Abstract
Objective
Treatment with a combination of d-β-hydroxybutyrate (BHB) and melatonin (M) improves survival in hemorrhagic shock models. Our objective was to find the most effective melatonin concentration in combination with 4 molar BHB (4 M BHB). Survival and markers of organ injury were analyzed in pigs exposed to pulmonary contusion, liver crush injury, and hemorrhagic shock and treated with lactated Ringer’s solution; 4 M BHB/43 mM M; 4 M BHB/20 mM M; 4 M BHB/10 mM M; 4 M BHB/4.3 mM M; or 4 M BHB/0.43 mM M. This work is an extension of a previously published research study.
Results
Survival was highest in pigs receiving 4 M BHB/43 mM M (13/14), followed by lactated Ringer’s solution (11/16) and BHB/M with decreased melatonin concentrations (4 M BHB/20 mM M 3/6, 4 M BHB/10 mM M 2/6, 4 M BHB/4.3 mM M 3/6, 4 M BHB/0.43 mM M 1/6, p = 0.011). High mortality was associated with increases in serum lactate, higher liver and muscle injury markers and decreases in PaO2:FiO2 ratios. Our study indicates that treatment with 4 M BHB and melatonin concentrations below 43 mM lack the survival benefit observed from 4 M BHB/43 mM melatonin in pigs experiencing hemorrhagic shock and polytrauma.
Electronic supplementary material
The online version of this article (10.1186/s13104-017-2975-0) contains supplementary material, which is available to authorized users.
Keywords: Blood loss, Resuscitation, Melatonin, Ketone bodies, Antioxidant, Hibernation
Introduction
Hemorrhagic shock, the state induced by severe blood loss, is the second leading cause of injury-related death [1]. Many deaths occur during the first hour after injury, often before bleeding is adequately controlled [2, 3]. In addition to bleeding control, patients receive resuscitation fluids to restore intravascular volume and tissue perfusion, however, the optimal resuscitation fluid and protocol has not been identified [4, 5]. It has been recognized that currently used resuscitation fluids can have adverse effects themselves [6, 7]. Hence, there is significant need for novel treatments for the early phase of hemorrhagic shock. Infusion of a combination of 4 M d-β-hydroxybutyrate/43 mM melatonin (BHB/M) during early hemorrhagic shock significantly decreased mortality in preclinical rat and pig models [8, 9]. The treatment was developed after the observation that levels of d-β-hydroxybutyrate (BHB), a ketone body, and melatonin (M), an antioxidant, increase in hibernators during torpor and arousal, respectively [10–13].
The goal of this study was to establish the melatonin concentration that in combination with 4 M BHB most effectively improves post-shock survival. We tested the effects of decreased melatonin concentrations in BHB/M in our established porcine hemorrhagic shock, trauma and resuscitation model [14]. We focused on melatonin, as preceding experiments showed that in rat hemorrhagic shock, the concentration of melatonin, but not BHB in the treatment could be decreased without loss of efficacy [15]. We hypothesized that solutions containing 4 M BHB and a melatonin concentration of 43 mM would be equally as effective at improving post-hemorrhagic shock survival as solutions with 4 M BHB in combination with 20, 10, 4.3, or 0.43 mM melatonin.
Main text
Methods
Shock, treatment and resuscitation
All procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee (Protocol # 1306-30703A) and in accordance with the National Institutes of Health guidelines for ethical animal research. Fifty-four (28 male, 26 female) Yorkshire-Landrace pigs (15–25 kg, Manthei Hog Farm, LLC, Elk River, Minnesota) were exposed to our established shock and injury protocol (Fig. 1). Induction of anesthesia, instrumentation, shock and injury, treatment infusion, limited resuscitation (R) and full resuscitation (FR), hemodynamic measurements, and analysis of blood gases, organ function markers and drug serum levels have been previously described in detail [14].
Fig. 1.
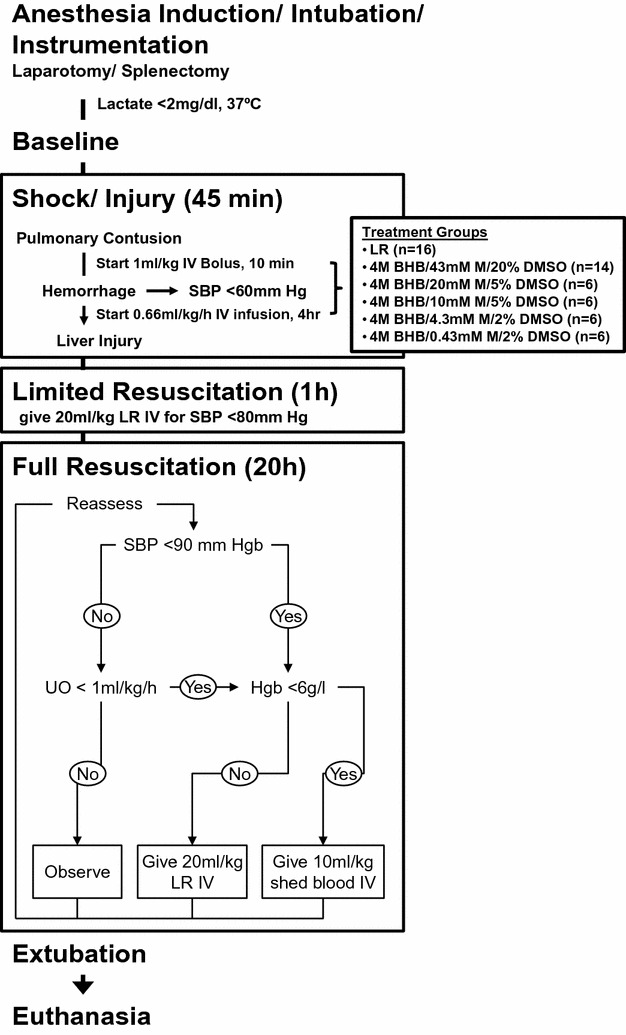
Shock, injury and resuscitation protocol. Pulmonary contusion was followed by blood withdrawal and creation of liver crush injuries with a Holcomb clamp [35]. Fifteen minutes after contusion, treatment solutions were administered as a 1 ml/kg bolus, immediately followed by 0.66 ml/kg/h continuous infusion over 4 h (3.64 ml total). Pigs received limited resuscitation, throughout which they were evaluated every 10 min and, if necessary, received boluses of lactated Ringer’s solution (LR). An hour later, full resuscitation was initiated, throughout pigs received intravenous LR and shed blood. BHB d-β-hydroxybutyrate, DMSO dimethyl sulfoxide, Hgb hemoglobin, IV intravenous, LR lactated Ringer’s solution, M melatonin, SBP systolic blood pressure, UO urine output
(Modified from [14])
Surviving animals were extubated and recovered for 24 h, 48 h or 14 days. Pigs received ceftiofur (5 mg/kg intravenous daily), analgesia was administered during anesthesia [buprenorphine (0.03 mg/kg subcutaneous every 4 h)] and after arousal [ketoprofen (2 mg/kg daily), buprenorphine (0.03 mg/kg twice daily)]. Animal care staff performed postoperative checks on wellness, body temperature, respiration and pulse at least twice daily. Pigs experiencing unrelieved pain or stress during recovery were sacrificed. Euthanasia was performed with beuthanasia solution (0.22 ml/kg intravenous).
8-Isoprostane ELISA
For 8-isoprostane analysis, urine samples were centrifuged (1000 RpM, 5 min), the supernatant was mixed 1:1 with 1 M acetate buffer (pH 4), extracted using C-18 columns and quantified with ELISA (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. 8-Isoprostane levels were normalized to urine output per body weight per hour [16]. Samples collected at FR 7 h were used when material from FR 8 h was not available.
Statistical analysis
The presented work is an extension of earlier experiments, and 24 of the pigs analyzed here were part of a previously published study (12 in the lactated Ringer’s (LR), 12 in the 4 M BHB/43 mM melatonin group) [14]. To increase the power of the original study, we increased both original groups. However, to save BHB, a significant cost factor in our study, we opted to add fewer pigs to the 4 M BHB/43 mM M than the LR group. Survival was analyzed via Kaplan–Meier analysis with generalized Wilcoxon test. AUCt was calculated using PKSolver from Baseline over five sampling time points (AUC0-FR20) using the trapezoidal rule [17]. Non-longitudinal data were analyzed via Kruskal–Wallis test with Dunn–Bonferroni corrections and are reported as medians with interquartile ranges (IQR). Longitudinal parameters were analyzed via Proc Mixed procedure in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and are depicted at key time points as least-squared means with 95% confidence intervals (CI). Group (G), Time (T) and group * time interaction (G * T) were modeled as fixed effects. The models used compound symmetry, autoregressive or no covariance structure and the between-within method for degrees of freedom. For parameters with significant interaction effects, differences at individual time points were analyzed by pairwise comparisons with Tukey adjustments.
Results
Shock induction and resuscitation
There were no significant differences in the amount of blood withdrawn, blood returned or total fluids administered. Pigs treated with 4 M BHB/10 mM melatonin received significantly less LR than those receiving 4 M BHB/0.43 mM melatonin (95% CIs [750, 2206], [2344, 4908] ml/kg, p = 0.029), which is likely due to the high early mortality in this group (Fig. 2).
Fig. 2.
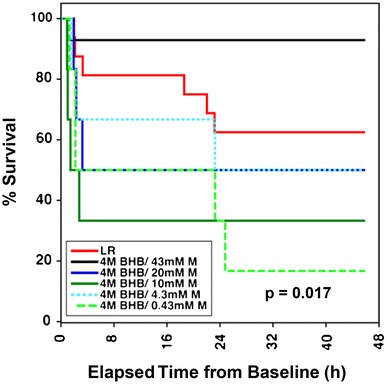
Survival in pigs experiencing hemorrhagic shock and injury. Mean survival in hours [95% CI]: LR 33.1 [24.5, 41.6], 4 M BHB/43 mM M 42.6 [36.5, 48.6], 4 M BHB/20 mM M 24.1 [6.8, 41.4], 4 M BHB/10 mM M 16.3 [0, 33.0], 4 M BHB/4.3 mM M 27.4 [11.7, 43.2], 4 M BHB/0.43 mM M 16.6 [3.5, 29.7]. BHB d-β-hydroxybutyrate, LR lactated Ringer’s solution, M melatonin
Survival
Twenty-four hours after extubation there was a significant difference in overall survival (p = 0.017, Fig. 2), with the highest rate observed in the 4 M BHB/43 mM melatonin group (13/14), followed by LR pigs (10/16) and those receiving lower doses of melatonin (4 M BHB/20 mM M 3/6, 4 M BHB/10 mM M 2/6, 4 M BHB/4.3 mM M 3/6, 4 M BHB/0.43 mM M 1/6). Survival between 4 M BHB/43 mM M and LR-treated pigs did not differ significantly (p = 0.094). There were significant differences between the 43 mM and the 20, 10 and 0.43 mM melatonin groups (p < 0.05), and between the LR and the 4 M BHB/10 mM melatonin group (p = 0.028).
Drug serum levels
BHB/M-treated pigs experienced dose-dependent increases in melatonin and BHB serum concentrations, which peaked at the end of shock and returned to control levels by the end of resuscitation (Additional file 1: Figure S1). Melatonin concentrations in 4 M BHB/43 mM melatonin pigs were significantly higher than in all other groups after shock and during early resuscitation. Differences were significant between pigs infused with 4 M BHB/20 mM melatonin versus those receiving LR, 4 M BHB/4.3 mM melatonin or 4 M BHB/0.43 mM melatonin at the end of shock. At the end of shock, BHB concentrations were higher in all BHB/M groups than in controls. We observed some variability in BHB levels which resulted in significant group differences, however, there was no obvious effect of melatonin dose on BHB serum concentrations. Total drug exposure over time followed the patterns observed for drug serum levels.
Hemodynamic physiologic parameters
Key hemodynamic and physiologic parameters are depicted in (Additional file 2: Table S1). Hemorrhage caused a drop in mean arterial pressure and cardiac output along with increases in heart rate in all groups, which recovered during resuscitation. Urine output did not differ significantly between groups at individual time points. BHB/M treatment increased sodium and decreased potassium levels during early resuscitation, a previously described effect that was independent of treatment melatonin concentration [9]. We observed shock-induced decreases in pH which returned towards baseline levels during resuscitation. There were no obvious BHB/M-treatment or melatonin dose-dependent effects on hemoglobin or serum levels of blood urea nitrogen and lactate dehydrogenase. There were no obvious treatment-dependent effects on body temperature, mean pulmonary artery pressure, pulmonary artery occlusion pressure, bladder pressure, mixed venous oxygen saturation, oxygen consumption, serum levels of alanine aminotransferase, albumin, total protein, bilirubin and alkaline phosphatase (not shown).
Lactate levels peaked during limited resuscitation but returned to baseline levels by the end of the experiment (Fig. 3a). BHB/M-treated pigs receiving low melatonin concentrations experienced dose-dependent decreases in PaO2:FiO2 ratios during early resuscitation (Fig. 3b). Pigs treated with BHB/M experienced increases in serum concentrations of aspartate aminotransferase (AST) and creatine kinase (CK) (Fig. 3b, c). The shock-induced disturbances were most prominent in groups with high early mortality rates, namely in pigs receiving 4 M BHB with 20, 10 and 0.43 mM melatonin.
Fig. 3.
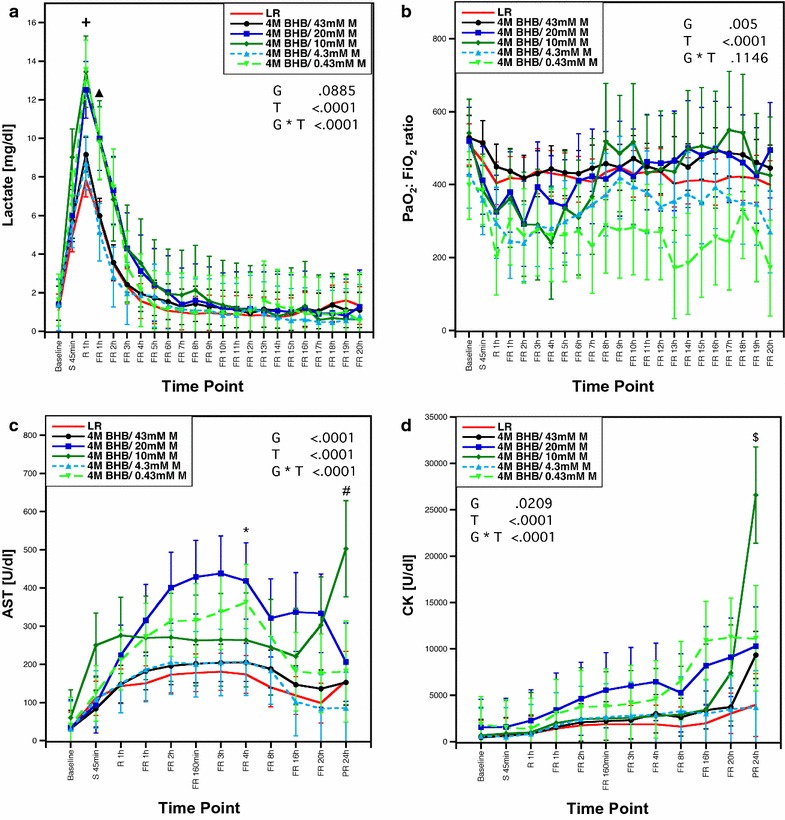
a Lactate levels, b PaO2:FiO2 ratios, c AST levels and d creatine kinase concentrations throughout the experiment. Data presented as least-squares means with 95% confidence intervals. +p < 0.05 for LR vs 4 M BHB/10mM M; ▲p < 0.05 for LR vs 4 M BHB/20 mM M and 4 M BHB/10 mM M and 4 M BHB/0.43 mM M, 4 M BHB/0.43 mM M vs 4 M BHB/43 mM M and 4 M BHB/4.3 mM M. *p < 0.05 for LR vs 4 M BHB/20 mM M; # for 4 M BHB/10 mM M versus 4 M BHB/4.3 mM M and 4 M BHB/43 mM M; $ for 4 M BHB/10 mM M vs LR and 4 M BHB/43 mM M and 4 M BHB/20 mM M and 4 M BHB/4.3 mM M. AST aspartate aminotransferase, BHB d-β-hydroxybutyrate, CK total creatine kinase, FiO 2 inspired fraction of oxygen, FR full resuscitation, G group effect, G * T group * time interaction effect, LR lactated Ringer’s solution, M melatonin, PaO 2 partial arterial pressure of oxygen, R limited resuscitation, S 45 min end of shock period, T time effect
8-Isoprostane urine levels were analyzed as markers of trauma-induced oxidative stress [18]. There was an insignificant trend towards lower 8-isoprostane levels in the groups treated with BHB/M during resuscitation (Additional file 3: Figure S2). This effect was independent of the melatonin concentration in the treatment.
Discussion
4 M BHB/43 mM M significantly improves survival in preclinical hemorrhagic shock models [8, 9]. Here, we describe experiments to optimize the melatonin concentration in the treatment. Earlier experiments showed that in pigs exposed to hemorrhagic shock and injury, doubling the dose of 4 M BHB/43 mM M resulted in increased mortality (unpublished data). In rats, lowering the BHB concentration in combination with 43 mM M resulted in a trend towards decreased survival, while survival times were retained when melatonin levels were lowered [15]. Based on these results, we used our porcine hemorrhage, injury and resuscitation model to evaluate treatment solutions containing 4 M BHB in combination with 0.43–43 mM melatonin. We hypothesized that the melatonin concentration could be decreased without loss of efficacy.
Mortality in pigs receiving BHB/M containing below-standard melatonin concentrations exceeded that in the control group. This was surprising, as previous studies suggest a beneficial effect of BHB in hemorrhagic shock in both rats and pigs, acting synergistically with melatonin. In rats experiencing hemorrhagic shock, 4 M BHB alone significantly improved survival, and lowering the BHB concentration in BHB/M was associated with a trend towards increased mortality [8, 15].
Our data suggests that treatment with BHB/M containing decreased melatonin levels increased shock-induced lung and organ injury. These animals experienced increased lactate levels and elevated serum concentrations of AST and CK, markers of liver and muscle injury. Pulmonary contusion and hemorrhagic shock induce pulmonary inflammation, which can lead to hypoxemia and acute respiratory distress syndrome [19, 20]. We observed melatonin dose-dependent decreases in PaO2:FiO2 ratios during early resuscitation, indicating increased lung injury. This was unexpected, as both melatonin and BHB exhibit anti-inflammatory effects and BHB decreases inflammation and apoptosis in rats and pigs exposed to severe blood loss [21–30]. However, melatonin effects in hemorrhagic shock can be dose-dependent, while ketone bodies may be pro-inflammatory at high doses [31–34]. With systemic melatonin levels insufficient to counteract the effects of shock and injury, the high BHB dose may have exacerbated trauma-induced inflammation. This was not associated with increased oxidative stress, as we observed a trend towards decreased 8-isoprostane levels in BHB/M treated pigs, which was independent of the melatonin concentration in the treatment.
Conclusions
Hemorrhagic shock is the leading cause of preventable death after injury, with many of these deaths occurring in the prehospital phase. 4 M BHB/43 mM M is a low-volume resuscitation fluid that significantly improves survival when administered during early hemorrhage in preclinical models of hemorrhagic shock and injury [8, 9, 15]. Optimization of treatment dose is an important step towards translation from preclinical to clinical use. Here, we tested the efficacy of 4 M BHB in combination with 0.43–43 mM melatonin in porcine hemorrhagic shock, injury and resuscitation. Treatment with below-standard melatonin concentrations resulted in mortality rates exceeding that in the control group. Lowered melatonin treatment concentrations resulted in increased markers of lung, liver and kidney injury, suggesting that decreased melatonin serum levels were insufficient to counteract BHB-induced increases in inflammation. Our research underlines the importance of reaching adequate systemic BHB and melatonin levels, while illustrating the narrow therapeutic window of the treatment in its current formulation.
Limitations
This study has several limitations. As the melatonin dose-ranging experiments were an extension of our previously published study, we expanded the original 4 M BHB/43 mM M and LR groups and added treatments to our original experiment [14]. Consequently, group sizes were uneven and animals were not completely randomized, rendering a risk for model variation over time. However, BHB/M at the standard dose exerted a robust beneficial effect and consistently outperformed LR in our model (2/2 additional BHB/M pigs survived, while only 1/4 of LR pigs survived).
As we did not include dimethyl sulfoxide (DMSO)-treated control groups in our experiments, it is unclear whether changes in DMSO concentrations affected efficacy in the low-dose melatonin groups. Previously, BHB/M- treatment was significantly more effective at increasing post-shock survival than treatment with isosmotic solutions containing equal DMSO concentrations [9]. As we concluded that it was unlikely that changes in DMSO concentration affected survival, we opted for LR as control to represent the standard of care.
Survival differences between 4 M BHB/43 mM M and the control group were not significant in this study (Fig. 2), which is likely due to the limited sample size used. As our experiments clearly showed that decreasing melatonin concentrations was detrimental, we limited our sample size to save animals and resources.
Additional files
Additional file 1. Average drug serum levels (a, b) and drug exposure over time (c, d) during hemorrhagic shock and injury.
Additional file 2. Physiologic parameters and markers of organ function in pigs exposed to hemorrhagic shock, injury and resuscitation.
Additional file 3. Urine 8-isoprostane levels during hemorrhagic shock and injury.
Authors’ contributions
GB and KM designed the study. KM, SM and AW conducted experiments. AW conducted the 8-isoprostane ELISA. AW ad KM analyzed data. AW wrote the manuscript and KM, SM and GB critically appraised and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
GB is an inventor on US patent 8728532 B2: “Ischemia/reperfusion protection compositions and methods of using”, which describes the use of BHB/M for ischemia/reperfusion injury. GB and AW are inventors on provisional Patent Application Number 62/397,211: “Resuscitation composition and methods of making and using”, which describes novel resuscitation compositions for BHB/M. The remaining authors declare that they have no competing interests.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval
All animal procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health guidelines for ethical animal research.
Funding
This work was supported by USDOD ARMY, Grant W81XWH-10-2-0121. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AST
aspartate aminotransferase
- AUC
area under the curve
- BHB
d-β-hydroxybutyrate
- CK
total creatinine kinase
- DMSO
dimethyl sulfoxide
- FiO2
inspired fraction of oxygen
- FR
full resuscitation
- G
group effect
- G * T
group * time interaction effect
- Hgb
hemoglobin
- IQR
interquartile range
- IV
intravenous
- LR
lactated Ringer’s solution
- M
melatonin
- PaO2
partial arterial pressure of oxygen
- PR
post resuscitation
- R
limited resuscitation
- SBP
systolic blood pressure
- SvO2
mixed venous oxygen saturation
- T
time effect
- UO
urine output
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13104-017-2975-0) contains supplementary material, which is available to authorized users.
Contributor Information
Andrea Wolf, Phone: 612-624-5889, Email: awolf@umn.edu.
Kristine E. Mulier, Email: groeh001@umn.edu
Sydne L. Muratore, Email: clark626@umn.edu
Gregory J. Beilman, Email: beilman@umn.edu
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–S9. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Demetriades D, Kimbrell B, Salim A, Velmahos G, Rhee P, Preston C, Gruzinski G, Chan L. Trauma deaths in a mature urban trauma system: is “trimodal” distribution a valid concept? J Am Coll Surg. 2005;201:343–348. doi: 10.1016/j.jamcollsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8:373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33:229–241. doi: 10.1097/SHK.0b013e3181c30f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 7.Pope AM. Fluid resuscitation: state of the science for treating combat casualties and civilian injuries. Washington: National Academies Press; 1999. [PubMed] [Google Scholar]
- 8.Klein AH, Wendroth SM, Drewes LR, Andrews MT. Small-volume d-β-hydroxybutyrate solution infusion increases survivability of lethal hemorrhagic shock in rats. Shock. 2010;34:565–572. doi: 10.1097/SHK.0b013e3181e15063. [DOI] [PubMed] [Google Scholar]
- 9.Mulier KE, Lexcen DR, Luzcek E, Greenberg JJ, Beilman GJ. Treatment with β-hydroxybutyrate and melatonin is associated with improved survival in a porcine model of hemorrhagic shock. Resuscitation. 2012;83:253–258. doi: 10.1016/j.resuscitation.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 11.Larkin JE, Yellon SM, Zucker I. Melatonin production accompanies arousal from daily torpor in Siberian hamsters. Physiol Biochem Zool. 2003;76:577–585. doi: 10.1086/375436. [DOI] [PubMed] [Google Scholar]
- 12.Andrews MT, Russeth KP, Drewes LR, Henry PG. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R383–R393. doi: 10.1152/ajpregu.90795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60:143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- 14.Wolf A, Mulier KE, Iyegha UP, Asghar JI, Beilman GJ. Safety of d-β-hydroxybutyrate and melatonin for the treatment of hemorrhagic shock with polytrauma. Shock. 2015;44(Suppl 1):79–89. doi: 10.1097/SHK.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 15.de Lara Rodriguez CEP, Drewes LR, Andrews MT. Hibernation-based blood loss therapy increases survivability of lethal hemorrhagic shock in rats. J Comp Physiol B. 2017;187(5–6):769–778. doi: 10.1007/s00360-017-1076-7. [DOI] [PubMed] [Google Scholar]
- 16.Lusczek E, Nelson T, Lexcen D, Witowski N, Mulier K, Beilman G. Urine metabolomics in hemorrhagic shock: normalization of urine in the face of changing intravascular fluid volume and perturbations in metabolism. J Bioanal Biomed. 2011;3:038–048. doi: 10.4172/1948-593X.1000041. [DOI] [Google Scholar]
- 17.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in microsoft excel. Comput Methods Programs Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 19.Ganie FA, Lone H, Lone GN, Wani ML, Singh S, Dar AM, Wani NU, Wani SN, Nazeer NU. Lung contusion: a clinico-pathological entity with unpredictable clinical course. Bull Emerg Trauma. 2013;1:7–16. [PMC free article] [PubMed] [Google Scholar]
- 20.Niesler U, Palmer A, Radermacher P, Huber-Lang MS. Role of alveolar macrophages in the inflammatory response after trauma. Shock. 2014;42:3–10. doi: 10.1097/SHK.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 21.Ayuste EC, Chen H, Koustova E, Rhee P, Ahuja N, Chen Z, Valeri CR, Spaniolas K, Mehrani T, Alam HB. Hepatic and pulmonary apoptosis after hemorrhagic shock in swine can be reduced through modifications of conventional Ringer’s solution. J Trauma. 2006;60:52–63. doi: 10.1097/01.ta.0000200156.05397.0b. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Koustova E, Shults C, Sailhamer EA, Alam HB. Differential effect of resuscitation on Toll-like receptors in a model of hemorrhagic shock without a septic challenge. Resuscitation. 2007;74:526–537. doi: 10.1016/j.resuscitation.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Jaskille A, Koustova E, Rhee P, Britten-Webb J, Chen HZ, Valeri CR, Kirkpatrick JR, Alam HB. Hepatic apoptosis after hemorrhagic shock in rats can be reduced through modifications of conventional Ringer’s solution. J Am Coll Surg. 2006;202:25–35. doi: 10.1016/j.jamcollsurg.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Ozdinc S, Oz G, Ozdemir C, Kilic I, Karakaya Z, Bal A, Koken T, Solak O. Melatonin: is it an effective antioxidant for pulmonary contusion? J Surg Res. 2016;204:445–451. doi: 10.1016/j.jss.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Takhtfooladi H, Takhtfooladi M, Moayer F, Mobarakeh S. Melatonin attenuates lung injury in a hind limb ischemia–reperfusion rat model. Rev Port Pneumol. 2006;2015(21):30–35. doi: 10.1016/j.rppnen.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Yang F-L, Subeq Y-M, Lee C-J, Lee R-P, Peng T-C, Hsu B-G. Melatonin ameliorates hemorrhagic shock-induced organ damage in rats. J Surg Res. 2011;167:E315–E321. doi: 10.1016/j.jss.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Zhao D, An H, Zhang H, Jiang C, Yang B. Melatonin prevents lung injury induced by hepatic ischemia–reperfusion through anti-inflammatory and anti-apoptosis effects. Int Immunopharmacol. 2015;29:462–467. doi: 10.1016/j.intimp.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Fu SP, Li SN, Wang JF, Li Y, Xie SS, Xue WJ, Liu HM, Huang BX, Lv QK, Lei LC, et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-κB activation. Mediat Inflamm. 2014;2014:983401. doi: 10.1155/2014/983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gambhir D, Ananth S, Veeranan-Karmegam R, Elangovan S, Hester S, Jennings E, Offermanns S, Nussbaum JJ, Smith SB, Thangaraju M, et al. GPR109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Investig Ophthalmol Vis Sci. 2012;53:2208–2217. doi: 10.1167/iovs.11-8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain SK, Kannan K, Lim G, McVie R, Bocchini JA., Jr Hyperketonemia increases tumor necrosis factor-alpha secretion in cultured U937 monocytes and type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes. 2002;51:2287–2293. doi: 10.2337/diabetes.51.7.2287. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan DJ, Shelby J, Shao YL, Affleck DG, Hinson DM, Barton RG. Melatonin and a 21-aminosteroid attenuate shock after hemorrhage but differentially affect serum cytokines. J Surg Res. 1996;64:13–18. doi: 10.1006/jsre.1996.0299. [DOI] [PubMed] [Google Scholar]
- 33.Kurepa D, Pramanik AK, Kakkilaya V, Caldito G, Groome LJ, Bocchini JA, Jain SK. Elevated acetoacetate and monocyte chemotactic protein-1 levels in cord blood of infants of diabetic mothers. Neonatology. 2012;102:163–168. doi: 10.1159/000339286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:E298–E306. doi: 10.1152/ajpendo.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holcomb JB, Pusateri AE, Harris RA, Charles NC, Gomez RR, Cole JP, Beall LD, Bayer V, MacPhee MJ, Hess JR. Effect of dry fibrin sealant dressings versus gauze packing on blood loss in grade V liver injuries in resuscitated swine. J Trauma. 1999;46:49–57. doi: 10.1097/00005373-199901000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Average drug serum levels (a, b) and drug exposure over time (c, d) during hemorrhagic shock and injury.
Additional file 2. Physiologic parameters and markers of organ function in pigs exposed to hemorrhagic shock, injury and resuscitation.
Additional file 3. Urine 8-isoprostane levels during hemorrhagic shock and injury.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


