Abstract
Background: Dietary essential omega-6 (n-6) and omega-3 (n-3) 18 carbon (18C-) polyunsaturated fatty acids (PUFA), linoleic acid (LA) and α-linolenic acid (ALA), can be converted (utilizing desaturase and elongase enzymes encoded by FADS and ELOVL genes) to biologically-active long chain (LC; >20)-PUFAs by numerous cells and tissues. These n-6 and n-3 LC-PUFAs and their metabolites (ex, eicosanoids and endocannabinoids) play critical signaling and structural roles in almost all physiologic and pathophysiologic processes. Methods: This review summarizes: (1) the biosynthesis, metabolism and roles of LC-PUFAs; (2) the potential impact of rapidly altering the intake of dietary LA and ALA; (3) the genetics and evolution of LC-PUFA biosynthesis; (4) Gene–diet interactions that may lead to excess levels of n-6 LC-PUFAs and deficiencies of n-3 LC-PUFAs; and (5) opportunities for precision nutrition approaches to personalize n-3 LC-PUFA supplementation for individuals and populations. Conclusions: The rapid nature of transitions in 18C-PUFA exposure together with the genetic variation in the LC-PUFA biosynthetic pathway found in different populations make mal-adaptations a likely outcome of our current nutritional environment. Understanding this genetic variation in the context of 18C-PUFA dietary exposure should enable the development of individualized n-3 LC-PUFA supplementation regimens to prevent and manage human disease.
Keywords: omega-3 fatty acids, polyunsaturated fatty acids, gene-diet interaction, human disease, inflammation, fatty acid desaturase genes, arachidonic acid, eicosanoids, endocannabinoids
1. Introduction
Modern humans emerged from Africa ~200,000 years ago and spread across the earth over the past 100,000 years. During this time, available food sources created evolutionary pressure that drove genetic architecture which allowed our species to adapt, survive and proliferate; this was coupled with a rapid expansion in brain size (particularly gray matter in the cerebral cortex) and, with it, advancements in human intelligence, socialization, and innovation.
The modern Western diet (MWD) has dramatically changed the nutritional content of ingested foods in developed countries, and given the rapid nature of these nutritional transitions, mal-adaptations and related human diseases are a likely outcome of our current nutritional environment [1,2]. For example, up to 72% of dietary calories consumed presently in the MWD did not exist in hunter-gatherer diets [3]. Changes in food type (quality) and quantity in the MWD have been largely driven by technological changes in food production and processing to provide high-calorie and appealing food (high in sugars, refined grains and oils) to large urban populations [1,4]. These have led to detrimental shifts in nutrient metabolism leading to gene-diet interactions responsible for more obesity and localized and systemic inflammation [2]. In turn, this inflammation contributes to the pathogenesis of a variety of disease states, including cardiovascular disease, diabetes and insulin resistance, cancer, autoimmunity, hypersensitivity disorders such as asthma and allergies, chronic joint disease, skin and digestive disorders, dementia and Alzheimer’s disease [5,6,7,8,9,10,11,12,13,14]. As challenging as these changes are for overall populations of developed countries such as the US, they are more negative for certain populations and ethnic groups [15,16,17,18,19,20], in whom a disproportionate burden of preventable disease, death, and disability now exists. However, the emergence of the field of precision nutrition that factors in individual- and population-based genetic variability in the context of human diets offers the promise to provide more specific and individualized dietary and supplement interventions that may prevent and mitigate many of the pro-inflammatory effects of the MWD [21].
With regard to fatty acid (FA) intake, there has been marked shift (due largely to recommendations from health agencies) to reduce levels of saturated fatty acids and replace them with polyunsaturated fatty acids (PUFAs) in an attempt to lower serum total cholesterol and LDL lipoproteins [22,23]. From a practical perspective, this meant a replacement of sources of saturated fat such as lard and butter with PUFA-containing vegetable oils (soybean, corn, and canola oils, as well as margarine and shortenings), which are rich in the 18 carbon (18C) omega-6 (n-6) PUFA linoleic acid (18:2n-6, LA) and poor in both the omega-3 (n-3) 18C-PUFA, α-linolenic acid (18:3n-3, ALA) and monounsaturated fatty acids. In fact, it has been estimated that soybean oil consumption alone (which contains 58 g LA/100 g oil) increased >1000-fold from 1909 to 1999 and now contributes to ~7% of daily energy of the MWD [1]. Over time, this progressive increase in the ingestion of vegetable oils has led to a 3-fold increase (to 6–8% energy) in dietary LA content of the MWD [1,3,24,25,26], as well as an estimated 40% reduction in total n-3 long chain (≥20 carbon; LC-) PUFA levels, and a large shift in the ratio of dietary n-6/n-3 C18 PUFAs consumed from ~5:1 to >10:1 [1,27].
The objectives of this review are first to point out how lifestyle variables and specifically our current dietary PUFA exposure together with ancestral-based genetic variation in the LC-PUFA biosynthetic pathway gives rise to distinct molecular profiles (levels of LC-PUFAs, LC-PUFA metabolites, inflammatory and other disease biomarkers) that enhance disease risk for certain individuals and populations. These gene-diet interactions may be particularly important to health in western countries as dietary n-6 and n-3 18C PUFAs comprise almost 10% of daily calories in the MWD. The second objective of the review is to describe how an understanding of PUFA-based gene-diet interactions can provide a scientific basis for the development of specific dietary and supplement strategies with n-3 LC-PUFAs to prevent and manage human diseases.
2. Long Chain Polyunsaturated Fatty Acid Biosynthesis and Biological Activities
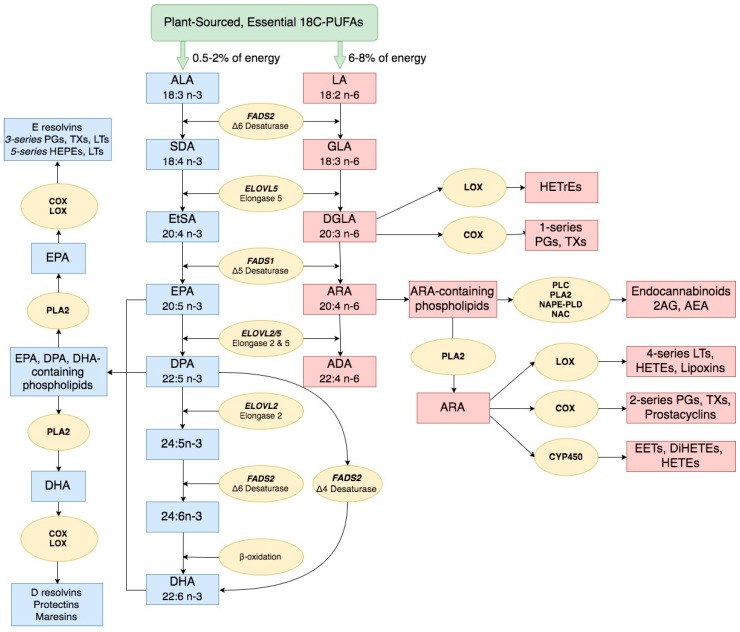
From the work of George and Mildred Burr almost 100 years ago [28,29], which was extended by the studies of Ralph Holman [30,31], it became clear that n-3 and n-6 18C-PUFAs were essential for human health. Furthermore, these 18C-PUFAs originated from the diet and were not synthesized from acetyl and malonyl CoA ester condensations catalyzed by fatty acid synthase. The two essential dietary PUFAs of shortest (18C) chain length, ALA and LA, are the key substrates that enter the biosynthetic pathways leading to biologically-active n-3 and n-6 LC-PUFAs, respectively. Figure 1 highlights the LC-PUFA biosynthetic pathway and genes known to encode for enzymes that play key roles in the two parallel and competing pathways that synthesize n-3 and n-6 LC-PUFAs. Two desaturation enzymes encoded by fatty acid desaturase 1 and 2 (FADS1 and FADS2) and one elongation enzyme encoded by ELOVL5 synthesize eicosapentaenoic acid (20:5n-3, EPA) and arachidonic acid (20:4n-6, ARA) from ALA and LA, respectively [32,33,34,35,36]. The n-3 LC-PUFA, docosapentaenoic acid (22:5n-3; DPA) and the n-6 LC-PUFA, adrenic acid (22:4n-6; ADA) can be generated from EPA and ARA, respectively, using an additional elongation enzyme (encoded by ELVOL 5/2), and finally docosahexaenoic acid (22:6n-3; DHA) can be produced from DPA with a ∆-4 desaturation enzyme also encoded by FADS2 [37]. EPA may also be converted to DHA utilizing three additional biosynthetic steps (2 elongation, 1 desaturation and 1 β-oxidation). Smaller quantities of LC-PUFAs can be obtained directly from the diet. For example, preformed ARA is found in organ meats, eggs, poultry, and fish, and various types of seafood such as cold-water fish are rich in preformed n-3 LC-PUFAs, EPA, DPA and DHA [26].
Figure 1.
Polyunsaturated fatty acid biosynthesis. n-3 and n-6 LC-PUFA are synthesized from dietary intake of essential fatty acids ALA and LA, respectively, through a series of enzymatic desaturation (FADS2 and FADS1) and elongation (ELOVL2 and ELOVL5) steps. This pathway gives rise to primary n-3 LC-PUFAs and n-6 LC-PUFAs such as EPA, DPA, DHA and ARA. These LC-PUFAs (as free fatty acids or complex lipids) and their metabolites impact a wide ranges of physiologic and pathophysiologic processes. Abbreviations: FADS1/2, fatty acid desaturase 1/2; ELOVL 2/5, fatty acid elongase 2/5; ALA, α-linolenic acid; SDA, stearidonic acid; EtSA, eicosatetraenoic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; LA, linoleic acid; GLA, γ-linolenic acid; DGLA, dihomo-γ-linolenic acid; ARA, arachidonic acid; ADA, adrenic acid; PG, prostaglandin; TX, thromboxane; LT, leukotriene; HEPE, hydroxyeicosapentaenoic acid; HETrE, hydroxyeicosatrienoic acid, HETE, hydroxyeicosatetraenoic acid; DiHETE, dihydroxyeicosatetraenoic acid; EET, epoxyeicosatetraenoic acid; 2AG, 2-arachidonoylglycerol; AEA, arachidonoyl ethanolamide/anandamide.
Once formed, LC-PUFAs have many roles as free fatty acids and esterified in complex lipids (Figure 1). These include biophysical properties essential for proper plasma membrane function, energy production by β-oxidation and specific biochemical roles as precursors of bioactive lipids [38,39]. For example, in the central nervous system, the n-3 LC-PUFA DHA is the most abundant FA in complex lipids constituting approximately 50% of the weight of neuronal plasma membranes. Its membrane status and signaling capacity directly impact brain development and function via several mechanisms, including maintaining membrane integrity, neurotransmission, neurogenesis, membrane receptor function and signal transduction [38,40,41,42,43,44].
Importantly, both n-6 and n-3 LC-PUFAs are also converted to a diverse family of metabolites including multiple forms of prostaglandins, thromboxanes, hydroxyeicosatetraenoic acids, epoxyeicosatrienoic acids, leukotrienes, lipoxins, resolvins, protectins, maresins and endocannabinoids (Figure 1) [45,46,47,48,49,50,51,52]. LC-PUFAs and their metabolites, along with their cellular receptors, are present in practically all cells and tissues of the body and act as potent signaling molecules that impact a wide range of physiologic and pathophysiologic processes [45,46,47,48,49,50,51,52].
Most evidence to date indicates that n-6 and n-3 LC-PUFAs and their metabolic products have not only different, but often opposing effects on immunity and inflammation [53,54,55,56,57]. In general, n-6 LC-PUFA metabolites and particularly ARA act as local hormones to promote acute and chronic inflammation [46,51,52]. In contrast to ARA, n-3 LC-PUFAs, such as EPA, DPA, and DHA, can be metabolized to anti-inflammatory mediators that have “pro-resolution” properties [47,58]. An exception to this principle are the ARA-derived lipoxins that exert anti-inflammatory, pro-resolution bioactions [59].
Over the past 20 years, one of the most fascinating areas of science has been the discovery of the pleiotropic effects of the endocannabinoid system [60,61,62]. Endocannabinoids have been shown to be complex lipids (such as 2-arachidonoyl glycerol and arachidonyl ethanolamide) derived from the n-6 LC-PUFA, ARA [60,61,62]. More recent studies have demonstrated that n-3 LC-PUFA derivatives of endocannabinoids also exist [63,64,65]. Endocannabinoid action via cannabinoid 1 and 2 receptors impacts a wide range of biological functions including energy balance and metabolism, mood, memory, sleep, reproduction, thermoregulation and immune function [63,64,65,66,67,68,69,70,71,72,73,74,75]. Endocannabinoids can also be metabolized by cyclooxygenases, lipoygenases, and p450 epoxygenases to form other biologically-active complex lipids [65,76,77].
It is clear from the aforementioned studies that n-6 and n-3 LC-PUFAs and their metabolites have structural and/or signaling roles throughout the human body (Figure 1). Additionally, maintaining a proper balance of n-6 and n-3 LC-PUFAs and their metabolites is critical to homeostasis in virtually every physiologic system. Consequently, environmental and genetic mechanisms that influence their levels and balance will impact human health and disease.
3. Impact of Dietary Linoleic Acid and α-Linolenic Acid Levels on n-3 LC-PUFA Biosynthesis
Several studies have warned against health and disease outcomes that could result from radical increases of dietary LA in the MWD in such a short period of time [26,78,79]. Given the shared enzymatic steps involved in the processing of LA and ALA, these n-6 and n-3 18C-PUFAs and their metabolic intermediates compete with each other in the liver and other tissues as substrates for synthetic enzymatic reactions that produce LC-PUFAs [80,81]. Additionally, there is an overall limited capacity of 18C-PUFAs that can be converted to LC-PUFAs [53]. As discussed in detail below, this biosynthetic limit in capacity is highly impacted at an individual level by genetic variation in the LC-PUFA biosynthetic pathway. Consequently, a dramatic increase in LA in the MWD observed over the past 75 years together with competition between n-6 and n-3 substrates within the pathway has been shown in animal models and humans to shift the pathway toward the biosynthesis of high levels n-6 LC-PUFAs and away from n-3 LC-PUFAs [53,82,83,84,85,86,87]. In 1992, Lands and colleagues described non-linear interactions between LA and ALA in forming LC-PUFAs utilizing a hyperbolic equation that fit for rats, mice and humans [53]. The equation points out the limitation of generating n-3 LC-PUFAs when n-3 ALA is ingested together with several-fold greater amounts of n-6 LA as is the case with the MWD. Wood and colleagues reviewed human studies that examined the effect of altering LA and ALA on n-6 and n-3 LC-PUFA biosynthesis and concluded that it is possible to increase n-3 LC-PUFAs by reducing LA or increasing ALA intake in humans [82]. However, LA levels need to be reduced to <2.5% energy before levels of DHA can be increased. Again, typical LA levels in the MWD reside between 6–8% energy; consequently, high levels of LA in the MWD would be predicted to markedly reduce, not increase DHA. In fact, it has been estimated that LA concentrations in the MWD have decreased the omega-3 index by 41%, from 6.51 to 3.84 [1].
A 1997 paper by Okuyama and colleagues made a compelling case that excess LA and the increase in the LA/ALA ratio as a result of moving away from traditional diets led to ‘Omega-3 Deficiency Syndrome’ in the elderly in Japan [84]. The paper summarized the “evidence which indicates that increased dietary LA and relative n-3 deficiency are major risk factors for western-type cancers, cardiovascular and cerebrovascular diseases and also for allergic hyper-reactivity.” They also suggest that n-3 LC-PUFAs deficiency created by excess LA and LA/ALA ratios in the MWD affects human behavior patterns in industrialized countries. Certainly these assertions are supported by a large body of scientific literature in both animal models and human studies discussed throughout this review.
4. The Genetics and Evolution of LC-PUFA Biosynthesis
Through a better understanding of genetic variation associated with the utilization of specific nutrients, precision nutrition approaches offer the potential to predict the physiological and pathological consequences of the interaction of individual genetic differences and diet to prevent and/or manage adverse outcomes. Until recently, it was assumed that the metabolic capacity of the LC-PUFA biosynthetic pathway was limited and fairly uniform in all humans. This premise was supported by metabolic studies in European ancestry populations, which suggest that only a small proportion of ingested dietary 18C PUFAs (typically 2–3% energy) are converted into LC-PUFAs [81,88,89,90]. However, studies over the past decade have demonstrated common genetic and epigenetic variation in genes (including FADS1, FADS2, ELOVL5 and ELOVL2) throughout the LC-PUFA biosynthetic pathway are highly associated with the levels of LC-PUFAs produced in human circulation, cells and tissues [91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116]. This body of evidence has challenged the concept that LC-PUFA biosynthesis from 18C-PUFAs is uniform among individuals and populations.
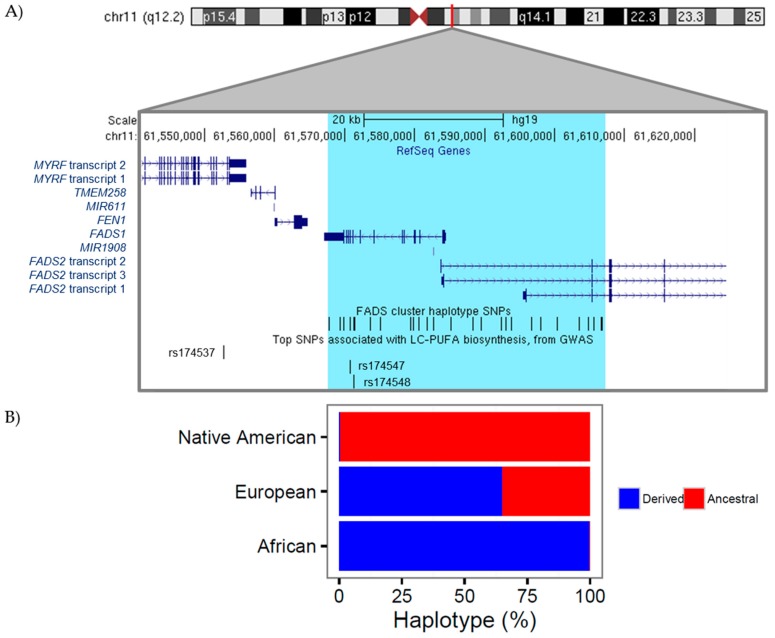
The desaturase enzymes within the pathway, encoded by the two genes (FADS1, FADS2) in the FADS cluster region (chr11: 61,540,615–61,664,170) have long been recognized as the rate-limiting steps in the conversion of 18C-PUFAs to LC-PUFA (Figure 1 and Figure 2). Our laboratory initially demonstrated that there are marked differences between African and European ancestry populations in the circulating levels of n-6 and n-3 LC-PUFAs [100,106]. This work also showed that ~80% of African Americans carry two copies of FADS alleles associated with more efficient biosynthesis of LC-PUFAs, compared to only ~45% of European Americans and these genetic differences explained a large proportion of the variability in LC-PUFA levels between African and European Americans. Numerous other studies have also revealed strong genetic influences within the FADS cluster region on circulating, cellular and tissue levels of LC-PUFAs [2,36,117]. This region of association comprises a linkage disequilibrium (LD) block covering the promoter regions of both FADS1 and FADS2 (Figure 2). Importantly, the derived haplotype that includes numerous genetic variants with common allele frequencies (when compared to the ancestral haplotype) is associated with higher levels of LC-PUFAs and an increased efficiency (as determined by product to precursor ratios within the LC-PUFA biosynthetic pathway) by which LC-PUFAs are synthesized [117]. One of the most surprising aspects of these genetic studies is the observation that there are dramatic differences in the frequencies of the ancestral and derived haplotypes and thus the efficiency of LC-PUFA biosynthesis among diverse global populations. For example, the ancestral haplotype is most common (97%) in Native Americans and virtually absent in Africa, suggesting that Native Americans and individuals of Native American ancestry have a more limited capacity than Africans to synthesize LC-PUFAs [118]. The derived haplotype is observed at varying frequencies (25–50%) in Europe and East Asia [117,119]. As described in detail below, extensive evolution of the FADS cluster and thus changes in the efficiency of LC-PUFA biosynthesis took place as early humans adapted to local environments as they moved from Africa to the Americas. These are reflected in the dramatic differences in the FADS haplotype frequencies and LC-PUFA biosynthetic efficiencies observed in diverse modern populations.
Figure 2.
Genetic variation near the FADS gene cluster. (A) A expanded depiction of the FADS gene cluster on chromosome 11 illustrates the genomic location (build hg19) of: genes in this region (shown in dark blue, from RefSeq), the FADS cluster haplotype region and single nucleotide polymorphisms (SNPs) (region highlighted in light blue with SNPs shown as black vertical bars), and three individual SNPs identified as the most significantly associated genetic variants genome-wide with LC-PUFA levels (rs174537, rs174547, and rs174548); (B) The observed percentage of derived vs. ancestral FADS cluster haplotype varies by ethnicity.
Numerous individual genetic variants within the FADS cluster (as illustrated in Figure 2) have been identified by genome-wide association studies (GWAS) to be highly associated with LC-PUFA levels as well a wide variety of important molecular and clinical phenotypes. For instance, GWAS of plasma n-3 and n-6 LC-PUFA levels in Italian, European, and Chinese populations have identified single nucleotide polymorphisms (SNPs) such as rs174537 and rs174547 located near FADS1, which are the strongest signals genome-wide associating with levels of n-3 and n-6 LC-PUFAs such as ARA and EPA [94,101,120,121,122]. Genetic variants in the FADS cluster are also associated with numerous human phenotypes including inflammatory and cardiovascular disorders, blood lipid levels including low-density lipoprotein (LDL) and triglyceride levels, coronary artery disease, insulin resistance, perinatal depression, atopic diseases, attention/hyperactivity, intelligence and memory in children [36,92,96,99,102,105,109,111,116,123,124,125,126,127,128]. The strong associations between genetic variation in the FADS cluster, LC-PUFA levels, and important clinical phenotypes support the concept that genetically-induced alterations in LC-PUFA levels may play important roles in several human diseases. However, due to the high degree of LD observed between genetic variants in this region, it is difficult to determine which variants have a casual role in altering FADS activity thus the efficiency of LC-PUFA biosynthesis.
An important question from this work is: why are there such distinct ancestral-based genetic variation within the FADS cluster? It is important to understand that nutrients and genomes interact reciprocally. As described above, genetic variation confers differences in nutrient utilization. However, changes in nutrient exposure throughout human development also created adaptive pressures that led to selection of genetic variation that better fit nutritional environments. Our laboratory initially examined evolutionary forces shaping patterns of variation in the FADS cluster by examining geographically diverse populations representing 14 populations and focused on a 300 kb region centered on the FADS loci [119]. This work confirmed that there are marked global differences in allele frequencies of variants in the FADS gene cluster that are strongly associated with the efficiency of conversion of LA and ALA to ARA and DHA, respectively. This study also provided evidence that alleles associated with LC-PUFA biosynthesis were driven to near fixation in African populations by positive selection ~85,000 years ago. The selection of these FADS variants would have enhanced LC-PUFA synthesis from plant-sourced 18C-PUFAs. We postulate that this enhanced the capacity to synthesize LC-PUFAs and particularly n-3 LC-PUFAs such as DHA, and was therefore an important advantage that would have facilitated the movement of humans away from marine sources of LC-PUFAs in isolated geographic regions and concomitant rapid expansion and migration throughout the African continent 60,000–80,000 years ago. That same time, Ameur and colleagues described the FADS haplotype patterns (ancestral and derived), with the derived haplotype found in Africa, that were associated with more efficient conversion of 18C-PUFAs into LC-PUFAs [117].
However, it was unclear from these initial papers: (1) why human populations migrating out of Africa appeared to carry the ancestral haplotype; (2) why Eurasian populations are polymorphic; and (3) why the ancestral haplotype is at near fixation in Native American populations. Fumagalli and colleagues provided an important clue to this puzzle by carrying out a genome-wide scan for positive selection in the Greenlandic Inuit and showing that genetic variation in the FADS cluster to be the strongest signatures for cold adaptation [129]. Interestingly, the two most highly differentiated SNPs (rs7115739 and rs174570) were associated with lower levels of LC-PUFAs and higher levels of 18C-PUFAs precursors. It was also demonstrated that these FADS cluster alleles were associated with a decrease in weight, height, fasting serum insulin, and fasting serum LDL cholesterol [129]. These investigators posited that the challenging environmental conditions of the Arctic likely imposed strong selective pressures on the Inuit and their ancestors and these physical and molecular phenotypes were important to cold adaptation. However, the PUFA-related molecular mechanism responsible for physical phenotypes such as weight and height are not yet understood. Very recently, we have demonstrated that the ancestral haplotype frequency is also correlated to Siberian populations’ geographic location, further suggesting the ancestral haplotype’s role in cold weather adaptation [130]. Additionally, this likely explains the high haplotype frequency of the ancestral haplotype within Native American populations [118,130].
There are several other recently published papers which have focused on the role that evolution of the FADS cluster played in the capacity of humans to adapt to varying global nutritional environments containing fluctuating levels of LC-PUFAs. For example, Mathieson and colleague carried out a genome-wide scan for positive selection from ancient and present-day European genomes and demonstrated strong selection for derived alleles over the past 4000 years [131]. Kothapalli and colleagues studied genomes from populations in South Asia and showed positive selection for an indel in FADS2 that is associated with more efficient LC-PUFA biosynthesis [132]. They suggest that this was an important adaptation as populations moved to more vegetarian diets with low levels of dietary LC-PUFAs [132]. Buckley and colleagues compared FADS sequencing data from present day to the Bronze Age and concluded that selection patterns in Europeans were driven by changes in dietary fat composition and specifically LC-PUFA levels following the transition to agriculture [133]. Taken together, all of these studies reflect the evolutionary importance for humans to regulate LC-PUFA biosynthesis. The complex interactions among local selective pressures in diverse local environments along global human migration patterns appears to have given rise to the global variation in frequencies of the derived and ancestral haplotypes that are observed today.
5. Anatomy of n-6 LC-PUFA Excesses and n-3 LC-PUFA Deficiencies
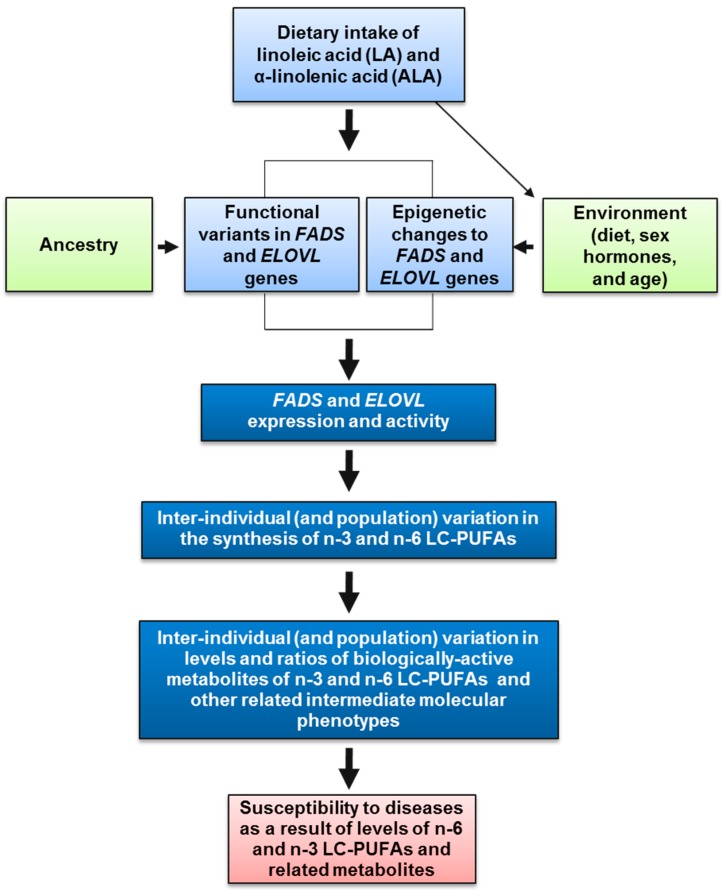
As outlined throughout this review, there are several components of the MWD and the diverse genetics of LC-PUFA biosynthesis among different human populations that could combine to create harmful gene-diet interactions, which in turn would impact levels of n-6 and n-3 LC-PUFAs, their metabolites and ultimately human disease (Figure 3). First, gene-diet interactions can arise when there is a major change in the exposure of a nutrient that is utilized by an important metabolic pathway. As discussed above, following recommendations to replace dietary saturated fatty acids with PUFAs, food production companies began replacing the saturated fatty acids, largely with n-6 18C-PUFAs, and this led to a dramatic increase (~3 fold) in the ingestion of LA. In contrast, the ingestion of the dietary n-3 18C-PUFA, ALA, has remained relatively constant [1]. This resulted in a significant change in not only LA exposure but also the ratio of dietary LA to ALA that enters the LC-PUFA biosynthetic pathway. As discussed above, LA and its n-6 metabolites directly compete with ALA and its n-3 metabolites in the synthesis of LC-PUFAs, and there is a limited capacity of the pathway to produce LC-PUFAs. Consequently, the ratio of LA to ALA has been altered by increasing dietary LA (to 6–8% of energy) and this dietary modification has shifted the pathway toward the biosynthesis of higher levels n-6 LC-PUFAs and away from n-3 LC-PUFAs.
Figure 3.
Anatomy of gene-diet interactions leading to n-6 LC-PUFA excesses and n-3 LC-PUFA deficiencies. Dietary intake of n-3 and n-6 18C-PUFAs, ALA and LA, respectively, interact with FADS or ELOVL genetic and epigenetic variation (that impacts FADS or ELOVL expression or resultant activity) to determine circulating and cellular levels of n-3 and n-6 LC-PUFAs. These interactions can result in an unhealthy balance of LC-PUFAs, with excess levels of n-6 LC-PUFAs or deficiencies of n-3 LC-PUFAs.
A second component of potentially harmful gene-diet interactions is exemplified in some individuals or human populations that have a greater genetic capacity to more efficiently utilize/metabolize a specific nutrient than others. A well-recognized example of this situation are the variants near the LCT locus that codes for the lactase enzyme, which metabolizes lactose in milk [134,135,136]. Cattle domestication ~10,000 years ago induced strong selection to be able to utilize lactose, the primary carbohydrate in milk, as adults. In most humans, levels of the lactase enzyme decreases after weaning, but certain populations that traditionally depended on milk have variants near the LTC locus associated with high levels of lactase and thus retain the capacity to utilize lactose into adulthood.
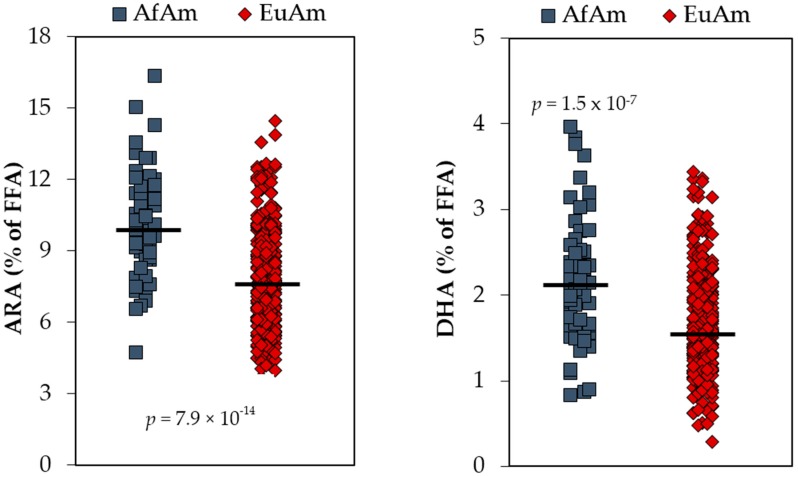
Similarly, studies over the past decade show that diverse global populations have differences in their capacity to utilize 18C-PUFAs to synthesize LC-PUFAs, and there is now strong evidence that common FADS variants form ancestral and derived haplotypes that account for these pathway efficiency differences. It has been recently proposed that the derived haplotype played a crucial role in human evolution under circumstances when dietary LC-PUFAs, especially dietary n-3 LC-PUFA levels, were low [119,131,132,133]. This included movement away from n-3 LC-PUFA-rich marine sources during the ‘great expansion’ in Africa 60,000–80,000 years ago and the adaptation to largely vegetarian diets after the development of agriculture in Europe and Asia ~12,000 years ago. Consequently, African, African ancestry and some south Asian populations have high frequencies of a derived haplotype that is associated with efficient LC-PUFA biosynthesis [117,119]. Additionally, the MWD provides very high levels of LA to this genetically more efficient pathway resulting in significantly higher levels of the n-6 LC-PUFA, ARA, when compared to most European or European ancestry populations [100,106]. Figure 4 illustrates this point by demonstrating that there are significant differences between circulating LC-PUFAs, ARA and DHA, in African and European American populations. A major question that arises from the data in Figure 4 is; what are the biological consequences and specifically the risk of human disease for individuals who are either at the upper (excess ARA levels) or lower (depressed DHA levels) end extremes?
Figure 4.
Serum Levels of ARA and DHA in African Americans (AfAm) and European Americans (EuAm). Both n-6 and n-3 LC-PUFAs (arachidonic acid, ARA; and docosahexaenoic acid, DHA) are elevated in serum from AfAm relative to EuAm from the same clinical diabetes study cohort [106].
Numerous studies have addressed the issue of excess ARA or efficient LA to ARA conversion in the context of cardiovascular disease (CVD). For example, Martinelli and colleagues [116] examined associations among 13 FADS genotypes, desaturase activity (as determined by ARA/LA ratios), inflammation (C-reactive protein (CRP)), and coronary artery disease (CAD) in 876 individuals with (n = 610) and without (n = 266) CAD. Individuals carrying certain haplotypes (the derived haplotype) had higher ARA/LA ratios in red blood cell membranes, corresponding to enhanced desaturase activity. Importantly, the ARA/LA ratio was an independent risk factor for CAD (Odds Ratio 2.55, p < 0.001), Furthermore, the pro-inflammatory marker, CRP increased progressively across tertiles of ARA/LA. This study provided a powerful example of how variants in the FADS cluster alter molecular phenotypes, which in turn alter disease risk. Li and colleagues also examined the association of FADS genotypes and plasma fatty acids in control (n = 510) and CAD patients (n = 505) from a Chinese Han population [111]. They also showed that the ARA/LA ratio was higher in CAD patients and the low pathway efficiency T allele at rs174537 was associated with a lower risk of CAD (Odds Ratio 0.74, p = 0.001). Similarly, Kwak and colleagues [105] carried out a case-control study in a Korean cohort and discovered that minor T allele at rs174537 was associated with lower risk of CAD (Odds Ratio 0.75, p = 0.006), and T allele carriers had significantly lower pathway efficiency as measured by ARA/LA and ARA/DGLA ratios. The T allele was also associated with lower total-and LDL-cholesterol and lipid peroxides. Importantly, Lettre and colleagues performed a meta-analysis on five African American cohorts (n ~ 8,000) and confirmed the association of FADS SNPs with not only lipid phenotypes, but CAD itself [137].
Other studies have demonstrated that high levels of ARA in adipose tissue are associated with elevated risk of acute myocardial infarction (AMI) [138,139]. For example, Kark and colleagues showed that ARA in adipose tissue was positively associated with AMI (O.R. 2.12, p = 0.004). Our laboratory has demonstrated that the genotypes at the FADS1 SNP, rs174537, which increases the efficiency of the LC-PUFA biosynthetic pathway, and thus circulating and cellular ARA levels, are also associated with higher levels of pro-inflammatory ARA-derived eicosanoids [140]. ARA-derived eicosanoids, such as urinary 8 epi-prostaglandin F(2α) that are independent risk factors for CAD, are also positively correlated with levels of ARA and ARA /LA ratios [105,141]. Together, these studies suggest that individuals and populations that have an enhanced genetic capacity to convert high levels of dietary LA to ARA are more likely to have high levels of ARA, ARA metabolites, inflammatory biomarkers, and diseases of inflammation such as CAD.
Although the aforementioned findings show genetic variation in the FADS cluster to be cross-sectionally associated with LC-PUFA and LC-PUFA metabolite levels, biomarkers of inflammation and CAD risk, few studies have investigated whether genetic variation within the FADS cluster is a significant mediator of the relationship between PUFA intake and CVD risk. One longitudinal cohort study [142] with a mean follow-up of 14 years, which included 24,032 participants aged 44–74 years, reported a borderline significant interaction by genotype of the FADS SNP rs174546 on the incidence of CVD by PUFA intake levels. Particularly, the ALA-to-LA intake ratio was inversely associated with CVD risk only among participants homozygous for the T-allele of FADS SNP rs174546 (HR for quintile 5 vs. quintile 1 = 0.72; 95% CI: 0.50, 1.04; p-trend = 0.049). Additionally, ALA intake inversely associated with ischemic stroke only among rs174546 TT genotype carriers (HR for quintile 5 vs. quintile 1 = 0.50; 95% CI: 0.27, 0.94; p-trend = 0.02). This study provides some evidence, albeit weak, that genetic variation in the FADS cluster may mediate the associations between PUFA intake and CVD risk, and that high ALA intake and a high ALA-to-LA intake ratio may be preferable to help prevent CVD and ischemic stroke particularly among those that are homozygous for the minor T-allele of rs174546. In summary, accumulating evidence suggests that the FADS locus may be useful in stratification and targeting of LC-PUFA recommendations for prevention of CVD; however, further research is necessary to better understand how genetic variation within the FADS cluster modifies the relationship between PUFA intake and CVD risk [143].
What about individuals/populations with low levels of LC-PUFAs such as DHA? This would be expected in those individuals/populations with FADS variants that make up the ancestral haplotype associated with inefficient LC-PUFA biosynthesis. As discussed above, the ancestral haplotype appears to have played a crucial role in the adaptation of Arctic populations to cold environments under conditions where high levels of preformed n-3 LC-PUFAs were ingested from the abundant marine sources [129]. Importantly, very high frequencies of this ancestral haplotype are also observed in Native American populations [118,130]. This genetic architecture, together with the current levels of LA, ALA, preformed n-3 LC-PUFAs, and specifically low levels of DHA in the MWD raises vital questions of how modern populations with high Native American ancestry acquire DHA. Does the near fixation of the ancestral haplotype, with its limited capacity to synthesize LC-PUFAs in Native American ancestry individuals, together with the PUFA composition (high levels of LA and low levels of ALA and DHA) give rise to n-3 LC-PUFA deficiencies and resulting diseases/disorders in Native American populations? DHA is critical for brain function throughout the human life span, but its accumulation is especially important to healthy brain development during gestation and infancy [144,145,146]. Additionally, as described above, n-3 LC-PUFAs such as DHA, DPA and EPA and their metabolites have potent anti-inflammatory properties [47,58]. There are no studies to date that have compared circulating and cellular levels of LC-PUFAs in a Native American ancestry cohort to other human populations. Future studies will be necessary to determine the risk of n-3 LC-PUFA deficiency in this population and whether n-3 LC-PUFA supplementation could provide important health benefits to Native American ancestry populations.
Most European and Asian ancestry populations are polymorphic for derived and ancestral haplotypes and individual genetic variants within the FADS cluster that impact LC-PUFA biosynthesis. Consequently, these populations appear to be more diverse with respect to their capacity to synthesize LC-PUFAs. For example, we have demonstrated in three European ancestry cohorts that ~45% of individuals have the homozygous GG genotype at the FADS1 SNP rs174537, which is associated with efficient LC-PUFA biosynthesis [36,97,100,106]. The TT genotype, which is associated with low efficiency LC-PUFA biosynthesis, is found in ~11% of the individuals in these cohorts and the balance (~44%) have the GT genotype. Similar to modern Native American ancestry populations, these studies raise important questions of how the 11% of individuals of European ancestry with the TT genotype and thus less efficient LC-PUFA biosynthesis acquire n-3 LC-PUFAs and particularly DHA when they are consuming a MWD.
Studies are just now beginning to emerge that indicate that epigenetics and specifically the methylation status of specific CpG sites within the FADS cluster (in a regulatory region between FADS1 and FADS2 that contains the FADS1 and FADS2 promoters and an regulatory enhancer) impacts the transcription of FADS cluster genes, LC-PUFA biosynthesis, and in one study, both immediate and delayed memory performance in toddlers [147,148]. A separate human study shows that previous PUFA exposure impacts the methylation status of GpG sites within this region [149]. We performed genome-wide allele-specific methylation (ASM) with the FADS1 SNP, rs174537 in 144 human liver samples and identified highly significant ASM with CpG sites between FADS1 and FADS2 in a enhancer signature region [150], leading to the hypothesis that the associations of rs174537 with LC-PUFA levels may be impacted by the methylation status of that enhancer region. Additionally, a study in rats indicates that maternal fat intake alters ARA and DHA status and the epigenetic regulation of FADS2 in offspring liver [151]. Although these studies are still early, collectively, they suggest that there are likely to be factors such as age, sex, pregnancy and prior PUFA exposure that impact epigenetic-mediated regulatory mechanisms of the FADS cluster leading to alterations in FADS gene transcription, LC-PUFA biosynthesis from 18C-PUFAs and ultimately LC-PUFA status [149]. Understanding the ramifications of these epigenetic modifications will likely be important in discerning how LC-PUFA levels are regulated both at individual and population levels.
6. Implications of Non-Uniform n-3 LC-PUFA Biosynthesis on the Efficacy of n-3 LC-PUFA Supplementation Trials
Pioneering studies 40 years ago showed that high dietary intake of n-3 LC-PUFA-enriched foods reduced mortality from myocardial infarction in Greenland Eskimos [152,153]. Since then, a growing body of evidence has shown that dietary n-3 LC-PUFAs may impact cardiovascular disease by numerous mechanisms including reducing circulating triglyceride concentrations, inflammatory processes, platelet aggregation and the incidence of arrhythmias and improving endothelial cell function [154]. Studies from predominantly secondary prevention trials and meta-analyses of observational studies in the 1980s through the early 2000s demonstrated a cardioprotective effect of fish consumption and n-3 PUFA supplementation [155,156,157,158,159,160]. Additionally, high circulating and dietary levels of n-3 LC-PUFAs were shown to associated with lower total mortality, especially deaths due to coronary artery disease [161,162]. However, more recent RCTs and meta-analyses indicate that supplementation with n-3 LC-PUFAs is not associated with lower risk of adverse outcomes such as all-cause mortality, cardiac death, sudden death, myocardial infarction, or stroke [163,164,165,166,167].
Similarly, several studies have shown associations between low levels of DHA and/or altered ratios of n-6 to n-3 LC-PUFAs and cognitive function (memory and Alzheimer’s disease) as well as psychological disorders (attention-deficit/hyperactivity-ADHD, schizophrenia, autism spectrum and major depressive disorders) in children, adolescents or adults [168,169,170,171,172,173,174,175,176,177,178,179,180]. However, systematic reviews and meta-analyses reveal that RCTs show inconsistent results for the therapeutic benefit of n-3 LC-PUFAs [181,182,183,184,185,186,187]. This pattern of erratic clinical results with n-3 LC-PUFAs is also observed in several inflammatory diseases including asthma [188,189], rheumatoid arthritis [190,191] and cancer [192,193,194,195].
Together, these studies and the controversies stemming from them have led to great confusion among clinicians and consumers alike about the efficacy of n-3 LC-PUFAs for the prevention and treatment of human disease. These varying clinical results have been particularly difficult to comprehend in light of the vast numbers of studies that have examined the mechanism by which n-3 LC-PUFAs exert their effects and convincing in vivo data with LC-PUFAs in animal models. Recently, experts at the International Society for the Study of Fatty Acids and Lipids discussed experimental design issues that may contribute to inconsistent results with n-3 LC-PUFA interventions in cardiovascular studies and thus must be considered in future clinical designs [196]. These included: (1) the potential of current CVD drug treatments to hide n-3 LC-PUFA benefits; (2) the potential impact of high background intakes of LC-PUFAs; (3) small sample sizes; (4) short treatment durations; (5) insufficient dosages of n-3 LC-PUFAs; (6) increase in n-6 PUFA intake; and (7) failure to measure baseline n-3 status. This current review has emphasized the potential impact of the latter three.
First, as discussed in detail above, the dramatic increase in dietary levels of the n-6 18C-PUFA, LA, in the MWD has resulted in an imbalance of LA and ALA entering the LC-PUFA biosynthetic pathway (Figure 1). Because these 18C-PUFAs compete for the desaturase and elongase steps in the pathway, the imbalance in LA and ALA together with the limited capacity of the pathway results in a disproportionate synthesis of n-6 LC-PUFAs at the expense of n-3 LC-PUFAs. The other major consideration is the overall capacity of the pathway to synthesize LC-PUFAs, and a large body of evidence (summarized above) indicates that this pathway capacity is variable in individuals and diverse populations and strongly linked to ancestry. For example, African, African ancestry and most south Asian populations contain evolutionary-driven genetic variants, particularly in the FADS cluster, that are strongly associated with efficient LC-PUFA biosynthesis and thus high levels of LC-PUFAs and particularly ARA. We have demonstrated that genetic variants associated with elevated levels of ARA are also associated with the capacity to generate high concentrations of pro-inflammatory eicosanoids in whole blood [140]. Consequently, we hypothesize that the combination of a marked increase in dietary LA as a result of the MWD, together with an enhanced capacity to convert that LA to ARA gives rise to elevated levels of a diverse family of ARA-derived mediators, which promote obesity, inflammation and related diseases. In this case, it may be the excess in ARA and ARA-derived mediators, created by the aforementioned gene–diet interaction, that limits the capacity of n-3 LC-PUFAs to impact inflammatory diseases such as CVD and cancer.
On the other hand, individuals of Arctic and Native American ancestry as well as a significant percentage of European and Asian populations have evolutionary-driven FADS variants associated with a less efficient LC-PUFA biosynthetic pathway [130]. In this case, we postulate that high levels of LA (relative to ALA) in the MWD are converted to sufficient levels of n-6 LC-PUFAs such as ARA and ARA metabolites. However, because of the constraints of the less efficient LC-PUFA biosynthetic pathway, it would be predicted that low quantities of n-3 LC-PUFAs and specifically DHA and DHA metabolites would be generated from dietary ALA. Such gene–diet interactions in these individuals/populations could lead to n-3 LC-PUFA deficiency, and like other nutrient deficiencies, these may be the patients that most benefit from supplementation with n-3 LC-PUFAs. It is important to emphasize that this is a hypothetical scenario at this point in time, and future studies will be necessary to determine actual levels of n-3 LC-PUFAs in individuals/populations with the ancestral FADS haplotype.
Superko and colleagues reviewed clinical investigations that actually measured blood or plasma levels of n-3 LC-PUFAs [197]. Not surprising, their data suggest that diet and geography play a critical role in levels of n-3 LC-PUFAs. For example, the lowest 5th percentile of Japanese living in Japan had higher levels of n-3 LC-PUFAs than whites living in Pennsylvania and Japanese Americans living in Honolulu. In an analysis of nine studies, Hawkey and Nigg found lower overall blood levels of the n-3 LC-PUFAs, EPA and DHA, in individuals with ADHD versus controls [183]. They suggest that there may be “a disruption in the conversion process from ALA to EPA/DHA in the ADHD population”. Several GWAS combined with metabolomics analyses have now examined associations between FADS variants and circulating levels of n-3 LC-PUFAs. Table 1 shows that FADS1 variants (rs174547, rs174537, and rs174548) are strongly associated with n-3 LC-PUFAs, EPA, DPA or DHA either as free fatty acids or in complex lipids [198,199,200] and further suggest that much of the variation in background n-3 LC-PUFA levels is due to genetic variation within the FADS cluster.
Table 1.
Association of key FADS SNPS with serum LC-PUFAs.
| SNPs | ||||
|---|---|---|---|---|
| n-3 PUFA/Metabolite | rs174547 p-Value | rs174537 p-Value | rs174548 p-Value | Data Source |
| 1-hexadecanoyl-2-docosapentaenoyl-GPC (16:0/22:5n3) | 2.97 × 10−95 | 3.83 × 10−93 | 9.17 × 10−88 | Draisma et al. [198] |
| 1-tetradecanoyl-2-docosapentaenoyl-GPC (14:0/22:5n3) | 2.76 × 10−58 | 1.94 × 10−57 | 5.08 × 10−54 | Draisma et al. [198] |
| 1-octadecanoyl-2-docosapentaenoyl-GPC (18:0/22:5n-3) | 2.23 × 10−42 | 4.61 × 10−41 | 2.53 × 10−40 | Draisma et al. [198] |
| 1-O-docosanyl-2-docosahexaenoyl-GPC (o-22:0/22:6n-3) | 1.67 × 10−40 | 6.03 × 10−40 | 3.15 × 10−37 | Draisma et al. [198] |
| 1-O-hexadecyl-2-docosahexaenoyl-GPC (o-16:0/22:6n-3) | 1.35 × 10−25 | 4.90 × 10−24 | 1.36 × 10−23 | Draisma et al. [198] |
| 1-eicosanoyl-2-docosahexaenoyl-GPC (20:0/22:6n-3) | 2.19 × 10−23 | Draisma et al. [198] | ||
| eicosapentaenoate (EPA; 20:5n3) | 1.12 × 10−21 | 2.55 × 10−21 | 3.71 × 10−22 | Shin et al. [199] |
| 1-octadecanoyl-2-docosahexaenoyl-GPC (18:0/22:6n-3) | 8.48 × 10−20 | 3.88 × 10−19 | 1.70 × 10−18 | Draisma et al. [198] |
| 1-tetradecanoyl-2-docosahexaenoyl-GPC (14:0/22:6n-3) | 9.86 × 10−18 | 2.72 × 10−17 | 1.38 × 10−15 | Draisma et al. [198] |
| 1-octadecanoyl-2-docosapentaenoyl-GPC (18:0/22:5n3) | 4.43 × 10−14 | 9.83 × 10−14 | 9.99 × 10−16 | Long et al. [200] |
| octadecatetraenoic acid (stearidonate) (18:4n3) | 1.63 × 10−15 | 1.07 × 10−15 | 1.97 × 10−13 | Shin et al. [199] |
| 1-O-octadecyl-2-docosahexaenoyl-GPC (o-18:0/22:6n-3) | 2.02 × 10−15 | 5.04 × 10−15 | 1.47 × 10−14 | Draisma et al. [198] |
| 1-hexadecanoyl-2-docosahexaenoyl-GPC 16:0/22:6 | 1.21 × 10−14 | 4.16 × 10−14 | 3.02 × 10−13 | Draisma et al. [198] |
| 1-eicosatrienoyl-GPC (ETA; 20:3n-3) | 1.30 × 10−14 | Shin et al. [199] | ||
| docosapentaenoate (DPA; 22:5n-3) | 2.93 × 10−13 | 5.07 × 10−13 | Shin et al. [199] | |
| 1-eicosapentaenoyl-GPC (20:5n-3) | 1.59 × 10−12 | Long et al. [200] | ||
| 1-octadecenoyl-2-eicosapentaenoyl-GPC (18:1/20:5n-3) | 1.78 × 10−12 | Long et al. [200] | ||
| 1-palmitoyl-2-eicosapentaenoyl-GPC (16:0/20:5n-3) | 1.01 × 10−11 | Long et al. [200] | ||
Association of three key FADS SNPS with levels of serum n-3 LC-PUFAs either as free fatty acids or esterified complex lipids. Serum levels of n-3 LC-PUFAs and glycerol-3-phosphocholine (GPC) containing n-3 LC-PUFAs were associated with three SNPS in FADS1 gene region. Data are derived from SNiPA analysis [201] of studies by Draisma et al. [198]; Long et al. [200]; and Shin et al. [199]. Significant associations are shown for Bonferroni adjusted p-values for each of three representative studies.
In addition to blood levels, the review by Superko and colleagues also pointed out that there are marked differences in the impact of n-3 LC-PUFA supplementation on circulating levels of LC-PUFAs and altering ratios of n-3 to n-6 LC-PUFAs [197]. Consequently, large diverse RCTs typically have sizeable subsets of individuals with high, intermediate, and low blood levels of LC-PUFAs; and the degree to which these levels are altered appears highly variable. Thus, it is little wonder that RCTs with n-3 LC-PUFAs have yielded perplexing results. Providing individuals with n-3 LC-PUFA supplements who do not have LC-PUFA deficiencies, or diverse groups of individuals where diet–gene interactions have created markedly different n-6 to n-3 LC-PUFA levels and ratios is unlikely to provide clear results. Given the genetic diversity in the LC-PUFA biosynthetic pathway, it may be too much to expect that supplementation strategies that fail to stratify participants in some manner (ex, genetics, background n-3 LC-PUFA levels, or ratio of n-6 LC-PUFAs to n-3 LC-PUFAs) will show statistical efficacy for complex human diseases.
7. Conclusions
A primary goal of the emerging field of precision nutrition is to have the capacity to predict physiological and pathological outcomes of human diets based on a better understanding of interacting parameters such as an individual’s genetic capacity to utilize/metabolize certain dietary nutrients and that same individual’s dietary nutrient exposure. Insights gained from such studies also offer the potential to tailor nutritional recommendations and interventions to individuals and populations throughout the life span. The FADS cluster and ELOVL2/5 genes are highly polymorphic, with considerable ancestry-based variation in the frequencies of common variants. These variants are associated with different conversion efficiencies of ALA and LA to n-3 LC-PUFAs and n-6 LC-PUFAs, respectively, and also important molecular and clinical phenotypes linking them to the pathogenesis of numerous human diseases. Additionally, the imbalance of LA and ALA in the MWD alone has been demonstrated to induce higher levels of n-6 LC-PUFA relative n-3 LC-PUFAs in circulation, cells and tissues. Considering the high heritability of LC-PUFA biosynthetic capacity (i.e., the strong genetic regulation), marked epigenetic regulation (i.e., potential gene environment interplay in regulation) and differences in dietary PUFA intake (i.e., variability in the environment), it is highly likely that n-3 LC-PUFA supplementation efficacy is individualized with a complex interplay of genetics, epigenetics and environment.
What are the implications of all this genetic and dietary complexity from a practical application perspective? It is clear that there have been incredible increases in consumption of LA-containing oils, such as soybean oil, in the evolution of the MWD over the past 75 years, and that these dietary changes have led to dramatic increases in LA/ALA ratios and ARA-derived metabolites, and reductions in both circulating and tissue levels of n-3 LC-PUFAs. This has occurred during a time of marked increases in obesity and obesity-related inflammatory diseases. Indeed, the concept of ‘Omega-3 Deficiency Syndrome’ introduced by Okuyama and colleagues [84] as Japanese populations moved from their native to a Western diet, may apply to most individuals exposed to the MWD. Consequently, we believe it is safe to say that a reduction in the dietary intake of LA and ARA, together with an increase in n-3 LC-PUFAs would benefit most individuals. However, the fact that there are such individual- and population-based genetic differences in the metabolism of dietary 18C-PUFAs, resulting in high, intermediate, and low blood levels of n-6 and n-3 LC-PUFAs and LC-PUFA metabolites, suggests that some populations and individuals will respond to n-3 LC-PUFA supplementation better than others. Based on this premise, it is important for future investigations that focus on n-3 LC-PUFA supplementation for the prevention and management of human diseases to develop therapeutic strategies taking into consideration the genetic heterogeneity in their study populations.
Acknowledgments
Funding support includes National Institutes of Health grants, P50-AT002782 and R01-AT008621 (F.H.C.) and the WFHS Department of Urology.
Author Contributions
F.H.C. conceived and designed the review. M.C.S., R.D., L.M.R., S.S., R.A.M. and F.H.C. analyzed published data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blasbalg T.L., Hibbeln J.R., Ramsden C.E., Majchrzak S.F., Rawlings R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chilton F.H., Murphy R.C., Wilson B.A., Sergeant S., Ainsworth H., Seeds M.C., Mathias R.A. Diet-gene interactions and pufa metabolism: A potential contributor to health disparities and human diseases. Nutrients. 2014;6:1993–2022. doi: 10.3390/nu6051993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O’Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 4.Popkin B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006;84:289–298. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Ferrante A.W., Jr. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamminga E.A., van der Lely A.J., Neumann H.A., Thio H.B. Chronic inflammation in psoriasis and obesity: Implications for therapy. Med. Hypotheses. 2006;67:768–773. doi: 10.1016/j.mehy.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Forsythe L.K., Wallace J.M., Livingstone M.B. Obesity and inflammation: The effects of weight loss. Nutr. Res. Rev. 2008;21:117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen X.-M.T., Lane J., Smith B.R., Nguyen N.T. Changes in inflammatory biomarkers across weight classes in a representative US population: A link between obesity and inflammation. J. Gastrointest. Surg. 2009;13:1205–1212. doi: 10.1007/s11605-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calle E.E., Thun M.J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 10.Beuther D.A., Weiss S.T., Sutherland E.R. Obesity and asthma. Am. J. Respir. Crit. Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naderali E.K., Ratcliffe S.H., Dale M.C. Obesity and Alzheimer’s disease: A link between body weight and cognitive function in old age. Am. J. Alzheimers Dis. Other Demen. 2009;24:445–449. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leveille S.G., Wee C.C., Iezzoni L.I. Trends in obesity and arthritis among baby boomers and their predecessors, 1971–2002. Am. J. Public Health. 2005;95:1607–1613. doi: 10.2105/AJPH.2004.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serini S., Calviello G. Reduction of oxidative/nitrosative stress in brain and its involvement in the neuroprotective effect of n-3 pufa in Alzheimer’s disease. Curr. Alzheimer Res. 2016;13:123–134. doi: 10.2174/1567205012666150921101147. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.J., Han S.B., Nam S.Y., Oh K.W., Hong J.T. Inflammation and Alzheimer’s disease. Arch. Pharmcal Res. 2010;33:1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 15.Sankar P., Cho M.K., Condit C.M., Hunt L.M., Koenig B., Marshall P., Lee S.S., Spicer P. Genetic research and health disparities. JAMA. 2004;291:2985–2989. doi: 10.1001/jama.291.24.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankar P., Cho M.K., Mountain J. Race and ethnicity in genetic research. Am. J. Med. Genet. 2007;143A:961–970. doi: 10.1002/ajmg.a.31575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mensah G.A., Mokdad A.H., Ford E.S., Greenlund K.J., Croft J.B. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 18.Krieger N., Chen J.T., Waterman P.D., Soobader M.J., Subramanian S.V., Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am. J. Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 19.Braveman P. Health disparities and health equity: Concepts and measurement. Annu. Rev. Public Health. 2006;27:167–194. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- 20.Kuzawa C.W., Sweet E. Epigenetics and the embodiment of race: Developmental origins of US racial disparities in cardiovascular health. Am. J. Hum. Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- 21.De Toro-Martin J., Arsenault B.J., Despres J.P., Vohl M.C. Precision Nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients. 2017;9:913. doi: 10.3390/nu9080913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Heart Association & American Stroke Association 50 Years of American Heart Association Dietary Fats Recommendations. [(accessed on 9 February 2017)];2015 Available online: http://www.heart.org/idc/groups/heart-public/@wcm/@fc/documents/downloadable/ucm_475005.pdf.
- 23.Miller M., Stone N.J., Ballantyne C., Bittner V., Criqui M.H., Ginsberg H.N., Goldberg A.C., Howard W.J., Jacobson M.S., Kris-Etherton P.M., et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 24.Nutrient Content of the U.S. Food Supply, 1909-99: A Summary Report. U.S. Department of Agriculture, Center for Nutrition Policy and Promotion 2002, Home Economics Research Report No. 55. [(accessed on 9 February 2017)]; Available online: https://www.cnpp.usda.gov/sites/default/files/nutrient_content_of_the_us_food_supply/FoodSupply1909-1999Report.pdf.
- 25.Eaton S.B. Humans, lipids and evolution. Lipids. 1992;27:814–820. doi: 10.1007/BF02535856. [DOI] [PubMed] [Google Scholar]
- 26.Hibbeln J.R., Nieminen L.R., Blasbalg T.L., Riggs J.A., Lands W.E. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 27.Simopoulos A.P. Importance of the omega-6/omega-3 balance in health and disease: Evolutionary aspects of diet. World Rev. Nutr. Diet. 2011;102:10–21. doi: 10.1159/000327785. [DOI] [PubMed] [Google Scholar]
- 28.Burr G.O., Burr M.M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 29.Burr G.O., Burr M.M. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 1930;86:587–621. doi: 10.1016/0163-7827(81)90004-7. [DOI] [Google Scholar]
- 30.Holman R.T. Essential fatty acids. Nutr. Rev. 1958;16:33–35. doi: 10.1111/j.1753-4887.1958.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 31.Holman R.T. The slow discovery of the importance of omega 3 essential fatty acids in human health. J. Nutr. 1998;128:427S–433S. doi: 10.1093/jn/128.2.427S. [DOI] [PubMed] [Google Scholar]
- 32.Sprecher H. Biochemistry of essential fatty acids. Prog. Lipid Res. 1981;20:13–22. doi: 10.1016/0163-7827(81)90009-6. [DOI] [PubMed] [Google Scholar]
- 33.Sprecher H., Luthria D.L., Mohammed B.S., Baykousheva S.P. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J. Lipid Res. 1995;36:2471–2477. [PubMed] [Google Scholar]
- 34.Sprecher H., Chen Q. Polyunsaturated fatty acid biosynthesis: A microsomal-peroxisomal process. Prostaglandins Leukot. Essent. Fat. Acids. 1999;60:317–321. doi: 10.1016/S0952-3278(99)80006-4. [DOI] [PubMed] [Google Scholar]
- 35.Park W.J., Kothapalli K.S., Lawrence P., Tyburczy C., Brenna J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009;50:1195–1202. doi: 10.1194/jlr.M800630-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathias R.A., Pani V., Chilton F.H. Genetic variants in the FADS gene: Implications for dietary recommendations for fatty acid intake. Curr. Nutr. Rep. 2014;3:139–148. doi: 10.1007/s13668-014-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park H.G., Park W.J., Kothapalli K.S., Brenna J.T. The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015;29:3911–3919. doi: 10.1096/fj.15-271783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spector A.A. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J. Mol. Neurosci. 2001;16:159–165. doi: 10.1385/JMN:16:2-3:159. [DOI] [PubMed] [Google Scholar]
- 39.Lands W.E., Hart P. Metabolism of glycerolipids.VI. Specificities of acyl coenzyme A: Phospolipid acyltransferases. J. Biol. Chem. 1965;240:1905–1911. [PubMed] [Google Scholar]
- 40.Gibson R.A., Muhlhausler B., Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 2011;7(Suppl. 2):17–26. doi: 10.1111/j.1740-8709.2011.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNamara R.K., Carlson S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fat. Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.McCann J.C., Ames B.N. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005;82:281–295. doi: 10.1093/ajcn.82.2.281. [DOI] [PubMed] [Google Scholar]
- 43.Weiser M.J., Butt C.M., Mohajeri M.H. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. 2016;8:99. doi: 10.3390/nu8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hibbeln J.R., Davis J.M., Steer C., Emmett P., Rogers I., Williams C., Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 45.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D.C., Liao J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith W.L. The eicosanoids and their biochemical mechanisms of action. Biochem. J. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Marzoa V.M.D., Bisogno T., De Petrocellis L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;22:521–528. doi: 10.1016/S0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- 49.Buczynski M.W., Dumlao D.S., Dennis E.A. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chilton F.H., Fonteh A.N., Surette M.E., Triggiani M., Winkler J.D. Control of arachidonate levels within inflammatory cells. Biochim. Biophys. Acta. 1996;1299:1–15. doi: 10.1016/0005-2760(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 51.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 52.Haeggstrom J.Z., Funk C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 53.Lands W.E., Libelt B., Morris A., Kramer N.C., Prewitt T.E., Bowen P., Schmeisser D., Davidson M.H., Burns J.H. Maintenance of lower proportions of (n-6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n-3) fatty acids. Biochim. Biophys. Acta. 1992;1180:147–162. doi: 10.1016/0925-4439(92)90063-S. [DOI] [PubMed] [Google Scholar]
- 54.James M.J., Gibson R.A., Cleland L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 55.Lands B. A critique of paradoxes in current advice on dietary lipids. Prog. Lipid Res. 2008;47:77–106. doi: 10.1016/j.plipres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Lands B. Omega-3 PUFAs lower the propensity for arachidonic acid cascade overreactions. Biomed. Res. Int. 2015;2015:285135. doi: 10.1155/2015/285135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitz G., Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Serhan C.N. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romano M., Cianci E., Simiele F., Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 60.Berghuis P., Rajnicek A.M., Morozov Y.M., Ross R.A., Mulder J., Urban G.M., Monory K., Marsicano G., Matteoli M., Canty A., et al. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 61.Matias I., Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol. Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Batkai S., Pacher P., Osei-Hyiaman D., Radaeva S., Liu J., Harvey-White J., Offertaler L., Mackie K., Rudd M.A., Bukoski R.D., et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown I., Cascio M.G., Rotondo D., Pertwee R.G., Heys S.D., Wahle K.W. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 2013;52:80–109. doi: 10.1016/j.plipres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Brown I., Cascio M.G., Wahle K.W., Smoum R., Mechoulam R., Ross R.A., Pertwee R.G., Heys S.D. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis. 2010;31:1584–1591. doi: 10.1093/carcin/bgq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDougle D.R., Watson J.E., Abdeen A.A., Adili R., Caputo M.P., Krapf J.E., Johnson R.W., Kilian K.A., Holinstat M., Das A. Anti-inflammatory omega-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA. 2017;114:E6034–E6043. doi: 10.1073/pnas.1610325114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Araque A., Castillo P.E., Manzoni O.J., Tonini R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24. doi: 10.1016/j.neuropharm.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ashton J.C., Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skaper S.D., Di Marzo V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:3193–3200. doi: 10.1098/rstb.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sloan M.E., Gowin J.L., Ramchandani V.A., Hurd Y.L., Le Foll B. The endocannabinoid system as a target for addiction treatment: Trials and tribulations. Neuropharmacology. 2017;124:73–83. doi: 10.1016/j.neuropharm.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Murillo-Rodriguez E., Poot-Ake A., Arias-Carrion O., Pacheco-Pantoja E., Fuente-Ortegon Ade L., Arankowsky-Sandoval G. The emerging role of the endocannabinoid system in the sleep-wake cycle modulation. Cent. Nerv. Syst. Agents Med. Chem. 2011;11:189–196. doi: 10.2174/187152411798047780. [DOI] [PubMed] [Google Scholar]
- 71.Malek N., Starowicz K. Dual-acting compounds targeting endocannabinoid and endovanilloid systems-a novel treatment option for chronic pain management. Front. Pharmacol. 2016;7:257. doi: 10.3389/fphar.2016.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lau B.K., Cota D., Cristino L., Borgland S.L. Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology. 2017;124:38–51. doi: 10.1016/j.neuropharm.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 73.Kruk-Slomka M., Dzik A., Budzynska B., Biala G. Endocannabinoid system: The direct and indirect involvement in the memory and learning processes-a short review. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hedlund P., Gratzke C. The endocannabinoid system—A target for the treatment of LUTS? Nat. Rev. Urol. 2016;13:463–470. doi: 10.1038/nrurol.2016.110. [DOI] [PubMed] [Google Scholar]
- 75.Giaginis C., Lakiotaki E., Korkolopoulou P., Konstantopoulos K., Patsouris E., Theocharis S. Endocannabinoid system: A promising therapeutic target for the treatment of haematological malignancies? Curr. Med. Chem. 2016;23:2350–2362. doi: 10.2174/0929867323666160530144934. [DOI] [PubMed] [Google Scholar]
- 76.Fonseca B.M., Costa M.A., Almada M., Correia-da-Silva G., Teixeira N.A. Endogenous cannabinoids revisited: A biochemistry perspective. Prostaglandins Other Lipid Mediat. 2013;102–103:13–30. doi: 10.1016/j.prostaglandins.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Rouzer C.A., Marnett L.J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramsden C.E., Hibbeln J.R., Majchrzak S.F., Davis J.M. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2010;104:1586–1600. doi: 10.1017/S0007114510004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramsden C.E., Zamora D., Majchrzak-Hong S., Faurot K.R., Broste S.K., Frantz R.P., Davis J.M., Ringel A., Suchindran C.M., Hibbeln J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968-73) BMJ. 2016;353:i1246. doi: 10.1136/bmj.i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lands B. Dietary omega-3 and omega-6 fatty acids compete in producing tissue compositions and tissue responses. Mil. Med. 2014;179:76–81. doi: 10.7205/MILMED-D-14-00149. [DOI] [PubMed] [Google Scholar]
- 81.Liou Y.A., King D.J., Zibrik D., Innis S.M. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J. Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- 82.Wood K.E., Mantzioris E., Gibson R.A., Ramsden C.E., Muhlhausler B.S. The effect of modifying dietary LA and ALA intakes on omega-3 long chain polyunsaturated fatty acid (n-3 LCPUFA) status in human adults: A systematic review and commentary. Prostaglandins Leukot. Essent. Fat. Acids. 2015;95:47–55. doi: 10.1016/j.plefa.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Macintosh B.A., Ramsden C.E., Faurot K.R., Zamora D., Mangan M., Hibbeln J.R., Mann J.D. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br. J. Nutr. 2013:1–10. doi: 10.1017/S0007114512005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okuyama H. Dietary fatty acids—The N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog. Lipid Res. 1996;35:409–457. doi: 10.1016/S0163-7827(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 85.Cleland L.G., James M.J., Neumann M.A., D’Angelo M., Gibson R.A. Linoleate inhibits EPA incorporation from dietary fish-oil supplements in human subjects. Am. J. Clin. Nutr. 1992;55:395–399. doi: 10.1093/ajcn/55.2.395. [DOI] [PubMed] [Google Scholar]
- 86.Blank C., Neumann M.A., Makrides M., Gibson R.A. Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J. Lipid Res. 2002;43:1537–1543. doi: 10.1194/jlr.M200152-JLR200. [DOI] [PubMed] [Google Scholar]
- 87.Wood K.E., Lau A., Mantzioris E., Gibson R.A., Ramsden C.E., Muhlhausler B.S. A low omega-6 polyunsaturated fatty acid (n-6 PUFA) diet increases omega-3 (n-3) long chain PUFA status in plasma phospholipids in humans. Prostaglandins Leukot. Essent. Fat. Acids. 2014;90:133–138. doi: 10.1016/j.plefa.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brenna J.T., Salem N., Jr., Sinclair A.J., Cunnane S.C., International Society for the Study of Fatty, Acids and Lipids, ISSFAL alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 89.Arterburn L.M., Hall E.B., Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 90.Goyens P.L., Spilker M.E., Zock P.L., Katan M.B., Mensink R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006;84:44–53. doi: 10.1093/ajcn/84.1.44. [DOI] [PubMed] [Google Scholar]
- 91.Schaeffer L., Gohlke H., Muller M., Heid I.M., Palmer L.J., Kompauer I., Demmelmair H., Illig T., Koletzko B., Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 92.Malerba G., Schaeffer L., Xumerle L., Klopp N., Trabetti E., Biscuola M., Cavallari U., Galavotti R., Martinelli N., Guarini P., et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids. 2008;43:289–299. doi: 10.1007/s11745-008-3158-5. [DOI] [PubMed] [Google Scholar]
- 93.Xie L., Innis S.M. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr. 2008;138:2222–2228. doi: 10.3945/jn.108.096156. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka T., Shen J., Abecasis G.R., Kisialiou A., Ordovas J.M., Guralnik J.M., Singleton A., Bandinelli S., Cherubini A., Arnett D., et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5:e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rzehak P., Heinrich J., Klopp N., Schaeffer L., Hoff S., Wolfram G., Illig T., Linseisen J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br. J. Nutr. 2009;101:20–26. doi: 10.1017/S0007114508992564. [DOI] [PubMed] [Google Scholar]
- 96.Xie L., Innis S.M. Association of fatty acid desaturase gene polymorphisms with blood lipid essential fatty acids and perinatal depression among Canadian women: A pilot study. J. Nutrigenet. Nutrigenom. 2009;2:243–250. doi: 10.1159/000255636. [DOI] [PubMed] [Google Scholar]
- 97.Mathias R.A., Vergara C., Gao L., Rafaels N., Hand T., Campbell M., Bickel C., Ivester P., Sergeant S., Barnes K.C., et al. FADS genetic variants and omega-6 polyunsaturated fatty acid metabolism in a homogeneous island population. J. Lipid Res. 2010;51:2766–2774. doi: 10.1194/jlr.M008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bokor S., Dumont J., Spinneker A., Gonzalez-Gross M., Nova E., Widhalm K., Moschonis G., Stehle P., Amouyel P., De H.S., et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J. Lipid Res. 2010;51:2325–2333. doi: 10.1194/jlr.M006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rzehak P., Thijs C., Standl M., Mommers M., Glaser C., Jansen E., Klopp N., Koppelman G.H., Singmann P., Postma D.S., et al. Variants of the FADS1 FADS2 gene cluster, blood levels of polyunsaturated fatty acids and eczema in children within the first 2 years of life. PLoS ONE. 2010;5:e13261. doi: 10.1371/journal.pone.0013261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mathias R.A., Sergeant S., Ruczinski I., Torgerson D.G., Hugenschmidt C.E., Kubala M., Vaidya D., Suktitipat B., Ziegler J.T., Ivester P., et al. The impact of FADS genetic variants on omega6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet. 2011;12:50. doi: 10.1186/1471-2156-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lemaitre R.N., Tanaka T., Tang W., Manichaikul A., Foy M., Kabagambe E.K., Nettleton J.A., King I.B., Weng L.C., Bhattacharya S., et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morales E., Bustamante M., Gonzalez J.R., Guxens M., Torrent M., Mendez M., Garcia-Esteban R., Julvez J., Forns J., Vrijheid M., et al. Genetic variants of the FADS gene cluster and ELOVL gene family, colostrums LC-PUFA levels, breastfeeding, and child cognition. PLoS ONE. 2011;6:e17181. doi: 10.1371/journal.pone.0017181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lattka E., Rzehak P., Szabo E., Jakobik V., Weck M., Weyermann M., Grallert H., Rothenbacher D., Heinrich J., Brenner H., et al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am. J. Clin. Nutr. 2011;93:382–391. doi: 10.3945/ajcn.110.004515. [DOI] [PubMed] [Google Scholar]
- 104.Koletzko B., Lattka E., Zeilinger S., Illig T., Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: Findings from the Avon Longitudinal Study of Parents and Children. Am. J. Clin. Nutr. 2011;93:211–219. doi: 10.3945/ajcn.110.006189. [DOI] [PubMed] [Google Scholar]
- 105.Kwak J.H., Paik J.K., Kim O.Y., Jang Y., Lee S.H., Ordovas J.M., Lee J.H. FADS gene polymorphisms in Koreans: Association with omega6 polyunsaturated fatty acids in serum phospholipids, lipid peroxides, and coronary artery disease. Atherosclerosis. 2011;214:94–100. doi: 10.1016/j.atherosclerosis.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 106.Sergeant S., Hugenschmidt C.E., Rudock M.E., Ziegler J.T., Ivester P., Ainsworth H.C., Vaidya D., Case L.D., Langefeld C.D., Freedman B.I., et al. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br. J. Nutr. 2012;107:547–555. doi: 10.1017/S0007114511003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steer C.D., Hibbeln J.R., Golding J., Davey S.G. Polyunsaturated fatty acid levels in blood during pregnancy, at birth and at 7 years: Their associations with two common FADS2 polymorphisms. Hum. Mol. Genet. 2012;21:1504–1512. doi: 10.1093/hmg/ddr588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Porenta S.R., Ko Y.A., Gruber S.B., Mukherjee B., Baylin A., Ren J., Djuric Z. Interaction of fatty acid genotype and diet on changes in colonic fatty acids in a Mediterranean diet intervention study. Cancer Prev. Res. 2013;6:1212–1221. doi: 10.1158/1940-6207.CAPR-13-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong S.H., Kwak J.H., Paik J.K., Chae J.S., Lee J.H. Association of polymorphisms in FADS gene with age-related changes in serum phospholipid polyunsaturated fatty acids and oxidative stress markers in middle-aged nonobese men. Clin. Interv. Aging. 2013;8:585–596. doi: 10.2147/CIA.S42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harslof L.B., Larsen L.H., Ritz C., Hellgren L.I., Michaelsen K.F., Vogel U., Lauritzen L. FADS genotype and diet are important determinants of DHA status: A cross-sectional study in Danish infants. Am. J. Clin. Nutr. 2013;97:1403–1410. doi: 10.3945/ajcn.113.058685. [DOI] [PubMed] [Google Scholar]
- 111.Li S.W., Lin K., Ma P., Zhang Z.L., Zhou Y.D., Lu S.Y., Zhou X., Liu S.M. FADS gene polymorphisms confer the risk of coronary artery disease in a Chinese Han population through the altered desaturase activities: Based on high-resolution melting analysis. PLoS ONE. 2013;8:e55869. doi: 10.1371/journal.pone.0055869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gillingham L.G., Harding S.V., Rideout T.C., Yurkova N., Cunnane S.C., Eck P.K., Jones P.J. Dietary oils and FADS1-FADS2 genetic variants modulate (13C)alpha-linolenic acid metabolism and plasma fatty acid composition. Am. J. Clin. Nutr. 2013;97:195–207. doi: 10.3945/ajcn.112.043117. [DOI] [PubMed] [Google Scholar]
- 113.Freemantle E., Lalovic A., Mechawar N., Turecki G. Age and haplotype variations within FADS1 interact and associate with alterations in fatty acid composition in human male cortical brain tissue. PLoS ONE. 2012;7:e42696. doi: 10.1371/journal.pone.0042696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lattka E., Koletzko B., Zeilinger S., Hibbeln J.R., Klopp N., Ring S.M., Steer C.D. Umbilical cord PUFA are determined by maternal and child fatty acid desaturase (FADS) genetic variants in the Avon Longitudinal Study of Parents and Children (ALSPAC) Br. J. Nutr. 2013;109:1196–1210. doi: 10.1017/S0007114512003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tintle N.L., Pottala J.V., Lacey S., Ramachandran V., Westra J., Rogers A., Clark J., Olthoff B., Larson M., Harris W., et al. A genome-wide association study of saturated, mono- and polyunsaturated red blood cell fatty acids in the Framingham Heart Offspring Study. Prostaglandins Leukot. Essent. Fat. Acids. 2015;94:65–72. doi: 10.1016/j.plefa.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martinelli N., Girelli D., Malerba G., Guarini P., Illig T., Trabetti E., Sandri M., Friso S., Pizzolo F., Schaeffer L., et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am. J. Clin. Nutr. 2008;88:941–949. doi: 10.1093/ajcn/88.4.941. [DOI] [PubMed] [Google Scholar]
- 117.Ameur A., Enroth S., Johansson A., Zaboli G., Igl W., Johansson A.C., Rivas M.A., Daly M.J., Schmitz G., Hicks A.A., et al. Genetic adaptation of fatty-acid metabolism: A human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 2012;90:809–820. doi: 10.1016/j.ajhg.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amorim C.E., Nunes K., Meyer D., Comas D., Bortolini M.C., Salzano F.M., Hunemeier T. Genetic signature of natural selection in first Americans. Proc. Natl. Acad. Sci. USA. 2017;114:2195–2199. doi: 10.1073/pnas.1620541114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mathias R.A., Fu W.Q., Akey J.M., Ainsworth H.C., Torgerson D.G., Ruczinski I., Sergeant S., Barnes K.C., Chilton F.H. Adaptive evolution of the FADS gene cluster within africa. PLoS ONE. 2012;7:e44926. doi: 10.1371/journal.pone.0044926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dorajoo R., Sun Y., Han Y., Ke T., Burger A., Chang X., Low H.Q., Guan W., Lemaitre R.N., Khor C.C., et al. A genome-wide association study of n-3 and n-6 plasma fatty acids in a Singaporean Chinese population. Genes Nutr. 2015;10:53. doi: 10.1007/s12263-015-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guan W., Steffen B.T., Lemaitre R.N., Wu J.H.Y., Tanaka T., Manichaikul A., Foy M., Rich S.S., Wang L., Nettleton J.A., et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ. Cardiovasc. Genet. 2014;7:321–331. doi: 10.1161/CIRCGENETICS.113.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu Y., Li H., Lu L., Manichaikul A., Zhu J., Chen Y.D., Sun L., Liang S., Siscovick D.S., Steffen L.M., et al. Genome-wide meta-analyses identify novel loci associated with n-3 and n-6 polyunsaturated fatty acid levels in Chinese and European-ancestry populations. Hum. Mol. Genet. 2016;25:1215–1224. doi: 10.1093/hmg/ddw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lattka E., Illig T., Heinrich J., Koletzko B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clin. Nutr. 2010;29:277–287. doi: 10.1016/j.clnu.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 124.Chambers J.C., Zhang W., Sehmi J., Li X., Wass M.N., Van der Harst P., Holm H., Sanna S., Kavousi M., Baumeister S.E., et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat. Genet. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mirkov S., Myers J.L., Ramirez J., Liu W. SNPs affecting serum metabolomic traits may regulate gene transcription and lipid accumulation in the liver. Metabolism. 2012;61:1523–1527. doi: 10.1016/j.metabol.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dupuis J., Langenberg C., Prokopenko I., Saxena R., Soranzo N., Jackson A.U., Wheeler E., Glazer N.L., Bouatia-Naji N., Gloyn A.L., et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burghardt K.J., Gardner K.N., Johnson J.W., Ellingrod V.L. Fatty Acid desaturase gene polymorphisms and metabolic measures in schizophrenia and bipolar patients taking antipsychotics. Cardiovasc. Psychiatry Neurol. 2013;2013:596945. doi: 10.1155/2013/596945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Standl M., Lattka E., Stach B., Koletzko S., Bauer C.P., von B.A., Berdel D., Kramer U., Schaaf B., Roder S., et al. FADS1 FADS2 gene cluster, PUFA intake and blood lipids in children: Results from the GINIplus and LISAplus studies. PLoS ONE. 2012;7:e37780. doi: 10.1371/journal.pone.0037780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fumagalli M., Moltke I., Grarup N., Racimo F., Bjerregaard P., Jorgensen M.E., Korneliussen T.S., Gerbault P., Skotte L., Linneberg A., et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343–1347. doi: 10.1126/science.aab2319. [DOI] [PubMed] [Google Scholar]
- 130.Harris D.N., Ruczinski I., Yanek L.R., Becker L.C., Guio H., Cui T., Chilton F.H., Mathias R.A., O’Conner T. Evolution of hominim polyunsatruated fatty acid metabolism; from Africa to the New World. BioRxiv. 2017 doi: 10.1101/175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mathieson I., Lazaridis I., Rohland N., Mallick S., Patterson N., Roodenberg S.A., Harney E., Stewardson K., Fernandes D., Novak M., et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kothapalli K.S., Ye K., Gadgil M.S., Carlson S.E., O’Brien K.O., Zhang J.Y., Park H.G., Ojukwu K., Zou J., Hyon S.S., et al. Positive selection on a regulatory insertion-deletion polymorphism in FADS2 influences apparent endogenous synthesis of arachidonic acid. Mol. Biol. Evol. 2016;33:1726–1739. doi: 10.1093/molbev/msw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buckley M.T., Racimo F., Allentoft M.E., Jensen M.K., Jonsson A., Huang H., Hormozdiari F., Sikora M., Marnetto D., Eskin E., et al. Selection in europeans on fatty acid desaturases associated with dietary changes. Mol. Biol. Evol. 2017;34:1307–1318. doi: 10.1093/molbev/msx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sabeti P.C., Varilly P., Fry B., Lohmueller J., Hostetter E., Cotsapas C., Xie X., Byrne E.H., McCarroll S.A., Gaudet R., et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449:913–918. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Enattah N.S., Sahi T., Savilahti E., Terwilliger J.D., Peltonen L., Jarvela I. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 2002;30:233–237. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- 136.Tishkoff S.A., Reed F.A., Ranciaro A., Voight B.F., Babbitt C.C., Silverman J.S., Powell K., Mortensen H.M., Hirbo J.B., Osman M., et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat. Genet. 2007;39:31–40. doi: 10.1038/ng1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lettre G., Palmer C.D., Young T., Ejebe K.G., Allayee H., Benjamin E.J., Bennett F., Bowden D.W., Chakravarti A., Dreisbach A., et al. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: The NHLBI CARe Project. PLoS Genet. 2011;7:e1001300. doi: 10.1371/journal.pgen.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kark J.D., Kaufmann N.A., Binka F., Goldberger N., Berry E.M. Adipose tissue n-6 fatty acids and acute myocardial infarction in a population consuming a diet high in polyunsaturated fatty acids. Am. J. Clin. Nutr. 2003;77:796–802. doi: 10.1093/ajcn/77.4.796. [DOI] [PubMed] [Google Scholar]
- 139.Baylin A., Campos H. Arachidonic acid in adipose tissue is associated with nonfatal acute myocardial infarction in the central valley of Costa Rica. J. Nutr. 2004;134:3095–3099. doi: 10.1093/jn/134.11.3095. [DOI] [PubMed] [Google Scholar]
- 140.Hester A.G., Murphy R.C., Uhlson C.J., Ivester P., Lee T.C., Sergeant S., Miller L.R., Howard T.D., Mathias R.A., Chilton F.H. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J. Biol. Chem. 2014;289:22482–22489. doi: 10.1074/jbc.M114.579557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park J.Y., Paik J.K., Kim O.Y., Chae J.S., Jang Y., Lee J.H. Interactions between the APOA5-1131T>C and the FEN1 10154G>T polymorphisms on omega6 polyunsaturated fatty acids in serum phospholipids and coronary artery disease. J. Lipid Res. 2010;51:3281–3288. doi: 10.1194/jlr.M010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hellstrand S., Ericson U., Gullberg B., Hedblad B., Orho-Melander M., Sonestedt E. Genetic variation in FADS1 has little effect on the association between dietary PUFA intake and cardiovascular disease. J. Nutr. 2014;144:1356–1363. doi: 10.3945/jn.114.192708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O’Neill C.M., Minihane A.M. The impact of fatty acid desaturase genotype on fatty acid status and cardiovascular health in adults. Proc. Nutr. Soc. 2017;76:64–75. doi: 10.1017/S0029665116000732. [DOI] [PubMed] [Google Scholar]
- 144.Miller L.R., Jorgensen M.J., Kaplan J.R., Seeds M.C., Rahbar E., Morgan T.M., Welborn A., Chilton S.M., Gillis J., Hester A., et al. Alterations in levels and ratios of n-3 and n-6 polyunsaturated fatty acids in the temporal cortex and liver of vervet monkeys from birth to early adulthood. Physiol. Behav. 2016;156:71–78. doi: 10.1016/j.physbeh.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kitson A.P., Stark K.D., Duncan R.E. Enzymes in brain phospholipid docosahexaenoic acid accretion: A PL-ethora of potential PL-ayers. Prostaglandins Leukot. Essent. Fat. Acids. 2012;87:1–10. doi: 10.1016/j.plefa.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 146.Kuipers R.S., Luxwolda M.F., Offringa P.J., Boersma E.R., Dijck-Brouwer D.A., Muskiet F.A. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fat. Acids. 2012;86:13–20. doi: 10.1016/j.plefa.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 147.Cheatham C.L., Lupu D.S., Niculescu M.D. Genetic and epigenetic transgenerational implications related to omega-3 fatty acids. Part II: Maternal FADS2 rs174575 genotype and DNA methylation predict toddler cognitive performance. Nutr. Res. 2015;35:948–955. doi: 10.1016/j.nutres.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 148.Lupu D.S., Cheatham C.L., Corbin K.D., Niculescu M.D. Genetic and epigenetic transgenerational implications related to omega-3 fatty acids. Part I: Maternal FADS2 genotype and DNA methylation correlate with polyunsaturated fatty acid status in toddlers: An exploratory analysis. Nutr. Res. 2015;35:939–947. doi: 10.1016/j.nutres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 149.Hoile S.P., Clarke-Harris R., Huang R.C., Calder P.C., Mori T.A., Beilin L.J., Lillycrop K.A., Burdge G.C. Supplementation with N-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS ONE. 2014;9:e109896. doi: 10.1371/journal.pone.0109896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Howard T.D., Mathias R.A., Seeds M.C., Herrington D.M., Hixson J.E., Shimmin L.C., Hawkins G.A., Sellers M., Ainsworth H.C., Sergeant S., et al. DNA methylation in an enhancer region of the FADS cluster is associated with fads activity in human liver. PLoS ONE. 2014;9:e97510. doi: 10.1371/journal.pone.0097510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoile S.P., Irvine N.A., Kelsall C.J., Sibbons C., Feunteun A., Collister A., Torrens C., Calder P.C., Hanson M.A., Lillycrop K.A., et al. Maternal fat intake in rats alters 20:4n-6 and 22:6n-3 status and the epigenetic regulation of FADS2 in offspring liver. J. Nutr. Biochem. 2013;24:1213–1220. doi: 10.1016/j.jnutbio.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bang H.O., Dyerberg J., Nielsen A.B. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;1:1143–1145. doi: 10.1016/S0140-6736(71)91658-8. [DOI] [PubMed] [Google Scholar]
- 153.Dyerberg J., Bang H.O. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. 1979;2:433–435. doi: 10.1016/S0140-6736(79)91490-9. [DOI] [PubMed] [Google Scholar]
- 154.Ander B.P., Dupasquier C.M., Prociuk M.A., Pierce G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003;8:164–172. [PMC free article] [PubMed] [Google Scholar]
- 155.Burr M.L., Fehily A.M., Gilbert J.F., Rogers S., Holliday R.M., Sweetnam P.M., Elwood P.C., Deadman N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/S0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 156.Investigators G.-P. Dietray supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSIPrevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 157.Marchioli R., Barzi F., Bomba E., Chieffo C., Di Gregorio D., Di Mascio R., Franzosi M.G., Geraci E., Levantesi G., Maggioni A.P., et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.CIR.0000014682.14181.F2. [DOI] [PubMed] [Google Scholar]
- 158.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 159.Tavazzi L., Tognoni G., Franzosi M.G., Latini R., Maggioni A.P., Marchioli R., Nicolosi G.L., Porcu M., Investigators G.-H. Rationale and design of the GISSI heart failure trial: A large trial to assess the effects of n-3 polyunsaturated fatty acids and rosuvastatin in symptomatic congestive heart failure. Eur. J. Heart Fail. 2004;6:635–641. doi: 10.1016/j.ejheart.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 160.He K., Song Y., Daviglus M.L., Liu K., Van Horn L., Dyer A.R., Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 161.Chowdhury R., Warnakula S., Kunutsor S., Crowe F., Ward H.A., Johnson L., Franco O.H., Butterworth A.S., Forouhi N.G., Thompson S.G., et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 162.Del Gobbo L.C., Imamura F., Aslibekyan S., Marklund M., Virtanen J.K., Wennberg M., Yakoob M.Y., Chiuve S.E., Dela Cruz L., Frazier-Wood A.C., et al. Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: Pooling project of 19 cohort studies. JAMA Intern. Med. 2016;176:1155–1166. doi: 10.1001/jamainternmed.2016.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kromhout D., Giltay E.J., Geleijnse J.M., Alpha Omega Trial G. n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 164.Rauch B., Schiele R., Schneider S., Diller F., Victor N., Gohlke H., Gottwik M., Steinbeck G., Del Castillo U., Sack R., et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 165.Galan P., Kesse-Guyot E., Czernichow S., Briancon S., Blacher J., Hercberg S., Group S.F.O.C. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bosch J., Gerstein H.C., Dagenais G.R., Diaz R., Dyal L., Jung H., Maggiono A.P., Probstfield J., Ramachandran A., Riddle M.C., et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 167.Roncaglioni M.C., Tombesi M., Avanzini F., Barlera S., Caimi V., Longoni P., Marzona I., Milani V., Silletta M.G., Tognoni G., et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 168.Bourre J.M. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J. Nutr. Health Aging. 2004;8:163–174. [PubMed] [Google Scholar]
- 169.Van Elst K., Bruining H., Birtoli B., Terreaux C., Buitelaar J.K., Kas M.J. Food for thought: Dietary changes in essential fatty acid ratios and the increase in autism spectrum disorders. Neurosci. Biobehav. Rev. 2014;45:369–378. doi: 10.1016/j.neubiorev.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 170.James S., Montgomery P., Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD) Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD007992.pub2. [DOI] [PubMed] [Google Scholar]
- 171.Gow R.V., Hibbeln J.R. Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc. Psychiatr. Clin. N. Am. 2014;23:555–590. doi: 10.1016/j.chc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bondi C.O., Taha A.Y., Tock J.L., Totah N.K., Cheon Y., Torres G.E., Rapoport S.I., Moghaddam B. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol. Psychiatry. 2014;75:38–46. doi: 10.1016/j.biopsych.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim S.W., Schafer M.R., Klier C.M., Berk M., Rice S., Allott K., Bartholomeusz C.F., Whittle S.L., Pilioussis E., Pantelis C., et al. Relationship between membrane fatty acids and cognitive symptoms and information processing in individuals at ultra-high risk for psychosis. Schizophr. Res. 2014;158:39–44. doi: 10.1016/j.schres.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 174.Meyer B.J., Grenyer B.F., Crowe T., Owen A.J., Grigonis-Deane E.M., Howe P.R. Improvement of major depression is associated with increased erythrocyte DHA. Lipids. 2013;48:863–868. doi: 10.1007/s11745-013-3801-7. [DOI] [PubMed] [Google Scholar]
- 175.Gillies D., Sinn J., Lad S.S., Leach M.J., Ross M.J. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst. Rev. 2012;7 doi: 10.1002/14651858.CD007986.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lattka E., Klopp N., Demmelmair H., Klingler M., Heinrich J., Koletzko B. Genetic variations in polyunsaturated fatty acid metabolism--implications for child health? Ann. Nutr. Metab. 2012;60(Suppl. 3):8–17. doi: 10.1159/000337308. [DOI] [PubMed] [Google Scholar]
- 177.Horrobin D.F., Manku M.S., Hillman H., Iain A., Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biol. Psychiatry. 1991;30:795–805. doi: 10.1016/0006-3223(91)90235-E. [DOI] [PubMed] [Google Scholar]
- 178.Assies J., Lieverse R., Vreken P., Wanders R.J., Dingemans P.M., Linszen D.H. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol. Psychiatry. 2001;49:510–522. doi: 10.1016/S0006-3223(00)00986-0. [DOI] [PubMed] [Google Scholar]
- 179.McNamara R.K., Vannest J.J., Valentine C.J. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World J. Psychiatry. 2015;5:15–34. doi: 10.5498/wjp.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stevens L.J., Zentall S.S., Deck J.L., Abate M.L., Watkins B.A., Lipp S.R., Burgess J.R. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am. J. Clin. Nutr. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 181.Wu S., Ding Y., Wu F., Li R., Hou J., Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: A meta-analysis. Neurosci. Biobehav. Rev. 2015;48:1–9. doi: 10.1016/j.neubiorev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 182.Jiao J., Li Q., Chu J., Zeng W., Yang M., Zhu S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014;100:1422–1436. doi: 10.3945/ajcn.114.095315. [DOI] [PubMed] [Google Scholar]
- 183.Hawkey E., Nigg J.T. Omega-3 fatty acid and ADHD: Blood level analysis and meta-analytic extension of supplementation trials. Clin. Psychol. Rev. 2014;34:496–505. doi: 10.1016/j.cpr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bloch M.H., Hannestad J. Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry. 2012;17:1272–1282. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cooper R.E., Tye C., Kuntsi J., Vassos E., Asherson P. Omega-3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta-analysis. J. Psychopharmacol. 2015;29:753–763. doi: 10.1177/0269881115587958. [DOI] [PubMed] [Google Scholar]
- 186.Gould J.F., Smithers L.G., Makrides M. The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013;97:531–544. doi: 10.3945/ajcn.112.045781. [DOI] [PubMed] [Google Scholar]
- 187.Shulkin M.L., Pimpin L., Bellinger D., Kranz S., Duggan C., Fawzi W., Mozaffarian D. Effects of omega-3 supplementation during pregnancy and youth on neurodevelopment and cognition in childhood: A systematic review and meta-analysis. FASEB J. 2016;30(Suppl. 295):5. [Google Scholar]
- 188.Woods R.K., Thien F.C., Abramson M.J. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst. Rev. 2002 doi: 10.1002/14651858.CD001283. [DOI] [PubMed] [Google Scholar]
- 189.Best K.P., Gold M., Kennedy D., Martin J., Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am. J. Clin. Nutr. 2016;103:128–143. doi: 10.3945/ajcn.115.111104. [DOI] [PubMed] [Google Scholar]
- 190.Fortin P.R., Lew R.A., Liang M.H., Wright E.A., Beckett L.A., Chalmers T.C., Sperling R.I. Validation of a meta-analysis: The effects of fish oil in rheumatoid arthritis. J. Clin. Epidemiol. 1995;48:1379–1390. doi: 10.1016/0895-4356(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 191.Goldberg R.J., Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. doi: 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 192.Leitzmann M.F., Stampfer M.J., Michaud D.S., Augustsson K., Colditz G.C., Willett W.C., Giovannucci E.L. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am. J. Clin. Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 193.Chavarro J.E., Stampfer M.J., Li H., Campos H., Kurth T., Ma J. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol. Biomark. Prev. 2007;16:1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- 194.Sakai M., Kakutani S., Horikawa C., Tokuda H., Kawashima H., Shibata H., Okubo H., Sasaki S. Arachidonic acid and cancer risk: A systematic review of observational studies. BMC Cancer. 2012;12:606. doi: 10.1186/1471-2407-12-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hooper L., Thompson R.L., Harrison R.A., Summerbell C.D., Ness A.R., Moore H.J., Worthington H.V., Durrington P.N., Higgins J.P., Capps N.E., et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rice H.B., Bernasconi A., Maki K.C., Harris W.S., von Schacky C., Calder P.C. Conducting omega-3 clinical trials with cardiovascular outcomes: Proceedings of a workshop held at ISSFAL 2014. Prostaglandins Leukot. Essent. Fat. Acids. 2016;107:30–42. doi: 10.1016/j.plefa.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 197.Superko H.R., Superko A.R., Lundberg G.P., Margolis B., Garrett B.C., Nasir K., Agatston A.S. Omega-3 fatty acid blood levels clinical significance update. Curr. Cardiovasc. Risk Rep. 2014;8:407. doi: 10.1007/s12170-014-0407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Draisma H.H.M., Pool R., Kobl M., Jansen R., Petersen A.K., Vaarhorst A.A.M., Yet I., Haller T., Demirkan A., Esko T., et al. Genome-wide association study identifies novel genetic variants contributing to variation in blood metabolite levels. Nat. Commun. 2015;6:7208. doi: 10.1038/ncomms8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shin S.Y., Fauman E.B., Petersen A.K., Krumsiek J., Santos R., Huang J., Arnold M., Erte I., Forgetta V., Yang T.P., et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Long T., Hicks M., Yu H.C., Biggs W.H., Kirkness E.F., Menni C., Zierer J., Small K.S., Mangino M., Messier H., et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 2017;49:568–578. doi: 10.1038/ng.3809. [DOI] [PubMed] [Google Scholar]
- 201.Arnold M., Raffler J., Pfeufer A., Suhre K., Kastenmuller G. SNiPA: An interactive, genetic variant-centered annotation browser. Bioinformatics. 2015;31:1334–1336. doi: 10.1093/bioinformatics/btu779. [DOI] [PMC free article] [PubMed] [Google Scholar]