Abstract
Sleep spindles promote the consolidation of motor skill memory in young adults. Older adults, however, exhibit impoverished sleep-dependent motor memory consolidation. The underlying pathophysiological mechanism(s) explaining why motor memory consolidation in older adults fails to benefit from sleep remains unclear. Here, we demonstrate that male and female older adults show impoverished overnight motor skill memory consolidation relative to young adults, with the extent of impairment being associated with the degree of reduced frontal fast sleep spindle density. The magnitude of the loss of frontal fast sleep spindles in older adults was predicted by the degree of reduced white matter integrity throughout multiple white matter tracts known to connect subcortical and cortical brain regions. We further demonstrate that the structural integrity of selective white matter fiber tracts, specifically within right posterior corona radiata, right tapetum, and bilateral corpus callosum, statistically moderates whether sleep spindles promoted overnight consolidation of motor skill memory. Therefore, white matter integrity within tracts known to connect cortical sensorimotor control regions dictates the functional influence of sleep spindles on motor skill memory consolidation in the elderly. The deterioration of white matter fiber tracts associated with human brain aging thus appears to be one pathophysiological mechanism influencing subcortical–cortical propagation of sleep spindles and their related memory benefits.
SIGNIFICANCE STATEMENT Numerous studies have shown that sleep spindle expression is reduced and sleep-dependent motor memory is impaired in older adults. However, the mechanisms underlying these alterations have remained unknown. The present study reveals that age-related degeneration of white matter within select fiber tracts is associated with reduced sleep spindles in older adults. We further demonstrate that, within these same fiber tracts, the degree of degeneration determines whether sleep spindles can promote motor memory consolidation. Therefore, white matter integrity in the human brain, more than age per se, determines the magnitude of decline in sleep spindles in later life and, with it, the success (or lack thereof) of sleep-dependent motor memory consolidation in older adults.
Keywords: aging, DTI, motor memory, sleep, sleep spindles, WMH
Introduction
An established literature in young adults demonstrates that sleep, relative to wakefulness, supports the offline consolidation of motor sequence memory preferentially, affording stability that slows forgetting (Smith and MacNeill, 1994; Fischer et al., 2002; Walker et al., 2002, 2003b, 2005; Korman et al., 2003; Robertson et al., 2004; Fischer et al., 2005; Spencer et al., 2006, 2007; Nishida and Walker, 2007; Rickard et al., 2008; Barakat et al., 2011; Tucker et al., 2011; Wilson et al., 2012; Fogel et al., 2014; Nettersheim et al., 2015). Consistent with previous reports (but see Karni et al., 1994; Fischer et al., 2002), these overnight benefits in motor skill consolidation correlate with the amount of nonrapid eye movement (NREM) sleep, specifically stage 2 NREM sleep, particularly in the last quartile (Smith and MacNeill, 1994; Walker et al., 2002; Robertson et al., 2004; Nishida and Walker, 2007; Barakat et al., 2011; Fogel et al., 2014), and the fast-frequency sleep spindles that predominate during these stages (Nishida and Walker, 2007; Barakat et al., 2011; Fogel et al., 2014). The latter finding is of particular relevance considering a recent report establishing the causal role of stage 2 NREM fast-frequency sleep spindles in actively promoting motor skill memory consolidation (Lustenberger et al., 2016). This sleep spindle-dependent consolidation effect is associated with a network-level reorganization of motor memories throughout primary and secondary motor cortices, sensorimotor parietal cortex, basal ganglia, and cerebellum (Fischer et al., 2005; Walker et al., 2005; Fogel et al., 2014).
In contrast to young adults, multiple studies have demonstrated reduced sleep spindles (De Gennaro and Ferrara, 2003; Martin et al., 2013; Mander et al., 2014) and impoverished overnight motor skill memory consolidation in older adults, resulting in nominal retention differences relative to equivalent time periods spent awake (Spencer et al., 2007; Wilson et al., 2012; Fogel et al., 2014). Moreover, sleep spindles do not furnish the same functional reorganization of motor memories observed in young adults (Fogel et al., 2014). Although these findings reveal impairments in sleep-dependent motor memory consolidation processes, they do not explain why sleep spindles fail to transact a consolidation benefit to motor skill memories in older adults.
One candidate mechanism explaining the failure of sleep-dependent motor memory consolidation is age-related deterioration in the structural integrity of white matter in the brain. Major commissural and projection white matter fiber tracts support thalamocortical communication between ipsilateral and contralateral sensorimotor regions and subcortical striatal and hippocampal regions that are integral to motor function (Schmahmann et al., 2008). Moreover, integrity of these same tracts is necessary for learning and retention of skilled motor memory routines. For example, blocking myelination within the corpus callosum white matter tract after novel motor learning in rodents abolishes the subsequent long-term consolidation of those motor skill memories (McKenzie et al., 2014). Moreover, greater time devoted to motor skill learning results in higher corpus callosum white matter integrity (Bengtsson et al., 2005). In addition, white matter integrity in these commissural fiber tracts is degraded significantly in older adults (Salat et al., 2005), with the degree of degradation predicting the severity of cognitive and motor deficits (Ryberg et al., 2011).
White matter structure within these circuits is not only linked to motor skills. Importantly, white matter integrity within corticosubcortical tracts that encompass the corpus callosum predict the quantitative and qualitative features of sleep spindles in young adults (Piantoni et al., 2013)—loops that are causally necessary for the expression of sleep spindles (Steriade et al., 1987). Given that white matter regulates the expression of sleep spindle oscillations, it is therefore reasonable to posit that the structural integrity of these pathways also dictates the functional benefits associated with sleep spindles, including motor memory consolidation (Fischer et al., 2005; Walker et al., 2005; Fogel et al., 2014).
Building on this evidence, we tested the hypothesis that the severity of white matter atrophy predicts the severity of sleep spindle reduction in older relative to young adults. We further hypothesize that degeneration of specific portions of white matter, such as regions of the corpus callosum linking sensorimotor cortices, would additionally reduce effectiveness of sleep spindles in promoting overnight motor memory consolidation. We examined these hypotheses by combining overnight polysomnography (PSG) recording with full-head EEG, high-resolution structural and diffusion tensor imaging (DTI), and assessment of performance on a validated motor skill task (MST) in young and older adults.
Materials and Methods
Participants.
Ninety-one community-dwelling, healthy participants recruited through local advertisements completed the study (Table 1), with 31 healthy older adults (22 females, mean ± SD, 73.5 ± 5.2 years) and 20 healthy young adults (12 females, mean ± SD, 20.4 ± 2.0 years) training on a validated MST in the evening and retesting in the morning after sleep 10–12 h later. Twenty healthy older adults (17 females, mean ± SD, 74.1 ± 7.1 years) and 20 healthy young adults (9 females, mean ± SD, 21.7 ± 2.9 years) acted as wake controls, training in the morning and retesting 10–12 h later in the evening. The study was approved by the local human studies committee at University of California, Berkeley, with all participants providing written informed consent. Exclusion criteria included presence of neurologic, psychiatric or sleep disorders, current use of antidepressant or hypnotic medications, or being left handed. Participants were free of depressive symptoms (Yesavage et al., 1982) and all scored >25 on the Mini Mental State Examination (Folstein et al., 1975). In addition to neuroradiological assessments and medical interviews (Mander et al., 2013; Mander et al., 2014) obtained within one year of study entry, elderly participants performed within 2 SDs of their age-matched control group on tests of episodic memory (Wechsler, 1987; Delis et al., 2000) and frontal function (Reitan, 1958; Zec, 1986; Table 1). Before study entry, older participants underwent sleep disorders screening with a PSG recording night (described below) reviewed by a board-certified sleep medicine specialist (B.L.). Participants were excluded if they displayed evidence of a parasomnia or an apnea/hypopnea index ≥15 (Young et al., 2002). All participants abstained from caffeine, alcohol, and daytime naps for the 48 h before and during the study. Participants kept normal, habitual sleep–wake rhythms and averaged 7–9 h of reported time in bed per night before study participation, as verified by sleep logs (Table 1).
Table 1.
Demographic and neuropsychological measures (mean ± SD)
| Variable | Young (sleep; n = 20) | Young (wake; n = 20) | Older (sleep; n = 31) | Older (wake; n = 20) |
|---|---|---|---|---|
| Age (y) | 20.4 ± 2.0 | 21.7 ± 2.9 | 73.5 ± 5.2 | 74.1 ± 7.1 |
| Gender | 12 Female | 9 Female | 22 Female | 17 Female |
| MMSE | 29.6 ± 0.9 | 29.4 ± 1.0 | 29.5 ± 1.0 | 29.4 ± 0.9 |
| Mean prestudy bed time | 0:20 ± 0:54 | 23:43 ± 1:01 | 22:50 ± 1:15 | 22:56 ± 1:06 |
| Mean prestudy wake time | 8:26 ± 0:52 | 7:34 ± 0:53 | 7:16 ± 1:11 | 6:41 ± 1:09 |
| Mean prestudy time in bed (h) | 8.11 ± 0.58 | 8.01 ± 0.67 | 8.42 ± 0.73 | 7.83 ± 0.84 |
| Mean prestudy sleep time (h) | 7.75 ± 0.61 | 7.52 ± 0.85 | 7.14 ± 1.00 | 7.28 ± 1.00 |
| Mean prestudy sleep latency (min) | 15.7 ± 9.2 | 22.5 ± 26.4 | 35.7 ± 42.2 | 17.6 ± 19.1 |
| Mean prestudy sleep efficiency (%) | 95.6 ± 3.4 | 93.8 ± 6.8 | 86.0 ± 11.5 | 92.9 ± 6.7 |
| Neuropsychological measures | ||||
| CVLT (long delay, # free recalled) | 11.0 ± 3.1 | 12.9 ± 2.8 | ||
| WMS (visual reproduction %) | 75.0 ± 17.0 | 83.9 ± 21.7 | ||
| Trailmaking B (seconds) | 72.2 ± 34.5 | 80.0 ± 33.3 | ||
| Stroop (# correct in 60 s) | 50.2 ± 14.2 | 50.1 ± 11.8 |
MMSE, Mini Mental State Examination; CVLT, California Verbal Learning Test (number recalled after 20 min delay); WMS, Wechsler Memory Scale-Revised.
Experimental design and statistical analyses.
Young and older participants in the sleep groups entered the lab in the evening of the experimental night and were trained on a validated MST (described below). After training, participants were given an 8 h sleep opportunity measured with PSG starting at their habitual bed time based on sleep logs 5 d before the study date (Table 1). PSG recording included a 19-channel EEG array (details below). Approximately 2 h after awakening, participants underwent high-resolution structural MRI scanning and DTI, followed by the MST retest.
Young and older participants in the wake groups entered the laboratory in the morning and were trained on a MST sequence, returning 10–12 h later for the MST retest. Importantly, all wake group participants were instructed not to nap during this period and no participant reported napping during this interval.
To assess MST performance differences between groups, two three-way repeated-measures ANOVAs were used to compare MST speed (number of correct sequences typed in each 30 s trial) and accuracy (error rate in each 30 s trial) between young and older adults, with age group (young/older) and sleep condition group (sleep/wake) as between-subjects factors and trial (baseline: trials 1 and 2 mean, post-training: trials 11 and 12 mean, retest: trials 14–16 mean) as a within-subjects factor. This ANOVA was followed by eight post hoc tests comparing the difference between baseline and post-training (initial learning) and post-training and retest (motor skill memory consolidation) between sleep condition and age groups using false discovery rate (FDR) correction for multiple comparisons (Benjamini and Hochberg, 1995). Effect sizes associated with the impact of age on offline motor skill memory consolidation were compared statistically across sleep and wake condition groups to determine whether the relative degree of age-related impairment depended on the presence or absence of sleep. Assessing the impact of motor sequence on MST speed and accuracy during initial learning (baseline − post-training trials) and motor skill memory consolidation (post-training − retest trials), independent-samples t tests were used comparing between sequence A and B using FDR correction for multiple comparisons. The same approach was used to compare neuropsychological status variables between sleep and wake condition groups in older adults. If differences were detected, then these variables were correlated with MST measures of initial learning and motor skill memory consolidation to determine whether differences in neuropsychological status or motor sequence affected MST performance across trials.
Age effects, defined as the difference between young and older adults, were examined in sleep variables using ANOVA models. Specifically, differences in sleep staging variables between age groups were compared using independent-samples t tests with FDR correction for multiple comparisons (Benjamini and Hochberg, 1995). Stage 2 NREM fast sleep spindle density across the whole night were compared between age groups, with FDR correction used across all 19 EEG derivations. Differences in stage 2 NREM fast sleep spindle density were further examined by using a three-way repeated-measures ANOVA, with age group as a between-subjects factor and quartile (1–4) and EEG derivation (1–19) as within-subjects factors. Age effects at each electrode in each quartile were examined using post hoc tests FDR corrected across 76 comparisons (Benjamini and Hochberg, 1995).
Multiple regression analyses were used to examine associations between motor skill memory consolidation and stage 2 NREM sleep spindle density across the whole night and during the fourth quartile only, with FDR correction across all 19 channels. The correlation between motor skill memory consolidation and stage 2 NREM sleep spindle density at the EEG derivation demonstrating peak significance (CZ during fourth quartile) across all participants was then examined separately for young and older adults to determine whether both groups exhibited similar motor skill memory and sleep associations. The separate correlations for each group were not examined at other derivations and, for this reason, p-values were not corrected across the 19-channel EEG array. To determine the specificity of spindle and motor skill memory consolidation effects, fast sleep spindle density was also correlated with initial learning and motor skill memory consolidation was also correlated with sleep stage measures and subjective sleepiness variables. Finally, motor skill memory consolidation was also associated with fast sleep spindle density during all NREM sleep and NREM slow wave sleep to determine whether these associations were specific to sleep spindles during stage 2 NREM sleep.
DTI data were analyzed using FSL 5.0 to compare white matter mean diffusivity between young and older adults. As described in the DTI analysis section, an independent-samples t test was used to determine the degree to which mean diffusivity (MD) values were higher in older relative to young adults at a voxelwise, familywise error (FWE)-corrected level. An ANCOVA model with age group as a between-subjects factor and fourth quartile stage 2 NREM sleep fast sleep spindle density as a covariate was used identify which white matter regions were associated with fast sleep spindles even when controlling for the effects of age group. Therefore, incorporating age group as a factor in the analysis determines whether effects are driven by large age group differences, averting spurious correlations.
Mean MD values across significant clusters were then extracted and compared to determine whether white matter MD in these regions differed between young and older adults. To determine whether white matter associations with fast sleep spindles were linked to white matter degeneration in older adults, white matter lesion (WML) volume was correlated with mean sleep spindle-associated MD cluster values (nonparametric Kendall's τ correlation to accommodate the typical nature of WML data).
Moderation analyses were performed to determine whether the relationship between sleep spindles and motor skill performance was influenced by white matter brain structure. Here, moderation defines the interaction term that reflects the influence that white matter integrity has on dictating the strength of association between sleep spindles and motor skill memory consolidation. The moderation analysis therefore investigated the influence of white matter MD values on the ability of CZ fast sleep spindles to predict motor skill memory consolidation. Age group, CZ fast sleep spindles, and associated MD cluster values were included as additional regressors in the multiple regression model. White matter tract regional specificity was determined by parcellation of 20 anatomically defined subregions from the validated Johns Hopkins University (JHU) white matter tractography atlas (Wakana et al., 2004; Huang et al., 2005; Hua et al., 2008; Mori et al., 2008). FDR correction was applied to adjust for the number of moderation models examined after age correction.
All analyses were completed using SPSS version 24.0 software and R version 3.3.2 software (R Core Team, 2013).
MST.
The MST was chosen because of its sensitivity to sleep (Walker et al., 2002, 2003a, 2003b, 2005; Kuriyama et al., 2004; Nishida and Walker, 2007; Tucker et al., 2011). The task required subjects to press four numeric keys on a standard computer keyboard with the fingers of their left (nondominant) hand, repeating a five element sequence (4-1-3-2-4, sequence A) or (2-3-1-4-2, sequence B), “as quickly and as accurately as possible” for a period of 30 s. Sleep group participants learned one unique sequence in the evening before sleep, with the sequence (A or B) counterbalanced in session assignment across participants. The young and older adult wake group participants learned one unique sequence in the morning after sleep, with the sequence (A or B) counterbalanced across young and older adult group participants. The numeric sequence was displayed at the top of the screen at all times to exclude any working memory component to the task. Each key press produced a white dot on the screen, forming a row from left to right, rather than the number itself, so as not to provide accuracy feedback. The computer recorded the key press responses and each 30 s trial was scored automatically for the number of complete sequences achieved (speed) and the number of errors made (accuracy).
The encoding session consisted of 12 30 s trials with 30 s rest periods between trials and lasted a total of 12 min and the retest session consisted of 4 trials lasting 4 min. The efficiency of learning during training was measured by the subtracted difference between average performance (speed and accuracy, separately) at baseline (mean of trials 1–2) from the averaged performance post-training (mean of trials 11–12; Walker et al., 2002). Consistent with previous reports in older adults, offline consolidation was determined by the subtracted difference between average performance post-training from the average performance at retest (mean of trials 14–16). The first retest trials were excluded due to the recognized “warm-up” effect that, if considered, may obscure offline improvement in older adults (Tucker et al., 2011). Finally, to adjust for group differences in overall number of typed sequences associated with general slowing of reaction time in older adults on this task (Spencer et al., 2007; Tucker et al., 2011; Wilson et al., 2012; Pace-Schott and Spencer, 2013), differences across training and session were converted to percentage change from the first two trials in the case of training or from the last two training session trials in the case of sleep/wake session effects. MST data from two older adult participants were lost, leaving 29 older adults in the sleep group for all pertinent MST analyses.
MRI scanning.
Scanning was performed on a Siemens Trio 3 tesla scanner at the Henry H. Wheeler, Jr. Brain Imaging Center equipped with a 32-channel head coil. Two high-resolution T1-weighted anatomical images were acquired using a 3D MPRAGE protocol with the following parameters: TR, 1900 ms; TE, 2.52 ms; TI, 900 ms, nonselective inversion pulse; flip angle, 9°; FOV, 256 mm; matrix, 256 × 256; 176 1.0 mm isometric sagittal slices.
In addition, high-resolution, whole-head DTI data were acquired using a diffusion-weighted spin EPI method (TR/TE 7900/102 ms; 1 average; FatSat, echo spacing 0.83 ms; FOV 282 × 282 mm; matrix 128 × 128; 55 2.2 mm isometric axial slices; 9:45 scanning duration) using parallel imaging reconstruction (GRAPPA) with acceleration factor 2 and 6/8 partial fourier in the phase-encoding dimension. Diffusion-weighting along 64 separate directions was applied with a b value of 1000 s/mm2 and 6 images without diffusion weighting (b = 0 s/mm2) were also acquired to aid preprocessing.
Finally, in older adults only, a high-resolution T2-weighted fluid-attenuated inversion recovery (FLAIR) image designed to enhance white matter hyperintensity segmentation (Jack et al., 2001) was acquired with the following parameters: TR, 6000 ms; TE, 388 ms; TI, 2100 ms, nonselective inversion pulse; flip angle, 120°; FOV, 256 mm; matrix, 256 × 258; 160 1.0 mm isometric sagittal slices. This scan was not acquired in young adults because it is unlikely that healthy young adults free of a history of neurological disorders strokes, and traumatic brain injury would have white matter lesions.
DTI analysis.
DTI data underwent standard preprocessing using the FSL 5.0 processing pipeline (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki; Smith et al., 2004). First, the brain was extracted using the brain extraction toolbox (Smith, 2002) with an image without diffusion weighting (b = 0 s/mm2) used for all registrations due to its greater structural contrast. Images were corrected for motion and eddy current distortion. Fractional anisotropy (FA) and MD maps were then estimated for each participant by using the FMRIB diffusion toolbox to fit a tensor model to the corrected diffusion data. FA and MD maps were registered to standard template space using the nonlinear registration tool FNIRT. Next, a mean FA image was created and a threshold value of 0.2 was used to restrict analyses to voxels consistently identified as white matter. This image was then “skeletonized” to centers of tracts common across all participants. Registered MD maps were then projected onto this skeleton, with the resulting data used for voxelwise whole-brain analysis using the tract-based spatial statistics toolbox (TBSS; Smith et al., 2006). An independent-samples t test was used to determine the effects of age on MD measures and an ANCOVA model was used to determine where MD was correlated with frontal sleep spindles while controlling for age group.
Models were estimated using the “randomize” tool in FSL with 5000 permutations. Clusters were considered significant using the threshold-free cluster enhancement method using whole-brain FWE correction. Regional labels for significant clusters in all TBSS analyses were obtained from the JHU white matter tractography atlas (Wakana et al., 2004; Huang et al., 2005; Hua et al., 2008; Mori et al., 2008). Mean MD measures within significant clusters were extracted and used in the below described independent-samples t tests and regression analyses. Regional MD measures were extracted and examined by creating masks of significant voxels within distinct anatomically defined white matter regions using the JHU white matter tractography atlas (Wakana et al., 2004; Huang et al., 2005; Hua et al., 2008; Mori et al., 2008).
WML volumes, those associated with white matter hyperintensities observed in T2-weighted FLAIR images, were extracted from T2-weighted FLAIR images and quantified using the Quanta 2.0 software package, as described previously (DeCarli et al., 2005; Lockhart et al., 2014). Because WMLs are not expected in healthy young adults, FLAIR images were only collected in older adults. Images were traced manually along the dura mater to remove the cerebellum, subcortical brain tissue, and nonbrain tissue.
Images were then corrected for intensity nonuniformities and modeled as a combination of two Gaussian probability functions, with these functions corresponding to gray and white matter brain tissue and CSF. These two probability functions were used to segment out brain tissue for WML analysis, with the two exterior brain tissue voxels eroded to minimize the influence of partial volume effects on WML detection. An automated algorithm was then used to detect WMLs, defined as voxels with signal intensity >3.5 SDs above the mean of all brain voxels (DeCarli et al., 2005; Lockhart et al., 2014). Detected voxels were then inspected visually to remove artifacts. WMLs were considered for analysis only if they appeared on three consecutive slices visible on all three orientations. WML volumes were then estimated relative to the total intracranial volume. Images from two participants were lost due to computer error, leaving only 29 older participants for this analysis. Due to the non-normal distribution of WML values, the nonparametric Kendall's τ coefficient was derived to examine the association between WML and DTI-derived MD values in older adults.
Sleep monitoring and EEG analysis.
PSG on the experimental night was recorded using a Grass Technologies Comet XL system, including 19-channel EEG placed using the 10–20 system, EOG recorded at the right and left outer canthi (right superior; left inferior), and EMG. Reference electrodes were recorded at both the left and right mastoid (A1, A2). Data were digitized at 400 Hz and stored unfiltered (recovered frequency range of 0.1–100 Hz) except for a 60 Hz notch filter. Sleep was scored using standard criteria (Rechtschaffen and Kales, 1968). Sleep monitoring on the screening night was recorded using a Grass Technologies AURA PSG Ambulatory System and also included nasal/oral airflow, abdominal and chest belts, and pulse oximetry.
Sleep spindle analysis.
Before spindle analysis, each EEG channel was rereferenced to the average of the left and right mastoid, allowing for commonality of reference. Artifacts in the time series were removed by visual rejection. After artifact rejection, EEG was band-pass filtered using a finite impulse response function set between 12 and 15 Hz as described previously (Eschenko et al., 2006; Ferrarelli et al., 2007). Automatic sleep spindle detection analysis was then implemented using an established algorithm as described previously (Ferrarelli et al., 2007; Mander et al., 2011; Mander et al., 2014). In short, the amplitude of the rectified signal from stage 2 NREM sleep was used as a unique time series, identifying amplitude fluctuations exceeding threshold values, with the lower and upper values set at two and eight times the average amplitude. The peak amplitude for each spindle was defined as the local maximum above the threshold, with the beginning and end of the spindle defined as points immediately preceding or following this peak, when the amplitude of the time series dropped below the cutoff threshold. This method was used in part because it advantageously defines spindles based on deviations from the mean signal amplitude and thus is robust against global differences in EEG signal that may be expected when comparing across individuals (Ferrarelli et al., 2007).
The algorithm-determined spindles were restricted only to those events falling within the specified frequency range. After detection, sleep spindles were separated into slow (12–13.5 Hz) and fast (13.5–15 Hz) spindles for analysis, a separation consistent with the existence of two distinct peaks in sigma activity during sleep proximal to the threshold applied in the current study, with these distinct peaks expressing distinct topographical and developmental trajectories (De Gennaro and Ferrara, 2003). The combination of fast and slow spindle density represents total spindle density, with total spindle density values consistent with those reported in previous studies (De Gennaro and Ferrara, 2003). Given previous reports linking age effects on sleep spindles and showing that sleep effects on motor skill learning may differ by quartile of stage 2 NREM sleep (Walker et al., 2002; Martin et al., 2013), stage 2 NREM sleep was separated into quartiles and spindle density was calculated within each quartile. Sleep spindle density was used to predict motor learning effects, as per our a priori hypothesis.
Measures of subjective sleepiness.
Subjective sleepiness was measured using a validated visual analog scale (Monk, 1989) collected every 2 h throughout the study while subjects were awake, including at the beginning of each testing session. Subjective ratings were compared between testing sessions to assess the change in subjective sleepiness and its association with motor learning between and across groups.
Results
Impact of age and sleep on motor skill learning and offline consolidation
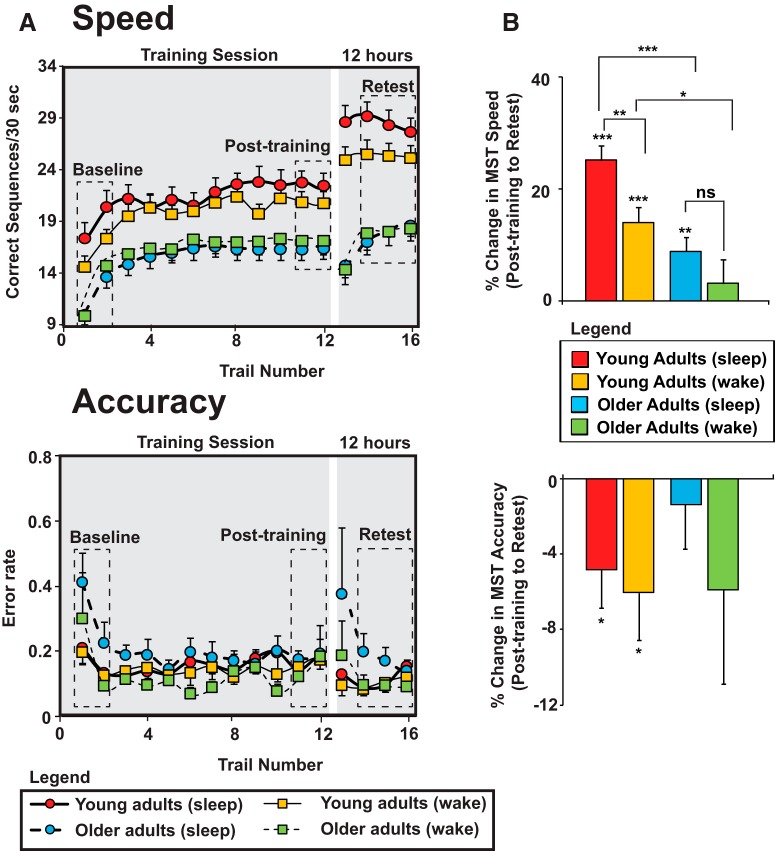
We first investigated whether age group (young/old) or wake/sleep condition group influenced MST speed (number of correctly typed sequences per 30 s block) or accuracy (error rate per 30 s block) across testing trials (Fig. 1A). For MST speed, a three-way repeated-measures ANOVA was conducted, with trial (baseline mean, post-training mean, retest mean) as a within-subject factor and age group (young/older) and wake/sleep condition group as between-subjects factors. There were main effects of age group (p < 0.001) and trial (p < 0.001) and, importantly, both age group × trial (p < 0.001) and wake/sleep condition group × trial interactions (p = 0.039). No other significant main or interaction effects were detected, including age group × wake/sleep condition group (p = 0.343) and age group × wake/sleep condition group × trial (p = 0.543) interaction effects. These findings indicate that age impairs motor skill performance, whereas brain state (wake/sleep) influences offline motor memory retention significantly.
Figure 1.
Behavioral performance on the MST in young (red) and older (light blue) adults who slept and young (orange) and older (green) adults who remained awake between training and retest. A, Performance speed (number of correct sequences typed per 30 s trial; top) and accuracy (error rate; bottom) during training (trials 1–12) and 10–12 h retest (trials 13–16). Baseline (trials 1–2), post-training (trials 11–12), and retest (trials 14–16) trials used in analysis are outlined by dashed boxes. B, Percentage change in speed (top) and accuracy (bottom) from post-training (trial 11–12 mean) to 10–12 h retest (trial 14–16 mean). Inclusion of measures of the change in reaction time across training (between-sequence mean reaction time, within-sequence mean reaction time, SD of reaction time) in an ANOVA model examining the change in MST speed from post-training to retest does not change the results appreciably. Significant trial by age group and trial by sleep condition group interactions still remain (all p < 0.01) despite no significant differences in MST speed during training. Values are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 on within- and between-subjects post hoc tests
Next, we examined our hypothesis that older adults exhibit a less robust benefit from sleep on motor skill memory. These post hoc tests compared directly the performance change from baseline to post-training and post-training to retest across sleep and age groups. We used an FDR correction for multiple comparisons (Benjamini and Hochberg, 1995); there were eight comparisons (Fig. 1B).
There were no significant differences in initial learning acquisition, as measured by the change in MST speed from baseline to post-training, across the four groups: (1) young sleep condition group, (2) young wake condition group, (3) older sleep condition group, and (4) older wake condition group (all p > 0.1 uncorrected, p > 0.2 FDR corrected).
Importantly, however, there were significant differences in terms of subsequent offline degree of motor skill memory consolidation (post-training vs retest or delayed retention) between groups across wake and sleep periods. As in prior studies (Fischer et al., 2002; Walker et al., 2002; Spencer et al., 2006), young adults showed superior offline memory retention across sleep relative to young adults across wake (p = 0.005, p = 0.017 FDR corrected, Cohen's d = 0.94). In contrast, no such typical sleep-dependent wake/sleep benefit was observed in older adults (p = 0.260, p = 0.347 FDR corrected, Cohen's d = 0.33). A direct comparison demonstrated that older adults showed significantly less overnight motor skill memory retention benefit relative to young adults in the sleep condition groups (p < 0.0001, p < 0.0001 FDR corrected, Cohen's d = 1.7). However, young adults also exhibited less motor skill memory forgetting than older adults in the wake condition groups (p = 0.006, p = 0.017 FDR corrected, Cohen's d = 0.92). To determine whether the impact of age on motor skill memory consolidation differed by sleep condition, the age group effect sizes in the sleep and wake condition groups were compared statistically. The size of the effect of age was significantly larger across sleep than across wake (Cohen's d = 1.7 vs 0.92, p = 0.049), demonstrating that older adults show the greatest relative impairment in offline consolidation across periods that include sleep.
The same three-way repeated-measures ANOVA of MST accuracy only revealed a main effect of trial (p = 0.004), indicating that the impact of age and sleep condition groups on the change in MST performance across trials was specific to MST speed. In addition, MST sequence (A/B) did not influence MST performance change across trials (speed or accuracy; all independent-samples t tests comparing baseline with post-training and post-training with retest p > 0.08 uncorrected, p > 0.18 FDR corrected).
Together, these data support the experimental hypothesis that overnight motor memory consolidation is diminished significantly in older relative to young adults.
Impact of age on fast sleep spindle density
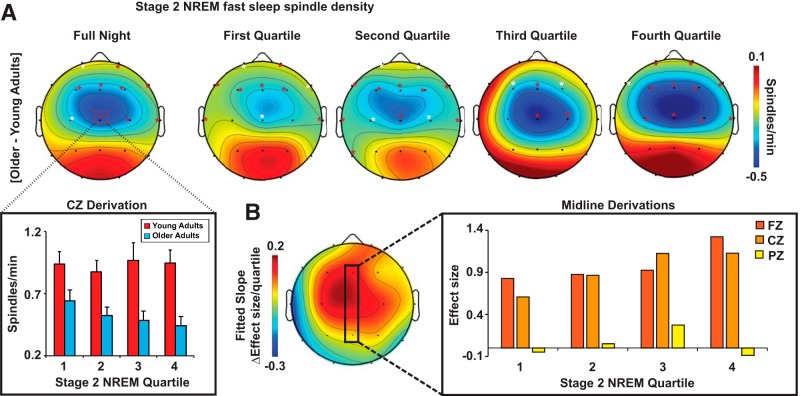
We next examined the impact of age on sleep, focusing a priori on fast sleep spindles (13.5–15 Hz) during stage 2 NREM sleep due to the recognized association between motor sequence consolidation and late night stage 2 NREM sleep and associated fast sleep spindles (Smith and MacNeill, 1994; Walker et al., 2002; Peters et al., 2008; Tamaki et al., 2009; Lustenberger et al., 2016; see Table 2 for age differences in sleep stage characteristics). Two-sample t tests, FDR corrected for 19 comparisons, revealed that fast sleep spindle density during stage 2 NREM sleep was significantly lower in older relative to young adults, primarily in frontal and central EEG derivations (Fig. 2, peak age difference detected at FZ, CZ, and T4). Because the consolidation of motor skill memory may depend on stage 2 NREM sleep quartile (Walker et al., 2002), as well as the impact of age on fast sleep spindles (Martin et al., 2013), we next explored the impact of age group on fast sleep spindles across the sleep period.
Table 2.
Sleep statistics (mean ± SEM)
| Variable | Young (n = 20) | Older (n = 31) |
|---|---|---|
| Total recording time (min) | 480.4 ± 0.2 | 479.5 ± 0.7 |
| Total sleep time (min) | 431.6 ± 6.1 | 340.8 ± 12.2*** |
| Sleep latency (min) | 16.6 ± 2.8 | 23.1 ± 4.7 |
| Wake after sleep onset | 27.9 ± 5.8 | 114.0 ± 11.2*** |
| Stage 1 (min) | 14.3 ± 1.6 | 22.4 ± 1.5** |
| Stage 2 (min) | 201.1 ± 6.7 | 192.1 ± 10.4 |
| Slow-wave sleep (min) | 117.9 ± 7.1 | 61.7 ± 6.5*** |
| Rapid eye movement sleep (min) | 98.3 ± 6.1 | 64.6 ± 5.5*** |
| Sleep efficiency (%) | 90.8 ± 1.3 | 71.4 ± 2.6*** |
*p < 0.05,
** p < 0.01,
***p < 0.001.
Figure 2.
A, Two-dimensional topoplots of the impact of age on stage 2 NREM fast sleep spindle density (13.5–15 Hz) across the entire night (left most topoplot) and during all four quartiles of stage 2 NREM sleep. Fast sleep spindle density during stage 2 NREM sleep for young (red) and older (light blue) adults at CZ, the derivation exhibiting the greatest difference in spindle density across age groups, during each quartile of stage 2 NREM sleep is plotted at the bottom left. B, Two-dimensional topoplot of the fitted slope of the change in effect size of the age difference in stage 2 NREM fast sleep spindle density across quartiles. Warmer colors reflect positive increases in effect size across the night; that is, the difference between young and older adults in stage 2 NREM fast sleep spindle density increases progressively across the night. The effect size of the difference in fast sleep spindle density during stage 2 NREM sleep between young and older adults at FZ (dark orange), CZ (light orange), and PZ (yellow) is plotted for each quartile at the bottom right. White asterisks, p < 0.05; red asterisks, p < 0.05 FDR corrected across 76 comparisons.
A three-way repeated-measures ANOVA with age group (young/older) as a between-subjects factor and quartile of the stage 2 NREM sleep period (first-fourth) and EEG derivation (channels 1–19) as within-subjects factors revealed the following: (1) significant age group × EEG derivation (p < 0.001) and age group × quartile × EEG derivation (p = 0.013) interactions, (2) a significant main effect of derivation (p < 0.001), and (3) a trend of a main effect of age group (p = 0.059; Fig. 2A). That is, older adults exhibited a reduction in stage 2 NREM fast sleep spindles at specific EEG derivations with the degree of reduction depending on the quartile of the stage 2 NREM sleep period. Using FDR correction for multiple comparisons (76 comparisons across four quartiles), we further identified that age effects (difference between young and older adults) centered on frontal and central EEG derivations. For raw differences, effect size, and significance, these age differences became more prominent as the night progressed, showing the largest differences in fast sleep spindle density during the fourth quartile (Fig. 2A,B).
Aging, fast sleep spindles, and motor memory consolidation
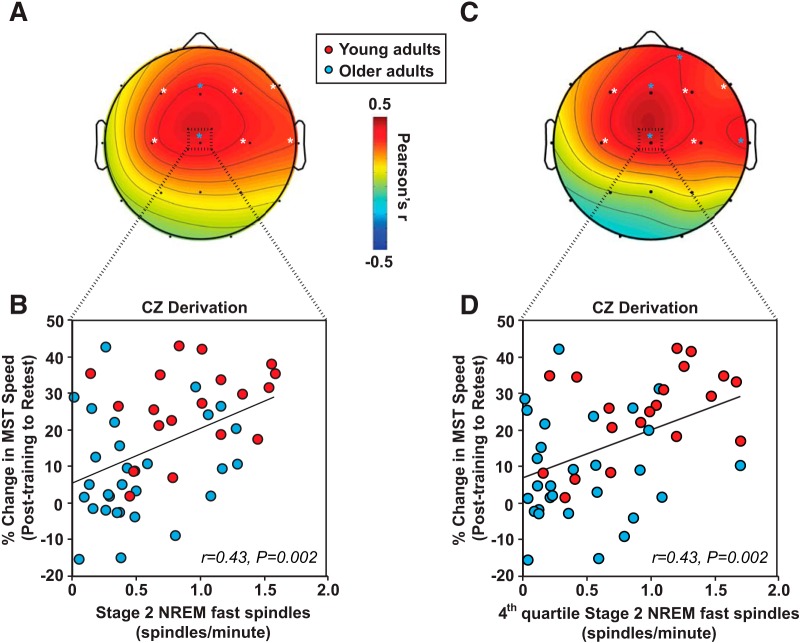
Next, we tested the hypothesis that stage 2 NREM fast sleep spindle density predicted the degree of overnight, offline motor skill memory consolidation. Across all participants, stage 2 NREM fast sleep spindles at multiple central, frontal, and temporal EEG derivations positively predicted success of overnight motor skill memory consolidation (Fig. 3A). Consistent with the impact of age on fast sleep spindles, FZ and CZ derivations demonstrated the most robust associations, remaining significant after FDR correction across the 19 electrode array (Fig. 3B; strongest at CZ; r = 0.43, p = 0.002). Because age-related reductions in stage 2 NREM fast sleep spindles were maximal in the fourth quartile, we then examined associations between stage 2 NREM fast sleep spindles in the fourth quartile and offline motor skill memory consolidation. Consistent with our hypothesis, fast sleep spindle and motor skill memory consolidation associations were stronger in the fourth quartile than collapsed across all stage 2 NREM sleep (Fig. 3C,D) and, consistent with the nature of the left-handed, unimanual task, also strongest in the contralateral (to hand) right hemisphere and midline derivations (Fp2, FZ, CZ, and T4), all of which remained significant after FDR correction (Fig. 3C,D; strongest at FZ and CZ).
Figure 3.
A, Two-dimensional topoplot of the association between stage 2 NREM fast sleep spindle density and percentage overnight change in MST performance. B, Scatter plot of this association with mean stage 2 NREM fast sleep spindle density over the entire sleep period at CZ is presented below (bottom left) for young (red) and older (light blue) adults. C, Two-dimensional topoplot of the association between fourth quartile stage 2 NREM fast sleep spindle density and percentage overnight change in MST performance. D, Scatter plot of this association with mean stage 2 NREM sleep spindle density during the fourth quartile at CZ is presented below (bottom right) for young (red) and older (light blue) adults. White asterisks, p < 0.05 uncorrected; light blue asterisks, FDR corrected (light blue).
Building on our planned comparisons between age groups, these significant midline relationships were present in young adults (r = 0.48 p = 0.033), but not in older adults (r = 0.08 p = 0.679). Total sleep time, sleep efficiency, and subjective sleepiness did not predict overnight motor memory consolidation effects for either young or older adults or for all subjects combined (all r2 < 0.08, p > 0.18). Performance improvement across initial training also did not correlate with fast sleep spindles in young, old, or all participants combined (p > 0.143). Therefore, fast sleep spindles, specifically and selectively in the last quartile of stage 2 NREM sleep, were associated with overnight motor skill memory retention in young but not older adults.
Beyond our a priori focus on stage 2 NREM sleep fast sleep spindles, we also examined associations across all adults between motor skill memory consolidation and fast sleep spindle density, as well as the durations of total sleep time, REM sleep, slow-wave sleep, and total NREM sleep in post hoc analyses. No significant associations were detected between motor skill memory consolidation and total sleep time, time spent in slow wave sleep, or overall NREM sleep time (all p > 0.07). Moreover, REM sleep time, percentage of the sleep period spent in REM sleep, and the time or percentage of the sleep period spent in REM sleep during the fourth quartile did not predict motor skill memory consolidation (all r2 < 0.03, p > 0.24). Critically, CZ fast sleep spindles remained a significant predictor of motor skill memory consolidation when stage 2 NREM sleep metrics were included in the regression models for analyses collapsed across all subjects (r = 0.43, fourth quartile sleep fast sleep spindles at CZ p = 0.002, fourth quartile stage 2 NREM sleep duration p = 0.697) and in young subjects alone (r = 0.59, fourth quartile sleep fast sleep spindles at CZ p = 0.037, fourth quartile stage 2 NREM sleep duration p = 0.091). Therefore, fast sleep spindles and not duration of REM and NREM sleep stages in general predicts the success of motor skill memory consolidation.
Aging, white matter structure, and fast sleep spindles
We next tested the experimental predictions that white matter degeneration in the brain, as assessed using DTI, was greater in older relative to young adults and accounted for the age-related reduction in fast sleep spindles. We focused on white matter because sleep spindles are expressed in cortico-thalamic loops (Steriade et al., 1987; De Gennaro and Ferrara, 2003), sleep spindle expression is associated with DTI white matter measures in young adults (Piantoni et al., 2013), and white matter measures affect the long-term retention of motor skills (Bengtsson et al., 2005; McKenzie et al., 2014). Analyses examined MD, with higher MD values in white matter regions (i.e., greater diffusivity of water molecules within white matter tissue) potentially reflecting lower white matter integrity due to white matter degeneration. MD is commonly used to estimate the degree of “white matter integrity.” However, without confirmation, differences in any DTI measure may merely reflect individual differences in the distribution of orientation of axons within white matter fiber tracts (Jones et al., 2013). To verify that MD measures reflected in part differences in white matter integrity, MD values were associated with WML volume derived from T2-FLAIR imaging acquired in older adults. Together, these analyses allowed us to evaluate whether age-related differences in white matter integrity tracked with changes in sleep spindle expression.
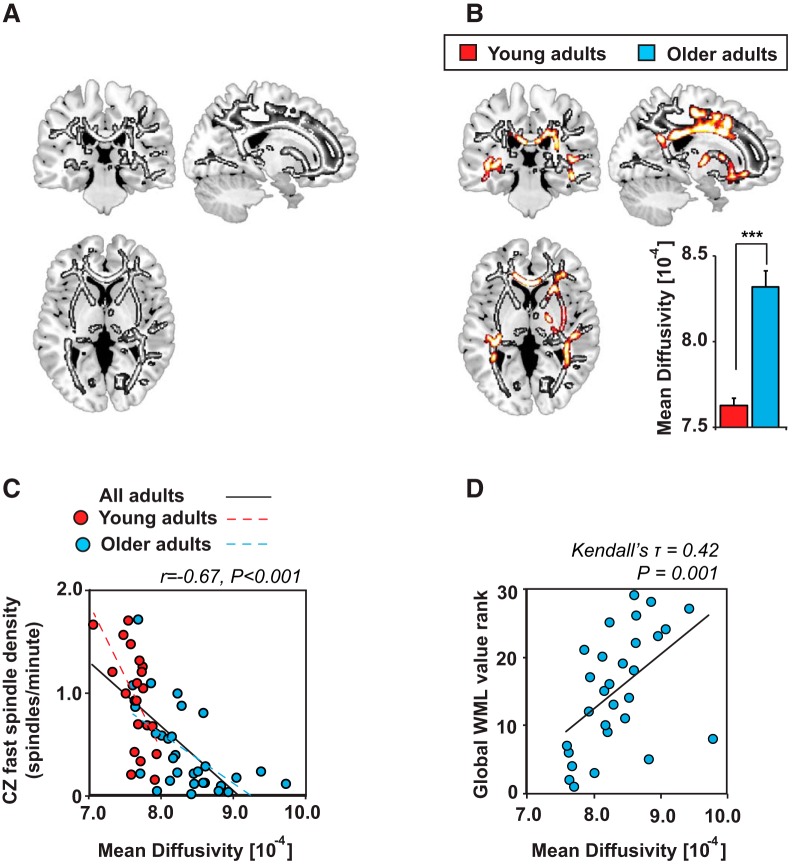
First, we compared white matter MD between young and older adult groups using an independent-samples t test with whole-brain FWE correction. Numerous white matter fiber tracts displayed significantly higher white matter MD in older relative to young adults, potentially reflecting reduced white matter integrity due to age-related degeneration within white matter fiber tracts (Fig. 4A). Results were overlaid on the JHU white matter atlas containing 48 distinct white matter regions to determine the specific anatomical tracts exhibiting age effects (Wakana et al., 2004; Huang et al., 2005; Mori et al., 2008).
Figure 4.
Associations among white matter mean diffusivity, age group, fourth quartile stage 2 NREM fast sleep spindle density at CZ, and WML volume. A, Voxelwise comparison of the increase in white matter MD in older relative to young adults (in grayscale). B, Fourth quartile stage 2 NREM fast sleep spindle density at CZ predicting white matter MD while controlling for age group (in hot colors) overlaid on clusters demonstrating age effects (in grayscale). Effects are presented at p < 0.05 FWE whole-brain corrected at the cluster level. The mean extracted white matter MD across all significant voxels associated with fourth quartile stage 2 NREM fast sleep spindle density at CZ in young (red) and older (light blue) adults is presented below. C, Scatter plot of the association between fourth quartile stage 2 NREM fast sleep spindle density at CZ and mean extracted MD in young (red) and older (light blue) adults. D, Scatter plot of the association between white matter lesion (WML) volume and the mean extracted white matter MD across all significant voxels associated with fourth quartile stage 2 NREM fast sleep spindle density at CZ in older adults. ***p < 0.001.
An ANCOVA model, using age group and midline (CZ) fast sleep spindle density in the fourth quartile to predict MD with whole-brain FWE correction applied demonstrated that commissural and projecting fiber tracts (Fig. 4B, Table 3), including the corpus callosum, which has a well recognized association with motor skill memory (McKenzie et al., 2014), were associated with fourth quartile fast sleep spindle density in both young and older adults. Specifically, higher MD in these regions was associated with a progressive diminution in fast sleep spindle density in the fourth quartile across all individuals combined (r = −0.67 p < 0.001; Fig. 4C) and in older (r = −0.59 p < 0.001) and young (r = −0.59 p = 0.006) adults separately (Fig. 4C). Critically, all of these significant clusters were detected in the previous age-group analysis, demonstrating that white matter MD predicted frontal fast sleep spindle density in regions showing age effects (Fig. 4C).
Table 3.
MD predicts sleep spindle density at CZ
| JHU atlas label | JHU no. | pFDR |
|---|---|---|
| Corpus callosum genu | 3 | 0.021* |
| Corpus callosum body | 4 | 0.005* |
| Corpus callosum splenium | 5 | 0.003* |
| R anterior limb of the internal capsule | 17 | 0.008* |
| R posterior limb of the internal capsule | 19 | 0.005 |
| R retrolenticular limb of the internal capsule | 21 | 0.005* |
| L retrolenticular limb of the internal capsule | 22 | 0.003 |
| R anterior corona radiata | 23 | 0.011* |
| R superior corona radiata | 25 | 0.009* |
| R posterior corona radiata | 27 | 0.005* |
| L posterior corona radiata | 28 | 0.008* |
| R posterior thalamic radiation | 29 | 0.003* |
| L posterior thalamic radiation | 30 | 0.003* |
| R sagittal stratum | 31 | 0.003* |
| R external capsule | 33 | 0.003* |
| R fornix/stria terminalis | 39 | 0.005* |
| L fornix/stria terminalis | 40 | 0.005* |
| R superior longitudinal fasciculus | 41 | 0.011* |
| R superior fronto-occipital fasciculus | 43 | 0.008* |
| R tapetum | 47 | 0.013* |
*Significant age effect in MD was also detected at p < 0.05 FDR corrected. Presented effects are adjusted for age group.
Further verifying that MD differed by age in these voxels, MD in these white matter regions was significantly higher (p < 0.001; Fig. 4B), whereas FA was significantly lower (p < 0.001) in older relative to younger adults, potentially representing lower white matter integrity in older adults. Consistent with this interpretation, WML volume also correlated with mean MD values (Kendall's τ = 0.42, p = 0.001; Fig. 4D). Therefore, the age difference in MD in these fiber tracts likely reflects in part age-dependent erosion of white matter integrity, which results in reduced expression of late night stage 2 NREM frontal fast sleep spindles.
To determine whether the association between white matter MD values and fast sleep spindles in the last quartile of the night detected at CZ was specific, MD values were extracted from the significant voxels presented in Figure 4B. The values were then compared with fast sleep spindle density during the fourth quartile at a posterior EEG derivation that did not exhibit an age effect on fourth quartile fast sleep spindle density (at O1 p = 0.267) and at a frontal EEG derivation that did exhibit an age effect (at F7 p = 0.045 FDR corrected), yet did not show a significant sleep spindle effect on motor memory consolidation (at F7 p = 0.238 uncorrected). In addition, white matter MD values were included in multiple regression models predicting fourth quartile fast sleep spindle density at O1 and F7 while controlling for age group. These models revealed that, although white matter MD averaged across all significant voxels was associated with fourth quartile CZ fast sleep spindle density, MD in this cluster was not associated with fourth quartile O1 (for MD p = 0.265, for age p = 0.119) or F7 (for MD p = 0.350, for age p = 0.247) fast sleep spindle density. Therefore, white matter integrity in these select commissural and largely right hemisphere projecting fiber tracts, including the corpus callosum, corona radiata, and thalamic radiations (see Table 3 for full list), specifically predicts fast sleep spindle density only at midline and right hemisphere EEG derivations exhibiting both an age effect and an effect on motor memory consolidation.
Moderation of sleep spindle-related motor memory consolidation by white matter structure
Finally, to test the hypothesis that the decline in white matter integrity associated with a reduction in midline frontal fast sleep spindles contributed significantly to impairments in offline motor memory consolidation, moderation analyses were performed. If the relationship between last quartile fast sleep spindles and overnight motor memory consolidation is independent, then the inclusion of white matter MD (the moderator) into a statistical model with frontal fast sleep spindles and motor memory retention should have no interacting influence. However, if white matter integrity moderates the relationship between spindles and overnight motor memory consolidation, white matter MD and sleep spindles should interact significantly.
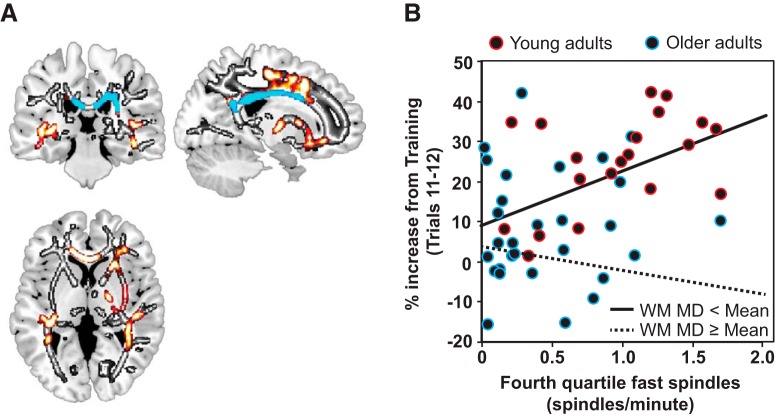
Consistent with the latter prediction, the experimental hypothesis, there was a significant moderation effect (interaction) between the mean MD within the white matter cluster reported in Table 3 and midline fast sleep spindles (MD × CZ fast spindles; p = 0.045). These findings indicate that the efficacy of sleep spindles to support motor memory consolidation may depend on the integrity of the white matter supporting their expression. However, when controlling for age group, it was reduced to a trend for MD (MD × CZ sleep spindles p = 0.106). Given this trend after the inclusion of age group for MD, it is possible that only select white matter regions within this cluster account significantly for the age effect on sleep spindle-dependent motor memory consolidation. To determine the specific anatomical white matter regions moderating the influence of sleep spindles on motor memory consolidation, after accounting for age, we used the JHU white matter atlas to parcellate the cluster predicting sleep spindle density (Table 3) into 20 distinct anatomical regions of interest (Wakana et al., 2004; Huang et al., 2005; Mori et al., 2008). Of these 20 individual regions of interest, four remained significant in demonstrating moderation when controlling for age group for MD, including the body and splenium of the corpus callosum (for MD p = 0.012, JHU-4; p = 0.020, JHU-5), the right posterior corona radiata (for MD p = 0.040, JHU-25), and the right tapetum (for MD p = 0.020, JHU-47), all of which remained significant after FDR correction (for JHU-4 p = 0.027 FDR corrected, for JHU-5 p = 0.027 FDR corrected, for JHU-27 p = 0.040 FDR corrected, for JHU-47 p = 0.027 FDR corrected; Fig. 5). It is critical to note that, by definition, all of these MD clusters demonstrating a significant moderation effect overlapped with clusters demonstrating an age effect and predicting fourth quartile frontal fast sleep spindle density (Fig. 5). Therefore, white matter integrity, particularly within sensorimotor tracts including posterior portions of the corpus callosum and posterior projection fibers containing connections that were degraded in older adults, influences statistically whether sleep spindles promote motor memory consolidation.
Figure 5.
White matter integrity moderates the association between fourth quartile stage 2 NREM fast sleep spindle density at CZ and motor memory consolidation. A, Age effect on white matter MD presented in grayscale. Fourth quartile stage 2 NREM fast sleep spindle density at CZ predicting white matter MD while controlling for age group is presented in hot colors. Voxels in this cluster that moderate significantly the relationship between fourth quartile stage 2 NREM fast sleep spindle density at CZ and motor memory consolidation while controlling for age group are presented in cool colors. B, Interaction plot demonstrating the influence of the mean white matter MD across the body and splenium of the corpus callosum, the right posterior corona radiata, and the right tapetum on the association between fourth quartile stage 2 NREM fast sleep spindle density at CZ and motor memory consolidation. Original data are plotted with color outlining young (red) and older (light blue) adults for reference, with regression lines shown for participants with white matter MD values (WM MD) less than the mean (black line) and greater than or equal to the mean (black dashed line) across all participants. This plot reveals that, as white matter integrity decreases, sleep spindles offer less of a benefit to motor memory consolidation even while controlling for age.
Discussion
Older adults have significantly impaired overnight motor memory consolidation relative to young adults and the reason for why this occurs has remained unknown. Here, we show that white matter degeneration within specific sensorimotor tracts represents at least one mechanism that accounts for both impaired frontal fast sleep spindle expression and sleep-dependent motor memory consolidation. That is, the integrity of white matter structure in the adult human brain, more than age per se, determines the presence or absence of sleep spindle-associated motor memory consolidation.
In the current study, we demonstrate that the association between fast sleep spindles and motor learning is strongest in the fourth quartile of stage 2 NREM sleep and this effect is diminished in older relative to young adults. Although this time of night sensitivity is consistent with previous work demonstrating a quartile effect of stage 2 NREM sleep on motor skill learning (Walker et al., 2002), fast sleep spindles throughout stage 2 NREM sleep also significantly predicted motor skill memory consolidation. It thus is unlikely that the last quartile is mechanistically unique with regard to sleep-dependent neuroplasticity.
However, the last quartile of the sleep period shows the highest sleep spindle activity and density and is also when sleep spindle expression is affected the most by age (Landolt et al., 1996; Carrier et al., 2011; Martin et al., 2013). Therefore, age-related reductions in sleep spindle-dependent motor skill memory consolidation may be especially sensitive to the effect of age at the time of night when sleep spindles typically predominate: the last quartile of stage 2 NREM sleep. The time of night effect may also relate to other NREM sleep oscillations. Slow waves support declarative memory consolidation causally (Marshall et al., 2006), predominate early in the night, and can often couple with sleep spindles during these phases. Sleep spindles later in the night appear to be less clustered with cortical slow waves and, with the lower incidence of cortical slow waves at this time of night, may be more able to support procedural motor skill memory consolidation. This is consistent with a recent causal manipulation using transcranial stimulation demonstrating that targeted sleep spindle enhancement benefits motor skill memory most significantly during stage 2 NREM sleep, rather than during slow-wave sleep (Lustenberger et al., 2016).
White matter and sleep spindles
Consistent with prior findings (De Gennaro and Ferrara, 2003; Martin et al., 2013; Mander et al., 2014), we identified reductions in fast sleep spindles in older relative to young adults that were topographically maximal over central and frontal EEG derivations. Furthermore, the extent of sleep spindle impairment, relative to young adults, increased as the night progressed, with the largest difference observed in the last quartile of the night. Extending prior work, we establish that one factor statistically predicting this now well replicated topographically and temporally specific spindle impairment is the degree of white matter pathway deterioration in older adults.
In young adults, white matter in multiple fiber tracks explains interindividual differences in sleep spindle expression (Piantoni et al., 2013). Our findings show considerable overlap with these previously reported fiber pathways, including the corpus callosum, suggesting that white matter integrity within regions that account for individual differences in sleep spindle expression in young adults also predicts age-related decline in fast sleep spindle expression. It should be noted that the association between task-related fast sleep spindles and white matter that we identified was topographically discrete, being unique to the frontocentral derivations. This is relevant because it was these derivations that similarly show age-specific reductions in sleep spindles in older relative to young adults and were also regions that further predicted the success of overnight motor memory consolidation in young adults.
This specificity supports the interpretation that sleep spindles that are expressed over each EEG derivation may also be expressed within, or at least modulated significantly by, regionally specific properties of white matter fiber pathways that are associated with different neurobehavioral functions and may exhibit age-related atrophy at distinct rates.
The current anatomical associations may further illuminate why the process of human aging reduces frontal sleep spindle expression selectively while leaving posterior sleep spindle expression relatively preserved (De Gennaro and Ferrara, 2003; Mander et al., 2014). Frontal white matter fiber tracts deteriorate early and more severely with increasing age than posterior and temporal white matter pathways (Salat et al., 2005). Based on the current findings, these selective degenerative influences on white matter brain structure in older age appear to represent one potentially important candidate mechanism explaining the regionally specific decline of the fast sleep spindle oscillation across the lifespan. This interpretation is consistent with the known anatomical basis of sleep spindles derived from animal studies linking cortico-thalamic white matter fiber integrity with sleep spindle expression (Steriade et al., 1987).
White matter as a moderator of sleep-dependent memory
Paralleling previous studies, we demonstrated that older adults have significantly impaired overnight motor memory consolidation relative to young adults. Prior studies have concluded from this evidence that sleep-dependent motor memory mechanisms are absent or significantly diminished in older adults (Spencer et al., 2007; Peters et al., 2008; Wilson et al., 2012; Fogel et al., 2014; Pace-Schott and Spencer, 2013). However, our white matter brain structure findings help to temper this claim and offer mechanistic insight into why this may be more the case in some older individuals than in others.
Although white matter integrity was degraded in a large collection of fiber tracts in older adults relative to young adults, it was age-related degeneration in a select subset of these fiber tracts that accounted for the topographic specificity and the magnitude of fast sleep spindle reduction in older adults. Critically, the degeneration of the posterior corpus callosum in particular, but also potentially the right tapetum and corona radiata white matter fibers, moderated significantly the influence of these same topographically specific fast sleep spindles on motor skill memory consolidation in older adults.
The impairment in sleep spindle-dependent motor skill memory consolidation was influenced by two nonmutually exclusive and co-occurring age-related impairments in sleep spindles. First, age reduces sleep spindle expression, particularly within motor memory-relevant white matter tracts, and this reduced expression may be insufficient to support motor memory consolidation. Second, age-related factors, including white matter degeneration, influences the ability of sleep spindles that are expressed within these motor memory-relevant tracts to facilitate the memory transformation necessary to promote motor skill memory consolidation.
It is unclear exactly how age-related degeneration of these specific white matter tracts contribute to disruptions in the transformation of motor skill memories supporting motor skill memory consolidation. The fiber paths demonstrating sleep- and age-related sensitivity in the current study are known to connect cortical regions over which sleep spindles are dominantly expressed. The deterioration of these pathways may therefore diminish the capacity of sleep spindles to influence the functional transformation of motor skill memories necessary to support procedural memory consolidation in the elderly. This is consistent with prior fMRI evidence demonstrating that aging alters the neural signature associated with sleep spindle-dependent offline motor skill memory transformation (Fogel et al., 2014). The relevance of these specific white matter tracts to sensorimotor function, and thus motor skill memory, is evident across five descriptive levels.
First, experimentally blocking myelination within the corpus callosum in rodents after novel motor learning abolishes long-term motor memory consolidation (McKenzie et al., 2014), indicating that properties of the corpus callosum are causally necessary to consolidate a newly acquired motor skill. Relatedly, increasing piano practice in human participants is associated with greater white matter integrity within the body and splenium of the corpus callosum (Bengtsson et al., 2005).
Second, cross-sectional and longitudinal studies in aging show that corpus callosum atrophy, particularly within the body and splenium of the corpus callosum, predicts deterioration of motor ability (Frederiksen et al., 2011; Ryberg et al., 2011; Koch et al., 2013).
Third, lesions within the corpus callosum can result in alien hand syndrome, a syndrome in which the control of complex goal-directed hand movements becomes aberrant, resulting in impaired motor performance (Banks et al., 1989; Aboitiz et al., 2003). This is aligned with the fact that the corpus callosum, as well as the right tapetum, is critical for integrating sensory information to aid motor planning and selection (Tanaka et al., 1996; Lindner et al., 2010).
Fourth, projection fibers in the posterior corona radiata communicates parietal sensory information that aids skilled motor actions, specifically those of the hand (Wakana et al., 2004; Kim and Pope, 2005; Iguchi et al., 2006).
Fifth, functional neuroimaging studies consistently implicate the primary motor, premotor, somatosensory cortex, parietal cortex, and basal ganglia in the overnight consolidation of this MST (Fischer et al., 2005; Walker et al., 2005; Fogel et al., 2014), which is relevant considering that communication between all these regions depends on sufficient white matter integrity within the body and splenium of the corpus callosum, the tapetum, and the posterior corona radiata (Tanaka et al., 1996; Wakana et al., 2004; Huang et al., 2005; Kim and Pope, 2005; Iguchi et al., 2006; Lindner et al., 2010).
Beyond the relevance for explaining white matter anatomical associations with sleep spindle oscillations, these same associations appear to have next-day functional outcome relevance, here in predicting the relative impairment in overnight motor skill memory consolidation in older adults. Age-dependent deterioration in these white matter pathways may therefore be one parsimonious factor explaining the discrepancy in the literature regarding why some studies in older individuals exhibit impaired sleep-dependent motor memory (Spencer et al., 2007; Fogel et al., 2014) whereas others show no evidence of overnight sleep-dependent memory deficits whatsoever (Tucker et al., 2011). Notably, the cohort in the latter study in which overnight improvement in MST performance was observed was composed of older adults that were significantly younger than the older adults in the current study (difference = 5.5 years, p = 0.008). Building on our results and based on the known association between age and white matter fiber tract integrity, it is possible that greater white matter degeneration in our older elderly cohort explains the lack of sleep-dependent motor skill memory consolidation reported.
Although these findings offer a first characterization of an age-related pathological mechanisms accounting for the late-life failure of sleep spindle-related motor memory consolidation, they raise several new questions. For example, it is now critical to know whether the structural integrity of the aging brain determines the efficacy of pharmacological and/or nonpharmacological sleep interventions for improving sleep in older adults and, as a consequence, sleep-dependent cognition. In addition, it remains unknown whether structural features of brain integrity predict the longitudinal decline in sleep physiology with the progression of age and, if so, how this relationship interacts with cognitive decline. Finally, clarifying whether these sleep physiology, brain structure, and memory associations are exaggerated in neurodegenerative diseases already known to display gray and white matter anomalies, sleep disruption, and cognitive impairment and how these relationships are distinct between dissociable conditions such as Alzheimer's disease, Parkinson's disease, and frontotemporal dementia is of critical importance (Petit et al., 2004; Terpening et al., 2013).
Footnotes
This work was supported by The National Institutes of Health (Grant R01-AG031164 to M.P.W., Grant R01-AG034570 to W.J.J., and Grant F32-AG039170 to B.A.M.) and the National Science Foundation (Major Research Instrumentation Program Grant BCS-0821855 to the Brain Imaging Center). We thank Maggie Belshe, Meghna Bhatter, Michelle Binod, Sam Bowditch, Catherine Dang, Amynta Hayenga, April Horn, Emily Hur, Candace Markeley, Elizabeth Mormino, Molly Nicholas, Sina Rashidi, Jacob Vogel, and Lily Zhang for assistance and Ben Inglis for help with the MR sequences.
The authors declare no competing financial interests.
References
- Aboitiz F, Carrasco X, Schröter C, Zaidel D, Zaidel E, Lavados M (2003) The alien hand syndrome: classification of forms reported and discussion of a new condition. Neurol Sci 24:252–257. 10.1007/s10072-003-0149-4 [DOI] [PubMed] [Google Scholar]
- Banks G, Short P, Martinez J, Latchaw R, Ratcliff G, Boller F (1989) The alien hand syndrome: clinical and postmortem findings. Arch Neurol 46:456–459. 10.1001/archneur.1989.00520400116030 [DOI] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, Carrier J (2011) Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res 217:117–121. 10.1016/j.bbr.2010.10.019 [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F (2005) Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150. 10.1038/nn1516 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological 57:289–300. [Google Scholar]
- Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Martin N, Barakat M, Paquet J, Filipini D (2011) Sleep slow wave changes during the middle years of life. Eur J Neurosci 33:758–766. 10.1111/j.1460-9568.2010.07543.x [DOI] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ (2005) Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 36:50–55. 10.1161/01.STR.0000150668.58689.f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M (2003) Sleep spindles: an overview. Sleep Med Rev 7:423–440. 10.1053/smrv.2002.0252 [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B (2000) California verbal learning test, Ed 2 San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Eschenko O, Mölle M, Born J, Sara SJ (2006) Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci 26:12914–12920. 10.1523/JNEUROSCI.3175-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G (2007) Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry 164:483–492. 10.1176/ajp.2007.164.3.483 [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J (2002) Sleep forms memory for finger skills. Proc Natl Acad Sci U S A 99:11987–11991. 10.1073/pnas.182178199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J (2005) Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci 25:11248–11255. 10.1523/JNEUROSCI.1743-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, Jbabdi S, Benali H, Karni A, Maquet P, Carrier J, Doyon J (2014) fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp 35:3625–3645. 10.1002/hbm.22426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12:189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Frederiksen KS, Garde E, Skimminge A, Barkhof F, Scheltens P, van Straaten EC, Fazekas F, Baezner H, Verdelho A, Ferro JM, Erkinjuntti T, Jokinen H, Wahlund LO, O'Brien JT, Basile AM, Pantoni L, Inzitari D, Waldemar G (2011) Corpus callosum tissue loss and development of motor and global cognitive impairment: the LADIS study. Dement Geriatr Cogn Disord 32:279–286. 10.1159/000334949 [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–347. 10.1016/j.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S (2005) DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage 26:195–205. 10.1016/j.neuroimage.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Iguchi Y, Kimura K, Ueno Y, Inoue T, Matusmoto N, Sunada Y (2006) Dysarthria-clumsy hand syndrome originating in the corona radiata. Eur J Neurol 13:e6. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, O'Brien PC, Rettman DW, Shiung MM, Xu Y, Muthupillai R, Manduca A, Avula R, Erickson BJ (2001) FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging 14:668–676. 10.1002/jmri.10011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage 73:239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D (1994) Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265:679–682. 10.1126/science.8036518 [DOI] [PubMed] [Google Scholar]
- Kim JS, Pope A (2005) Somatotopically located motor fibers in corona radiata: evidence from subcortical small infarcts. Neurology 64:1438–1440. 10.1212/01.WNL.0000158656.09335.E7 [DOI] [PubMed] [Google Scholar]
- Koch K, Wagner G, Schachtzabel C, Schultz CC, Güllmar D, Reichenbach JR, Sauer H, Schlösser RG (2013) Age-dependent visuomotor performance and white matter structure: a DTI study. Brain Struct Funct 218:1075–1084. 10.1007/s00429-012-0447-9 [DOI] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A (2003) Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci U S A 100:12492–12497. 10.1073/pnas.2035019100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Stickgold R, Walker MP (2004) Sleep-dependent learning and motor-skill complexity. Learn Mem 11:705–713. 10.1101/lm.76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbély AA (1996) Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res 738:205–212. 10.1016/S0006-8993(96)00770-6 [DOI] [PubMed] [Google Scholar]
- Lindner A, Iyer A, Kagan I, Andersen RA (2010) Human posterior parietal cortex plans where to reach and what to avoid. J Neurosci 30:11715–11725. 10.1523/JNEUROSCI.2849-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SN, Roach AE, Luck SJ, Geng J, Beckett L, Carmichael O, DeCarli C (2014) White matter hyperintensities are associated with visual search behavior independent of generalized slowing in aging. Neuropsychologia 52:93–101. 10.1016/j.neuropsychologia.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F (2016) Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol 26:2127–2136. 10.1016/j.cub.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Santhanam S, Saletin JM, Walker MP (2011) Wake deterioration and sleep restoration of human learning. Curr Biol 21:R183–R184. 10.1016/j.cub.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP (2013) Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci 16:357–364. 10.1038/nn.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP (2014) Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb Cortex 24:3301–3309. 10.1093/cercor/bht188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J (2006) Boosting slow oscillations during sleep potentiates memory. Nature 444:610–613. 10.1038/nature05278 [DOI] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, Carrier J (2013) Topography of age-related changes in sleep spindles. Neurobiol Aging 34:468–476. 10.1016/j.neurobiolaging.2012.05.020 [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD (2014) Motor skill learning requires active central myelination. Science 346:318–322. 10.1126/science.1254960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH. (1989) A Visual Analogue Scale technique to measure global vigor and affect. Psychiatry Res 27:89–99. 10.1016/0165-1781(89)90013-9 [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582. 10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettersheim A, Hallschmid M, Born J, Diekelmann S (2015) The role of sleep in motor sequence consolidation: stabilization rather than enhancement. J Neurosci 35:6696–6702. 10.1523/JNEUROSCI.1236-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP (2007) Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One 2:e341. 10.1371/journal.pone.0000341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM (2013) Age-related changes in consolidation of perceptual and muscle-based learning of motor skills. Front Aging Neurosci 5:83. 10.3389/fnagi.2013.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C (2008) Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res 17:23–33. 10.1111/j.1365-2869.2008.00634.x [DOI] [PubMed] [Google Scholar]
- Petit D, Gagnon JF, Fantini ML, Ferini-Strambi L, Montplaisir J (2004) Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res 56:487–496. 10.1016/j.jpsychores.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Piantoni G, Poil SS, Linkenkaer-Hansen K, Verweij IM, Ramautar JR, Van Someren EJ, Van Der Werf YD (2013) Individual differences in white matter diffusion affect sleep oscillations. J Neurosci 33:227–233. 10.1523/JNEUROSCI.2030-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rechtschaffen A, Kales A (1968) A manual of standardized terminology, techniques an scoring system of sleep stages in human subjects. Los Angeles: UCLA Brain Information Services. [Google Scholar]
- Reitan RM. (1958) Validity of the trail-making test as an indication of organic brain damage. Percept Mot Skills 8:271–276. [Google Scholar]
- Rickard TC, Cai DJ, Rieth CA, Jones J, Ard MC (2008) Sleep does not enhance motor sequence learning. J Exp Psychol Learn Mem Cogn 34:834–842. 10.1037/0278-7393.34.4.834 [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ (2004) Awareness modifies the skill-learning benefits of sleep. Curr Biol 14:208–212. [DOI] [PubMed] [Google Scholar]
- Ryberg C, Rostrup E, Paulson OB, Barkhof F, Scheltens P, van Straaten EC, van der Flier WM, Fazekas F, Schmidt R, Ferro JM, Baezner H, Erkinjuntti T, Jokinen H, Wahlund LO, Poggesi A, Pantoni L, Inzitari D, Waldemar G; LADIS study group (2011) Corpus callosum atrophy as a predictor of age-related cognitive and motor impairment: a 3-year follow-up of the LADIS study cohort. J Neurol Sci 307:100–105. 10.1016/j.jns.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM (2005) Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26:1215–1227. 10.1016/j.neurobiolaging.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM (2008) Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142:266–309. 10.1196/annals.1444.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, MacNeill C (1994) Impaired motor memory for a pursuit rotor task following Stage 2 sleep loss in college students. J Sleep Res 3:206–213. 10.1111/j.1365-2869.1994.tb00133.x [DOI] [PubMed] [Google Scholar]
- Smith SM. (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Spencer RM, Sunm M, Ivry RB (2006) Sleep-dependent consolidation of contextual learning. Curr Biol 16:1001–1005. 10.1016/j.cub.2006.03.094 [DOI] [PubMed] [Google Scholar]
- Spencer RM, Gouw AM, Ivry RB (2007) Age-related decline of sleep-dependent consolidation. Learn Mem 14:480–484. 10.1101/lm.569407 [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschênes M (1987) The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol 57:260–273. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T (2009) Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: a study using sLORETA. Clin Neurophysiol 120:878–886. 10.1016/j.clinph.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yoshida A, Kawahata N, Hashimoto R, Obayashi T (1996) Diagonistic dyspraxia: clinical characteristics, responsible lesion and possible underlying mechanism. Brain 119:859–873. 10.1093/brain/119.3.859 [DOI] [PubMed] [Google Scholar]
- Terpening Z, Naismith S, Melehan K, Gittins C, Bolitho S, Lewis SJ (2013) The contribution of nocturnal sleep to the consolidation of motor skill learning in healthy ageing and Parkinson's disease. J Sleep Res 22:398–405. 10.1111/jsr.12028 [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R (2011) Sleep optimizes motor skill in older adults. J Am Geriatr Soc 59:603–609. 10.1111/j.1532-5415.2011.03324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87. 10.1148/radiol.2301021640 [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R (2002) Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35:205–211. 10.1016/S0896-6273(02)00746-8 [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R (2003a) Dissociable stages of human memory consolidation and reconsolidation. Nature 425:616–620. 10.1038/nature01930 [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R (2003b) Sleep and the time course of motor skill learning. Learn Mem 10:275–284. 10.1101/lm.58503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G (2005) Sleep-dependent motor memory plasticity in the human brain. Neuroscience 133:911–917. 10.1016/j.neuroscience.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1987) Wechsler memory scale–revised. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM (2012) Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging 33:991–1000. 10.1016/j.neurobiolaging.2011.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17:37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165:1217–1239. 10.1164/rccm.2109080 [DOI] [PubMed] [Google Scholar]
- Zec RF. (1986) The Stroop color-word test: a paradigm for procedural learning. Arch Clin Neuropsychol 1:274–275. [Google Scholar]