Abstract
Purpose
The adrenal glands are a common site of metastases because of their rich blood supply. Previously, adrenal metastases were treated with systemic chemotherapy or, more rarely, with surgical resection or palliative radiation therapy. Stereotactic body radiation therapy (SBRT) has recently emerged as an attractive noninvasive approach to definitively treat these lesions. We present our experience in treating adrenal metastases using SBRT and review the current literature.
Methods and materials
This is a single-institution retrospective review of patients who received SBRT to adrenal metastases originating from various primary malignancies. Patients who were eligible for SBRT included those with limited metastatic disease (≤5 sites) with otherwise controlled metastatic disease and uncontrolled adrenal metastases.
Results
Ten patients met the study's inclusion criteria and received SBRT doses of 30 to 48 Gy in 3 to 5 fractions. Acute sequelae of SBRT treatment included 4 patients with grades 1 or 2 nausea, 3 patients with grade 1 fatigue, and 1 with grade 1 diarrhea. The median follow-up was 6 months with a median overall survival of 9.9 months. One patient demonstrated progressive adrenal gland disease 18.8 months after SBRT treatment. Seven patients developed new distant metastases after treatment, with a median progression-free survival of 3.4 months. Three months after SBRT to the adrenal gland, 1 patient developed a gastrointestinal bleed.
Conclusions
These results complement the limited existing body of literature by demonstrating that SBRT provides good control of treated adrenal gland metastasis; however, high-grade late toxicities may occur. More stringent dose constraint limits may prevent associated serious adverse events.
Summary.
Stereotactic body radiation therapy (SBRT) has recently emerged as an attractive noninvasive approach to definitively treat adrenal gland metastases. We reviewed the records of 10 patients with oligometastatic disease who received SBRT (30-48 Gy in 3-5 fractions) to adrenal gland metastases. Our conclusions support the safety and efficacy data of SBRT treatment to adrenal gland metastases.
Alt-text: Unlabelled box
Introduction
The adrenal glands are a common site for metastases, mainly because of their rich sinusoidal blood supply.1 Metastatic disease in the adrenal gland is most often from a lung primary malignancy but can also be due to multiple other primary malignancies, including breast, kidney, colon, and liver cancer.2 Historically, adrenal metastases were discovered symptomatically and treated with chemotherapy or, less often, with palliative external beam radiation therapy. However, with the increased use of imaging for cancer diagnosis and surveillance, occult adrenal metastases are more frequently identified. Many of these patients have a limited number of metastases (ie, oligometastatic disease). Aggressive use of definitive local therapies in oligometastatic disease may increase progression-free survival (PFS) and overall survival (OS).3, 4
Recently, stereotactic body radiation therapy (SBRT) has emerged as a noninvasive technique to treat metastatic adrenal lesions definitively.5 In contrast with conventional radiation therapy, SBRT allows for the delivery of large doses of radiation in a highly conformal manner. SBRT is frequently used as a definitive treatment modality for inoperable primary lung and liver malignancies,6 and a growing body of literature has explored SBRT for the definitive treatment of oligometastatic disease.3 The safety and tolerability of SBRT in treating adrenal gland metastases have only been reported in small case series; metastases typically originated from a single primary cancer site without a consensus on overall radiation dose or fractionation scheme.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20
Herein, we present our institutional experience with SBRT to adrenal gland metastases originating from various primary cancer sites and demonstrate that administering SBRT to adrenal gland metastases has favorable treatment outcomes, although associated serious side effects may occur.
Methods and materials
Patient population
After approval by our institutional review board, we reviewed a prospectively maintained database of patients who received SBRT to adrenal gland metastases. At our institution, the criteria for receiving SBRT to the adrenal gland include a limited metastatic disease burden and controlled systemic disease except for the adrenal metastasis.
Stereotactic body radiation therapy
Computed tomography (CT)-based treatment planning was used for all patients. Immobilization was obtained with either an arm board or a custom-made partial Vac-Lok (CIVCO Radiotherapy, Orange City, IA). Patients received a full expiration breath-hold contrast-enhanced CT scan with 2 mm slices and a 4-dimensional CT scan to quantify motion. The 4-dimensional CT data sets included 10 respiratory phases: 20%, 40%, 60%, 80%, and 100% inspiration phases, and 80%, 60%, 40%, 20%, and 0% expiration phases. If a motion greater than 1 cm was noted on the 4-dimensional CT scan, respiratory gating was used for treatment planning and delivery. In cases with ≤1 cm motion, an internal target volume (ITV) was created using the 100% inspiration, 0% expiration, and full-expiration breath-hold contrast-enhanced CT scan. In gated cases, beam-gating phases were designed to keep the tumor motion at ≤1 cm while the beam was on. The ITV included the full-expiration breath-hold contrast-enhanced CT scan and 4-dimensional CT data sets that corresponded to the gated phases. The planning target volume included an additional 5 mm margin around the ITV.
Patients were treated with either intensity modulated radiation therapy–based plans, volumetric-modulated arc therapy, or 3-dimensional conformal treatment. Dose and fractionation were determined on the basis of tumor size and location; patients received total doses of 30 to 48 Gy in 3 to 5 fractions (Table 1). The dose constraints that were used in our series were as follows: 1) spinal cord was allowed to receive a maximum dose of ≤13 Gy delivered over 3 fractions; dose of ≤16 Gy delivered over 4 fractions; or dose ≤25 Gy delivered over 5 fractions; 2) ipsilateral kidney dose was kept as low as possible with at least 150 cc receiving a cumulative dose of ≤12 Gy; 3) the large and small bowel maximum doses were kept as low as possible.
Table 1.
Patient characteristics
| Patient | Age | Sex | Primary Histology | Adrenal Met Size (cm3) | Additional Sites of Disease | Back or Flank Pain Prior to RT | Prescribed Dose | BED10 Delivered | Adrenal Disease Status Post SBRT (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | M | Esophagus adenocarcinoma | 18.3 | Bone, lung, inguinal lymph node | No | 35 Gy/5fx | 59.5 Gy | Stable (5.9) |
| 2 | 68 | F | NSCLC | 7.5 | bone | Yes | 30 Gy/5fx | 48 Gy | Stable (4.3) |
| 3 | 60 | M | HCC | 40.5 | — | No | 45 Gy/3fx | 112.5 Gy | Stable (7.4)a |
| 4 | 65 | M | SCLC | 25.4 | Liver, bone | Yes | 30 Gy/3fx | 60 Gy | Stable (11.1)a |
| 5 | 67 | M | Leiomyosarcoma | 50.8 | Bone, liver, pancreas | No | 48 Gy/4fx | 105.6 Gy | Stable (34.4)a |
| 6 | 59 | M | Unknown | 104.0 | bone | Yes | 30 Gy/3fx | 60 Gy | Progression (18.2)a |
| 7 | 79 | F | NSCLC | 17.2 | — | No | 30 Gy/5fx | 48 Gy | Stable (9.9)a |
| 8 | 36 | F | NSCLC | 3.9 | BRAIN | No | 36 Gy/3fx | 79.2 Gy | Stable (4.5)a |
| 9 | 59 | F | NSCLC | 19.9 | BRAIN | Yes | 36 Gy/3fx | 79.2 Gy | Stable (4)a |
| 10 | 67 | M | SCLC | 4.4 | BRAIN | No | 35 Gy/5fx | 59.5 Gy | Stable (6.6)a |
BED, biologically effective dose; fx, fraction; HCC, hepatocellular carcinoma; NSCLC, non-small cell lung cancer; RT, radiation therapy; SBRT, stereotactic body radiation therapy; SCLC, small cell lung cancer.
The patient is deceased. Stable and progression of disease were determined per Response Evaluation Criteria In Solid Tumors, Version 1.1.
Fractions were delivered every other day, with at least 40 hours between fractions. Prior to treatment delivery, either a megavoltage or kilovoltage cone beam CT scan was acquired for localization. No patient received concurrent chemotherapy during the SBRT. A biological effective dose (BED)10 for the tumor was calculated using the following equation:
where d is the radiation dose per fraction, n is the number of fractions, and α/β is equal to 10 Gy.
Follow-up and response evaluation
All patients were assessed at least once during treatment for acute side effects as well as 1 month after completion of treatment and then every 3 months to assess late toxicities. Our institutional practice is to evaluate patients with a CT scan 1 to 3 months after treatment for a response assessment. Additional CT scans were typically obtained every 3 months. A complete blood count with differential and electrolyte panels, including blood urea nitrogen and creatinine, were drawn at each follow-up visit to assess the ability to receive systemic therapy and risk for kidney injury.
Statistical analysis
The Kaplan-Meier method was used to estimate OS and PFS from the completion of radiation therapy. Treated adrenal gland metastasis control was determined by the patient's most recent CT scan and was defined as the absence of progression at the treatment site per Response Evaluation Criteria In Solid Tumors, Version 1.1. Statistical analysis was performed with SPSS, Version 24 (IBM Corporation, Armonk, NY).
Results
The baseline demographic and clinical data of 11 patients who underwent SBRT to treat adrenal gland metastases are summarized in Table 1. One patient died during the course of radiation therapy because of aspiration pneumonia and was removed from the analysis, leaving 10 eligible patients. The median patient age was 66 years, and the mean tumor volume was 31.4 cm3 (range, 3.88-104 cm3). The mean duration from the time of initial cancer diagnosis to adrenal metastases development was 18.8 months (range, 0-74 months). Two patients had adrenal metastases at diagnosis. Eight patients received chemotherapy before SBRT treatment, and 3 patients received chemotherapy after SBRT treatment. Five patients received immunotherapy either before or after SBRT treatment.
Four patients reported symptomatic back or flank pain as a result of the adrenal gland metastases before SBRT initiation. All of these patients reported improvement in their symptoms after SBRT treatment. One patient received SBRT after an unsuccessful palliative course of radiation treatment (30 Gy in 10 fractions) to adrenal metastases for pain control. This patient experienced an improvement in pain after completion of SBRT.
Regarding acute toxicities, 4 patients developed grade 1 or 2 nausea, 2 patients developed grade 1 fatigue, and 1patient reported grade 1 diarrhea (Table 2) in the acute period after treatment. The median follow-up duration after SBRT was 6 months (range, 1-34 months) with follow-up CT scans available for 10 patients. At the last follow-up, 8 of 10 patients had died. Median OS was 9.9 months (Fig 1a). Seven patients developed new distant metastases after SBRT treatment; median PFS was 3.4 months (Fig 1b). Nine patients (90%) achieved control of treated adrenal gland metastasis after completion of SBRT, with 1 patient experiencing progression of the adrenal gland metastasis 18.2 months after completion of treatment (Table 1). The other 9 patients had stable disease in treated adrenal glands at the time of the final follow-up visit or at death.
Table 2.
Acute and late toxicities during and after SBRT to adrenal gland metastases
| Toxicity | N (%) |
|---|---|
| Acute | |
| Nausea (Grade 1 and 2) | 4 (36.4) |
| Fatigue (Grade 1) | 3 (27.3) |
| Diarrhea (Grade 1) | 1 (9.1) |
| Late | |
| Gastrointestinal bleed | 1 (9.1) |
SBRT, stereotactic body radiation therapy.
Figure 1.
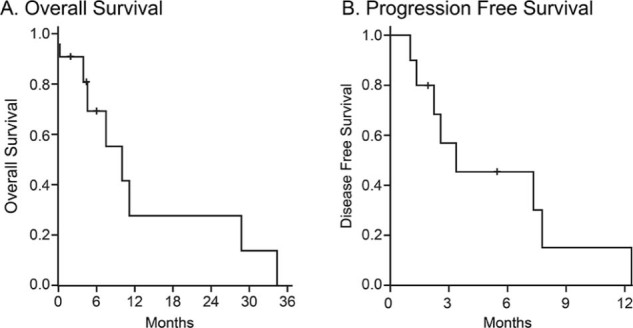
Overall survival and progression-free survival of patients with oligometastatic disease treated with stereotactic body radiation therapy to adrenal gland metastasis. Kaplan-Meier curve for overall survival (A) and progression-free survival (B). Median overall survival was 9.9 months, and median progression-free survival was 3.4 months. Patients alive at the time of analysis were censored to the date of last follow-up.
For chronic toxicities, 1 patient developed a gastrointestinal bleed 3 months after completion of SBRT treatment. An upper gastrointestinal endoscopy noted a subepithelial hemorrhage in the proximal jejunum, but no definitive source of bleeding was identified. This individual's maximum bowel radiation dose was calculated as 52.8 Gy delivered over 3 fractions.
Discussion
This study used a prospectively maintained database of patients who received SBRT to the adrenal gland for metastatic disease. We found that SBRT provides good control of treated adrenal gland metastasis with acceptable acute toxicity; OS and treated adrenal gland metastasis control rates were similar to other reports in the literature given the relatively short follow-up period (Table 3). However, 1 patient developed a gastrointestinal bleed 3 months after completion of SBRT to the adrenal gland.
Table 3.
Published adrenal SBRT case series
| Study | Patients | Lesions | Primary Histology | Median Follow-up (mo) | Total Dose (Gy) | Pain Relief | Local Control | Overall Survival (median, mo) | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Katoh et al, 200815 | 9 | 10 | 5 NSCLC 1 SCLC 2 HCC 1 prostate |
16 | 30-48 | yes, 1/1 patients | 100% | 15 | NR |
| Chawla et al, 20099 | 30 | 35 | 20 Lung 4 GI 3 breast 1 head and neck 1 melanoma 1 unknown |
9.8 | 16-50 | NR | 27% | 11 | Grade 1 nausea and fatigue “common” |
| Casamasssima et al, 20118 | 48 | 58 | 24 Lung 12 colon 4 melanoma 3 breast 1 uterus 1 unknown |
16.2 | 21.69-54.09 | yes, 4/4 patients | 94% | NR | 1 case grade 2 adrenal insufficiency |
| Torok et al, 201120 | 7 | 9 | 4 NSCLC 1 SCLC 2 HCC |
14 | 16 or 27 | yes, 1/2 patients | 89% | 8 | NR |
| Holy et al, 201114 | 13 | 13 | 13 NSCLC | 21 | 20-40 | yes, 6/8 patients | 77% | 23 | 46% grade 1 nausea, 15% gastric/duodenal ulcer |
| Oshiro et al, 201117 | 19 | 19 | 14 NSCLC 5 SCLC |
10.1 | 30-60 | NR | 72% | NR | 1 grade 2 duodenal ulcer |
| Guiou et al, 201213 | 9 | 10 | 4 NSCLC 5 SCLC |
7.3 (mean) | 40 | NR | NR | 10.2 | 22% nausea, vomiting |
| Scorsetti et al, 201219 | 34 | 36 | 22 NSCLC 3 SCLC 3 Melanoma 1 Sarcoma 1 Colorectal adenocarcinoma 1 RCC 1 Unknown |
41 | 20-37.5 | NR | 93% | 22 | 6% grade 2 nausea |
| Ahmed et al, 20137 | 13 | 13 | 4 NSCLC 2 RCC 1 melanoma 1 skin SCC 1 bladder 1 colon 1 cholangiocarcinoma |
12.3 | 33.75-60 | NR | 92% | 7.2 | 38% grade 1 fatigue 8% grade 1 abdominal pain 8% grade 1 diarrhea 15% grade 2 nausea 8% late grade 2 fatigue, abdominal pain, and nausea |
| Rudra et al, 201318 | 10 | 13 | 6 NSCLC 2 SCLC 2 RCC |
14.9 | 24-50 | NR | 73% | 17 | 80% grade 1-2 fatigue 40% grade 1-2 GI toxicity 1 case grade 2 adrenal insufficiency |
| Li et al, 201316 | 26 | 26 | 2 Pheochromocytoma 10 lung 6 bladder 4 unknown 4 RCC |
NR | 30-50 | yes, 15/16 patients | 77% | 17 | 88% grade 1-2 fatigue 69% grade 1-2 anorexia 38% grade 1-2 N/V 23% grade 1-2 skin rxn 4% grade 3 nausea/vomiting |
| Desai et al, 201510 | 14 | 14 | 6 NSCLC 2RCC 1 melanoma 1 primary adrenal 1 mixed Mullerian 1 GE junction 1 bladder 1 lymphoma |
NR | 20-30 | NR | 45.5% | NR | NR |
| Gamsiz et al, 201512 | 15 | 17 | 15 NSCLC | 16 | 30 | yes, 2/2 patients | 86.7% | NR | 46% grade 1 nausea 80% grade 1 fatigue 1 case dyspeptic disorder |
| Franzese et al 201611 | 46 | 46 | 30 lung 7 colorectal adenocarcinoma 9 other |
7.6 | 40 | NR | 78.3% | 28.5 (mean) | 13.1% pain, nausea, or vomiting 2 cases asthenia |
| Present study 2017 | 10 | 10 | 4 NSCLC 2 SCLC 1 esophageal carcinoma 1 HCC 1 leiomyosarcoma 1 unknown |
6 | 30-48 | yes, 4/4 patients | 70% | 9.9 | 36.4% nausea (grade 1 and 2) 27.3% fatigue (grade 1) 1 case diarrhea (grade 1) 1 case GI bleed |
GE, gastroesophageal; GI, gastrointestinal; HCC, hepatocellular carcinoma, NR, not reported; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; SCLC, small cell lung cancer.
There is considerable heterogeneity with respect to the SBRT technique, radiation dose, and radiation fractionation schemes in the adrenal gland SBRT literature. Published series have used a variety of radiation approaches, including 3-dimensional conformal intensity modulated radiation therapy, volumetric-modulated arc therapy, and robotic radiosurgery techniques. In addition, SBRT dose and fractionation have varied both among and within case series, with the radiation doses ranging from 16 to 60 Gy delivered in 1 to 10 fractions.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 In our series, 30 to 48 Gy was administered in 3 to 5 fractions, with all evaluable patients achieving treated adrenal gland metastasis control on initial follow-up imaging. Only 1 patient demonstrated adrenal gland progression 18.2 months after completion of SBRT per Response Evaluation Criteria In Solid Tumors, Version 1.1. Reported treated adrenal gland metastasis control rates have typically been high in published case series, with rates between 70% and 100%, with the exception of the studies by Chawla et al and Desai et al, who reported rates of 27% and 45.5%, respectively.9, 10
Several studies have explored the effect of BED on local tumor control and noted higher local tumor control as associated with higher BED values. Li et al noted greater treated adrenal gland metastasis control rates of 100% for tumors with a BED10 >100 Gy and 82% for tumors with a BED10 <100 Gy.16 Desai et al reported a mean BED10 of 96.7 Gy for nonlocally failing tumors and 76 Gy for locally failing tumors.10 In examining their 13-lesion cohort, Rudra et al noted that the 3 local failures had the lowest BED10, with a mean of 43.2 Gy.18 In our cohort, we did not find an association between BED10 and treated adrenal gland metastasis control. The patient who demonstrated adrenal gland progression post-SBRT had a BED10 of 105.6 Gy. Greater numbers of patients may allow for a better correlation between BED and treated adrenal gland metastasis control.
Dosimetry to the adrenal gland is complicated because these tumors can have considerable respiratory motion in addition to multiple nearby organs at risk, which could lead to selectively underdoing tumor coverage. For the credentialing of NRG BR001, a current ongoing multicenter phase 1 study investigating SBRT treatment for oligometastatic disease, conformity and underdosing of the target were affected because of the closeness of the stomach or other organs at risk.21 Of note, NRG BR001 has selected an initial dose of 45 Gy in 3 fractions to adrenal gland metastases with a decreased dose of 42 Gy in 3 fractions allowed if there are adjacent dose-limiting structures.22 This study has closed accrual to abdominal SBRT sites, including the adrenal glands, and the outcome report from the study is pending.
Although the reported treated adrenal gland metastasis control rates for adrenal SBRT are high, OS and distant control rates for patients receiving SBRT to the adrenal gland have varied more than control rates of treated adrenal gland metastasis. We reported a median OS of 9.9 months, which is within the literature-reported range of median OS (range, 7.2-23 months). Distant control has been evaluated by several case series, with 1 year distant control reported as 9% to 55%.7, 8, 9 Our median PFS rate was 3.4 months. Although both our median OS and PFS were at the lower end of reported values, this is likely because of differences in patient population histologies, prior treatment, and extent of disease (patient selection) at the time of SBRT treatment. Four of ten evaluable patients had non-small cell lung cancer primary disease. A recent report showed that local consolidative therapy of oligometastatic non-small cell lung cancer disease had improved PFS.23
Although SBRT is used for definitive treatment, symptom palliation can also be achieved through radiation therapy. Six studies evaluating SBRT's effects on pain control all reported an improvement in pain after SBRT, with response rates ranging from 50% to 100%.8, 12, 14, 15, 16, 20 This is consistent with our outcome of improvement in pain in all 4 patients who reported back or flank pain before SBRT, including 1 patient who did not achieve pain control after receiving palliative radiation to the adrenal gland (30 Gy in 10 fractions).
SBRT to adrenal gland metastasis provides high local control with acceptable acute toxicity, with most treatment-related toxicities being self-limited and occurring in the acute phase. The most commonly reported side effects are nausea, vomiting, and fatigue, with rates of nausea ranging from 6% to 40%7, 12, 13, 14, 18, 19 and fatigue ranging from 38% to 88%7, 12, 18 when reported, which is consistent with our outcomes of 36% (nausea) and 27% (fatigue). Late toxicities reported in the literature include adrenal insufficiency and gastric/duodenal ulcers.14, 16, 17 A case report also detailed late gastric perforation and hemorrhage leading to the death of a patient who received 60 Gy in 5 fractions to an adrenal lesion with concurrent vinorelbine.24 A review of the gastric radiation dose found that the stomach received >52.5 Gy to 2.9 mL of dose. Although these radiation doses met their predefined organ-at-risk dose limits, concurrent chemotherapy may have potentiated the radiation dose effect in that setting. Our series had 1 patient who developed an upper gastrointestinal bleed after SBRT to the left adrenal gland, with a maximum bowel dose of 52.8 Gy in 3 fractions. This serious adverse event occurred without the addition of chemotherapy. Bowel dose constraints should be strongly considered to prevent late toxicity. Given the lack of literature on abdominal SBRT and with most reports consisting of small case series, SBRT bowel dose volume constraints need further refinement.
Conclusions
Our results complement the existing literature by providing further safety and efficacy data on SBRT to adrenal gland metastases. Adrenal SBRT may provide better pain control than conventional palliative radiation therapy. However, associated serious adverse events may occur, and more stringent dose constraint limits and avoidance of concurrent chemotherapy should be considered. Further large-scale studies will be necessary to determine the acceptable dose parameters for both efficacy and avoidance of toxicity.
Footnotes
Sources of support: This study was supported in part by our institutional Department of Radiation Oncology and National Institute of Health grant CCSG 5P30-CA08686.
Conflicts of interest: The authors declare that no actual or potential conflict of interest exists.
References
- 1.Kung A.W., Pun K.K., Lam K., Wang C., Leung C.Y. Addisonian crisis as presenting feature in malignancies. Cancer. 1990;65:177–179. doi: 10.1002/1097-0142(19900101)65:1<177::aid-cncr2820650134>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Lam K.Y., Lo C.Y. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95–101. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 3.Alongi F., Arcangeli S., Filippi A.R., Ricardi U., Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012;17:1100–1107. doi: 10.1634/theoncologist.2012-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weichselbaum R.R., Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 5.Ippolito E., D'Angelillo R.M., Fiore M., Molfese E., Trodellla L., Ramella S. SBRT: A viable option for treating adrenal gland metastases. Rep Pract Oncol Radiother. 2015;20:484–490. doi: 10.1016/j.rpor.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubio C., Morera R., Hernando O., Leroy T., Lartigau S.E. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother. 2013;18:387–396. doi: 10.1016/j.rpor.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed K.A., Barney B.M., Macdonald O.K. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol. 2013;36:509–513. doi: 10.1097/COC.0b013e3182569189. [DOI] [PubMed] [Google Scholar]
- 8.Casamassima F., Livi L., Masciullo S. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82:919–923. doi: 10.1016/j.ijrobp.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 9.Chawla S., Chen Y., Katz A.W. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009;75:71–75. doi: 10.1016/j.ijrobp.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 10.Desai A., Rai H., Haas J., Witten M., Blacksburg S., Schneider J.G. A retrospective review of CyberKnife stereotactic body radiotherapy for adrenal tumors (primary and metastatic): Winthrop University Hospital experience. Front Oncol. 2015;5:185. doi: 10.3389/fonc.2015.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franzese C., Franceschini D., Cozzi L. Minimally invasive stereotactical radio-ablation of adrenal metastases as an alternative to surgery. Cancer Res Treat. 2017;49:20–28. doi: 10.4143/crt.2016.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamsiz H., Beyzadeoglu M., Sager O. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori. 2015;101:98–103. doi: 10.5301/tj.5000222. [DOI] [PubMed] [Google Scholar]
- 13.Guiou M., Mayr N.A., Kim E.Y. Stereotactic body radiotherapy for adrenal metastases from lung cancer. J Radiat Oncol. 2012;1:155–163. [Google Scholar]
- 14.Holy R., Piroth M., Pinkawa M., Eble M.J. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187:245–251. doi: 10.1007/s00066-011-2192-z. [DOI] [PubMed] [Google Scholar]
- 15.Katoh N., Onimaru R., Sakuhara Y. Real-time tumor-tracking radiotherapy for adrenal tumors. Radiother Oncol. 2008;87:418–424. doi: 10.1016/j.radonc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Shi Z., Wang Z. Treating adrenal tumors in 26 patients with CyberKnife: A mono-institutional experience. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0080654. e80654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshiro Y., Takeda Y., Hirano S., Ito H., Aruga T. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol. 2011;34:249–253. doi: 10.1097/COC.0b013e3181dbb727. [DOI] [PubMed] [Google Scholar]
- 18.Rudra S., Malik R., Ranck M.C. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol Cancer Res Treat. 2013;12:217–224. doi: 10.7785/tcrt.2012.500320. [DOI] [PubMed] [Google Scholar]
- 19.Scorsetti M., Alongi F., Filippi A.R. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: A retrospective analysis of 34 patients. Acta Oncol. 2012;51:618–623. doi: 10.3109/0284186X.2011.652738. [DOI] [PubMed] [Google Scholar]
- 20.Torok J., Wegner R.E., Burton S.A., Heron D.E. Stereotactic body radiation therapy for adrenal metastases: A retrospective review of a noninvasive therapeutic strategy. Future Oncol. 2011;7:145–151. doi: 10.2217/fon.10.165. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hallaq H.A., Chmura S.J., Salama J.K. Benchmark credentialing results for NRG-BR001: The first National Cancer Institute-sponsored trial of stereotactic body Radiation Therapy for Multiple Metastases. Int J Radiat Oncol Biol Phys. 2017;97:155–163. doi: 10.1016/j.ijrobp.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NRG Oncology . 2016. Stereotactic body radiation therapy in treating patients with metastatic breast cancer, non-small cell lung cancer, or prostate cancer. ClinicalTrials.gov. [Google Scholar]
- 23.Gomez D.R., Blumenschein G.R., Jr, Lee J.J. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onishi H., Ozaki M., Kuriyama K. Serious gastric ulcer event after stereotactic body radiotherapy (SBRT) delivered with concomitant vinorelbine in a patient with left adrenal metastasis of lung cancer. Acta Oncol. 2012;51:624–628. doi: 10.3109/0284186X.2012.671957. [DOI] [PubMed] [Google Scholar]


