Abstract
Background: Carnitine deficiency has been implicated as a potential pathway for cancer-related fatigue that could be treated with carnitine supplementation. The aim of this systematic literature review and meta-analysis was to evaluate the literature regarding the use of supplemental carnitine as a treatment for cancer-related fatigue. Methods: Using the PRISMA guidelines, an electronic search of the Cochrane Library, MEDLINE, Embase, CINAHL and reference lists was conducted. Data were extracted and independently assessed for quality using the Academy of Nutrition and Dietetics evidence analysis by two reviewers. In studies with positive quality ratings, a meta-analysis was performed using the random-effects model on Carnitine and cancer-related fatigue. Results: Twelve studies were included for review with eight reporting improvement in measures of fatigue, while four reported no benefit. However, many studies were non-randomized, open-label and/or used inappropriate dose or comparators. Meta-analysis was performed in three studies with sufficient data. Carnitine did not significantly reduce cancer-related fatigue with a standardized mean difference (SMD) of 0.06 points ((95% CI −0.09, 0.21); p = 0.45). Conclusion: Results from studies with lower risk of bias do not support the use of carnitine supplementation for cancer-related fatigue.
Keywords: carnitine, fatigue, cancer, dietary supplement, systematic review
1. Introduction
Cancer-related fatigue (CRF) is one of the most common side-effects of cancer treatment, affecting up to 91% of patients and is now considered one of the most distressing symptoms of cancer [1,2]. CRF is associated with worsened quality of life, depression and anxiety, inability to perform activities of daily living and can have significant financial costs to both patients and their caregivers [3,4]. Furthermore, CRF can persist for months or years after the completion of cancer treatment and is associated with reduced recurrence-free and overall survival [5,6,7,8].
Despite its high prevalence, the aetiology of CRF remains unclear. Mechanisms implicated in the development of CRF include inflammation, anaemia and altered neuroendocrine pathways [9]. In addition, most observational studies report that decreased serum carnitine has been associated with fatigue [10,11,12,13]. Carnitine is pivotal to energy production; facilitating the uptake of fatty acids into the mitochondria for beta-oxidation. Chemotherapy regimens including cisplatin and ifosfomide disrupt carnitine metabolism by increasing the renal excretion of carnitine [14,15]. Furthermore, muscle wasting, as seen in cancer cachexia, can further exacerbate fatigue. Carnitine may have multiple properties that can prevent or reduce muscle wasting including modulation of protein synthesis and degradation, as well as anti-apoptotic, antioxidant and anti-inflammatory properties [16]. Therefore, it is plausible that the restoration of serum carnitine levels via supplemental carnitine could ameliorate CRF.
Several trials have investigated the effectiveness of supplemental carnitine for the management of CRF. The aim of this review was to systematically evaluate intervention trials regarding the use of supplemental carnitine as a treatment for cancer-related fatigue to inform the clinical management of CRF.
2. Methods
This study was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
2.1. Eligibility Criteria
Eligible studies included those evaluating CRF as a primary or secondary outcome in patients of any cancer diagnosis and age, supplemented with carnitine as either a stand-alone intervention or in combination with other agents. Studies were limited to those published in English. Randomized and pseudo-randomized controlled trials were preferred; however, if there were less than five randomized studies, non-randomized and single arm studies were included.
2.2. Data Collection and Extraction
The CINAHL, Cochrane Library (i.e., Cochrane CENTRAL and Cochrane Database of Systematic Reviews), Embase and MEDLINE databases were searched from database inception to December 2016. Two reviewers (Wolfgang Marx and Laisa Teleni) independently screened the titles and abstracts for relevance. Relevant articles were retrieved and two review authors (Wolfgang Marx and Laisa Teleni) independently screened the full text for eligibility. The reference lists of eligible articles were screened for relevant publications. Data extraction was performed in duplicate (Wolfgang Marx and Rachelle S Opie) and a third author (Liz Isenring) was designated as referee.
Extracted data included the study design, inclusion and exclusion criteria, patient demographics (e.g., age, gender), dosing schedule (including dose and frequency), sample size (including dropout rates and reasons), method used to assess fatigue, study outcomes (including self-reported measures of fatigue and adverse events), funding details and potential conflict of interest. We extracted the mean (change from baseline or end-of-study value) and appropriate variance data (standard deviation, standard error or 95% confidence intervals) to perform the meta-analysis.
2.3. Assessment of Study Quality
The quality of the included studies was assessed independently by two authors (Wolfgang Marx and Rachelle S Opie) using the Academy of Nutrition and Dietetics Quality Criteria Checklist which assesses studies for selection, allocation, reporting and attrition bias as well as the level of external validity. Using this checklist, each study was assigned a positive (low risk of bias), negative (high risk of bias) or neutral (moderate risk of bias) rating.
2.4. Data Synthesis
Studies that were rated as positive quality were pooled into Revman for meta-analysis using the DerSimonian and Laird random-effects model [18,19]. Based on the quality rating of the included studies, meta-analysis was deemed inappropriate in 9 of the 12 studies and therefore, findings are presented in narrative form. Treatment effect was calculated as the standardized mean difference (SMD) due to the variability in fatigue measurement scales. One study was standardized to a negative score by multiplying the mean and standard deviation by −1 to reflect a directional score consistent with the other studies [20,21]. A statistically significant (p < 0.05) result was considered evidence of an effect.
3. Results
3.1. Study Characteristics
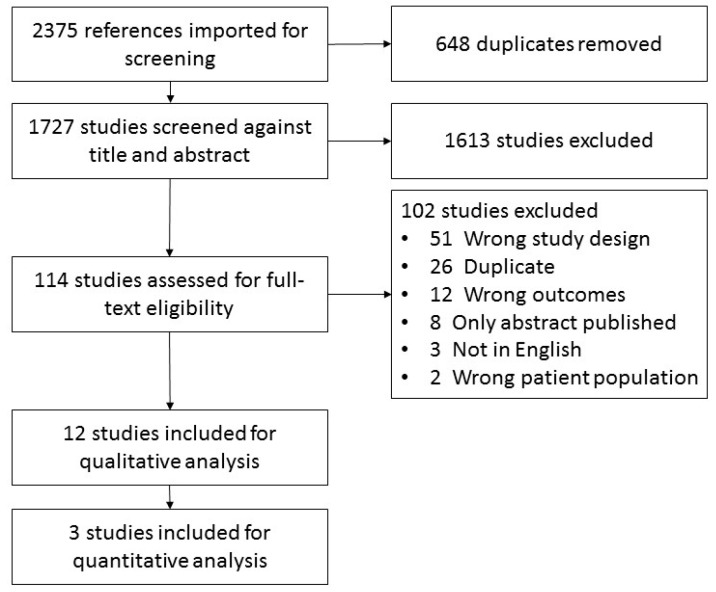
Of the 1727 articles screened, 12 met the eligibility criteria (Figure 1). Population groups included eight advanced cancer, seven mixed diagnoses, two breast cancer only and the remaining studies included pancreatic, gynaecological cancer or multiple myeloma patients only. Three studies included patients with carnitine deficiency only (as confirmed by blood test), five studies included patients with self-reported fatigue and six had no restriction on carnitine deficiency or fatigue in their eligibility criteria.
Figure 1.
Systematic review flow diagram.
The included studies used a variety of study designs. Three studies were single-arm trials and eight studies used a comparator arm, four of which used a placebo control while five used either standard care or various active ingredients (see Table 1). Eight studies were open-label, two studies were double-blinded throughout the intervention [20,22] and two studies incorporated both a double-blinded and an open-label phase [23,24]. Intervention characteristics and key findings are summarized in Table 1.
Table 1.
Study Design, Population and Quality of Included Studies.
| Study & Design | Study Design and Quality | Population and Attrition | Sample Size and Attrition |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CG, control group; COI, conflict of interest; IG, intervention group; MA, N/A, not applicable.
There was a large range of sample sizes with eight studies having a total sample size below 100 participants. However, four studies had moderate-to-large sample sizes that ranged from 144 to 409 participants [20,24,25,26].
3.2. Carnitine Regimens
All studies used an oral dose of carnitine, delivered in a range of forms including liquid (n = 5) [22,23,24,27,28] capsule (n = 1) and jelly (n = 1) formulations [29]. Nine studies used a dose between 2 to 6 g per day and three studies used <2 g/day, taken once or up to three divided doses per day, with Iwase et al. [29] using the smallest dose of 50 mg [23,27]. Most studies used carnitine as a stand-alone intervention—however, three studies used carnitine as a co-intervention with antioxidant supplements (e.g., coenzyme Q10, alpha lipoic acid), nonsteroidal anti-inflammatories (e.g., celecoxib) and steroids (e.g., megestrol acetate and medroxyprogesterone acetate) [25,29,30]. The intervention period varied from one week up to 24 weeks.
3.3. Outcome Measures
All studies used a self-reported measure to assess fatigue with most studies using the following validated questionnaires: The Multidimensional Fatigue Symptom Inventory—Short Form, Functional Assessment of Chronic Illness Therapy-Fatigue Scale, or the Brief Fatigue Index (BFI). Three studies calculated sample sizes a priori based on fatigue with one study, Cruciani et al. [24], achieving the required sample size [23,24,29]. Five studies did not report on fatigue as their primary outcome but provided powered calculations based on other outcomes (e.g., lean body mass, peripheral neuropathy and inflammation) and four provided no sample size calculation.
Other secondary outcomes included quality of life, anthropometric measures (e.g., lean body mass, grip strength, DEXA), pathology (e.g., reactive oxygen species, glutathione peroxidase, superoxide dismutase, pro-inflammatory cytokines and C-reactive protein), physical function, depression and mood scales, measures of peripheral neuropathy and treatment response (e.g., complete remission, partial response, minimal response).
3.4. Compliance Measures
Five studies reported on compliance with Hershman et al. [20] (pill count) and Kraft et al. [22] (serum carnitine) providing sufficient detail on the method of measuring compliance [25,26,30].
3.5. Quality Rating
Three studies included in this review received a positive quality rating with two receiving a neutral rating and seven receiving a negative rating (Table 1). The primary reasons for the neutral or negative rating were a lack of blinding, lack of inclusion of a placebo/control group, lack of adjustment of potential confounders and randomization, failure to conduct an intention-to-treat analysis.
3.6. Intervention Results on Cancer-Related Fatigue
Four studies reported no improvement in measures of CRF in response to carnitine supplementation and eight studies reported significant improvement in CRF (Table 2). Four single arm studies reported significant improvements when compared to baseline [27,28,30,31]. Iwase et al. [29] reported a significant improvement in worst level of and mean change in fatigue compared to the control group but not average fatigue. Maccio et al. [25] reported that a combination therapy that included carnitine (as well as celecoxib, alpha lipoic acid, carboxycysteine and megestrol acetate) resulted in a significantly improved CRF compared to the control group. Similarly, Mantovani et al. [26] reported a significant improvement in fatigue when using a combination intervention but not when participants received carnitine and an antioxidant supplement only. Cruciani et al. [23] found no significant difference between the intervention and placebo group during a blinded phase; however, after a second, open-label phase whereby all participants received the carnitine supplement, the participants who were originally allocated to the blinded intervention group reported significantly improved levels of fatigue.
Table 2.
Intervention and Results of Included Studies.
| Study & Design | Intervention | Results |
|---|---|---|
|
|
Fatigue: At 3-weeks post-baseline: FACT-Fatigue (scored 0–65; lower scores indicate more severe symptoms)
|
|
|
Fatigue: At 4-weeks post-baseline: MFSI-SF (scored 0–150; higher scores indicate more severe symptoms)
QoL-OS (scored 0–73; higher scores indicate more severe symptoms)
Lean body mass via BIA
Numerical scale (scored 0–10; lower scores indicate more severe symptoms)
Grip strength via dynamometer data not reported (P > 0.05 since baseline) Pathology: At 4-weeks post-baseline: ROS
|
|
|
For all patients (n = 27): Fatigue: At 1-week post-baseline: Brief Fatigue Inventory (scored 0–90; higher scores indicate more severe symptoms)
Centre for Epidemiologic Studies Depression Scale (scored 0–60; higher scores indicate more severe symptoms)
Epworth Sleeplessness Scale (scored 0–24; higher scores indicate more severe symptoms)
Haemoglobin
|
|
|
Fatigue: At end of treatment: FACT-Fatigue (scored 0–65; lower scores indicate more severe symptoms)
|
|
|
Fatigue: At 3-weeks post-baseline: Brief Fatigue Inventory global fatigue score (scored 0–10; higher scores indicate more severe symptoms)
EORTC-QLQ-C30 global health status sub-group (scoring unclear; lower scores indicate more severe symptoms)
Hospital Anxiety and Depression Scale—Anxiety (scored 0–21; lower scores indicate more severe symptoms)
Most common severe adverse events were leukopenia and neutropenia. Detailed list of adverse events included in Iwase et al. |
|
|
Fatigue: At 4-weeks post-baseline: FACT-Anaemia fatigue sub-scale (scoring unclear; lower scores indicate more severe symptoms)
FACT-Anaemia physical sub-scale (scoring unclear; lower scores indicate more severe symptoms)
KPS (scoring 0–100; lower scores indicate more severe symptoms)
|
|
|
Fatigue: At 3-months post-baseline: Brief Fatigue Inventory (scored 0–90; higher scores indicate more severe symptoms)
Body mass index via BIA
EORTC-QLQ-C30 global health status sub-group (scoring unclear; lower scores indicate more severe symptoms)
EORTC-QLQ-C30 cognitive function sub-group (scoring unclear; lower scores indicate more severe symptoms)
|
|
|
Fatigue: At 4-weeks post-baseline: Brief Fatigue Inventory (scored 0–90; higher scores indicate more severe symptoms)
Centre for Epidemiologic Studies Depression Scale (scored 0–60; higher scores indicate more severe symptoms)
ECOG PS (scoring 0–5; higher scores indicate more severe symptoms)
|
|
|
Fatigue: At 6-months post-baseline: FACT-Fatigue (scored 0–65; lower scores indicate more severe symptoms)
FACT-Taxane Trial Outcome Index (scoring unclear; lower scores indicate more severe symptoms)
|
|
|
Fatigue: At 4-months post-baseline: MFSI-SF (scored 0–150; higher scores indicate more severe symptoms)
EORTC-QLQ-C30 (scored 0–100; lower scores indicate more severe symptoms)
ECOG PS (scoring 0–5; higher scores indicate more severe symptoms)
Lean body mass via BIA
Grip strength via dynamometer
Visual analogue scale (scoring unclear; lower scores indicate more severe symptoms)
IL-6
|
|
|
Fatigue: At 4-months post-baseline: MFSI-SF (scored 0–150; higher scores indicate more severe symptoms)
EORTC-QLQ-C30 (scored 0–100; lower scores indicate more severe symptoms)
Lean body mass via DEXA
Visual analogue scale (scoring unclear; lower scores indicate more severe symptoms)
Grip strength via dynamometer
ECOG PS (scoring 0–5; higher scores indicate more severe symptoms)
CRP
IG-a n = 2 diarrhoea and n = 1 epigastria; IG-b n = 1 epigastria |
|
|
Fatigue: At 4-months post-baseline: MFSI-SF (scored 0–150; higher scores indicate more severe symptoms)
EORTC-QLQ-C30 (scored 0–100; lower scores indicate more severe symptoms)
Visual analogue scale (scoring unclear; lower scores indicate more severe symptoms)
Lean body mass via DEXA
Grip strength via dynamometer
6-min walk test
CRP
|
μm, micrometre; BCAA, branched chain amino acid; BIA, bioelectrical impedance analysis; CG, control group; Co-Q10, coenzyme Q10; COI, conflict of interest; CRP, c-reactive protein; CT, computed tomography; DEXA, dual-energy X-ray absorptiometry; ECOG PS, decilitre; Eastern Cooperative Oncology Group performance status; dL, decilitre; FACT, Functional Assessment of Cancer Therapy; g, gram; GPx, Glutathione peroxidase; IG, intervention group; IL, interleukin; kcal, kilocalorie; IU, international unit; IV, intravenous; kg, kilogram; KPS, Karnofsky Performance Status; L, litre; L3, third lumbar vertebrae; m, meter; megestrol acetate; MPA, medroxyprogesterone acetate; MFSI-SF, Multidimensional Fatigue Symptoms Inventory-Short Form; mg, milligrams; MPA, medroxyprogesterone acetate; N/A, not applicable; pg, picogram; QoL, quality of life; ROS, Reactive oxygen species; SOD, superoxide dismutase; TNF, tumour necrosis factor. Ω Data reported in Cruciani et al. 2009 [23] was a mean of 117.2 ± 4.9; however, due to the scoring of the assessment tool sub-scale and other data points reported in using this tool the review authors believe this to be an error and that the mean was 17.2.
All studies with a low risk of bias reported no significant difference in measures of fatigue while most studies with a moderate to high risk of bias reported significant improvements in fatigue.
3.7. Adverse Events
Eight studies provided data on adverse events. Commonly reported adverse events included diarrhoea (n = 7 studies) [20,23,24,25,26,29,32] and haematological toxicities (n = 3 studies) [24,29,32] with both symptoms reported in approximately less than five patients in each study reporting ≥grade 3 symptoms. Of the studies that included a control group, no significant increase in adverse events in the intervention group was reported.
3.8. Meta-Analysis
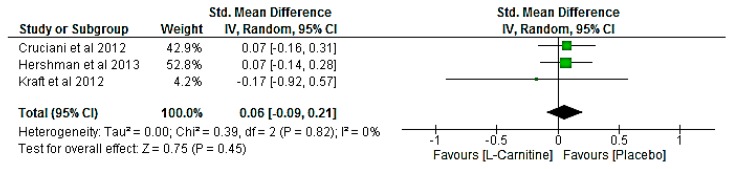
In three studies involving a total of 659 participants [20,22,24], carnitine did not significantly reduce CRF (SMD of 0.06 points (95% CI −0.09, 0.21); p = 0.45; Figure 2). There was no evidence of statistical heterogeneity (I2 = 0%). Clinical heterogeneity was evident from the three studies in regards to the dose (2–4 g of carnitine per day), patient demographics (40–100% females included) and carnitine status. However, there were not enough studies to conduct sensitivity analyses to isolate these potential sources of heterogeneity and test the robustness of findings.
Figure 2.
Forest plot of the effect of Carnitine dietary supplement on Cancer-related fatigue. CI = confidence interval; IV = inverse variance.
4. Discussion
Our review identified 12 studies that investigated the use of orally administered carnitine for the treatment of CRF. Despite most (8/12) studies reporting a significant improvement in measures of CRF, most (9/12) studies contain significant limitations that require consideration. Many studies were open-label, single-arm trials which introduces significant performance, selection and detection bias, particularly for fatigue, a self-reported measure that is likely to be susceptible to a placebo response. Some studies either did not analyse or did not find statistical improvements in fatigue when compared to a control or other intervention groups and instead reported improvements in fatigue at the final time point when compared to baseline. The lack of significant improvements compared to a parallel control group further limits the confidence that these improvements are attributed to the intervention alone.
The two largest studies, both randomized controlled trials, reported no significant difference in CRF between intervention and control groups [20,24]. Hershman et al. [20] reported no significant difference in fatigue; however, CRF was a secondary outcome and did not exclusively recruit patients reporting carnitine deficiency or fatigue. The only included study that was sufficiently powered to detect significant differences in fatigue, Cruciani et al. [24], found no effect in any measure of fatigue despite promising results from their earlier studies [27,33]. A possible explanation for these differences is that earlier studies from the same authors recruited carnitine deficient patients only (defined as free carnitine <35 mM/L for males or <25 mM/L for females, or acyl/free carnitine ratio >0.4) which is in contrast to their largest study which recruited patients with moderate to severe fatigue, irrespective of carnitine status [24]. However, an included subgroup analysis of carnitine deficient patients reported no statistically significant differences in CRF despite mean CRF levels in the carnitine group being consistently lower at follow-up time points. The authors noted that the dose used in their study was lower than doses used in studies that have reported improvements in fatigue (1 g versus up to 6 g) [25]. However, there are also studies that have investigated higher doses that have reported no significant improvements [20,22,32].
While the lower doses of carnitine used in some included studies (e.g., 50 mg reported by Iwase et al. [29]) are unlikely to deliver a sufficient dose of absorbable carnitine to provide a therapeutic effect, pharmacokinetic research suggests that higher doses may also be suboptimal [34,35]. Carnitine supplementation has relatively poor oral bioavailability, with 5–16% of carnitine being absorbed after a single dose of 2 g and 6 g of carnitine in healthy participants [34]. Furthermore, plasma concentrations of carnitine are non-linear with one study reporting that doses of 0.5 g, 1 g and 2 g resulted in similar plasma concentrations of carnitine while the 2 g dose resulted in significantly increased plasma concentrations of a proatherogenic metabolite of carnitine, trimethylamine-N-oxide [36], indicating that absorption had been saturated and that doses exceeding 2 g may not provide further therapeutic effect [35]. It should be noted, however, that the cited pharmacokinetic studies have all been conducted in healthy participants and the dose of carnitine required to saturate absorption pathways may differ in carnitine deficient populations.
There are multiple risk factors for CRF including but not limited to, inflammation, anaemia and altered neuroendocrine pathways [9]. The lack of control for these potential mechanisms of fatigue in the included studies may have confounded the results. This is partially supported by the positive results of studies that investigated carnitine as part of a multi-ingredient intervention that targeted multiple pathways [25,26,30].
Follow-up periods and patient populations were also inconsistent across studies. Carnitine status fluctuates both during the course of cancer treatment and in response to different chemotherapy regimens [11,12]. For example, doxorubicin is reported to affect carnitine levels to a greater extent than other treatments and Heuberger et al. [37] reported carnitine levels to increase one week after chemotherapy while studies that have measured carnitine status with longer time points have reported a decrease [11,12]. Hence, future studies are required to investigate carnitine fluctuations over the course of chemotherapy treatment and intervention trials may benefit from recruiting homogenous cancer populations.
Future Directions and Clinical Implications
Due to the dearth of clinical studies with low risk of bias, clinical recommendations for the use of carnitine in treating CRF are premature. Only 3 of the 12 studies were suitable for meta-analysis which reduces the conclusions which can be drawn from the analysis. The available evidence indicates carnitine supplementation is unlikely to provide a clinically meaningful benefit for CRF in the chemotherapy cancer-population.
Further research is required to elucidate the safety profile of carnitine in the cancer setting. While the included studies reported carnitine supplementation to be well tolerated, Hershman et al. [20] found symptoms of peripheral neuropathy increased in participants receiving carnitine supplementation, which should be considered before clinical use. Furthermore, carnitine supplementation may increase risk of cardiovascular disease via the increase in proatherogenic microbiota-derived metabolites trimethylamine, trimethylamine-N-oxide and γ-butyrobetaine [36] These metabolites have also been shown in animal models to increase concentrations of the carcinogenic compound N-nitrosodimethylamine [38]. Therefore, long term carnitine supplementation may increase risk of chronic diseases, particularly at higher doses which provides a greater amount of substrates for these proatherogenic metabolites.
Biases inherent in intervention study designs with high-risk of bias mean it is difficult to determine if the reported adverse events can be attributed solely to carnitine supplementation. Future intervention studies should utilize trial designs with the lowest risk of bias (e.g., randomized controlled trials), implement methods of measuring adherence to intervention (e.g., pill counts and/or serum carnitine), investigate carnitine as a stand-alone intervention and ensure study populations are controlled for aetiology of fatigue and chemotherapy regimens.
5. Conclusions
Of the 12 studies included in this systematic review, eight reported carnitine supplementation to significantly improve measures of cancer-related fatigue. However, due to the significant bias of many included studies, the null findings of the two largest studies and our meta-analysis and the potential increase in peripheral neuropathy, there is currently insufficient evidence to recommend its use in the cancer setting. Future studies should include rigorous study design methods to reduce bias and focus on population subsets with confirmed carnitine deficiency.
Acknowledgments
Publications fees were funded by the Bond University APC Funding Scheme. The authors received no other funding for this publication.
Author Contributions
W.M. and L.T. conceptualized the systematic review, designed and implemented the protocol. R.S.O. and W.M. completed the quality appraisal, J.K. conducted the meta-analysis. All authors contributed to the manuscript development. All authors have read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lawrence D.P., Kupelnick B., Miller K., Devine D., Lau J. Evidence report on the occurrence, assessment and treatment of fatigue in cancer patients. J. Natl. Cancer Inst. Monogr. 2004 doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 2.Carelle N., Piotto E., Bellanger A., Germanaud J., Thuillier A., Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155–163. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]
- 3.Curt G.A., Breitbart W., Cella D., Groopman J.E., Horning S.J., Itri L.M., Johnson D.H., Miaskowski C., Scherr S.L., Portenoy R.K., et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 4.Broeckel J.A., Jacobsen P.B., Horton J., Balducci L., Lyman G.H. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 5.Curran S.L., Beacham A.O., Andrykowski M.A. Ecological momentary assessment of fatigue following breast cancer treatment. J. Behav. Med. 2004;27:425–444. doi: 10.1023/B:JOBM.0000047608.03692.0c. [DOI] [PubMed] [Google Scholar]
- 6.Groenvold M., Petersen M.A., Idler E., Bjorner J.B., Fayers P.M., Mouridsen H.T. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res. Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 7.Quinten C., Maringwa J., Gotay C.C., Martinelli F., Coens C., Reeve B.B., Flechtner H., Greimel E., King M., Osoba D., et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J. Natl. Cancer Inst. 2011;103:1851–1858. doi: 10.1093/jnci/djr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bower J.E., Ganz P.A., Desmond K.A., Bernaards C., Rowland J.H., Meyerowitz B.E., Belin T.R. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 9.Bower J.E. Cancer-related fatigue mdash mechanisms, risk factors and treatments. Nat. Rev. Clin. Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter S.C., Szabo-Aczel S., Curry C.J., Hutchinson H.T., Hogue R., Shug A. Plasma carnitine deficiency. Clinical observations in 51 pediatric patients. Am. J. Dis. Child. 1987;141:660–665. doi: 10.1001/archpedi.1987.04460060076039. [DOI] [PubMed] [Google Scholar]
- 11.Hockenberry M.J., Hooke M.C., Gregurich M., McCarthy K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J. Pediatr. Hematol. Oncol. 2009;31:664–669. doi: 10.1097/MPH.0b013e3181b259a7. [DOI] [PubMed] [Google Scholar]
- 12.Endo K., Tsuji A., Kondo S., Wakisaka N., Murono S., Yoshizaki T. Carnitine is associated with fatigue following chemoradiotherapy for head and neck cancer. Acta Oto-Laryngol. 2015;135:846–852. doi: 10.3109/00016489.2015.1030769. [DOI] [PubMed] [Google Scholar]
- 13.Marx W., Teleni L., Ferguson M., Walpole E., Isenring E. The Effect of Chemotherapy on Serum Carnitine Levels and Fatigue in Chemotherapy Naïve Medical Oncology Patients: A Pilot Study. Carnitine, Chemotherapy and Fatigue. J. Nutr. Disord. Ther. 2014 doi: 10.4172/2161-0509.1000131. [DOI] [Google Scholar]
- 14.Mancinelli A., D’Iddio S., Bisonni R., Graziano F., Lippe P., Calvani M. Urinary excretion of l-carnitine and its short-chain acetyl-l-carnitine in patients undergoing carboplatin treatment. Cancer Chemother. Pharmacol. 2007;60:19–26. doi: 10.1007/s00280-006-0341-3. [DOI] [PubMed] [Google Scholar]
- 15.Marthaler N.P., Visarius T., Kupfer A., Lauterburg B.H. Increased urinary losses of carnitine during ifosfamide chemotherapy. Cancer Chemother. Pharmacol. 1999;44:170–172. doi: 10.1007/s002800050963. [DOI] [PubMed] [Google Scholar]
- 16.Ringseis R., Keller J., Eder K. Mechanisms underlying the anti-wasting effect of l-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013;52:1421–1442. doi: 10.1007/s00394-013-0511-0. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Review Manager (RevMan) [(accessed on 7 October 2017)]; Available online: http://community.cochrane.org/tools/review-production-tools/revman-5.
- 20.Hershman D.L., Unger J.M., Crew K.D., Minasian L.M., Awad D., Moinpour C.M., Hansen L., Lew D.L., Greenlee H., Fehrenbacher L., et al. Randomized Double-Blind Placebo-Controlled Trial of Acetyl-l-Carnitine for the Prevention of Taxane-Induced Neuropathy in Women Undergoing Adjuvant Breast Cancer Therapy. J. Clin. Oncol. 2013 doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 22.Kraft M., Kraft K., Gärtner S., Mayerle J., Simon P., Weber E., Schütte K., Stieler J., Koula-Jenik H., Holzhauer P., et al. l-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—A randomized multicentre trial. Nutr. J. 2012;11:52. doi: 10.1186/1475-2891-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruciani R.A., Dvorkin E., Homel P., Culliney B., Malamud S., Lapin J., Portenoy R.K., Esteban-Cruciani N. l-carnitine supplementation in patients with advanced cancer and carnitine deficiency: A double-blind, placebo-controlled study. J. Pain Symptom Manag. 2009;37:622–631. doi: 10.1016/j.jpainsymman.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Cruciani R.A., Zhang J.J., Manola J., Cella D., Ansari B., Fisch M.J. l-carnitine Supplementation for the Management of Fatigue in Patients With Cancer: An Eastern Cooperative Oncology Group Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Oncol. 2012;30:3864–3869. doi: 10.1200/JCO.2011.40.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maccio A., Madeddu C., Gramignano G., Mulas C., Floris C., Sanna E., Cau M.C., Panzone F., Mantovani G. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynaecological cancers: Evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol. Oncol. 2012;124:417–425. doi: 10.1016/j.ygyno.2011.12.435. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani G., Maccio A., Madeddu C., Serpe R., Massa E., Dessi M., Panzone F., Contu P. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15:200–211. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruciani R.A., Dvorkin E., Homel P., Malamud S., Culliney B., Lapin J., Portenoy R.K., Esteban-Cruciani N. Safety, tolerability and symptom outcomes associated with l-carnitine supplementation in patients with cancer, fatigue and carnitine deficiency: A phase I/II study. J. Pain Symptom Manag. 2006;32:551–559. doi: 10.1016/j.jpainsymman.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Gramignano G., Lusso M.R., Madeddu C., Massa E., Serpe R., Deiana L., Lamonica G., Dessi M., Spiga C., Astara G., et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006;22:136–145. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Iwase S., Kawaguchi T., Yotsumoto D., Doi T., Miyara K., Odagiri H., Kitamura K., Ariyoshi K., Miyaji T., Ishiki H., et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and l-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: A multi-institutional, randomized, exploratory trial (JORTC-CAM01) Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2016;24:637–646. doi: 10.1007/s00520-015-2824-4. [DOI] [PubMed] [Google Scholar]
- 30.Madeddu C., Dessi M., Panzone F., Serpe R., Antoni G., Cau M.C., Montaldo L., Mela Q., Mura M., Astara G., et al. Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib +/− megestrol acetate for patients with cancer-related anorexia/cachexia syndrome. Clin. Nutr. 2012;31:176–182. doi: 10.1016/j.clnu.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Graziano F., Bisonni R., Catalano V., Silva R., Rovidati S., Mencarini E., Ferraro B., Canestrari F., Baldelli A.M., De Gaetano A., et al. Potential role of levocarnitine supplementation for the treatment of chemotherapy-induced fatigue in non-anaemic cancer patients. Br. J. Cancer. 2002;86:1854–1857. doi: 10.1038/sj.bjc.6600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callander N., Markovina S., Eickhoff J., Hutson P., Campbell T., Hematti P., Go R., Hegeman R., Longo W., Williams E., et al. Acetyl-l-carnitine (ALCAR) for the prevention of chemotherapy-induced peripheral neuropathy in patients with relapsed or refractory multiple myeloma treated with bortezomib, doxorubicin and low-dose dexamethasone: A study from the Wisconsin Oncology Network. Cancer Chemother. Pharmacol. 2014;74:875–882. doi: 10.1007/s00280-014-2550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruciani R.A., Zhang J., Manola J.B., Cella D., Ansari B., Fisch M.J. Phase III randomized, placebo-controlled trial of l-carnitine supplementation for fatigue in patients with cancer. J. Clin. Oncol. 2009;27:e20532. doi: 10.1200/JCO.2011.40.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harper P., Elwin C.-E., Cederblad G. Pharmacokinetics of bolus intravenous and oral doses of l-carnitine in healthy subjects. Eur. J. Clin. Pharmacol. 1988;35:69–75. doi: 10.1007/BF00555510. [DOI] [PubMed] [Google Scholar]
- 35.Bain M.A., Milne R.W., Evans A.M. Disposition and metabolite kinetics of oral l-carnitine in humans. J. Clin. Pharmacol. 2006;46:1163–1170. doi: 10.1177/0091270006292851. [DOI] [PubMed] [Google Scholar]
- 36.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heuberger W., Berardi S., Jacky E., Pey P., Krahenbuhl S. Increased urinary excretion of carnitine in patients treated with cisplatin. Eur. J. Clin. Pharmacol. 1998;54:503–508. doi: 10.1007/s002280050504. [DOI] [PubMed] [Google Scholar]
- 38.Empl M.T., Kammeyer P., Ulrich R., Joseph J.F., Parr M.K., Willenberg I., Schebb N.H., Baumgartner W., Rohrdanz E., Steffen C., et al. The influence of chronic l-carnitine supplementation on the formation of preneoplastic and atherosclerotic lesions in the colon and aorta of male F344 rats. Arch. Toxicol. 2015;89:2079–2087. doi: 10.1007/s00204-014-1341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]