacizumab, topotecan | 2 months | New lung metastases |
| 2 | 65 | F | Endometrial adenocarcinoma | Surgery, pelvic external beam radiation therapy +brachytherapy, carboplatin/paclitaxel | 4 years | New liver metastases |
| 3 | 77 | M | Uveal melanoma | Enucleation, palliative external beam radiation therapy, ipilimumab, pembrolizumab | 4 days | Progression elsewhere in the liver |
| 4 | 62 | M | Hepatocellular carcinoma | Transarterial chemoembolization ×3 | 3 months | No additional disease |
| 5 | 60 | M | Hepatocellular carcinoma | None | NA | No additional disease |
| 6 | 68 | M | Esophageal adenocarcinoma | Thoracic external beam radiation therapy + carboplatin/paclitaxel, surgery (esophagectomy), capecitabine/oxaliplatin/trastuzumab, radiofrequency ablation to liver | 1 month | Progression elsewhere in the liver |
| 7 | 73 | F | Hilar cholangio-carcinoma | Gemcitabine/cisplatin | 3 weeks | No additional disease |
| 8 | 69 | M | Hepatocellular carcinoma | Transarterial chemoembolization ×3 | 2 months | Peritoneal metastases |
| 9 | 81 | F | Hepatocellular carcinoma | None | NA | No additional disease |
| 10 | 71 | F | Hepatocellular carcinoma | Transarterial chemoembolization ×2 | 2.5 months | No additional disease |
SBRT, stereotactic body radiation therapy.
Immunophenotyping by flow cytometry
Unmanipulated whole blood samples were stained directly with antibodies. Flow cytometry was performed with 7 flow protocols on the 3-laser, 10-color Gallios Flow Cytometer (Beckman Coulter, Brea, CA). All 10-color procedures, antibodies, flow protocols, instrument settings, analysis software, dot plots, and gating strategies have been previously described.10, 11
Statistics
Prism Version 7.0 (GraphPad Software, LaJolla, CA) was used to create all graphical representations and for statistical comparisons. For multiple t test comparisons between HVs and the entire cohort, HVs and patients with liver cancer (HCC/CCA), HVs and patients with cancer and metastases to the liver, and patients with liver cancer versus patients with metastases, a false discovery rate approach was performed via the multiple t test analysis and the 2-stage setup method of Benjamini, Krieger, and Yekutieli using Prism software. Individual P-values were determined with no assumption of consistent standard deviation between the phenotypes with each row (representing 1 phenotype) analyzed independently from other phenotypes and the false discovery rate (Q-value) set at 10%. Therefore, the criteria to determine whether the change in an immunophenotype met the discovery threshold was set at P < .05 and Q < .10. The Wilcoxon matched-pairs signed rank test was used to test for differences for patients who received SBRT treatment.
Results
To identify circulating immune cell alterations in liver cancer and to understand the effects of SBRT on peripheral blood leukocytes, we measured more than 110 immunophenotypes by flow cytometry in patients with liver cancer and liver metastases (Supplemental Table S1). These protocols were specifically designed to survey the landscape of patients' immune systems prior to treatments and to identify biomarkers for response (or lack thereof) to treatment in a manner that follows good laboratory practices, including standard operating procedures, standard sample processing, and qualified reagents (analyte-specific reagent/in vitro diagnostic-grade antibodies where possible) and equipment.
In this hypothesis-generating preliminary study, we examined whether there were any immunophenotypic differences between HV controls (n = 40) and the entire cohort (n = 10) or patients with primary liver cancer (n = 6) or patients with liver metastases (n = 4). We found 12 immunophenotypes that differed between the controls and the entire cohort, 8 between the controls and patients with liver cancer, and 8 between the controls and patients with liver metastasis (Table 2). Naïve T cells and circulating dendritic cells (Lineage−HLA-DR+ DCs) appeared to represent the most prominent changes among the 3 groups of patients. The CD62L+CD27+ subset of completely naïve CD4+CD45RA+ T cells were decreased by more than 25% in all 3 patient groups. The flow data and gating strategies for these phenotypes from representative subjects are shown in Supplemental Figure S1. Some CD4+ T cell phenotypes were only different in the entire cohort. The percentage of CD25+CD4+ T cells was reduced and the percentage of PD-1+CD4+ T cells was elevated in the entire cohort, whereas these changes were not observed in CD8+ T cells. The reduction of circulating dendritic cells was more substantial in patients with liver metastasis than in patients with liver cancer, but patients with liver cancer exhibited an abnormal distribution of some DC subsets (LIN−DR+CD11cdim were higher and LIN−HLA-DR+CD33+CD11c++CD16+ were lower than those of the controls). Although we acknowledge that the cohort is small, these data suggest numerous leukocyte abnormalities with both common and distinct phenotype alterations between patients with primary liver cancer and liver metastases.
Table 2.
List of immunophenotypic differences between HVs and patients prior to SBRT
| Mean | Mean | P-value | Q-value | |
|---|---|---|---|---|
| HV vs Cohort | HV | Cohort | ||
| CD4+CD45RA+CD62L+CD27+ Naïve (% of CD4+CD45RA+ T cells) | 92.04 | 60.59 | <.0001 | <.0001 |
| LIN−HLA-DR+ dendritic cells (% of MNCs) | 3.15 | 1.73 | .0002 | .0101 |
| LIN−HLA-DR+CD33+CD11c++CD16+ (% of LIN−HLA-DR+) | 42.61 | 15.71 | .0012 | .0446 |
| CD4+CD45RA+ Naïve T cells (% of CD4+) | 41.76 | 27.21 | .0017 | .0455 |
| CD4+CD25+ (% of CD4+ T cells) | 23.49 | 9.88 | .0021 | .0455 |
| LIN−DR+CD11cdim (% of LIN−HLA-DR+) | 3.74 | 7.65 | .0025 | .0455 |
| CD4+PD-1+ (% of CD4+ T cells) | 24.32 | 35.02 | .0041 | .0657 |
| CD15+CD16−CD49d+CCR3+ Eosinophils CD66b FITC MFI | 18.91 | 12.79 | .0048 | .0669 |
| CD142+ Monocytes (% of CD14+) | 2.44 | 9.07 | .0095 | .0998 |
| LIN-DR+CD33+CD11c++CD16− (% of LIN−HLA-DR+) | 35.99 | 55.67 | .0102 | .0998 |
| CD19+CD21− (% of CD19+ B cells) | 9.49 | 18.79 | .0108 | .0998 |
| CD19+CD21+ (% of CD19+ B cells) | 90.51 | 81.21 | .0108 | .0998 |
| HV vs HCC/CCA | HV | HCC/CCA | ||
| CD4+CD45RA+CD62L+CD27+ Naïve (% of CD4+CD45RA+ T cells) | 92.04 | 51.75 | <.0001 | <.0001 |
| CD142+ Monocytes (% of CD14+) | 2.44 | 15.83 | <.0001 | .0051 |
| LIN−DR+CD11cdim (% of LIN−HLA-DR+) | 3.74 | 9.54 | .0003 | .0101 |
| CD19+CD21+ (% of CD19+ B cells) | 90.51 | 74.62 | .0013 | .0283 |
| CD19+CD21− (% of CD19+ B cells) | 9.49 | 25.38 | .0013 | .0283 |
| LIN−HLA-DR+CD33+CD11c++CD16+ (% of LIN−HLA-DR+) | 42.61 | 9.21 | .0017 | .0309 |
| CD14+CD16+ Intermediate Monocytes (cells/µl) | 47.53 | 104.00 | .0034 | .0545 |
| CD4+CD45RA+ Naïve T cells (% of CD4+) | 41.76 | 25.18 | .0041 | .0568 |
| HV vs Liver Metastasis | HV | Liver Mets | ||
| CD4+CD45RA+CD62L+CD27+ Naïve (% of CD4+CD45RA+ T cells) | 92.04 | 69.43 | <.0001 | .0049 |
| CD15+CD16+ Neutrophils (cells/µl) | 2931.00 | 5067.00 | .0012 | .0329 |
| LIN−HLA-DR+ Dendritic cells (% of MNCs) | 3.15 | 1.45 | .0013 | .0329 |
| gamma delta T cells (% of CD3+ T cells) | 1.23 | 3.09 | .0015 | .0329 |
| gamma delta T cells (cells/µl) | 13.27 | 35.07 | .0015 | .0329 |
| CD15+SSChi Granulocytes (cells/µl) | 3127.00 | 5198.00 | .0021 | .0386 |
| CD4+CD8+ T cells (cells/µl) | 12.19 | 59.07 | .0033 | .0515 |
| CD4−CD8− T cells (cells/µl) | 22.64 | 43.23 | .0100 | .0995 |
CCA, cholangiocarcinoma; CD, cluster of differentiation; FITC, fluorescein isothiocyanate; HCC, hepatocellular carcinoma; HVs, health volunteers; LIN, lineage marker; MFI, mean fluorescence intensity; PD-1, programmed cell death 1; SBRT, stereotactic radiation therapy.
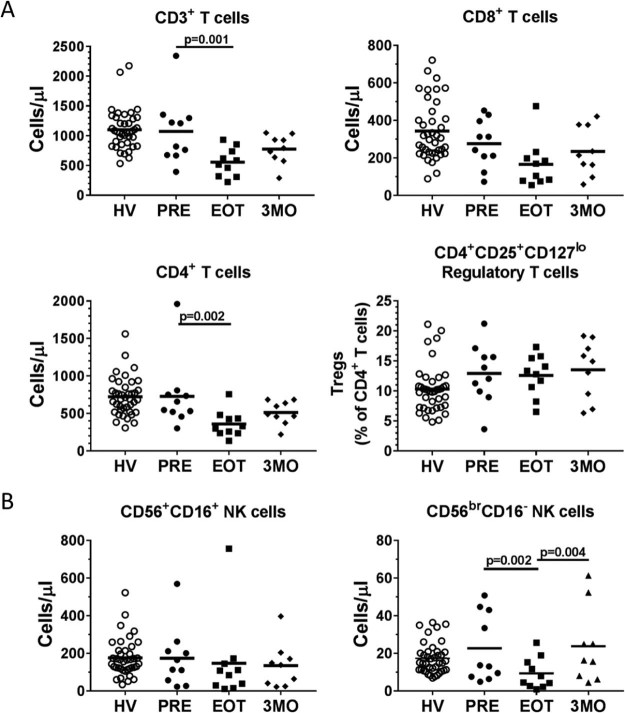
Next, we tested how many phenotypes were sensitive to SBRT. Almost all of the phenotypes measured did not change between baseline and end of treatment samples. However, we found that circulating T cell counts dropped an average of nearly 2-fold at the end of SBRT (1074 cells/µL to 556 cells/µL) and subsequently returned to near baseline levels after 3 months (Fig 1a). CD3+ T cell counts declined in all but 1 patient after SBRT. CD4+ T cells appeared to be the most affected with no statistically significant difference in the levels of CD8+ T cells after treatment. Within the CD4+ T cell compartment, SBRT did not alter the levels of CD4+CD25+CD127lo regulatory T cells. SBRT also appeared to have differential effects on natural killer (NK) cells. For the most prominent NK cell subset, CD56+CD16+ mature, cytotoxic NK cells, SBRT had no effect on the cell counts after treatment (Fig 1b). CD56brCD16− NK cells, which are precursor cells to CD56+CD16+ NK cells and less cytotoxic but release more cytokines,12 dropped to approximately 40% of levels at baseline (from 23 cells/µL at baseline to 9 cells/µL at end of treatment; P = .002) and returned to baseline levels at the 3-month time point. These results highlight the differential sensitivity of immune cell populations to SBRT.
Figure 1.
Stereotactic body radiation therapy decreases distinct peripheral blood immunophenotypes. Peripheral blood cell counts of (A) CD3+, CD4+, and CD8+ T cells and the percentages of CD4+CD25+CD127lo regulatory T cells and (B) CD56+CD16+ and CD56brCD16− natural killer cells in all patients pretreatment/baseline (PRE), end of treatment (EOT), and 3 months posttreatment (3MO) samples, with healthy volunteers (HV) included as reference data.
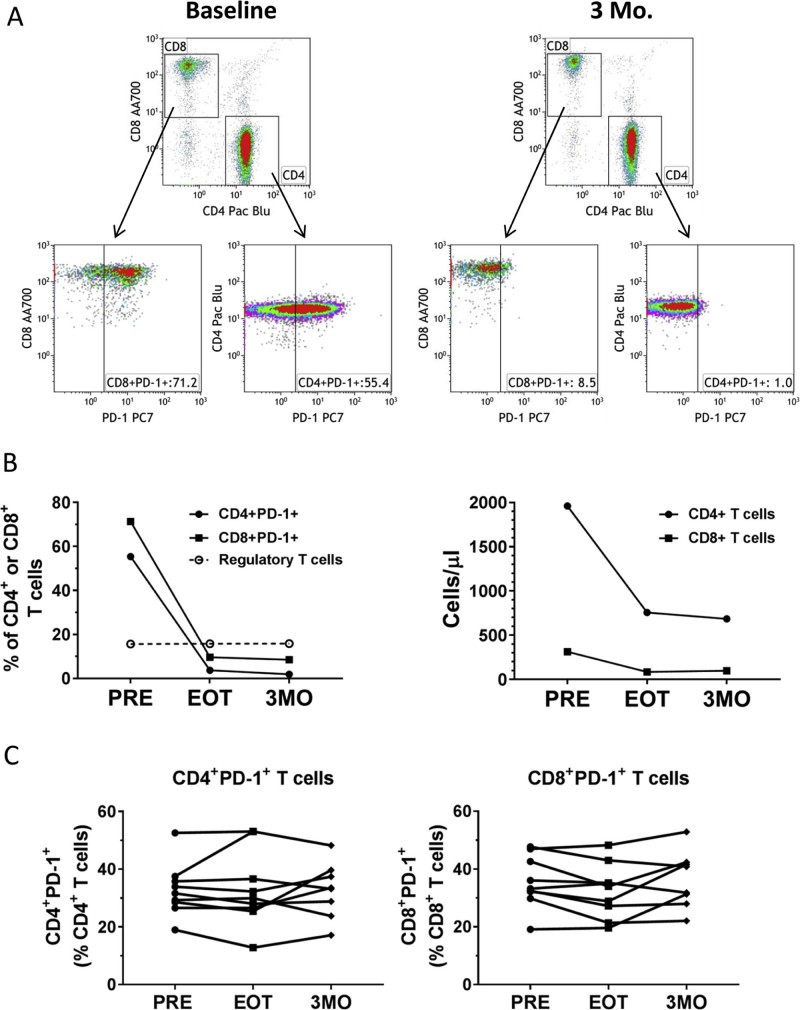
As shown in Table 2, the percentage of PD-1+ CD4+ T cells was elevated in the 10-patient cohort versus the HV controls. One of the patients with liver metastases also received pembrolizumab concurrently with SBRT. This additional treatment provided a unique opportunity to examine the potential changes in the T cell populations when exposed to PD-1 blockade. Figure 2a shows the gating strategies for measuring PD-1 levels on CD4+ and CD8+ T cells. In the baseline sample, 71.2% of CD8+ T cells and 55.4% of CD4+ T cells were positive for PD-1 expression. Not surprisingly, PD-1 levels nearly disappeared with pembrolizumab treatment and stayed low through the 3-month sample (Fig 2b, left graph). The drop in CD4+ and CD8+ T cells observed in this patient was similar to that of the other patients who were treated (Fig 2b, right graph). Other T cell phenotypes, such as regulatory T cells (CD4+CD25+CD127lo) as shown in Figure 2b (left graph), memory (CD45RO+), and naïve (CD45RA+) CTLA-4+, CD25+, or CD28+ cells as a percentage of CD4+ T cells or CD8+ T cells, did not unequivocally change when compared with the behavior of T cell phenotypes after SBRT alone (data not shown). For the entire cohort, excluding the patient on pembrolizumab, SBRT did not affect surface expression of PD-1 on circulating CD4+ T cells or CD8+ T cells (Fig 2c).
Figure 2.
Phenotypic analysis of a patient who received stereotactic body radiation therapy with concurrent pembrolizumab. A, Gating strategies for assessing the expression of PD-1 on CD4+ and CD8+ T cells. Gates for PD-1+ expression were based on fluorescence minus one (FMO) for PD-1 staining. Baseline and 3 months posttreatment (3MO) samples are shown. B, Graphs showing PD-1+ cells, Tregs, and cell counts of CD4+ and CD8+ T cells from one patient. C, PD-1+ CD4 and CD8 T cells in all patients through treatment. PRE, pretreatment/baseline; EOT, end of treatment.
Discussion
Unlike conventional RT, SBRT delivers high doses of radiation in a small number of fractions to the targeted tumor microenvironment while minimizing radiation exposure to the surrounding tissue. Because there is still some discussion with regard to the immunosuppressive effects of conventional RT, SBRT may be better than conventional RT because of its ability to induce abscopal effects, whereby distant tumors may be eradicated by the immune system from only locally treated tumors,5 and for the potential of SBRT to synergize with emerging immunotherapies.13 The paucity of human data, however, has limited the development of effective combinatorial approaches.
To better understand the immunological changes after SBRT, we wanted to know the immunological landscape of patients with cancer prior to treatment. Although we acknowledge that the cohort is small and the patient population is heterogeneous, this was a hypothesis-generating study to examine the changes to the immune system after radiation to the liver. Additionally, we identified numerous leukocyte abnormalities that occur in patients with liver cancer that may provide mechanistic clues to disease progression and/or phenotypes that may be targeted through specifically tailored therapies. We previously used this systems-based approach to understand the interplay of tumor and immune system interactions.14, 15 Additionally, immune biomarkers have been identified in patients with non-small cell lung cancer that predict both survival and treatment-related toxicity after SBRT treatment.16 In this study, high pretreatment neutrophil-to-lymphocyte ratios and lymphocytopenia were associated with poor outcomes. Although our cohort was not large enough to test whether the measured phenotypic differences correlated with survival, we found a broad range of phenotypes from T cells, B cells, and myeloid cells that were altered in these patients. The most striking phenotypic difference was the decrease in the percentage of CD4+CD45RA+CD62L+CD27+ naïve T cells of total CD4+CD45RA+ naïve T cells in both patients with liver cancer and patients with liver metastases. The presumed downregulation of CD62L and CD27 suggests that these patients may have impaired antigen-dependent T cell responses, T cell homing, and/or maturation into memory cells.17, 18, 19
Because SBRT targets radiation locally, damage to bone marrow and circulating leukocytes is thought to be minimized. In this cohort, CD3+ T cells counts dropped by nearly half after SBRT, whereas other lymphocytes, monocytes, and granulocytes only had insignificant declines. Interestingly, immature CD56brCD16− NK cells declined dramatically, but mature CD56+CD16+ NK cells did not. CD56brCD16− NK cells traffic to lymph nodes and participate in the early events of an adaptive immune response.20 Although we cannot rule out the possibility that radiation-induced cellular death or myelosuppression may contribute to the drop in T cell and CD56brCD16− NK counts, a very plausible scenario is that the T cells are trafficking to the lymph nodes and the tumor microenvironment, both at the irradiated site and potentially to metastatic sites. Indeed, several lines of evidence from mouse models support the hypothesis that RT induces the trafficking of lymphocytes.21, 22
The panel of immunophenotypes included many that act as barriers to immunotherapy, including the checkpoint inhibitors PD-1 and CTLA-4, regulatory T cells, and various myeloid-derived suppressor cells. Although only the percentage of PD-1+ CD4+ T cells was increased in the entire cohort at pretreatment, levels of circulating PD-1+ T cells did not change after SBRT. One patient was treated with pembrolizumab concurrently with SBRT. Although we did not detect meaningful changes in the distribution of T cell subsets after pembrolizumab (ie, changes in regulatory T cells or memory cells), the specific flow protocol for PD-1 demonstrated valuable utility in the measurement of PD-1 surface expression and will likely be useful for measuring the persistence of therapeutic antibody-bound T cells in peripheral blood.
Conclusion
The immunosuppressive environment of the liver creates challenges for effective immunotherapeutic approaches. The immune profiling of patients with liver cancer and the potential identification of biomarkers reported here are tangible first steps in the development of rational combinatorial approaches that combine immunotherapy with SBRT. This approach to identify biomarkers in patients with liver cancer justifies additional studies with expanded, disease-specific cohorts that can potentially guide patient selection in clinical trials, match the optimal immunotherapy with SBRT, optimize the timing of immunotherapy with SBRT, and identify biomarkers that are related to treatment-induced toxicities.
Footnotes
Sources of support: This study was funded with institutional internal sources.
Conflicts of interest: M.P.G. and A.B.D. report associated intellectual property and 2 patents pending: treating patients based on immune subtypes issued and methods and materials for assessing immune system profiles issued. L.R.R. reports receipt of personal fees from Bayer and grants from BTG, Gilead Sciences, and Wako Diagnostics outside of the submitted work. S.B., S.S.P., D.A.G., and C.L.H. have nothing to disclose.
Supplementary material related to this article can be found at https://doi.org/10.1016/j.adro.2017.08.003.
Supplementary data
The following is the supplementary data to this article:
Flow cytometric dot plots and gating strategies for selected immune phenotypes. A. For CD4+CD45RA+CD62L+CD27+ Naïve T cells (% of CD4+CD45RA+ T cells), CD3+ T cells were gated from mononuclear cells (MNCs) (data not shown). CD4+ T cells were gated from CD3+ T cells. CD25+CD127− regulatory T cells (Tregs) were isolated from non-regulatory T cells. Non-Tregs were separated into naïve (CD45RA+) and (CD45RO+) memory cells. CD62L+ and CD27+ naïve cells were measured as a percent of naïve CD4 T cells. B. LINDR+ circulating dendritic cells and LIN-DR- immature myeloid derived suppressor cells were gated from MNCs by negative LIN (CD3+CD14+CD19+CD56+) staining and for positive or negative staining for HLA-DR. Representative subjects from the cancer patient cohort (PT) or healthy volunteer (HV).
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bujold A., Massey C.A., Kim J.J. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 3.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 4.Wo J.Y., Dawson L.A., Zhu A.X., Hong T.S. An emerging role for radiation therapy in the treatment of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:353–368. doi: 10.1016/j.soc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Demaria S., Ng B., Devitt M.L. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Park S.S., Dong H., Liu X. PD-1 Restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3:610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow M.A., Callahan M.K., Barker C.A. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamell E.F., Wolchok J.D., Gnjatic S., Lee N.Y., Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrell K.W., Johnson J.E., Mou B., Barney B.M., Nelson K.E. Stereotactic body radiotherapy for primary and metastatic liver tumors—the Mayo Clinic experience. J Radiosurg SBRT. 2016;4:133–144. [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafson M.P., Lin Y., Maas M.L. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0121546. e0121546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson M.P., DiCostanzo A.C., Wheatley C.M. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J Immunother Cancer. 2017;5:30. doi: 10.1186/s40425-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poli A., Michel T., Theresine M., Andres E., Hentges F., Zimmer J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharabi A.B., Tran P.T., Lim M., Drake C.G., Deweese T.L. Stereotactic radiation therapy combined with immunotherapy: Augmenting the role of radiation in local and systemic treatment. Oncology. 2015;29:331–340. [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson M.P., Lin Y., Bleeker J.S. Intratumoral CD14+ cells and circulating CD14+HLA-DRlo/neg monocytes correlate with decreased survival in patients with clear cell renal cell carcinoma. Clin Cancer Res. 2015;21:4224–4233. doi: 10.1158/1078-0432.CCR-15-0260. [DOI] [PubMed] [Google Scholar]
- 15.Javeed N., Gustafson M.P., Dutta S.K. Immunosuppressive CD14+HLA-DRlo/neg monocytes are elevated in pancreatic cancer and “primed” by tumor-derived exosomes. Oncoimmunology. 2017;6:e1252013. doi: 10.1080/2162402X.2016.1252013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaverdian N., Veruttipong D., Wang J., Schaue D., Kupelian P., Lee P. Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-small-cell lung cancer patients treated with stereotactic body radiation therapy. Clin Lung Cancer. 2016;17:39–46. doi: 10.1016/j.cllc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Hendriks J., Gravestein L.A., Tesselaar K., van Lier R.A., Schumacher T.N., Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 18.Hengel R.L., Thaker V., Pavlick M.V. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol. 2003;170:28–32. doi: 10.4049/jimmunol.170.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Kobata T., Agematsu K., Kameoka J., Schlossman S.F., Morimoto C. CD27 is a signal-transducing molecule involved in CD45RA+ naive T cell costimulation. J Immunol. 1994;153:5422–5432. [PubMed] [Google Scholar]
- 20.Fehniger T.A., Cooper M.A., Nuovo G.J. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 21.Lugade A.A., Moran J.P., Gerber S.A., Rose R.C., Frelinger J.G., Lord E.M. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura S., Wang B., Kawashima N. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometric dot plots and gating strategies for selected immune phenotypes. A. For CD4+CD45RA+CD62L+CD27+ Naïve T cells (% of CD4+CD45RA+ T cells), CD3+ T cells were gated from mononuclear cells (MNCs) (data not shown). CD4+ T cells were gated from CD3+ T cells. CD25+CD127− regulatory T cells (Tregs) were isolated from non-regulatory T cells. Non-Tregs were separated into naïve (CD45RA+) and (CD45RO+) memory cells. CD62L+ and CD27+ naïve cells were measured as a percent of naïve CD4 T cells. B. LINDR+ circulating dendritic cells and LIN-DR- immature myeloid derived suppressor cells were gated from MNCs by negative LIN (CD3+CD14+CD19+CD56+) staining and for positive or negative staining for HLA-DR. Representative subjects from the cancer patient cohort (PT) or healthy volunteer (HV).