Abstract
Purpose
Limited data exist on fractionated stereotactic radiation therapy (FSRT) for brain metastases. We sought to evaluate the safety and efficacy of FSRT and further define its role in brain metastasis management.
Methods and materials
A total of 72 patients were treated with linear accelerator–based FSRT to 182 previously untreated, intact brain metastases. Targets received 25 or 30 Gy in 5 fractions. All targets within the same course received the same prescription regardless of size. Toxicity was recorded per Radiation Therapy Oncology Group central nervous system toxicity criteria.
Results
The median follow-up was 5 months (range, 1-71 months). The Kaplan-Meier estimate of 12-month local control was 86%. Tumors <3 cm in diameter demonstrated improved 12-month local control of 95% compared with 61% in tumors ≥3 cm (P < .001). The Kaplan-Meier estimate of 12-month local control was 91% in tumors treated with 30 Gy and only 75% in tumors treated with 25 Gy (P = .015). Tumor diameter ≥3 cm resulted in increased local failure, and a 30 Gy prescription resulted in decreased local failure on multivariate analysis (hazard ratio [HR], 8.11 [range, 2.09-31.50; P = .003] and HR, 0.26 [range, 0.07-0.93; P = .038]). Grade 4 central nervous system toxicity occurred in 4 patients (6%) requiring surgery, and no patient experienced irreversible grade 3 or 5 toxicity. Increasing tumor diameter was associated with increased toxicity risk (HR, 2.45 [range, 1.04-5.742; P = .04]).
Conclusions
FSRT for brain metastases appears to demonstrate a high rate of local control with minimal risk of severe toxicity. Local control appears to be associated with smaller tumor sizeand a higher prescription dose. FSRT is a viable option for those who are poor single-fraction candidates.
Summary.
Given the limitations and toxicity of whole brain radiation therapy, extending the paradigm of focal therapy to patients who are not candidates for single-fraction stereotactic radiation surgery due to size or location represents an important clinical challenge. We retrospectively evaluated patients with intact, previously untreated brain metastases who were treated with fractionated stereotactic radiation therapy over 5 fractions. We found that local control was dose dependent, and a smaller tumor size was associated with improved tumor control.
Alt-text: Unlabelled box
Introduction
Brain metastases are among the most commonly encountered complications of cancer and represent the most common intracranial neoplasm in adults. Brain metastases are a well-established cause of morbidity and mortality, affecting 20% to 40% of patients with cancer.1, 2, 3 Due to the significance of brain metastases, a great deal of focus has been placed on the appropriate treatment of these lesions.
The management of patients with brain metastases is evolving. Focal techniques are gaining favor as the initial radiation therapy technique for patients with brain metastases,4 and whole brain radiation therapy (WBRT) is commonly deferred due to toxicity concerns5, 6 and a lack of proven survival advantage.7 Unfortunately, not all patients are good candidates for stereotactic radiosurgery (SRS) because large tumors and those in unfavorable locations have been associated with unacceptable rates of treatment-related toxicity.8 Given the limitations of WBRT, extending the paradigm of focal therapy to patients who are not candidates for SRS represents an important clinical challenge.
A longstanding principle of radiation biology is that fractionating a course of radiation therapy may reduce effects on normal tissue while maintaining tumor control. Fractionated stereotactic radiation therapy (FSRT) combines the steep dose gradients and small treatment margins of SRS with the radiobiologic advantages of fractionation. Data are limited on FSRT for brain metastases, and many questions remain. We hypothesize that FSRT offers a safe alternative to SRS for larger tumors and can also be used effectively for smaller lesions. We report on our single institution experience, which to our knowledge represents the largest series of previously untreated, intact brain metastases treated with 5-fraction FSRT.
Methods and materials
We retrospectively reviewed the records of all patients with brain metastases who were treated at our institution between August 2008 and November 2015. All patients who underwent FSRT for previously untreated, intact brain metastases with at least 1 posttreatment magnetic resonance imaging (MRI) were included in this study. Patients who underwent FSRT after surgical resection were included if they also had intact lesions that were treated; for such patients, only the intact lesions were included in estimates of local control. Patients who underwent FSRT for new metastases that developed after prior WBRT were also included. This study was approved by our institutional review board.
Treatment
The cases of all potential radiosurgery candidates with brain metastases were reviewed at a multidisciplinary conference. The decision to use FSRT was individualized and based on physician preference, but FSRT was generally considered if a tumor was ≥3 cm in diameter, near a critical or eloquent structure, or if the proximity of moderately sized tumors would lead to dose bridging in a single-fraction SRS plan (Fig 1). For patients in whom FSRT was recommended, all targets received the same dose schedule regardless of size; therefore, many tumors <3 cm were treated with FSRT.
Figure 1.
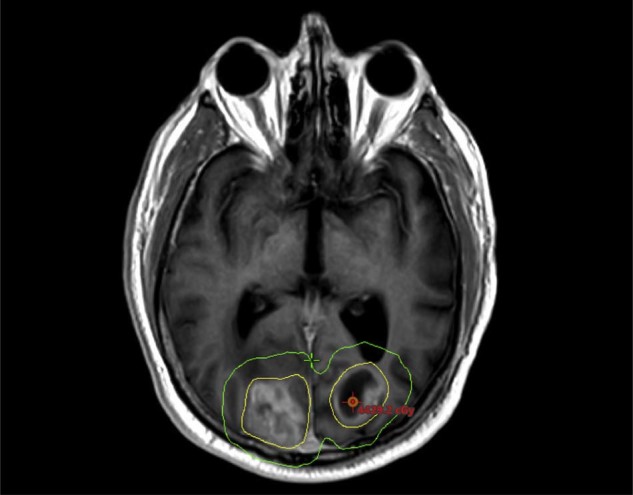
Tumors were selected for hypofractionation based on size and proximity to a critical structure or other tumors. The yellow and green isodose lines represent 100% and 50% of the prescription, respectively. This is a patient with 2 moderately sized metastases in close proximity with significant bridging of the 50% isodose line.
Patients were simulated in the supine position with an Aquaplast mask (WFR/Aquaplast Corp., Wyckoff, NJ) used for immobilization. A thin slice (≤2 mm slice thickness) MRI was obtained and registered with the simulation computed tomography (CT) scan for improved target delineation and normal structure identification. The gross tumor volume was defined as the enhancing abnormality identified on the T1 postcontrast MRI sequence and CT scan. An optional 1 to 3 mm planning target volume (PTV) expansion was infrequently used at the discretion of the treatingphysician to account for setup inaccuracy prior to the installation of a couch capable of 6 degrees of freedom correction. The total prescription dose was 25 or 30 Gy in 5 fractions prescribed volumetrically such that at least 99% of the PTV received the entire prescription dose delivered over a 7- to 14-day period.
Single isocenter treatment plans were generated in Varian Eclipse (Varian Medical Systems, Palo Alto, CA) after a standardized optimization protocol.9 Institutionally defined treatment planning goals were used to assess the quality of the plans (Table 1). All treatments were delivered with a linear accelerator, initially with a Varian 2100iX via sliding window intensity modulated radiation therapy using 6X or 15X photons and later with a Varian TrueBeam via volumetrically modulated arc therapy in flattening filter free mode with 10X photons (up to 2400 MU/min). Daily patient alignment was confirmed with a combination of kV orthogonal radiographs and cone beam CT for precise positioning immediately prior to treatment.
Table 1.
Central nervous system treatment planning organs at risk goals for 5-fraction treatments
| Organ | Constraint (maximum dose) | Prioritya |
|---|---|---|
| Brainstem | 31 Gy, V26 Gy <1 cc | I |
| Chiasm/Optic Nerve | 25 Gy, V20 Gy <0.2 cc | I |
| Cochlea | 27.5 Gy | II |
| Lens | 3-7 Gy | II |
| Retina | 5-15 Gy | II |
| Spinal Cord | 30 Gy, V22.5 Gy <0.25 cc | I |
| Cauda Equina | 34 Gy, V30 Gy <5 cc | I |
I = Do not violate, achieving constraint is more important than target coverage; II = Planning goal but less important than target coverage.
Endpoint definitions and statistical analysis
Local tumor progression was defined as the radiographic presence of a 25% increase in tumor diameter or the presence of more than scant tumor cells at the time of salvage surgery.10, 11, 12 Grade 3 or higher toxicity events as defined by the Radiation Therapy Oncology Group 90-05 central nervous system (CNS) toxicity criteria were recorded.8
Overall survival and freedom from toxicity were estimated on a per-patient basis using the Kaplan-Meier method and were measured from the initiation of radiation therapy. Living patients were censored at the time of the most recent clinical encounter, and patients without evidence of toxicity were censored at the time of most recent MRI or time of death. Estimation of local tumor control was performed on a per-tumor basis. Locally controlled tumors were censored from the analysis at the time of death or most recent MRI; to reduce the chance of overestimating the effect of FSRT, tumors were also censored from the local control analysis if patients underwent additional radiation therapy to this area (eg, if a patient received salvage WBRT for distant brain progression). Kaplan-Meier estimates between the groups were compared with the log-rank test. The effect of tumor and treatment parameters on local control was assessed with univariate and multivariate Cox proportional hazards models. All statistical tests were performed with SPSS software (IBM SPSS version 22.0, Chicago, IL).
Results
Patient and treatment characteristics
A total of 131 patients who underwent FSRT in 5 fractions for brain metastases between August 2008 and November 2015 were identified. Fifty-nine patients were excluded, including 19 patients who had received previous radiation to the lesions of interest, 26 who had no follow-up information available, 13 who had undergone prior surgery to the only treated lesion, and 1 patient who had a nonstandard radiation dose schedule. Thus, 72 patients met the inclusion criteria for this analysis resulting in a total of 182 tumors. Patient and treatment characteristics are presented in Table 2.
Table 2.
Patient, tumor, and treatment characteristics
| Characteristics | n |
|---|---|
| No. of patients | 72 |
| No. of tumors | 182 |
| Sex (M/F) | 48/24 |
| Median age (range), y | 63 (23-93) |
| Histology, n (%) | |
| Lung | 84 (46) |
| Breast | 17 (9) |
| Melanoma | 27 (15) |
| Gastrointestinal | 15 (8) |
| Genitourinary | 28 (16) |
| Other | 11 (6) |
| Median Karnofsky Performance Score (range) | 80 (50-90) |
| Median number of metastases (range) | 2 (1-16) |
| Patients with a single metastasis | 31 |
| Patients with multiple metastases | 41 |
| RPA I | 6 |
| RPA II | 58 |
| RPA III | 8 |
| Patients with prior focal RT (not to tumors of interest) | 9 (13%) |
| Patients with prior WBRT (not to tumors of interest) | 5 (7%) |
| Patients with prior Surgery (not to tumors of interest) | 14 (19%) |
| FSRT with FFF beam (up to 2400 MU/min) | 66 (92%) |
| 6 Gy × 5 = 30 Gy | 134 tumors |
| 5 Gy × 5 = 25 Gy | 48 tumors |
| PTV Margin | |
| None | 141 (78%) |
| 1 mm | 13 (7%) |
| 2 mm | 15 (8%) |
| 3 mm | 13 (7%) |
| Median Tumor volume (range) | 2.02 cc (0.01-39.00 cc) |
| Median Tumor diameter (range) | 1.68 cm (0.31-5.50 cm) |
| Median conformity index (range) | 1.11 (1.01-2.09) |
FFF, flattening filter-free; FSRT, fractionated stereotactic radiation therapy; PTV, planning target volume; RPA, recursive partitioning analysis; RT, radiation therapy; WBRT, whole brain radiation therapy.
Survival and local control outcomes
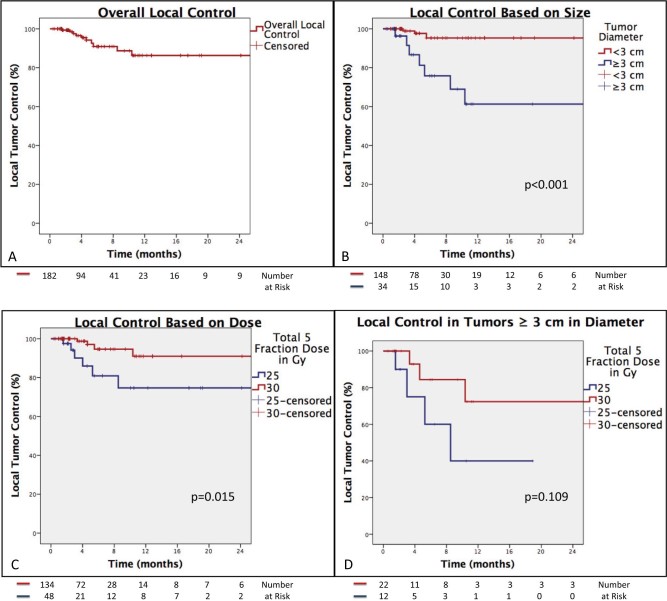
The median clinical and radiographic follow-up was 5 months (range, 1-71 months) and 5 months (range, 1-71 months), respectively. The median overall survival was 7 months with a 6- and 12-month estimate of overall survival of 63% and 29%, respectively. Local progression was observed in 10 tumors among 10 patients, resulting in a 12-month local control estimate of 86% (Fig 2).
Figure 2.
Kaplan-Meier estimates of local tumor control. A, Entire cohort. B, Local control based on tumor diameter. C, Improved local tumor control with a higher prescription dose. D, Trend toward improved tumor control with a higher prescription among tumors ≥3 cm in diameter.
The 12-month local control estimate of tumors measuring <3 cm (n = 146) in greatest dimension was 95% compared with 61% for tumors ≥3 cm (n = 36) in diameter (P < .001). The 12-month local control estimate of tumors <2 cm in diameter was 100% compared with 74% for tumors ≥2 cm in diameter. Tumors that were prescribed 30 Gy had a 12-month local control estimate of 91% compared with 75% in tumors prescribed 25 Gy (P = .015). Among tumors ≥3 cm (n = 36), a 30 Gy prescription was associated with a 72% 12-month local control estimate compared with 40% for tumors receiving 25 Gy (P = .109).
Table 3 presents the results of the Cox proportional hazards models for potential predictors of local failure. Smaller tumor size and higher radiation dose were significantly associated with improved local tumor control on univariate analysis; therefore, tumor diameter and dose were evaluated for significance in a multivariate analysis. Tumor diameter ≥3 cm was significantly associated with increased risk of local failure on multivariate analysis (hazard ratio [HR], 8.11 [range, 2.09-31.50; P = .003]). Furthermore, a total tumor dose of 30 Gy compared with 25 Gy was associated with a decreased risk of local failure on multivariate analysis (HR, 0.26 (range, 0.07-0.93; P = .038]).
Table 3.
Cox Proportional Hazard Model analysis of covariates that contribute to local failure
| Variable | Univariate hazard ratio (range) | P value | Multivariate hazard ratio (range) | P value |
|---|---|---|---|---|
| Tumor Diameter (≥3 cm) | 8.71 (2.24-33.90) | .002 | 8.11 (2.09-31.50) | .003 |
| Total Dose (30 vs. 25 Gy) | 0.24 (0.07-0.84) | .025 | 0.26 (0.07-0.93) | .038 |
| PTV margin (No vs. Yes) | 1.11 (0.23-5.22) | .899 | ||
| Histology (NSCLC) | Reference = 1 | .732 | ||
| Histology (Breast) | 0.94 (0.11-8.05) | |||
| Histology (Melanoma) | 1.78 (0.34-9.38) | |||
| Histology (Other) | 0.57 (0.11-2.94) |
NSCLC, non-small cell lung cancer; PTV, planning target volume.
Toxicity
No patient experienced irreversible grade 3 toxicity, and no patient died due to FSRT. Four patients required surgical resection of a single treated tumor. The pathologic findings of these resections are summarized in Table 4. Two patients with grade 4 toxicity were also scored as a local failure due to the presence of malignancy in the surgical specimen. Across all patients, the Kaplan-Meier estimate of 6-month freedom from irreversible grade 3 or grade 4 CNS toxicity rate was 95%. Among the 31 patients with 6 months of follow-up, 2 toxicity events occurred, resulting in a rate of 6% at 6 months. Only 12 patients had 12-month follow-up, and 4 toxicity events occurred within this timeframe with a resultant rate of 33% at 12 months. The 6-month Kaplan-Meier estimate of grade ≥3 toxicity was 5% for patients who were prescribed 30 Gy versus 7% for patients who were prescribed 25 Gy (P = .48). All 4 toxicity events occurred in tumors ≥2.8 cm in diameter, and 3 of the events occurred in tumors >3 cm in diameter. Increasing tumor diameter was significantly associated with an increased risk of CNS toxicity (HR, 2.45 [range, 1.04-5.742; P = .04]).
Table 4.
Pathology results from surgically resected tumors after previous FSRT
| Patient | Histology | Dose | Tumor no. | Tumor diameter (cm) | Previous intracranial treatment (radiation or surgery) | Pathology results | Scored as |
|---|---|---|---|---|---|---|---|
| 1 | Breast | 5 Gy × 5 | 2 | 2.80, 2.27 | None | A. Metastatic carcinoma consistent with breast cancer primary B. Radiation-induced damage |
Local Failure + Grade 4 toxicity |
| 2 | NSCLC | 6 Gy × 5 | 3 | 4.16, 3.40, 2.47 | None | A. Extensive radiation necrosis and radiation damage B. Residual metastatic adenocarcinoma comprises <5% of tissue |
Grade 4 toxicity |
| 3 | Unknown primary | 6 Gy × 5 | 2 | 3.43, 1.07 | Surgery to other brain metastasis | Metastatic adenocarcinoma exhibiting post-treatment effect | Local Failure + Grade 4 toxicity |
| 4 | Breast | 6 Gy × 5 | 3 | 3.55, 2.07, 1.18 | None | Extensive radiation necrosis (rare aggregates of recognizable adenocarcinoma of questionable viability) | Grade 4 toxicity |
FSRT, fractionated stereotactic radiation therapy; NSCLC, non-small cell lung cancer.
Discussion
FSRT offers the opportunity to use a focal treatment in patients who are poor candidates for treatment with single-fraction SRS due to toxicity concerns, but clinical outcomes data remain sparse. Therefore, we performed this retrospective analysis of FSRT for previously untreated brain metastases and found that FSRT resulted in high rates of local control for both small and large targets. Smaller tumor diameter and delivery of a higher radiation dose were associated with higher rates of local control. Treatment was well tolerated, with CNS toxicity rates lower than those reported for single-fraction SRS among patients and tumors with comparable characteristics.5, 8, 13, 14 Furthermore, 30 Gy over 5 fractions was not associated with increased toxicity compared with 25 Gy.
Previously reported 12-month local control estimates of FSRT have ranged from 52% to 95%.10, 13, 15, 16, 17, 18, 19 Although direct comparisons across studies are difficult due to heterogeneous patient populations, treatment techniques, and definitions of local control, the 12-month local control estimate of 86% in our study appears to compare favorably. Among larger tumors in particular, the 12-month estimate of local control among tumors ≥3 cm in diameter in our study was 61% overall, which improved to 72% when 30 Gy was prescribed. One important distinction in our study is that tumors treated with prior focal radiation, WBRT, and surgery were specifically excluded, unlike in other studies16, 20, 21 that reported very high rates of local control. Exclusion of patients with prior radiation or surgery to the treated tumors in our study makes the true local control of FSRT more apparent by removing the potential bias of other treatments on tumor control.
Another unique aspect of our study is that a relatively large number of smaller tumors were treated with FSRT. The 12-month local control estimate for tumors <3 cm in diameter was 95%, and we observed no failures in tumors <2 cm in diameter, results that are similar to previous reports on delivering ≥20 Gy in a single fraction.8, 22, 23 The high level of local control observed in this study among tumors <3 cm in diameter supports the limited FSRT literature, which identifies small tumor size as a predictor of improved local tumor control, and FSRT appears to be a reasonable alternative for small brain metastases.24, 25 Given these encouraging results, our practice for patients who are treated with FSRT is to use the same dose and fractionation across all targets, regardless of target size. Therefore, all tumors are treated with a single radiosurgery plan using a single isocenter, which significantly improves the efficiency of treatment planning and delivery.
The improved local tumor control with a higher dose schedule observed in our study is consistent with reports on single-fraction SRS, which have demonstrated a dose dependent effect on local tumor control.22, 26 Among studies examining FSRT in particular, there have been conflicting reports on the relationship between dose and tumor control. Whereas some studies have demonstrated improved control with dose escalation,18, 26, 27 particularly in tumors receiving an equivalent dose in 2 Gy fractions (EQD2) >35 Gy (alpha/beta = 10) or biological effective dose using an alpha/beta = 12 (BED12) >40 Gy (linear quadratic cubic model), others have failed to show a dose dependent response.10, 13, 24 This study demonstrated a 12-month tumor control estimate of 75% with 25 Gy in 5 fractions compared with 91% with 30 Gy in 5 fractions. By comparison, 25 Gy in 5 fractions results in an EQD2 of 31.25 Gy, and 30 Gy in 5 fractions results in an EQD2 of 40 Gy (alpha/beta = 10). The current study further supports the idea that local tumor control is dependent on radiation dose, even with use of multiple fractions.
Only 4 patients in this study developed severe CNS toxicity, yielding a Kaplan-Meier estimate of 5% at 6 months across all patients. Only 12 patients could be followed for 1 year, which resulted in a 33% risk of toxicity among this cohort. The limited follow-up in this study makes estimation of the true CNS toxicity somewhat challenging. A larger target size was associated with an increased risk of toxicity; however, we recognize that the small number of events limited the analytic power to identify other possible contributing factors. There may be a tumor size above which treatment with 30 Gy increases the risk of toxicity, necessitating the use of a lower dose, but this potential size cutoff remains unknown. We did not observe increased toxicity rates among patients who were prescribed 30 Gy compared with those who were prescribed 25 Gy, in contrast to other reports of higher rates of toxicity with higher doses.18, 28
One possible explanation for the favorable toxicity profile in our study despite dose escalation is the limited follow-up and survival of our cohort, which potentially underestimates the toxicity of FSRT because radiation necrosis is often a late effect. An additional explanation for the favorable toxicity profile in the study is that the majority of patients were treated without additional PTV margin because higher toxicity rates have been suggested with larger margins for patients receiving single-fraction SRS.29 Initial concern for rotational error in treating multiple metastases with a single isocenter resulted in the addition of a PTV margin to some targets; however, the installation of a 6 degree of freedom couch essentially eliminated the potentially observed rotational error, making zero-margin treatments consistently reproducible. Furthermore, the rate of CNS toxicity in this study was lower than has typically been reported for single-fraction SRS, particularly for larger tumors, but prospective studies are needed for a better comparison.5, 8, 13, 14
The limitations of this study include its retrospective nature, limited follow-up, and evaluation of only 2 fractionation schemes. We used well-defined inclusion criteria and endpoints to reduce bias, but the retrospective design of this study makes it potentially susceptible to bias that can only be addressed adequately in a prospective trial. Despite following the majority of patients until death, our follow-up is limited with a median follow-up of 5 months. This is likely attributable to the relatively short median survival of 7 months from FSRT in our cohort due to some patients, but not tumors, receiving other treatments prior to FSRT. Our institution has a relatively standardized dose schedule across the practice, which aids in analyzing the efficacy and safety of hypofractionation; however, the optimal dosing schedule remains unknown because many potential fractionations were not used in the treatment of our patients.
Conclusion
The results of our study suggest that FSRT for brain metastases is associated with a high rate of local control while maintaining acceptable toxicity rates. In this unique series in which all of a patient's tumors were treated with the same dose fractionation regardless of size, smaller tumors appeared to have significantly better local control compared with larger tumors. Use of the same FSRT prescription for large and small tumors in the same patient is feasible, but the diminished efficacy of FSRT observed among large tumors highlights the need for improved strategies and techniques in the treatment of these targets. Local control appears to be dose dependent with significantly better control observed in tumors receiving 30 Gy in 5 fractions; therefore, additional studies of dose escalation are needed to reveal the appropriate balance of FSRT efficacy and toxicity among larger brain metastases.
Footnotes
Meeting information: Portions of this work were presented in poster form at the American Society of Radiation Oncology Annual Meeting in Boston, MA, from September 25-28, 2016.
Sources of support: This research did not receive any specific funding from agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Patchell R.A. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 2.Fox B., Cheung V., Patel A., Suki D., Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011;22:1–6. doi: 10.1016/j.nec.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Schouten L., Rutten J., Huveneers H., Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network Clinical practice guidelines: Central nervous system cancers. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf Available at:
- 5.Chang E., Wefel J., Hess K. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kocher M., Soffietti R., Abacioglu U. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 Study. J Clin Oncol. 2010;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 9.Clark G., Popple R., Prendergast B. Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Pract Radiat Oncol. 2012;2:306–313. doi: 10.1016/j.prro.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Rajakesari S., Arvold N., Jimenez R. Local control after fractionated stereotactic radiation therapy for brain metastases. J Neurooncol. 2014;120:339–346. doi: 10.1007/s11060-014-1556-5. [DOI] [PubMed] [Google Scholar]
- 11.Dyer M., Kelly P., Chen Y. Importance of extracranial disease status and tumor subtype for patients undergoing radiosurgery for breast cancer brain metastases. Int J Radiat Oncol Biol Phys. 2012;83:479–486. doi: 10.1016/j.ijrobp.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Kelly P., Lin N., Claus E., Quant E., Weiss S., Alexander B. Salvage stereotactic radiosurgery for breast cancer brain metastases. Cancer. 2011;118:2014–2020. doi: 10.1002/cncr.26343. [DOI] [PubMed] [Google Scholar]
- 13.Fokas E., Henzel M., Surber G., Kleinert G., Hamm K., Engenhart-Cabillic R. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: Comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol. 2012;109:91–98. doi: 10.1007/s11060-012-0868-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y., Cho K., Kim J. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 2011;81:483–489. doi: 10.1016/j.ijrobp.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Matsuyama T., Kogo K., Oya N. Clinical outcomes of biological effective dose-based fractionated stereotactic radiation therapy for metastatic brain tumors from non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:984–990. doi: 10.1016/j.ijrobp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Fahrig A., Ganslandt O., Lambrecht U. Hypofractionated stereotactic radiotherapy for brain metastases. Strahlenther Onkol. 2007;183:625–630. doi: 10.1007/s00066-007-1714-1. [DOI] [PubMed] [Google Scholar]
- 17.Minniti G., Angelillo R., Scaringi C. Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol. 2014;117:295–301. doi: 10.1007/s11060-014-1388-3. [DOI] [PubMed] [Google Scholar]
- 18.Märtens B., Janssen S., Werner M. Hypofractionated stereotactic radiotherapy of limited brain metastases: A single-centre individualized treatment approach. BMC Cancer. 2012;12:497–505. doi: 10.1186/1471-2407-12-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton B., Gebhardt B., Prabhu R., Shu H., Curran W., Crocker I. Hypofractionated radiosurgery for intact or resected brain metastases: Defining the optimal dose and fractionation. Radiat Oncol. 2013;8:135–141. doi: 10.1186/1748-717X-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning M., Cardinale R., Benedict S. Hypofractionated stereotactic radiotherapy as an alternative to radiosurgery for the treatment of patients with brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:603–608. doi: 10.1016/s0360-3016(00)00475-2. [DOI] [PubMed] [Google Scholar]
- 21.Giubilei C., Ingrosso G., Andrea M., Benassi M., Santoni R. Hypofractionated stereotactic radiotherapy in combination with whole brain radiotherapy for brain metastases. J Neurooncol. 2008;91:207–212. doi: 10.1007/s11060-008-9700-8. [DOI] [PubMed] [Google Scholar]
- 22.Vogelbaum M., Angelov L., Lee S.Y., Li L., Barnett G., Suh J. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104:907–912. doi: 10.3171/jns.2006.104.6.907. [DOI] [PubMed] [Google Scholar]
- 23.Shehata M., Young B., Reid B. Stereotactic radiosurgery of 468 brain metastases < or = 2 cm: Implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Kwon A., Dibiase S., Wang B., Hughes S., Milcarek B., Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer. 2009;115:890–898. doi: 10.1002/cncr.24082. [DOI] [PubMed] [Google Scholar]
- 25.Bhatnagar A., Flickinger J., Kondziolka D., Lunsford L.D. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Wiggenraad R., Verbeek-De Kanter A., Kal H., Taphoorn M., Vissers T., Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011;98:292–297. doi: 10.1016/j.radonc.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Aoyama H., Shirato H., Onimaru R. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003;56:793–800. doi: 10.1016/s0360-3016(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 28.Ernst-Stecken A., Ganslandt O., Lambrecht U., Sauer R., Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: Results and toxicity. Radiother Oncol. 2006;81:18–24. doi: 10.1016/j.radonc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Kirkpatrick J., Wang Z., Sampson J. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91:100–108. doi: 10.1016/j.ijrobp.2014.09.004. [DOI] [PubMed] [Google Scholar]