Abstract
Purpose
We retrospectively analyzed late small bowel toxicity in patients who received abdominal or pelvic intensity modulated radiation therapy (IMRT) to the small bowel with a maximum dose greater than the generally accepted maximal tolerable dose of 45 Gy.
Methods and materials
All patients (N = 94) who received IMRT with a point dose of at least 45 Gy to tightly contoured small bowel between 2005 and 2014 at our institution were included. The median prescribed treatment dose was 70.2 Gy. The median follow-up was 20.1 months. Late small bowel toxicity was assessed using the Common Terminology Criteria for Adverse Events Version 3.0. Dosimetric variables and clinical factors were assessed for their relationship to small bowel toxicity.
Results
The median maximal small bowel point dose (Dmax) was 6546.5 cGy. The estimated 5-year rates of freedom from at least grade 1, at least grade 2, and at least grade 3 late small bowel toxicity were 72.4% (95% confidence interval [CI], 60.7%-86.5%), 91.9% (95% CI, 84.1%-100%), and 93.6% (95% CI, 86.2%-100%), respectively. One patient (1.1%) developed grade 3 late toxicity, and 2 patients (2.1%) developed grade 4 late toxicity. Use of capecitabine/5-fluorouracil treatment was a significant predictor (P < 0.001) of at least grade 1 and at least grade 2 small bowel toxicity. No other clinical factors were associated with toxicity. None of the dose-volume parameters were significant predictors of small bowel toxicity.
Conclusion
It may be possible with IMRT to deliver high doses to small volumes of small bowel with low rates of significant long-term complications. Further studies should explore tolerable dose-volume relationships in cases in which aggressive abdominal or pelvic treatment may be warranted to treat the underlying malignancy.
Summary.
Dose-volume thresholds that are strongly associated with late small bowel toxicity to guide clinical care are not well established, especially at the low volume–high dose range. A retrospective analysis of associations between dose-volume statistics, clinical factors, and the incidence of late small bowel toxicity in a cohort of 94 patients was conducted. Our data suggest that it may be possible to treat small volumes of small bowel above 45 to 50 Gy with acceptably low long-term toxicity risk.
Alt-text: Unlabelled box
Introduction
Radiation therapy for abdominal or pelvic malignancies can result in small bowel toxicity. Previous estimatesof the incidence of late small bowel toxicity, based on expert opinions in the pre–intensity modulated radiation therapy (IMRT) era, suggest that the complication probability of 5% after 5 years (TD5/5) for severe toxicity is at a dose of 50 Gy for irradiating one third of the small bowel.1
Relationships between dose-volume statistics and the incidence of acute small bowel toxicity have been assessed in patients with rectal and gynecologic cancers who received concurrent chemotherapy.2, 3, 4, 5 On the basis of these earlier studies, the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) guidelines recommend limiting V15 to <120 cc if individual loops are contoured and V45 to <195 cc if the entire peritoneal cavity is contoured.6 Although the QUANTEC guidelines also found some consistency with the aforementioned TD5/5 estimate for late small bowel toxicity, detailed dose-volume data for late small bowel toxicity were lacking.
Additionally, data with regard to late toxicity involving patients with irradiation of the small bowel to doses above 45 Gy are extremely limited. Previously published studies involved different fractionation schedules and non-IMRT radiation therapy, thus creating challenges in establishing guidelines that minimize late small bowel toxicity in IMRT treatment with higher maximum point dosages than the generally accepted 45 Gy.7, 8, 9, 10 Such guidelines are especially important in cases in which treatment of the underlying malignancy might benefit from more aggressive abdominal or pelvic radiation therapy.
Ambiguity also exists regarding whether contouring small bowel loops or the bowel bag is a more accurate predictor of late small bowel toxicity. Although some studies have used the entire bowel bag to account for bowel motion, 1 recent study has demonstrated that volumes measured by loop contouring were better predictors of overall gastrointestinal toxicity in a cohort of patients with cervical cancer.11 Other factors, such as the volume of liquid in the bladder, may also affect bowel motion and the accuracy of loop or bowel bag contouring methods.12
The aim of this study was to characterize the dose-volume relationship of late small bowel toxicity in patients who were treated with IMRT and whose malignancies warranted a maximum point dosage to the small bowel that was higher than 45 Gy. Additionally, we aimed to characterize any relationships between clinical variables or treatment factors and late small bowel toxicity.
Methods and materials
After institutional review board approval was obtained, we retrospectively reviewed 338 patients who were diagnosed with pelvic or abdominal malignancies and treated by one of the investigators (RDE) with IMRT alone (no brachytherapy) to prescribed doses of more than 45 Gy between January 2005 and June 2014. A total of 94 patients met the inclusion criteria of receiving a maximum point dose of at least 45 Gy to the small bowel.
Clinical data were extracted from the radiation oncology department records. Data on previous malignancies, presence of diabetes mellitus, vascular disease, previous gastrointestinal conditions, previous abdominal or pelvic surgeries, receipt of chemotherapy or hormone therapy (concurrent or sequential), date of completion of IMRT, prescribed dose of IMRT, use of daily image guidance, and the nature and severity of gastrointestinal symptoms in follow-up were collected. Toxicity was assessed using Common Terminology Criteria for Adverse Events Version 3.0. Small bowel toxicities included diarrhea, small bowel obstruction, enteritis, fistula, and stool incontinence. Any stool incontinence recorded was not confirmed to be a small bowel complication, but we elected to be conservative and assumed that any stool incontinence was a consequence of the radiation to the small bowel. Follow-up time was defined as the time between the completion of IMRT treatment and either the most recent follow-up or the incidence of small bowel toxicity. Severity of late toxicity was grouped into at least grade 1, at least grade 2, and at least grade 3 toxicity.
The individual loops of the small bowel were contoured on each computed tomography slice of the treatment-planning computed tomography scan. All patients received small bowel contrast to distinguish the small bowel from the large bowel. All contours were performed by a single experienced physician (RDE). Patients were treated with 180 to 200 cGy per fraction to a total prescribed dose between 5400 and 7560 cGy (median, 7020 cGy). A small bowel dose-volume histogram (DVH) was generated for each patient. We applied an internally developed guideline of 60 Gy to a maximum of 10 cc and 70 Gy to 5 cc when the physician felt this aggressive treatment was clinically warranted. These guidelines often were exceeded, however, with 25 instances above the 60 Gy limit and 4 of those 25 instances above the 70 Gy limit. The total volume of small bowel irradiated and the volume of small bowel receiving at least 10 Gy up to at least 80 Gy at 5 Gy intervals (V10, V15, etc.) were recorded. The maximum, minimum, and median point doses of the small bowel (Dmax, Dmin, and Dmean) were also recorded.
To assess the incidence over time, Kaplan-Meier analyses were conducted. Patients were divided into groups based on whether they did or did not have V80 >0 cc, V75 >1 cc, V75 >0 cc, V70 >1 cc down to V60. Groups were also constructed by dividing patients into those who were above and those who were below the cohort median of V10 up to V60 and of Dmax, Dmin, and Dmean. One group was created for V15 >120 cc to test the QUANTEC guideline on our patient cohort. Two groups were also created to evaluate toxicity in patients whose doses exceeded the internally developed guidelines of V60 <10 cc and V70 <5 cc. Toxicity differences between groups on the basis of the clinical variables, treatment factors, and the constructed dose groups described were conducted using the Kaplan-Meier estimator and the log-rank test.
For each clinical variable, treatment factor, or dosimetric group that showed a statistically significant difference with the log-rank test, a subset analysis of DVH statistics was performed to compare patients with or without that condition. The Kaplan-Meier method was also used to assess the time course of late small bowel toxicity for at least grade 1, at least grade 2, and at least grade 3 toxicity. Two patients had incomplete medical histories; their data were excluded from analyses that involved the missing information. One patient had an incomplete DVH and was excluded from analyses involving the missing DVH statistics. Additionally, 2 patients received multiple sets of abdominopelvic IMRT treatment and were excluded from all DVH statistics analyses because an accurate summation of the 2 treatment plans was not possible.
The Kaplan-Meier comparison was done for all constructed groups described and all clinical variables listed in Table 1 that could be divided into nonzero groupings. We considered a P-value <.05 as our significance threshold for the clinical variables, treatment factors, and binary dosimetric comparisons. However, because 26 separate dosimetric and 28 separate clinical comparisons were made, we applied the Bonferroni correction and set a significance level at P < .0019 for the dosimetric comparisons and at .0018 for the clinical comparisons. Statistical analyses were performed with R, Version 3.2.5.
Table 1.
Patient/treatment characteristics
| Clinical variable/treatment factor | Number of patients | % |
|---|---|---|
| Sex | ||
| Male | 63 | 67.0 |
| Female | 31 | 33.0 |
| Malignancy | ||
| Prostate | 50 | 53.2 |
| Primary | 37 | 39.4 |
| Postoperative | 11 | 11.7 |
| Recurrence | 2 | 2.1 |
| Bladder | 12 | 12.8 |
| Primary | 8 | 8.5 |
| Postoperative | 0 | 0 |
| Recurrence | 4 | 4.3 |
| Uterus | 7 | 7.4 |
| Primary | 5 | 5.3 |
| Postoperative | 0 | 0 |
| Recurrence | 2 | 2.1 |
| Ovary | 6 | 6.4 |
| Primary | 1 | 1.1 |
| Postoperative | 0 | 0 |
| Recurrence | 5 | 5.3 |
| Pancreas | 6 | 6.4 |
| Primary | 4 | 4.3 |
| Postoperative | 1 | 1.1 |
| Recurrence | 1 | 1.1 |
| Other | 13 | 13.8 |
| Image Guided Radiation Therapy | ||
| Yes | 32 | 34.0 |
| Cone beam computed tomography | 23 | 24.5 |
| kV | 9 | 9.6 |
| No | 62 | 66.0 |
| Diabetes Mellitus | ||
| Yes | 23 | 24.5 |
| No | 69 | 73.4 |
| Not available | 2 | 2.1 |
| Hypertension | ||
| Yes | 36 | 38.3 |
| No | 56 | 59.6 |
| Not available | 2 | 2.1 |
| Chemotherapy | ||
| Any | 37 | 39.4 |
| Cisplatin | 17 | 18.1 |
| Capecitabine/5-fluorouracil | 12 | 12.8 |
| Other | 8 | 8.5 |
| Concurrent | 29 | 30.9 |
| Cisplatin | 14 | 14.9 |
| Capecitabine/5-fluorouracil | 9 | 9.6 |
| Other | 6 | 6.4 |
| None | 57 | 60.6 |
|
Androgen Deprivation Therapy (Male Patients Only) |
||
| Any | 34 | 36.2 |
| Concurrent | 32 | 34.0 |
| None | 29 | 30.1 |
| Previous Gastrointestinal Conditions | ||
| Yes | 35 | 37.2 |
| No | 57 | 60.6 |
| Not available | 2 | 2.1 |
| Previous Abdominal Surgery | ||
| Yes | 37 | 39.4 |
| No | 55 | 58.5 |
| Not available | 2 | 2.1 |
| Vascular Disease | ||
| Yes | 16 | 17.0 |
| No | 76 | 80.9 |
| Not available | 2 | 2.1 |
| Constructed Dose-Volume Histogram Groups | ||
| V80 >0 cc | 2 | 2.2a |
| V75 >1 cc | 5 | 5.4a |
| V75 >0 cc | 12 | 13.0a |
| V70 >1 cc | 13 | 14.1a |
| V70 >0 cc | 22 | 23.9a |
| V65 >1 cc | 33 | 35.9a |
| V65 >0 cc | 50 | 54.3a |
| V60 >1 cc | 49 | 53.3a |
| V60 >0 cc | 65 | 70.7a |
These percentages were calculated using 92 in the denominator because 2 patients received multiple sets of radiation therapy and their dose-volume histograms could not be appropriately summed together.
Results
Patient characteristics and treatment factors are summarized in Table 1. DVH statistics are summarized in Table 2. Of the 92 patients who received 1 set of abdominopelvic IMRT treatment, 2 patients (2.2%) received point doses to the small bowel of >80 Gy; 22 (23.9%) received point doses to the small bowel >70 Gy.
Table 2.
Small bowel dose-volume histogram statistics summary
| Dose-volume histogram statistic | n | Minimum | Median | Maximum |
|---|---|---|---|---|
| Dmax, cGy | 92 | 4530.1 | 6546.5 | 8142.2 |
| Dmina, cGy | 92 | 51.6 | 346.0 | 1531.1 |
| Dmeana, cGy | 92 | 890.5 | 2622.2 | 4486.1 |
| Total Small Bowel Volume Contoured, cc | 92 | 7.8 | 145.7 | 1554.7 |
| V80, cc | 91 | 0.0 | 0.0 | 0.1 |
| V75, cc | 91 | 0.0 | 0.0 | 22.2 |
| V70, cc | 91 | 0.0 | 0.0 | 37.5 |
| V65, cc | 91 | 0.0 | 0.001 | 49.9 |
| V60, cc | 91 | 0.0 | 1.8 | 62.1 |
| V55, cc | 91 | 0.0 | 5.0 | 101.3 |
| V50, cc | 91 | 0.0 | 11.4 | 127.8 |
| V45, cc | 91 | 0.001 | 19.3 | 231.9 |
| V40, cc | 91 | 0.006 | 26.7 | 430.6 |
| V35, cc | 91 | 0.03 | 39.3 | 518.0 |
| V30, cc | 91 | 0.09 | 52.1 | 691.0 |
| V25, cc | 91 | 0.2 | 66.0 | 899.0 |
| V20, cc | 91 | 0.3 | 81.3 | 1231.2 |
| V15, cc | 91 | 0.6 | 95.8 | 1379.4 |
| V10, cc | 91 | 1.6 | 112.1 | 1419.8 |
These are minimum and mean doses to the small bowel that was contoured.
Overall, 17 patients (18.1%) experienced at least grade 1 late small bowel toxicity. Five patients (5.3%) experienced at least grade 2 late small bowel toxicity. Of the cases with at least grade 2 toxicity, 2 patients (2.1%) experienced grade 4 late toxicity with small bowel obstruction. The small bowel obstruction in one of these patients was in the high-dose area, but we could not assess the site of obstruction in the other patient because the images were not retrievable. One other patient (1.1%) experienced grade 3 stool incontinence. As previously mentioned, it was unclear if the stool incontinence was a small bowel complication, but we elected to be conservative and assumed that any stool incontinence was a consequence of radiation to the small bowel. If these stool incontinence cases were not small bowel complications, then our small bowel toxicity rates would be even lower than reported.
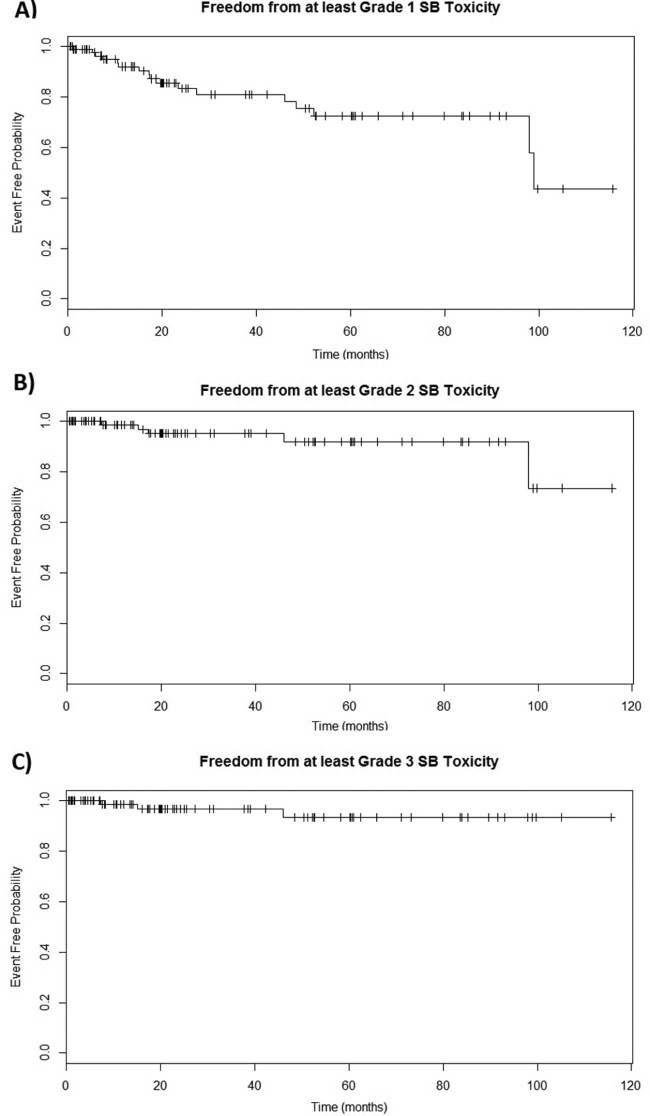
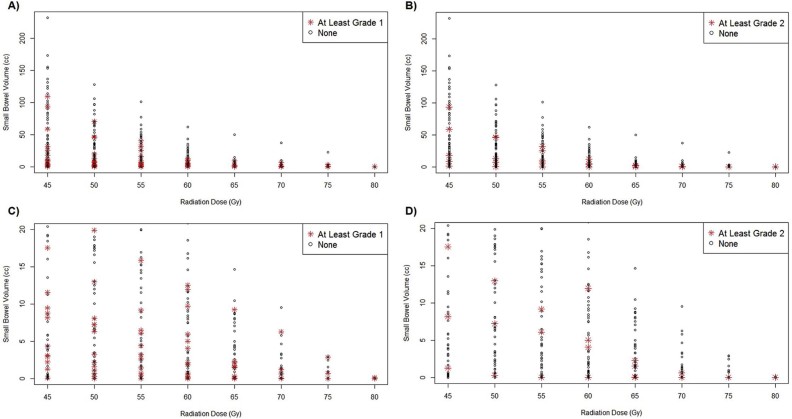
Relevant patient characteristics, treatment factors, and DVH statistics for the 3 patients who experienced at least grade 3 late small bowel toxicity are summarized in Table 3. Kaplan-Meier estimates of freedom and 95% confidence intervals (CIs) from at least grade 1, at least grade 2, and at least grade 3 late small bowel toxicity at 5 years were 72.4% (95% CI, 60.7%-86.5%), 91.9% (95% CI, 84.1%-100%), and 93.6% (95% CI, 86.2%-100%), respectively (Fig 1). Scatter plot of dose vs at least grade 1 and at least grade 2 toxicity is displayed in Fig 2.
Table 3.
Summary of patients with at least grade 3 late small bowel toxicity
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age, y | 53 | 72 | 87 |
| Treatment Setting | Postoperative | Primary treatment | Local recurrence |
| Malignancy | Prostate | Pancreas | Rectum |
| Prescribed IMRT Dose, cGy | 6480 | 5400 | 6300 |
| Drug Treatment | Prior 5-FU + cisplatin for esophageal cancer; leuprolide before prostate IMRT | Prior gemcitabine treatment; concurrent 5-FU treatment | Concurrent 5-FU treatment |
| Time of Complication after End of Radiation Therapy, mo | 15.1 | 7.3 | 46.0 |
| Complication Details | Grade 3 stool incontinence | Grade 4 small bowel obstruction + resection | Grade 4 small bowel obstruction |
| Medical History | Hypercholesterolemia; past esophageal cancer with IMRT treatment | Diarrhea; past breast cancer with IMRT treatment | GERD; aortic valve disease; HTN |
| Prior Surgeries | Radical prostatectomy; esophagectomy | None | Abdominal perineal resection |
| Dmax, cGy | 6813.3 | 5928.9 | 6548.9 |
| Dmeana, cGy | 3354.9 | 3193.4 | 3286.8 |
| V80, cc | 0.0 | 0.0 | 0.0 |
| V75, cc | 0.0 | 0.0 | 0.0 |
| V70, cc | 0.0 | 0.0 | 0.0 |
| V65, cc | 1.5 | 0.0 | 0.05 |
| V60, cc | 5.0 | 0.0 | 12.0 |
| V55, cc | 9.2 | 31.9 | 25.3 |
| V50, cc | 13.0 | 46.4 | 45.8 |
| V45, cc | 17.6 | 58.5 | 93.4 |
5-FU, 5-fluorouracil; IMRT, intensity modulated radiation therapy; GERD, gastroesophageal reflux disease; HTN, hypertension.
This is the mean dose to the small bowel that was contoured.
Figure 1.
Kaplan-Meier plots for freedom from (A) at least grade 1, (B) at least grade 2, and (C) at least grade 3 late small bowel toxicity. Tick marks denote censored observations.
Figure 2.
Volume of small bowel receiving 45 to 80 Gy in 5-Gy intervals. Red asterisks denote patients who experienced (A) at least grade 1 late small bowel toxicity and (B) at least grade 2 late small bowel toxicity. Magnified plots of small bowel volume up to 20 cc of the same dose range with patients who experienced (C) at least grade 1 late small bowel toxicity and (D) at least grade 2 small bowel toxicity marked similarly.
Log-rank tests for toxicity of all clinical variables, treatment factors, and dosimetric divisions showed only 1 statistically significant association (Table 4). Specifically, capecitabine/5-fluorouracil (5-FU) treatment (concurrent or sequential) was a significant predictor for at least grade 1 and at least grade 2 toxicity (Table 4), with 4 of 12 patients experiencing any toxicity, 3 of whom had higher than grade 2 toxicity. One of these patients developed grade 3 stool incontinence and had received 5-FU treatment for an esophageal malignancy within 6 months of initiating abdominopelvic radiation therapy (Table 3). Even after excluding this patient, capecitabine/5-FU treatment (concurrent or sequential) was still significant for at least grade 1 and at least grade 2 toxicity (P < .001 for both, even when excluding this patient). However, cisplatin treatment or the use of any chemotherapy (concurrent or sequential) were not significant (Table 4). Three of 12 patients who received capecitabine/5-FU treatment experienced at least grade 2 toxicity, versus only 1 of 16 patients who received cisplatin who experienced at least grade 2 toxicity.
Table 4.
Log-rank test for small bowel toxicity on groups
| Predictor | Grade ≥1 (P-value) |
Grade ≥2 (P-value) |
|---|---|---|
| Capecitabine/5-FU | < .001 | < .001 |
| Cisplatin | .808 | .4 |
| Any Chemotherapy | .191 | .00589 |
| Dmax >median | .789 | .47 |
| Dmina >median | .681 | .878 |
| Dmeana >median | .13 | .0866 |
| V15 >120 cc | .784 | .447 |
| V60 >10 cc | .511 | .85 |
| V70 >5 cc | .289 | .787 |
| V10 >median | .844 | .484 |
| V15 >median | .774 | .447 |
| V20 >median | .78 | .447 |
| V25 >median | .819 | .457 |
| V30 >median | .814 | .457 |
| V35 >median | .827 | .457 |
| V40 >median | .961 | .522 |
| V45 >median | .94 | .518 |
| V50 >median | .991 | .0929 |
| V55 >median | .31 | .0183 |
| V60 >median | .935 | .458 |
| V80 >0 cc | .257 | .767 |
| V75 >1 cc | .699 | .334 |
| V75 >0 cc | .681 | .672 |
| V70 >1 cc | .904 | .697 |
| V70 >0 cc | .873 | .394 |
| V65 >1 cc | .627 | .692 |
| V65 >0 cc | .663 | .74 |
| V60 >1 cc | .193 | .986 |
| V60 >0 cc | .898 | .649 |
These are minimum and mean doses to the small bowel that was contoured.
Notably, none of our dosimetric comparisons were statistically significant, including the groups for V15 >120 cc and for our internally developed guidelines. However, some of the variables, including the use of any chemotherapy, were close to our significance threshold. The log-rank test of any chemotherapy for higher than grade 2 toxicity had a P-value of .00589, but this likely was driven by the capecitabine patients (Table 4). Of the dosimetric variables, only the V55 median group came relatively close to our Bonferroni significance threshold.
A separate subset analysis was conducted for patients who did or did not receive capecitabine/5-FU treatment. These tests also showed no significant associations between the median DVH groups and small bowel toxicity.
Discussion
We found that despite delivering doses to the small bowel that were above the generally accepted maximum of 45 Gy, our patients experienced low rates of toxicity. Although past studies have quantified a relationship between acute small bowel toxicity and certain DVH parameters, we did not find that relationship with late small bowel toxicity in our overall patient cohort. Other studies that have explored late small bowel toxicity have had large inconsistencies with fractionation schedule and the type of radiation therapy used.7, 8, 9, 10
A sizeable number of patients received point doses of >70 Gy (n = 22), warranting an examination of the variables at least through V70 in our dataset. The insignificance of V60, V65, and V70 in the log-rank tests for the constructed groups (Table 1) is especially worth noting. These results suggest that it may be possible to deliver these doses without high rates of significant complications to the small bowel. Similarly, Green et al found a low incidence of grade 3 + acute or chronic small bowel toxicity when using IMRT or VMAT techniques in a cohort of patients with prostate cancer, 25 of whom received doses of >52 Gy to the small bowel with no incidence of late toxicity.13 Although it may be desirable to limit the maximum small bowel dose to <45 Gy if possible, it may be safe to increase the dose above this threshold without significant additional complications if treatment of the underlying malignancy would benefit. In particular, limiting the volume that receives 60 Gy to 10 cc and the volume that receives 70 Gy to 5 cc, as we have tried with most patients, may be appropriate in clinical situations that warrant aggressive treatment. Our results suggest that it may even be reasonable to raise these thresholds in certain situations.
Additionally, we observed that the 3 patients who experienced grade 3 or higher complications all received 5-FU treatment and had a prior gastrointestinal condition. As mentioned previously, 1 of these patients received 5-FU within 6 months of abdominopelvic radiation therapy for a different malignancy, but capecitabine/5-FU treatment remained significantly associated with at least grade 2 toxicity even when this patient was excluded. Past studies have also supported the potential need for clinicians to consider a wider variety of clinical variables and treatment factors when planning pelvic IMRT treatment.14 However, statistical analyses are greatly limited without a larger number of patients with these severe complications and unique medical histories.
It must also be noted that the retrospective nature of this study, with treatment by a single investigator (RDE) and a small number of grade 2 or higher complications, makes it difficult to draw definitive conclusions. The large 10-year range in which patients were considered, inconsistent periods between follow-up visits, patients who were lost to follow-up, and the inhomogeneity of our patient cohort further complicate any potential conclusions. The use of physician-reported outcomes, which are routinely lower than patient-reported outcomes, particularly for lower-grade toxicities, is also a limitation.
Future prospective studies from other investigators should consider including patient-reported outcomes and ideally should be conducted with larger cohorts using standard fractionation, uniform use of IMRT and image guidance, and a defined long-term follow-up schedule. These prospective studies are necessary to eliminate potential confounding variables and correlations between different factors in a retrospective study that may have biased our results. These studies are important to more accurately define the dose-volume relationship of small bowel toxicity and interactions with clinical variables and to establish appropriate thresholds for elevating radiation dosage in severe cases. Additionally, although many of our treatment plans show maximum small bowel doses well above 60 or 70 Gy, the true maximum small bowel dose may be substantially lower than reported in the plan due to the mobility of most of the small bowel. Patients who have fixed loops of small bowel within the high-dose region may be more susceptible to high levels of small bowel injury.
Finally, it must be noted that various genetic factors are likely to underlie individual patient risk of late radiation therapy toxicity. Variants in genes associated with DNA repair pathways, cell cycle arrest, and immune response have been thought to possibly increase the radiosensitivity of certain cells that are exposed during treatment.15 With the formation of a radiogenomics consortium, genome-wide association studies are becoming increasingly important in identifying single nucleotide polymorphisms that may contribute to the increased risk of radiation toxicity.16 Although replication of results and false positives remain large problems, recent analyses with increased power have found greater success in identifying single nucleotide polymorphism associations.15, 17 One recent study identified an additional risk locus for radiation toxicity compared with an earlier genome-wide association study of patients with prostate cancer, which highlights the need for more collaborative efforts.18 Further investigation into any differences in dose tolerance, specifically in patients receiving high maximal doses to the small bowel, should take these genetic risk factors into consideration. This type of prospective study remains extremely important in accurately evaluating the risk-reward tradeoff for patients whose underlying malignancies warrant aggressive pelvic or abdominal radiation treatment.
Conclusion
Our retrospective, hypothesis-generating data suggest that small volumes of small bowel can be treated above 45 to 50 Gy with acceptable long-term toxicity risk, but extra caution is advised in the setting of capecitabine/5-FU. The widespread acceptance of 45 to 50 Gy as the maximal dose to the small bowel may not be warranted and indeed might impede optimal patient care in situations in which a higher dose might provide improved tumor control. Additional research is needed, ideally with large numbers of patients to account for confounding factors and with prospectively collected data to minimize selection bias, to better determine the optimal dose-volume constraints for long-term small bowel toxicity.
Acknowledgments
The authors thank Frieda Trichter DSc, Rosemary Giuliano MSN, Omar Morales, Ira Leykin, and Anissa Muller for their assistance with data collection.
Footnotes
Conflicts of interest: None.
References
- 1.Emami B., Lyman J., Brown A. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 2.Robertson J.M., Lockman D., Yan D., Wallace M. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:413–418. doi: 10.1016/j.ijrobp.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 3.Baglan K.L., Frazier R.C., Yan D., Huang R.R., Martinez A.A., Robertson J.M. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176–183. doi: 10.1016/s0360-3016(01)01820-x. [DOI] [PubMed] [Google Scholar]
- 4.Roeske J.C., Bonta D., Mell L.K., Lujan A.E., Mundt A.J. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69:201–207. doi: 10.1016/j.radonc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Huang E.Y., Sung C.C., Ko S.F., Wang C.J., Yang K.D. The different volume effects of small-bowel toxicity during pelvic irradiation between gynecologic patients with and without abdominal surgery: A prospective study with computed tomography-based dosimetry. Int J Radiat Oncol Biol Phys. 2007;69:732–739. doi: 10.1016/j.ijrobp.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Kavanagh B.D., Pan C.C., Dawson L.A. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:101–107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 7.Nuyttens J.J., Prevost J.B., Van der Voort van Ziip N.C., Hoogeman M., Levendag P.C. Curative stereotactic robotic radiotherapy treatment for extracranial, extrapulmonary, extrahepatic, and extraspinal tumors: Technique, early results, and toxicity. Technol Cancer Res Treat. 2007;6:605–610. doi: 10.1177/153303460700600603. [DOI] [PubMed] [Google Scholar]
- 8.Kelly P., Das P., Pinnix C.C. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:143–149. doi: 10.1016/j.ijrobp.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Schneider R.A., Vitolo V., Albertini F. Small bowel toxicity after high dose spot scanning-based proton beam therapy for paraspinal/retroperitoneal neoplasms. Strahlenther Onkol. 2013;189:1020–1025. doi: 10.1007/s00066-013-0432-0. [DOI] [PubMed] [Google Scholar]
- 10.Sprawka A., Pietrzak L., Garmol D., Tyc-Szczepaniak D., Kepka L., Bujko K. Definitive radical external beam radiotherapy for rectal cancer: Evaluation of local effectiveness and risk of late small bowel damage. Acta Oncol. 2013;52:816–823. doi: 10.3109/0284186X.2012.707786. [DOI] [PubMed] [Google Scholar]
- 11.Isohashi F., Mabuchi S., Akino Y. Dose-volume analysis of predictors for chronic gastrointestinal complications in patients with cervical cancer treated with postoperative concurrent and whole-pelvic radiation therapy. J Radiat Res. 2016;57:668–676. doi: 10.1093/jrr/rrw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M.J., Kirk M., Zhai H., Lin L.L. Bag and loop small bowel contouring strategies differentially estimate small bowel dose for post-hysterectomy women receiving pencil beam scanning proton therapy. Acta Oncologia. 2016;55:900–908. doi: 10.3109/0284186X.2016.1142114. [DOI] [PubMed] [Google Scholar]
- 13.Green G., Williams R., Zhang J., Azawi S. Dose-volume relationship of acute and late small bowel toxicity from radiation therapy for prostate cancer: A veteran affairs study. Int J Radiat Oncol Biol Phys. 2014;90:S456. [Google Scholar]
- 14.Kuku S., Fragkos C., McCormack M., Forbes A. Radiation-induced bowel injury: The impact of radiotherapy on survivorship after treatment for gynaecological cancers. Br J Cancer. 2013;109:1504–1512. doi: 10.1038/bjc.2013.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West C.M., Bartnett G.C. Genetics and genomics of radiotherapy toxicity: Towards prediction. Genome Med. 2011;3:52. doi: 10.1186/gm268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West C., Rosenstein B.S. Establishment of a radiogenomics consortium. Int J Radiat Oncol Biol Phys. 2010;76:1295–1296. doi: 10.1016/j.ijrobp.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Bartnett G.C., Coles C.E., Elliot R.M. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: A prospective analysis study. Lancet Oncol. 2012;13:65–77. doi: 10.1016/S1470-2045(11)70302-3. [DOI] [PubMed] [Google Scholar]
- 18.Kerns S.L., Dorling L., Fachal L. Meta-analysis of genome wide association studies identifies genetic markers of late toxicity following radiotherapy for prostate cancer. EBioMedicine. 2016;10:150–163. doi: 10.1016/j.ebiom.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]