Abstract
Subungual melanoma (SUM) is an uncommon form of acral melanoma that arises within the nail matrix. The incidence for acral melanomas is similar worldwide, however, the proportion is higher in dark-skinned individuals. The subungual form represents approximately 2% of cutaneous non-sun-induced melanomas in the western world and up to 75% in Africans, 10% in Japanese, and 25% in the Chinese. No specific figures are available from the Indian subcontinent; however, the authors could trace three anecdotal case reports published over the last two decades. A general reluctance to biopsy a nail lesion to confirm the diagnosis may be contributing to the missed diagnosis. We report four cases of SUM of the big toenails seen over a period of 2.5 years. They were three women and one man with an age ranging from the 4th to 7th decade and disease duration of 6–18 months. The lesion involved the big toe in all and two patients had liver and lymph node metastasis at the time of presentation. Awareness among dermatologists regarding clinical manifestations, high index of suspicion for acral pigmented lesions, and nail bed biopsy may help in the early diagnosis and management and can prevent mortality.
Keywords: Hutchinson's sign, malignant, metastasis, subungual melanoma
Introduction
Subungual melanoma (SUM) are uncommon malignancy of the nail which represent 0.7–3.5% of cutaneous melanomas diagnosed in the general population.[1] SUM is a distinct variant of acral melanomas, with histology characteristic of acral lentiginous-type melanomas. SUM have varied clinical presentation and survival rates remains poor compared to melanoma of other parts of the body.[2] Low survival rates are usually attributable to late detection. Diagnosis requires a high index of clinical suspicion in pigmented nail lesion by documentation of the characteristic onychoscopic findings of longitudinal pigmented band (LPB) of the nail plate in early lesions and confirmatory histology on biopsy. Treatment of SUM remains surgical, with wide local excision and amputation as primary modalities. We report a small series of SUM in four cases of Indian origin seen over a period of 2.5 years.
Case Reports
Case 1
A 45-year-old woman presented with progressive blackish discoloration of the right big toe nail of 6 months duration. The lesion was asymptomatic to begin with but developed signs of secondary ulceration with mucopurulent discharge and mild pain after 4 months that resolved with oral antibiotics. The nail became dystrophic, and she was referred for dermatology consultation. Patient denied any history of preceding trauma. Examination revealed a black colored, dystrophic big toe nail with complete destruction of nail plate and minimal mucoid discharge. Hutchinson's sign was positive [Figure 1a]. There was no regional lymphadenopathy. Systemic examination was normal. A differential diagnosis of benign nail matrix nevus was considered. To confirm the diagnosis and to exclude the possibility of SUM, a biopsy from the nail bed was performed that revealed sheets of cells varying in shape from polygonal to spindle. The spindle cells had scanty cytoplasm, pleomorphic oval-to-spindle hyperchromatic nuclei. Some of these nuclei were angulated. Some cells were polygonal with large coarse nuclei and prominent macronucleoli. Many cells contained intracytoplasmic golden brown pigment. Areas of necrosis and mitotic figures were seen [Figure 2a–c]. Hematological investigations, X-ray of the right foot and chest, and ultrasonography of abdomen and pelvis were normal. Positron emission tomography (PET) scan did not reveal any evidence of distant metastasis. The TNM staging for the tumor was T3N0M0. She underwent excision of the entire lesion with digit amputation at the metatarsophalangeal joint [Figure 1b]. The Breslow tumor thickness was 3 mm and the resected margins were found to be free of tumor tissue [Figure 2d]. There was no evidence of recurrence at 2.5-year follow-up.
Figure 1.
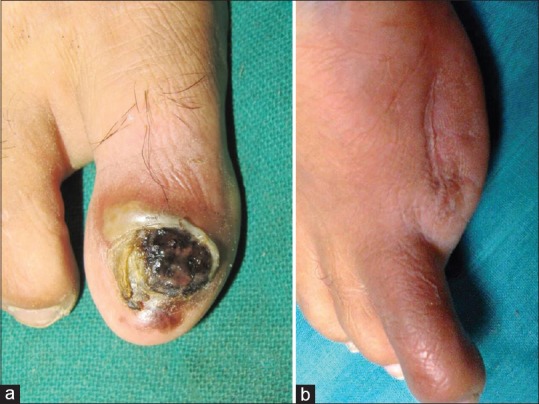
(a) Pigmented, dystrophic right big toenail with positive Hutchinson's sign. (b) Patient after the digit amputation at the metatarsophalangeal joint
Figure 2.
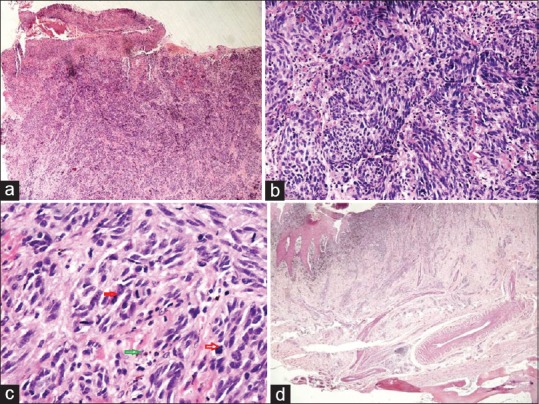
(a) An ulcerated tumor diffusely infiltrating the dermis (H and E, ×40). (b) Extensive proliferation of sheets of spindle-shaped atypical melanocytes. The cells have scanty cytoplasm and pleomorphic oval-to-spindle hyperchromatic nuclei and prominent nucleoli (H and E, ×200). (c) Spindle-shaped tumor cells with occasional cell showing intracytoplasmic golden brown pigment and atypical mitoses (H and E, ×400). (d) Resected margins are seen free of tumor tissue (H and E, ×40)
Case 2
A 70-year-old, ill looking man presented with ulcerated lesion on the left big toe present since 3 months. The lesion began as a painless linear brownish discoloration of the proximal edge of the nail plate approximately 14 months back that covered the entire length of the nail in approximately 3 months. This was followed by ulceration at the distal end of the toe. Patient also complained of recent onset low grade fever, malaise, loss of weight and appetite, and marked easy fatigability. Cutaneous examination revealed an exuberant ulcerative lesion covered with seropurulent discharge present at the distal end of the toe destroying distal half of the nail plate and a broad (>5 mm) irregular melanonychia on the proximal nail plate with positive Hutchinson's sign [Figure 3a]. Differential diagnosis of SUM and squamous cell carcinoma was considered, and biopsy confirmed the diagnosis of SUM. The TNM staging and histological details are presented in Table 1. Ultrasonography of the abdomen revealed liver metastasis. Patient was referred to oncologist but succumbed to the disease after 7 months of irregular follow up.
Figure 3.
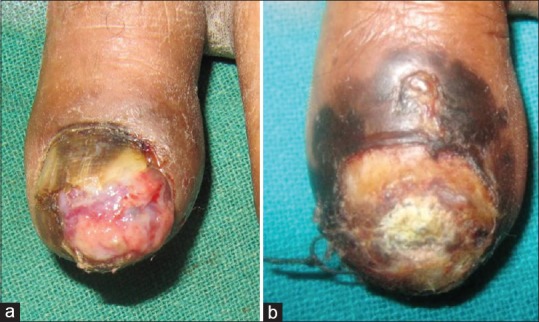
(a) Exuberant ulcerative lesion of the left big-toe with a broad (>5 mm) irregular melanonychia on the proximal nail plate and positive Hutchinson's sign in patient 2. (b) Completely dystrophic nail plate and irregular pigmentary changes around the left big toe (patient 3)
Table 1.
The TNM staging and histological details of the patients
Case 3
A 45-year-old otherwise healthy looking woman presented with completely dystrophic nail plate and irregular pigmentary changes all around the left big toe [Figure 3b]. The lesion began approximately a year and half ago as diffuse pigmentation of the nail plate starting proximally, and gradually involving the entire nail plate followed by the loss of nail plate. Patient did not have any systemic symptoms and denied any preceding trauma. The nail biopsy from the nail bed and proximal nail fold confirmed the clinical diagnosis of SUM [Table 1]. Investigations did not reveal any evidence of regional lymph node or distant metastasis. She was advised toe amputation for her localized disease. Despite adequate counselling about the disease progression and expected fatality, patient remained in denial and was lost to follow-up.
Case 4
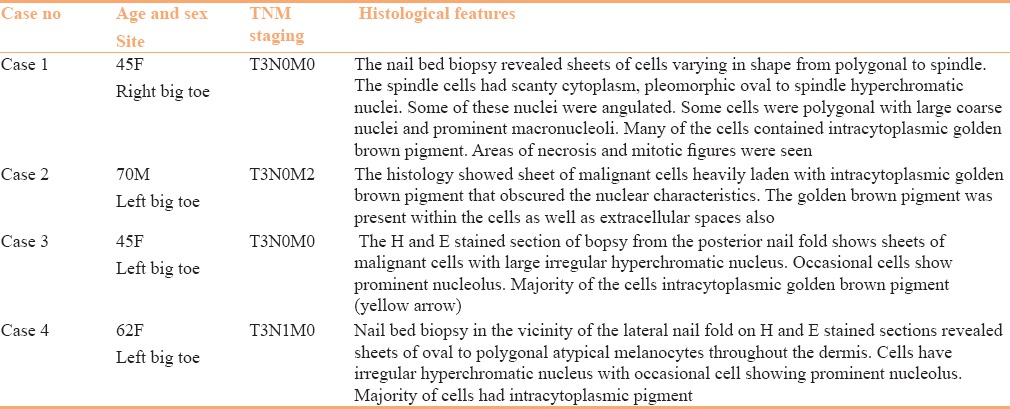
A 62-year-old cachexic women presented to dermatology outpatient with a painful, ruptured swelling in the left inguinal region discharging blood [Figure 4a]. The swelling was present as a firm mass for few months. Subsequently, it became fluctuant in the last 4 weeks and ruptured 1 week back that prompted her to seek medical consultation. Examination revealed the swelling to be inguinal lymph nodes. On examination of the left leg and toenails, an ulcerated nail bed with retention of 2 mm of proximal nail plate of left big toe and irregular blackish discoloration all around the nail apparatus [Figure 4b] was observed. The nail lesions had been present for the past 1.5 years. A nail bed biopsy performed adjacent to the lateral nail-fold was consistent with SUM [Table 1]. Fine needle aspiration cytology of the inguinal lymph nodes revealed melanoma metastases. X-ray chest and USG abdomen were within normal limit. Patient was referred to the oncology department for chemotherapy.
Figure 4.
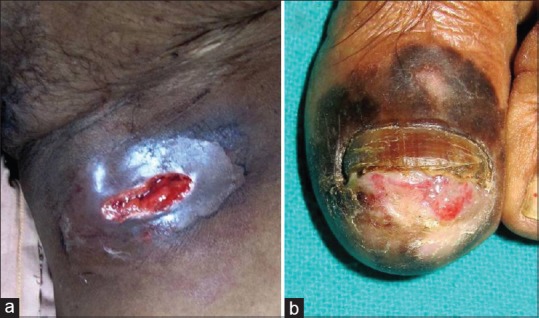
(a) Ruptured left inguinal node metastasis from SUM lesion. (b) Classic presentation of SUM with ulcerated nail bed and diffuse melanonychia
Discussion
SUM is a rare skin cancer. Diagnosing SUM is challenging owing to the diversity of its clinical presentations, including band-like black nail discolorations, subungual (amelanotic) masses, and splits of the nail plate. Differential diagnoses include benign melanocytic nevi, subungual hematoma, pyogenic granuloma, discoloration caused by drugs, and onychomycosis.[3] SUM may also masquerade a common nail entity such as an ingrown toenail.[4] ABCDEF rule improves early detection and survival of SUM.[1] In addition to careful clinical examination, dermoscopy (onychoscopy) is becoming an important noninvasive and reliable tool to differentiate between early benign and malignant pigmented nail lesions. Signs on dermoscopy of SUM presenting with LPB include irregular bands, disruption in parallelism, micro-Hutchinson sign, and pigment on the ridges of the hyponychium.[5] Onychoscopy of the diffusely pigmented nail plate yields only little information. However, as with cutaneous melanoma, the absolute diagnosis of SUM is made on histopathology.
In one study on SUM, finger nails were affected in 62% and toe nails in 38%. The thumb and great toe nails were affected in 73%.[6] In a clinicopathologic analysis of 177 acral melanomas in Koreans, 53 (29.9%) were reported in the subungual location.[7] There was no gender predilection with equal involvement of male and females. Sites affected by the long-term physical stress and trauma such as the thumb and great toe were most commonly affected. As expected, fingernail involvement was present in 32 (18.1%) and toenails in 21 (11.9%). All three previously reported cases of SUM from India had fingernail involvement.[8,9,10] All were males and the disease duration ranged from 10 to 38 months. On the contrary, big toe was involved in all four patients of the present series. Three patients out of the four were women, and the disease duration was relatively shorter (6–18 months), with liver and lymph node metastasis in one patient each. Hutchinson's sign was consistently positive in all cases. Thus, it may be considered as a useful guide to suspect malignant nature of the lesion.
Diagnosis of SUM is often delayed and carries a poor prognosis. The estimated 5-year survival is between 16% and 87%.[6] The prognosis depends upon the thickness of tumor termed the Breslow thickness, stage of the tumor, and degree of tumor invasion. Breslow thickness is considered a good prognostic indicator for SUM, even though it is less accurate than that for cutaneous melanoma. Up to 25% of the patients can present with lymph node or distant metastases. One each of our patient had liver and lymph node metastasis [Table 1].
Amputation through the proximal phalanx or the metatarsophalangeal joint is required in the hallux and toes. Fingers require resection through the distal interphalangeal joint. Recently, function-preserving resections in the thumb with nail removal, partial distal phalanx resection, and volar flap reconstruction has been advocated to maximize joint and sensory function, quality of life, and improve cosmesis.[11]
In contrast to cutaneous melanoma, occurrence of subungual SUM has been reported to be higher from Asian countries. However, with few anecdotal case reports from India, under reporting of SUM from Indian subcontinent remains a plausible explanation.
Conclusion
SUM has been considered a rare entity in Indian scenario. Hence, a high index of suspicion in acral pigmented lesions should be exercised by the dermatologists. With increasing patient awareness to seek medical attention for even subtle pigmented lesions and ease of performing onychoscopy and nail unit biopsy, an early diagnosis of SUM might be expected.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Levit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol. 2000;42:269–74. doi: 10.1016/S0190-9622(00)90137-3. [DOI] [PubMed] [Google Scholar]
- 2.Hudson DA, Krige JE, Strover RM, King HS. Subungual melanoma of the hand. J Hand Surg Br. 1990;15:288–90. doi: 10.1016/0266-7681_90_90005-o. [DOI] [PubMed] [Google Scholar]
- 3.Chokoeva AA, Tchernev G, Patterson JW, Lotti T, Wollina U. Life-threatening onychomycosis imitator. J Biol Regul Homeost Agents. 2015;29(1 Suppl):31–2. [PubMed] [Google Scholar]
- 4.Adnan A, Bajuri MY, Shukur MH, Subanesh S, Das S. Malignant melanoma masqueraded as ingrown toe nail. Clin Ter. 2014;165:41–5. doi: 10.7471/CT.2014.1660. [DOI] [PubMed] [Google Scholar]
- 5.Patel GA, Ragi G, Krysicki J, Schwartz RA. Subungual melanoma: A deceptive disorder. Acta Dermatovenerol Croat. 2008;16:236–42. [PubMed] [Google Scholar]
- 6.Kato T, Suetake T, Sugiyama Y, Tabata N, Tagami H. Epidemiology and prognosis of subungual melanoma in 34 Japanese patients. Br J Dermatol. 1996;134:383–387. [PubMed] [Google Scholar]
- 7.Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177acral melanomas in Koreans: Relevance of spreading pattern and physical stress. JAMA Dermatol. 2013;149:1281–8. doi: 10.1001/jamadermatol.2013.5853. [DOI] [PubMed] [Google Scholar]
- 8.Krishna K, Sharma P. Subungual malignant melanoma. Indian J Dermatol Venereol Leprol. 2002;68:354. [PubMed] [Google Scholar]
- 9.Rathi S, Dogra D, Khanna N. Subungual malignant melanoma clinically resembling granuloma pyogenicum. Indian J Dermatol Venereol Leprol. 1995;61:373–4. [PubMed] [Google Scholar]
- 10.Verma R, Kakkar S, Vasudevan B, Rana V, Mitra D, Neema S. A Rare Case of Subungual Melanoma. Indian J Dermatol Venereol Leprol. 2015;60:188–90. doi: 10.4103/0019-5154.152526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen JT, Bakri K, Nguyen EC, Johnson CH, Moran SL. Surgical management of subungual melanoma: Mayo clinic experience of 124 cases. Ann Plast Surg. 2013;7:346–54. doi: 10.1097/SAP.0b013e3182a0df64. [DOI] [PubMed] [Google Scholar]


